Abstract
A four-year longitudinal study of the structure of sylvatic transmission cycles of Trypanosoma cruzi, reservoir host competence and parasite discrete typing units was conducted in a disturbed rural area of the humid Chaco in Argentina. Among 190 mammals examined by xenodiagnosis and polymerase chain reaction amplification, the composite prevalence of infection was substantially higher in Dasypus novemcinctus armadillos (57.7%) and Didelphis albiventris opossums (38.1%) than in Euphractus sexcinctus (20.0%), Tolypeutes matacus (12.5%), and Chaetophractus vellerosus (6.3%) armadillos. Trypanosoma cruzi was detected for the first time in Thylamys pusilla small opossums and in two unidentified small rodents. Infection was spatially aggregated only in armadillos. All Didelphis were infected with T. cruzi I and all armadillo species were infected with T. cruzi III, implying two distinct sylvatic cycles with no inputs from the domestic cycle. Dasypus armadillos and Didelphis opossums were much more infectious to vectors than other armadillos, small opossums, or rodents.
Introduction
Trypanosoma cruzi is the etiologic agent of Chagas disease, a vector-borne zoonosis widely distributed in the Americas that infects approximately 10 million persons and causes approximately 15,000 deaths annually.1 Trypanosoma cruzi circulates in at least two broadly defined transmission cycles occurring in domestic and sylvatic habitats. The domestic cycle involves humans, domestic or synanthropic mammals, and domiciliated triatomines, whereas the sylvatic cycle includes multiple sylvatic triatomine species and up to 180 species of wild mammals infected with T. cruzi.2 Some of these species act as primary reservoir hosts in different ecologic environments, whereas others play secondary or dead-end roles.
Trypanosoma cruzi has been classified into six discrete typing units (DTUs) ranging from T. cruzi I (TcI) to T. cruzi VI (TcVI).3 TcI occurs in sylvatic cycles throughout the Americas in Didelphis sp. opossums and in many domestic cycles.4,5 TcIII has been detected in Dasypus novemcinctus armadillos throughout the Americas,6 whereas TcIV has been isolated mostly from sylvatic mammals in the northern Amazon basin and in the United States.4,7–9 TcII, TcV, and TcVI occur in domestic cycles in Brazil and in the southern cone countries of South America.7,8 Molecular methods uncovered the impressive genetic diversity of T. cruzi and heterogeneity in transmission cycles at the micro-scale spatial level5 and opened the way for a more detailed understanding of complex parasite transmission cycles.
Located in the southern cone countries of South America, the Gran Chaco is the most biodiverse ecoregion other than the Amazon10 and is also hyperendemic for Chagas disease.11 During recent decades, continuing logging combined with rapid expansion of intensified agriculture have caused accelerated deforestation, habitat degradation, and fragmentation of natural forests leading to significant biodiversity losses in the Gran Chaco.12 In this region, the most frequently identified sylvatic hosts of T. cruzi are didelphid marsupials and various species of armadillos, followed by carnivores (including skunks, ferrets, foxes, and coatis) and a few species of small rodents.7,9,13–20 In general, host and parasite distributions may be modified by the combined effects of alterations in climate, landscape and biodiversity, which may create new habitats suitable for the development of parasites and vectors and increase the risk of transmission of pathogens.21 The sylvatic distribution of triatomine bugs and transmission of T. cruzi appear to be closely associated with vegetation cover and landscape use.22 Ongoing large-scale changes in land use and habitat fragmentation throughout Latin America may impact sylvatic transmission cycles in unforeseen ways, either reducing or increasing parasite prevalence.19,23
Little is known about the structure and spatiotemporal dynamics of sylvatic transmission cycles of T. cruzi in the Gran Chaco. Few areas have been investigated in recent decades, usually through cross-sectional or convenience surveys; sampling efforts have focused on a limited number of species and environments, and identification of parasite DTUs from wild hosts has been sparse.7,9,16–20,24 Identification of the triatomine species acting as sylvatic vectors is lagging.7,8,20,25 Assessing the impacts of ongoing environmental changes requires more information on the structure of transmission cycles over time. A small-scale, baseline survey conducted in the interphase between the dry and humid Chaco identified white-eared opossums (Didelphis albiventris) and nine-banded armadillos (Da. novemcinctus) as the only sylvatic hosts of T. cruzi, and they were infected with TcI and TcIII, respectively.20 As part of a multi-site research project on the eco-epidemiology and control of Chagas disease in the Gran Chaco, we conducted a longitudinal study to investigate further the structure and spatiotemporal dynamics of sylvatic transmission cycles of T. cruzi in disturbed habitats, by expanding the range of species examined through targeting bats, tree-dwelling rodents, and small marsupials; assessing reservoir host competence, and identifying parasite DTUs in a wider range of hosts.
Materials and Methods
Study area.
Field work was conducted in a well-defined rural area (450 km2) in the municipality of Pampa del Indio (25°55′S, 56°58′W), Chaco Province, Argentina, which has been described elsewhere.26 For present purposes, the study area included six neighboring villages (Santa Rita, Santos Lugares, El Salvaje, La Loma, Campo Los Toros, and Los Ciervos). The area is in the dry sub-humid region.20 Small patches of native dry forest occur near houses; they usually are subjected to intense human pressure through intentional fires, hunting, logging, and deforestation for agriculture and goat, sheep, or cattle ranching. Before a residual insecticide spraying of all houses in the area in November 2007, the prevalence of house infestation with Triatoma infestans (46%) and infection with T. cruzi in domestic bugs (22%), dogs (26%), and cats (29%) indicated the occurrence of active domestic transmission (Cardinal MV and others, unpublished data).26
Capture and handling of wild mammals.
A team of at least four persons (including a veterinarian, a biologist and two or more assistants) caught wild mammals in forest patches during four three-week surveys conducted in August 2008, August 2009, August 2010, and March 2011. Captures made at the baseline study in August 2008 (45 specimens) were reported elsewhere.20 Six local collaborators with hunting skills and extensive knowledge of the area assisted us in the captures. The study area was divided into 10 square areas (quadrats). Two quadrats were selected at random, and 2–6 line transects (1–4 km in length) sampled in each survey. Captures were carried out in two environments (forest and grassland) by using traps, manual catches, and, in a few cases, through chemical immobilization with a blowgun and anesthetic darts. Trap locations were georeferenced (Garmin Legend C; Garmin, Olathe, KS), and traps were checked every morning and re-baited when appropriate. A Landsat satellite image of the study area was used to digitize the location of capture sites.
National traps and similar home-made traps were used to capture medium-sized mammals (white-eared opossums and armadillos). Traps were deployed every 50 meters along transects lines and were baited with beef or chicken scraps. Terrestrial micro-mammals were sampled by using Sherman (H. B. Sherman Traps, Tallahassee, FL) and pitfall traps. Sherman traps were located at ground level and on tree branches to capture small rodents and fat-tailed opossums (micro-mammals). Sherman traps were baited with seeds (oats, maize, seeds, and local fruits), fresh (apples and banana) and dry (walnuts, grape raisins) fruits, and pellets of a mixture of peanut butter, oatmeal and vanilla essence. Pitfall traps, comprising a 20-liter plastic bucket with drain holes in the bottom and the open top flush with the ground, were set up along transect lines in trap stations every 10 meters. Each trap station consisted of a drift fence (constructed with wooden stakes positioned every 2 meters and a 50 cm-wide, fine-gauge, plastic mesh) and 5 pitfall traps.
Bats were captured by using mist nets (6.0 meters wide, 2.6 meters high, 38.0 mm black mesh; AFO Banding Supplies, Manomet, MA). Nets were opened at dusk and monitored every 30–40 minutes for 6 hours. Mist nets were also placed where local villagers indicated the occurrence of bats (near water wells, hollow trees). Four mist nets were placed in a zigzag formation at each capture site. Tranquilizing darts (Dan Inject®, 3 mL; DanWild LLC, Austin, TX) shot with a home-made blowgun were used for distance catches of giant anteaters and crab-eating raccoons. Biosafety and animal processing procedures were performed according to protocols approved by the Argentinean Dr. Carlos Barclay Ethical Committee. Capture and transit permits were also obtained from the provincial government.
Captured animals were transported to the field laboratory where each individual was subjected to a preanesthetic examination. In most cases, animals were given parenteral anesthesia for induction and inhalatory anesthesia for maintenance. Parenteral anesthesia was performed with tiletamine clorhydrate and zolacepan clorhydrate (Zelazol®; Fort Dodge, Buenos Aires, Argentina) or ketamine clorhydrate (Vetaset®; Fort Dodge) combined with xylacine (Rompun®; Bayer, Leverkusen, Germany) at the minimum dose appropriate to species and weight.27 Inhalatory anesthesia with Isoflurane® was delivered with a vaporizer (IsoTec®; Datex-Ohmeda GE Healthcare, Little Chalfont, United Kingdom) and medicinal O2 (0.25–3 liters/minute). Anesthetized animals were maintained on thermic cushioned surfaces in a quiet and comfortable environment, and their eyes were protected with ophthalmic lubricant solutions and covered with home-made eyecups. The general condition of each animal was determined. All animals were sexed and weighed with Pesola®, marked with numeric metal tags (National Band and Tag Company, Newport, KY), and then released at the capture site once fully recovered from anesthesia. Didelphis opossums were assigned to stage classes based on tooth eruption.17
Animals were bled by venipuncture and each blood sample was separated in three aliquots. An aliquot was diluted 1:1 in guanidine hydrochloride-EDTA buffer (GEB) for polymerase chain reaction (PCR) amplification; another one was centrifuged at 3,000 rpm for 15 minutes for serum collection, and the remainder was used to determine total solids and packed cell volume (PCV). Animals were also examined by xenodiagnosis using 5–20 uninfected fourth-instar nymphs of Triatoma infestans (depending on body size) contained in wooden boxes applied on the belly of the host for 25 minutes.19,28 Five specimens could not be examined for T. cruzi infection (1 Nasua nasua, 1 Di. albiventris, 1 Leopardus geoffroyi, 1 Th. Pusilla, and 1 unidentified small rodent) because the animals were found dead or blood samples were insufficient.
Xenodiagnosis, parasite isolation and culture.
Pools of rectal contents from five bugs used in the xenodiagnosis of a given specimen were diluted with physiological saline solution and examined microscopically (Zeiss, Oberkochen, Germany) at 400× magnification 30 and 60 days post-exposure.28 If a pool was positive, bugs were re-examined individually to assess the individual host's infectiousness to the vector (defined as the proportion of infected bugs fed on a given individual relative to the total number of insects examined for infection at least once, excluding bugs that died prior to the first examination). Infectiousness was calculated for all animals positive by xenodiagnosis or kinetoplast DNA (kDNA) from GEB samples.
The rectal contents from two microscope-positive bugs from each xenodiagnosis-positive animal were inoculated separately into two tubes each that contained biphasic medium and incubated at 28°C and a relative humidity of 50%.24 Parasite growth was monitored weekly during four months until reaching 3 × 105 parasites/mL; a total of 188 culture tubes was followed. Isolates used in this study are cryopreserved in liquid nitrogen in the trypanosomatid culture collection of the Laboratory of Eco-Epidemiology in Buenos Aires.
Molecular diagnosis and genotyping.
Parasite DNA was extracted from blood samples mixed with GEB and boiled for 15 minutes by using the DNeasy Blood & Tissue Kit (QIAGEN, Valencia, CA) according to manufacturer's instructions. In addition, in samples positive by kDNA-PCR and negative by nuclear satellite DNA-PCR (SAT DNA-PCR), DNA was extracted from the rectal contents of xenodiagnostic bugs. The individual gut contents were obtained by cutting the abdomen below the third tergite and extracting the bugs' rectal ampoule, which was stored in a labeled vial. The hindgut contents were diluted in 50 mL of deionized sterile water, boiled for 15 minutes, and the DNA was extracted with DNAzol (Invitrogen, Carlsbad, CA).24
Infections with T. cruzi were identified by PCR amplification of the 330-basepair fragment from the kDNA minicircles of T. cruzi (kDNA-PCR) using primers and cycling conditions described.29 Aliquots of 12 μL of PCR products were visualized under ultraviolet light after electrophoresis in 3% agarose gels containing GelRed® (Biotium, Inc., Hayward, CA). For further confirmation, samples from individual hosts that were xenodiagnosis-negative and kDNA-PCR-positive (in GEB samples) were subsequently tested by SAT DNA-PCR or kDNA-PCR from xenodiagnosis-negative rectal contents of triatomine bugs, except for one Thylamys sp. in which infection was confirmed by means of a second round of DNA extraction and kDNA-PCR of GEB samples.
Parasite DTUs were identified in culture-derived DNA samples of each infected animal by PCR-based strategies specific for the intergenic region of spliced-leader genes (SL-IRac) with primers UTCC/TCac, SL-IRII with primers UTCC/TC1, SL-IRI with primers UTCC/TC2 with the incorporation of Taq Polimerase (Invitrogen).29
Statistical analysis.
Random-intercept logistic regression analysis implemented in Stata version 10.1 (StataCorp LP, College Station, TX) was used to test whether there was a significant (P < 0.05) effect of host species and other individual attributes (age, sex, body mass) on bug infection in xenodiagnostic tests of white-eared opossums and nine-banded armadillos positive by xenodiagnosis or kDNA-PCR because these were the only infected hosts with > 5 positive individuals. Analyses were clustered on individual hosts to account for overdispersion caused by between-host variability.
Spatial analysis.
Spatial analysis was performed separately for marsupials and armadillos. Marsupials included Di. albiventris, Th. pusilla, and Monodelphis sp., and armadillos included Da. novemcinctus, To. matacus, Euphractus sexcinctus, Ch. vellerosus, and Ch. villosus. Most of the captured animals were distributed in two main zones, one in the northern section of the study area and one in the southern section (Figures 1 and 2). Isolated capture points located more than 5 km from the main zones were excluded from the analysis because the sampling effort in these areas was substantially smaller; we used 55 location points for marsupials and 56 for armadillos. The spatial pattern of infected (i.e., xenodiagnosis positive or kDNA-PCR positive) and non-infected marsupials and armadillos was assessed by using the random labeling method with the pair-correlation function (ring width = 300 meters) implemented in Programita.30 A heterogeneous Poisson null model was used with intensity varying proportionally to the observed prevalence of T. cruzi infection for each species because prevalence differed substantially between species, especially in armadillos. Confidence envelopes were obtained through 1,000 Montecarlo simulations, using the upper and lower 25th simulations for a 95% confidence envelope.
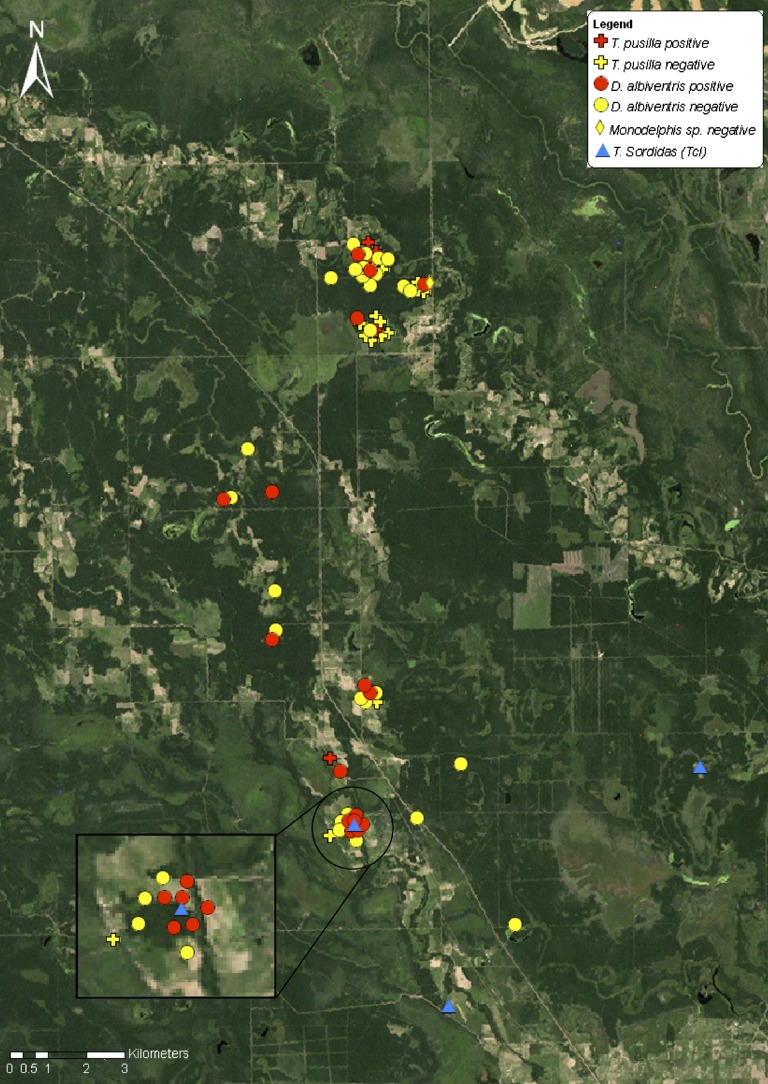
Figure 1.
Landsat image showing the location of Trypanosoma cruzi–infected and non-infected marsupials and infected Triatoma sordida in Pampa del Indio, Argentina, 2008–2011.
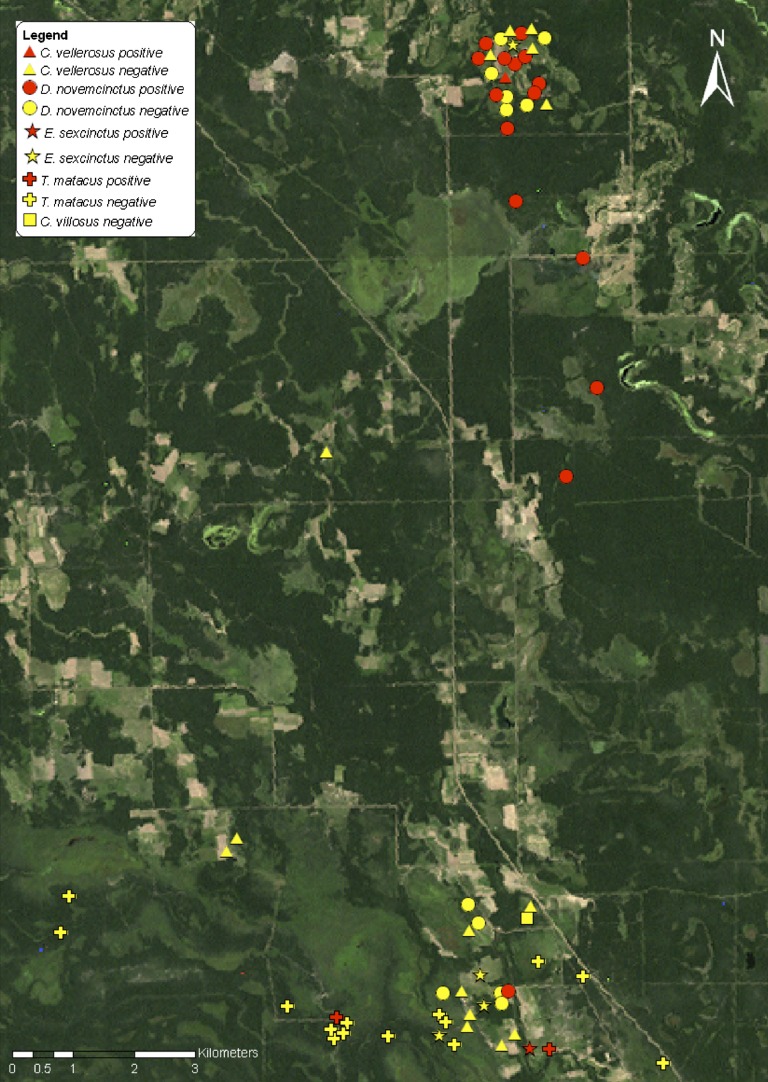
Figure 2.
Landsat image showing the location of Trypanosoma cruzi–infected and non-infected armadillos in Pampa del Indio, Argentina, 2008–2011.
Results
Capture of wild mammals.
A total of 195 mammals from at least 20 genus or species (including unidentified rodents) was captured using 7,746 trap-nights (Tables 1 and 2). White-eared opossums were the most frequently captured species (22.1%), followed by small rodents (19.5%), nine-banded armadillos (13.3%), and small fat-tailed opossums (10.8%). Male:female sex ratios deviated substantially from a 1:1 ratio in white-eared opossums Di. albiventris, Na. nasua, Cerdocyon thous, Sylvilagus brasiliensis, all species of armadillos (except Eu. sexcinctus), Th. pusilla, and Procyon cancrivorus. All animals were clinically normal by physical examination. Total solids and PCV values were normal for all species and similar to reference ranges published for captive species (Table 1).
Table 1.
Catch of wild mammals by species, sex ratio, mean packed cell volume, and mean total solids in Pampa del Indio, Chaco, Argentina, 2008–2011
| Host order | Scientific name | No. captured (%) | Male: female ratio | Packed Cell Volume (%) | Total solids (g/dL) | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. examined | Mean ± SD | Range | No. examined | Mean ± SD | Range | ||||
| Cingulata | Dasypus novemcinctus | 26 (13.3) | 1.5 | 13 | 40.0 ± 7.6 | 26.5–56 | 12 | 7.5 ± 1.0 | 5.5–8.8 |
| Tolypeutes matacus | 16 (8.2) | 2.2 | 12 | 37.7 ± 8.0 | 20–49 | 9 | 7.2 ± 0.8 | 5.7–8.2 | |
| Chaetophractus vellerosus | 16 (8.2) | 1.29 | 11 | 48.5 ± 7.8 | 33–57 | 10 | 6.3 ± 0.8 | 5.2–8.0 | |
| Euphractus sexcinctus | 5 (2.6) | 0.25 | 5 | 33.2 ± 5.5 | 24–38 | 4 | 6.3 ± 1.5 | 5.3–8.5 | |
| Chaetophractus villosus | 1 (0.5) | ||||||||
| Didelphimorphia | Didelphis albiventris | 43 (22.1) | 1.8 | 17 | 35.7 ± 5.5 | 20–43 | 15 | 7.0 ± 0.9 | 5.1–8.5 |
| Thylamys pusilla | 21 (10.8) | 0.67 | 4 | 42.5 ± 5.7 | 38–50 | 3 | 6.2 ± 0.7 | 5.7–7.0 | |
| Monodelphis sp. | 1 (0.5) | ||||||||
| Rodentia | Various species | 38 (19.5) | 1.18 | 12 | 42.3 ± 7.9 | 27–55 | 6 | 6.3 ± 0.8 | 5.0–7.0 |
| Galea musteloides | 1 (0.5) | ||||||||
| Ctenomys sp. | 1 (0.5) | ||||||||
| Carnivora | Nasua nasua | 5 (2.6) | 4 | 1 | 26 | 1 | 6.8 | ||
| Procyon cancrivorus | 4 (2.1) | 0.33 | 2 | 43.5 ± 4.9 | 40–47 | 2 | 7.5 ± 0.7 | 7.0–8.0 | |
| Cerdocyon thous | 4 (2.1) | 2 | 2 | 43.0 ± 1.4 | 42–44 | 2 | 7.4 ± 0.6 | 7.0–7.8 | |
| Leopardus geoffroyi | 4 (2.1) | 1 | 1 | 36.0 | |||||
| Chiroptera | Myotis sp. | 2 (1.0) | 1 | 1 | 37.5 | 1 | 6.0 | ||
| Eumops perotis | 1 (0.5) | 1 | 58.0 | 1 | 6.0 | ||||
| Dasypterus ega | 1 (0.5) | 1 | 57.0 | 1 | 8.5 | ||||
| Molossops temminckii | 1 (0.5) | ||||||||
| Lagomorpha | Sylvilagus brasiliensis | 3 (1.5) | 2 | 1 | 44.0 | ||||
| Pilosa | Myrmecophaga tridactyla | 1 (0.5) | 1 | 35.0 | |||||
| Total | 195 | 85 | 67 | ||||||
Table 2.
Catch per unit effort over time (CPUE) in Pampa del Indio, Chaco, Argentina, 2008–2011
| Date of survey | National traps | Sherman traps | ||||
|---|---|---|---|---|---|---|
| No. trap-nights | No. captured | CPUE* ± SE | No. trap-nights | No. captured | CPUE* ± SE | |
| August 2008 | 1,599 | 23 | 1.44 ± 0.29 | 440 | 13 | 2.95 ± 0.80 |
| August 2009 | 1,660 | 11 | 0.66 ± 0.20 | 1,413 | 12 | 0.85 ± 0.24 |
| August 2010 | 226 | 5 | 2.21 ± 0.97 | – | – | – |
| March 2011 | 1,130 | 28 | 2.48 ± 0.46 | 1,278 | 31 | 2.43 ± 0.43 |
| Total | 4,615 | 67 | 1.45 ± 0.18 | 3,131 | 56 | 1.79 ± 0.23 |
Animals per 100 trap-nights.
The mean catch per unit effort of micro-mammals (1.79 animals per 100 Sherman trap-night) was slightly, though not significantly higher, than that of medium-sized mammals (1.45 animals per 100 National or home-made trap-night) (Table 2). The mean catch per unit effort of micro-mammals and medium-size mammals decreased steeply from 2008 to 2009 (during a prolonged drought preceded by severe rainfalls occurring the spring-summer of 2008), and then recovered or exceeded previous levels (Table 2). No animals were captured with pitfall traps, and only three bats were captured in 60 mist net-nights. The remaining specimens were captured manually by local collaborators (n = 64) or with a blowgun (n = 5).
Trypanosoma cruzi infection.
The overall infection prevalence of 14.6% (95% CI = 9.5–19.7%) determined by xenodiagnosis was lower than the 20.3% (95% CI = 14.5–26.1%) detected by kDNA-PCR from blood samples, whereas the composite prevalence of infection (determined by either method) was 22.1% (95% CI = 16.2–28.0%) (Table 3). The composite prevalence of infection was substantially higher in Da. novemcinctus armadillos (57.7%) and Di. albiventris opossums (38.1%) than in Eu. sexcinctus (20.0%), To. matacus (12.5%), and Chaetophractus vellerosus (6.3%) armadillos.
Table 3.
Prevalence of Trypanosoma cruzi infection determined by xenodiagnosis and kDNA-PCR in sylvatic mammals from Pampa del Indio, Chaco, Argentina, 2008–2011*
| Species | % Infection prevalence (no. examined) | Positive by both methods | Positive only by xenodiagnosis | Positive only by kDNA-PCR | Negative by both methods | ||
|---|---|---|---|---|---|---|---|
| Xenodiagnosis | kDNA-PCR | Composite | |||||
| Dasypus novemcinctus | 48.0 (25) | 56.0 (25) | 57.7 (26)† | 11 | 0 | 3‡ | 11 |
| Tolypeutes matacus | 12.5 (16) | 0.0 (16) | 12.5 (16) | 0 | 2 | 0 | 14 |
| Chaetophractus vellerosus | 6.3 (16) | 6.3 (16) | 6.2 (16) | 1 | 0 | 0 | 15 |
| Euphractus sexcinctus | 0.0 (5) | 20.0 (5) | 20.0 (5) | 0 | 0 | 1§ | 4 |
| Chaetophractus villosus | 0.0 (1) | 0.0 (1) | 0.0 (1) | 0 | 0 | 0 | 1 |
| Didelphis albiventris | 29.3 (41) | 35.7 (42) | 38.1 (42)† | 11 | 1 | 4¶ | 25 |
| Thylamys pusilla | 0.0 (20) | 25.0 (20) | 25.0 (20) | 0 | 0 | 5# | 15 |
| Monodelphis sp. | 0.0 (1) | 0.0 (1) | 0.0 (1) | 0 | 0 | 0 | 1 |
| Unidentified small rodents | 0.0 (37) | 5.7 (35) | 5.4 (37)** | 0 | 0 | 2†† | 33 |
| Galea musteloides | 0.0 (1) | 0.0 (1) | 0.0 (1) | 0 | 0 | 0 | 1 |
| Ctenomys sp. | 0.0 (1) | 0.0 (1) | 0.0 (1) | 0 | 0 | 0 | 1 |
| Nasua nasua | 0.0 (1) | 0.0 (4) | 0.0 (4)§§ | 0 | 0 | 0 | 1 |
| Procyon cancrivorus | 0.0 (4) | 0.0 (4) | 0.0 (4) | 0 | 0 | 0 | 4 |
| Cerdocyon thous | 0.0 (4) | 0.0 (4) | 0.0 (4) | 0 | 0 | 0 | 4 |
| Leopardus geoffroyi | 0.0 (3) | 0.0 (3) | 0.0 (3) | 0 | 0 | 0 | 3 |
| Myotis sp. | 0.0 (2) | 0.0 (2) | 0.0 (2) | 0 | 0 | 0 | 2 |
| Eumops perotis | 0.0 (1) | 0.0 (1) | 0.0 (1) | 0 | 0 | 0 | 1 |
| Dasypterus ega | 0.0 (1) | 0.0 (1) | 0.0 (1) | 0 | 0 | 0 | 1 |
| Molossops temminckii | 0.0 (1) | 0.0 (1) | 0.0 (1) | 0 | 0 | 0 | 1 |
| Sylvilagus brasiliensis | 0.0 (3) | 0.0 (3) | 0.0 (3) | 0 | 0 | 0 | 3 |
| Myrmecophaga tridactyla | 0.0 (1) | 0.0 (1) | 0.0 (1) | 0 | 0 | 0 | 1 |
| Total | 14.6 (185) | 20.3 (187) | 22.1 (190) | 23 | 3 | 15 | 142 |
kDNA = kinetoplast DNA; PCR = polymerase chain reaction.
Includes one negative sample evaluated only by kDNA-PCR.
Two negative samples (1 positive by kDNA-PCR from bugs tested by xenodiagnosis) and 1 positive sample by nuclear satellite DNA-PCR.
One negative sample by nuclear satellite DNA-PCR and positive by kDNA-PCR from bugs tested by xenodiagnosis.
Three negative samples (2 positive by kDNA-PCR from bugs tested by xenodiagnosis) and 1 positive by nuclear satellite DNA-PCR.
Three negative samples (1 positive by kDNA-PCR from bugs tested by xenodiagnosis and 1 with two positive results by kDNA from a blood-guanidine mixture in different DNA extractions) and two positive by nuclear satellite DNA-PCR.
Includes two negative samples evaluated only by xenodiagnosis.
Two negative samples by nuclear satellite DNA-PCR (both positive by kDNA-PCR from bugs tested by xenodiagnosis).
Includes three negatives samples evaluated only by kDNA-PCR.
The kDNA-PCR detected significantly more infections than xenodiagnosis at the aggregate level (McNemar's χ2 = 8.00, degrees of freedom [df] = 1, P < 0.001) (Table 3). Xenodiagnosis-positive animals included 12 (29.3%) Di. albiventris opossums, and among armadillos, 12 (48.0%) Da. novemcinctus, 2 (12.5%) To. matacus, and one (6.3%) Ch. vellerosus. Trypanosoma cruzi parasites from all xenodiagnosis-positive individuals were successfully isolated, except in one To. matacus. The kDNA-PCR detected infections in 23 of 27 xenodiagnosis-positive animals and in 14 xenodiagnosis-negative animals; the latter included 5 Th. pusilla, 4 Di. albiventris, 2 Da. novemcinctus, 1 Eu. sexcinctus, and 2 unidentified rodent species. Using SAT DNA-PCR, we confirmed infections with T. cruzi in one Di. albiventris, one Da. novemcinctus, and two Th. pusilla that had been positive by kDNA-PCR only. Using kDNA-PCR in rectal contents from xenodiagnosis-negative triatomine bugs, we further confirmed infections with T. cruzi only detected by kDNA-PCR in blood samples in two Di. albiventris, one Da. novemcinctus, one Eu. sexcinctus, two unidentified rodents, and one Th. pusilla. Infection with T. cruzi was confirmed in another Th. pusilla specimen (in which bugs tested by xenodiagnosis had insufficient rectal contents) by performing two independent rounds of DNA extractions from blood and kDNA-PCR. No T. cruzi-infected individuals were detected in Lagomorpha, Chiroptera, Carnivora, and Pilosa.
In white-eared opossums, the annual prevalence of T. cruzi infection fluctuated between 36.4% (4 of 11) in 2008, 33.3% (1 of 3) in 2009, 0% (0 of 4) in 2010, and 45.8% (11 of 24) in 2011. Infection was fairly constant in juveniles (44.4%, 4 of 9) and pre-adults (45.0%, 9 of 20) and decreased slightly to 23.1% (3 of 13) in adults, and there were no statistically significant differences between stages (χ2 = 1.80, df = 2; P > 0.40). In nine-banded armadillos, the annual infection prevalence varied between 66.7% (6 of 9) in 2008, 60.0% (3 of 5) in 2009, 66.7% (4 of 6) in 2010, and 33.3% (2 of 6) in 2011.
The spatial distribution patterns of T. cruzi infection in all species of marsupials and armadillos combined separately are shown in Figures 1 and 2, respectively. Aggregation of infection occurred at a distance of 600 meters for armadillos, whereas no significant aggregation was detected for marsupials. Capture locations of domestic or peridomestic Triatoma sordida specimens infected with TcI are shown in Figure 1.31
All 12 opossums tested were infected with TcI, as indicated by the 150-basepair band for the SL-IRac leader sequence and the 475-basepair band for the SL-IRI leader sequence. All parasite isolates from 12 Da. novemcinctus, 1 Ch. vellerosus, and 1 To. matacus armadillos were TcIII, as indicated by the 200-basepair band for the SL-IRac leader sequence and the 125-basepair band for the 24sα ribosomal DNA-HnPCR sequence. The DTUs of kDNA-PCR-positive and xenodiagnosis-negative samples could not be identified.
Infectiousness to the vector.
The mean infectiousness to the vector in hosts positive by kDNA-PCR or xenodiagnosis peaked in Da. novemcinctus and Di. albiventris, and was zero in kDNA-PCR-positive Th. pusilla, Eu. sexcinctus, and the two unidentified rodents (Table 4). On average, Da. novemcinctus infected a larger fraction of bugs than Di. albiventris, but logistic regression analysis clustered by individual detected no statistically significant differences between host species (odds ratio = 2.13, 95% CI = 0.58–7.82).
Table 4.
Mean infectiousness to Triatoma infestans in hosts positive by kDNA-PCR or xenodiagnosis in Pampa del Indio, Chaco, Argentina, 2008–2011*
| Species | Xenodiagnosis or kDNA-PCR | No. positive by xenodiagnosis | Mean infectiousness (95% CI) | |
|---|---|---|---|---|
| No. examined | No. positive | |||
| Dasypus novemcinctus | 26 | 14 | 12 | 73.8 (49.5–96.1) |
| Tolypeutes matacus | 16 | 2 | 2 | 8.1 |
| Chaetophractus vellerosus | 16 | 1 | 1 | 35.3 |
| Euphractus sexcinctus | 5 | 1 | 0 | 0 |
| Didelphis albiventris | 42 | 16 | 12 | 55.7 (31.3–80.0) |
| Thylamys pusilla | 20 | 5 | 0 | 0 |
| Unidentified small rodents | 37 | 2 | 0 | 0 |
kDNA = kinetoplast DNA; PCR = polymerase chain reaction; CI = confidence interval.
The infectiousness to the vector of white-eared opossums did not decrease significantly with stage (χ2 = 1.28, df = 2, P > 0.5), body mass index (χ2 = 1.28, df = 2, P > 0.3), or sex (χ2 = 3.19, df = 1, P > 0.05). For nine-banded armadillos, infectiousness was not significantly associated with sex (χ2 = 0.50, df = 2, P > 0.5) or body mass index (χ2 = 0.50, df = 2, P > 0.5).
Discussion
Our longitudinal study documents that Da. novemcinctus armadillos and Di. albiventris opossums were the main sylvatic hosts of T. cruzi and had large reservoir competence; a wider range of hosts of T. cruzi than previous surveys conducted in the Gran Chaco ecoregion, including four armadillo and two marsupial species, and the first findings of T. cruzi in Th. pusilla small opossums and in two small rodents. The wider host range recorded is probably related to the large catch effort invested at ground and tree-branch levels over several years in a disturbed, heterogeneous environment with persistent transmission, combined with enhanced parasite detection by means of kDNA-PCR and xenodiagnosis.
The infection prevalence in Da. novemcinctus (57.7%) and Di. albiventris (38.1%) is among the largest documented in the region. Both species fit the definition of key reservoir hosts32 because they are capable of maintaining T. cruzi indefinitely and independently of domestic transmission cycles. This finding is shown by their frequent infection, persistent infectiousness to triatomine bugs,33 and ability to maintain viable populations in peridomestic or semi-sylvatic (disturbed) habitats despite being killed by domestic dogs.17,19
After being incriminated as the first sylvatic host of T. cruzi by Carlos Chagas, Da. novemcinctus has been found infected throughout the Americas.9 In the Argentinean Chaco, pioneering studies showed that it was more frequently infected (17–29%) than To. matacus (8%) using fresh blood examination, histopathologic analysis, and mouse inoculation,34 whereas more recent xenodiagnostic surveys reported no infection in several armadillo species.35 In Paraguay, 45% of 38 Da. novemcinctus armadillos examined by xenodiagnosis and hemoculture were infected with T. cruzi, whereas Eu. sexcinctus (17%), Ch. vellerosus (4%), and Ch. villosus (4%) were less frequently infected.7 Our results show large differences in infection prevalence between Da. novemcinctus and other armadillo species. The insectivorous habits of Da. novemcinctus and To. matacus suggest they are more likely to contract the infection by eventually eating triatomine bugs infected with T. cruzi than other species of omnivorous armadillos such as Ch. vellerosus and Ch. villosus, which are less insectivorous.36 The substantial variations in T. cruzi infection between different species of armadillos may also be associated with variations in the type and use of burrows between species.9 Unlike other species, the typical burrows of Da. novemcinctus are repeatedly used by the same individual and may act as long-term refuge for triatomine bugs.9
The prevalence of T. cruzi infection in Di. albiventris opossums (38%) was as high as in the past37 or elsewhere in Chaco (36%)18 and Santiago del Estero Provinces (32–36%).16,17 However, in Santiago del Estero Province, infection prevalence decreased to 9% more recently.19 Elsewhere in the Americas, infection prevalence in Didelphis opossums was as high as 52% in areas where the main vector species were Panstrongylus sp. and Rhodnius sp.38,39
An unexpected result was the absence of a stage-related increase in the prevalence of infection (produced by cumulative exposure) in white-eared opossums that did not fit a non-reversible catalytic model, in contrast to previous studies of opossums and dogs.19,28 The decrease in infections recorded in adult opossums may not be explained by an age-related decrease in infectiousness because we also used a more sensitive detection method (kDNA-PCR). Whether differential mortality of T. cruzi-infected adult opossums in disturbed habitats may create the age-related decrease in infection is unknown.
Our study identified Th. pusilla small opossums as a new host of T. cruzi by means of kDNA-PCR and SAT DNA-PCR. In Argentina, the few specimens of Th. pusilla examined with xenodiagnosis or hemoculture yielded negative results.14–16,19 In Chile, the related species Thylamys elegans was also infected with T. cruzi as determined by PCR and hybridization assays.40
One of the strengths of our study is the simultaneous use of xenodiagnosis (to assess reservoir host competence, i.e., infectiousness) and kDNA-PCR (for enhanced sensitivity). Nine-banded armadillos and white-eared opossums were highly infectious to Triatoma infestans nymphs after a single blood meal, whereas other armadillo species were quantitatively less infectious. This finding may be the first quantification of the infectiousness to the vector of the major sylvatic reservoir hosts. In contrast, small fat-tailed opossums and rodents with confirmed infections were not infectious to bugs, indicating they had a much lower reservoir host competence. Dasypus novemcinctus was far more abundant (or catchable) and had larger infection prevalence and infectiousness than other local armadillo species and Di. albiventris opossums.
The kDNA-PCR detected more infections than xenodiagnosis, although xenodiagnosis identified some infections not detected by kDNA-PCR, and both methods showed high levels of co-positivity and co-negativity. The kDNA-PCR enabled an independent confirmation of xenodiagnosis-negative specimens, which otherwise would have included false-negative results, given the limited sensitivity of xenodiagnosis. In Didelphis opossums and armadillo species positive by xenodiagnosis or kDNA-PCR, the false-negative rate of xenodiagnosis was 24% (8 of 34).
Several infections diagnosed only by kDNA-PCR from blood samples (i.e., negative by xenodiagnosis and SAT DNA-PCR) were confirmed by kDNA-PCR of rectal contents of bugs xenodiagnostic; these included samples from one six-banded armadillo, one Th. pusilla, two small rodents, and other species. We recorded three discordant cases positive by kDNA-PCR that could not be further confirmed by kDNA-PCR of bug rectal contents or SAT DNA-PCR of blood samples. The SAT DNA-PCR is expected to be more specific than kDNA-PCR because it amplifies nuclear DNA of the parasite, but fewer copies of the target nuclear gene reduce substantially the sensitivity of SAT DNA-PCR in TcI strains.41 This issue is further compounded by the limited amount of blood obtained from small opossums and rodents. Therefore, the three specimens that were only kDNA-positive are considered likely to have T. cruzi infections.
Armadillos and opossums were infected by different DTUs. The occurrence of TcIII in all infected armadillo species (including the first reports of TcIII in Ch. vellerosus and To. matacus from Argentina) are consistent with current knowledge,7,9 and was further confirmed by the analysis of sequence polymorphisms within the 200-basepair amplicons obtained in the SL-IRac PCR (Cura C and Schijman A, unpublished data).20 Infections with TcIII in armadillo species and with TcI in white-eared opossums imply separate, independent parasite transmission cycles. Our empirical results therefore support the theory that arboreal transmission cycles that include opossums are usually associated with TcI, whereas terrestrial transmission cycles with armadillos as hosts include TcIII.7,9,24,42 This separation is not complete because other DTUs have occasionally been found in opossums and armadillos.4,7,23,43 The most likely local vectors of TcI and TcIII, as yet not identified conclusively, are Triatoma sordida and Panstrongylus geniculatus, respectively. Triatoma sordida is locally abundant and was the only bug species found infected with TcI31 whereas P. geniculatus was found in local armadillo burrows.20 The concomitant findings of Di. albiventris opossums and an adult Triatoma sordida infected with TcI in the southern section (Figure 1) suggest that this species may be a putative sylvatic vector of TcI.
The two main capture zones in our study area (separated by 10 km) showed contrasting results. However, the landscape was similar in terms of vegetation cover, nearby water courses, and use of fields. In the northern section, a sizable number of armadillos was positive for TcIII and these infections were spatially aggregated. In the southern section, a substantial number of marsupials were infected (mainly white-eared opossums with TcI) although not in an aggregated manner. The reasons for such differences remain unknown.
The prospective study design provided evidence for large variations in the relative abundance of micro-mammals and medium-sized mammals over a four-year period, with a sudden decrease in numbers during a prolonged drought during 2008–2009, and a subsequent rebound in the abundance of medium-sized mammals. Most specimens were caught in small forest fragments (up to 2–3 hectares) 1–3 km from houses, which suggests a potential for close contact between wild and domestic animals, triatomine bugs, and human hosts. Most of the species caught were generalists that were found in peridomestic and semi-sylvatic areas. These species included opossums, which adapt easily to rural, suburban, and urban environments, and armadillos such as Da. novemcinctus, Ch. vellerosus, and To. matacus.44 Although armadillos prefer preserved habitats not disturbed by human activities, they adapt easily to degraded habitats where their tracks are more visible.44
Trypanosoma cruzi-infected armadillos pose a substantial risk for human or dog infection with T. cruzi because they are frequently hunted by rural villagers in the Gran Chaco.45 Villagers make unprotected contact with armadillo blood during skinning; dogs are fed their viscera and other remains, and armadillos are sometimes kept in captivity for extended periods during which they may become exposed to peridomestic or domestic bugs, as we observed during field work. All of these factors are potential entry points of TcIII into domestic transmission cycles.7,20,24 Two of 44 T. cruzi-infected domestic dogs from our study area had TcIII,46 and evidence of TcIII in domestic Triatoma infestans and dogs was obtained elsewhere in the Chaco24,47 and in the Amazon.9,43
Local wild mammals had no evidence of the parasite DTUs (TcV and TcVI) that infected local domestic dogs, cats, or Triatoma infestans.24,46 Therefore, the local occurrence of a spillover of domestic or peridomestic triatomine bugs carrying domestic DTUs to the sylvatic environment and subsequent creation of sylvatic foci of transmission was not recorded by our study to date. This process apparently occurred in southern Brazil and included Didelphis opossums and T. sordida.48 The repeated findings of sylvatic colonies of Triatoma infestans in the Gran Chaco49–51 suggest that domestic or peridomestic parasite or vector spillovers occur.
Our study had some limitations. The frequent adaptation of the main reservoir host species to degraded habitats combined with ability and knowledge of forest fragments of local villagers may have resulted in the large catch of armadillos and opossums, and likely biased estimates of relative host abundance. In addition, trapping efforts were heterogeneous among sites and years because of logistical reasons. The occurrence of mixed infections of T. cruzi DTUs in mammalian hosts might be masked by genotype selection associated with specific culture methods,52 the sensitivity of PCRs used to identify parasite genotypes, and differential parasite histotropism.53
The ongoing accelerated deforestation of the Gran Chaco (including our study area) has led to fragmented forest patches that may increase the contact rates between sylvatic or domestic hosts of T. cruzi and triatomine bug species. Residual forest fragments act as a refuge for some host species. The effects of habitat fragmentation on disease prevalence vary with the specific host-parasite relationship,23,54 and new foci of transmission may be created at intermediate levels of degradation.23 Some of the main reservoir hosts of T. cruzi adapt easily to peridomestic rural environments where they maintain sizable numbers and high prevalence of infection with T. cruzi. The observed large infection prevalence in white-eared opossums and nine-banded armadillos combined with their rather large abundance in small forest fragments nearby peridomestic sites may facilitate parasite transmission, especially in disturbed areas.
ACKNOWLEDGMENTS
We thank Leonardo Ceballos, Francisco Petrocco, Flavia Netto, Marina Leporace, Mariano Arias, Juan Pablo Arrabal, Lucía Maffey, Leonardo Lanati, Marta Lauricella, Carolina Cura, Margarita Bisio, Juan M. Burgos, Rubén Bárquez, Pablo Teta, Emiliano Muschetto, Alfonso Bros, Lescano Bros, Blanco Bros, and Raúl Stariolo for field and laboratory assistance; Fernando Garelli for advice on spatial analysis; and Patricio Diosque, Miguel A. Basombrío, and Michel Tibayrenc for providing reference strains of TcI-TcVI.
Footnotes
Financial support: This study was supported by awards from the International Development Research Center (EcoHealth Program), Tropical Disease Research (UNICEF/PNUD/WB/WHO), University of Buenos Aires to Ricardo E. Gürtler, and National Institutes of Health/National Science Foundation Ecology of Infectious Disease program award R01 TW05836 funded by the Fogarty International Center and the National Institute of Environmental Health Sciences to Uriel Kitron, Ricardo E. Gürtler, and Joel Cohen. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Ricardo E. Gürtler, M. Victoria Cardinal, and Alejandro G. Schijman are members of Consejo Nacional de Investigaciones Científicas y Técnicas Researcher's Career.
Authors' addresses: M. Marcela Orozco, Gustavo F. Enríquez, Julián A. Alvarado-Otegui, M. Victoria Cardinal, and Ricardo E. Gürtler, Laboratory of Eco-Epidemiology, Department of Ecology, Genetics and Evolution, Universidad de Buenos Aires, Buenos Aires, Argentina, E-mails: marcelaorozco.vet@gmail.com, gustavoenriquez@ege.fcen.uba.ar, lavizcachasp@hotmail.com, mvcardinal@ege.fcen.uba.ar, and gurtler@ege.fcen.uba.ar. Alejandro G. Schijman, Laboratorio de Biología Molecular de la Enfermedad de Chagas, Instituto de Investigaciones en Ingeniería Genética y Biología Molecular, Buenos Aires, Argentina, E-mail: aleschijman@gmail.com. Uriel Kitron, Department of Environmental Studies, Emory University, Atlanta, GA, E-mail: ukitron@emory.edu.
References
- 1.Schofield CJ, Jannin J, Salvatella R. The future of Chagas disease control. Trends Parasitol. 2006;22:583–588. doi: 10.1016/j.pt.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Control of Chagas Disease: Second Report of the WHO Expert Committee. Geneva: World Health Organization; 2002. [Google Scholar]
- 3.Zingales B, Andrade SG, Briones MR, Campbell DA, Chiari E, Fernandes O, Guhl F, Lages-Silva E, Macedo AM, Machado CR, Miles MA, Romanha AJ, Sturm NR, Tibayrenc M, Schijman AG. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz. 2009;104:1051–1054. doi: 10.1590/s0074-02762009000700021. [DOI] [PubMed] [Google Scholar]
- 4.Barnabe C, Brisse S, Tibayrenc M. Population structure and genetic typing of Trypanosoma cruzi, the agent of Chagas disease: a multilocus enzyme electrophoresis approach. Parasitology. 2000;120:513–526. doi: 10.1017/s0031182099005661. [DOI] [PubMed] [Google Scholar]
- 5.Miles MA, Feliciangeli MD, de Arias AR. American trypanosomiasis (Chagas' disease) and the role of molecular epidemiology in guiding control strategies. BMJ. 2003;326:1444–1448. doi: 10.1136/bmj.326.7404.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis MD, Ma J, Yeo M, Carrasco HJ, Llewellyn MS, Miles MA. Genotyping of Trypanosoma cruzi: systematic selection of assays allowing rapid and accurate discrimination of all known lineages. Am J Trop Med Hyg. 2009;81:1041–1049. doi: 10.4269/ajtmh.2009.09-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeo M, Acosta N, Llewellyn M, Sanchez H, Adamson S, Miles GA, Lopez E, Gonzalez N, Patterson JS, Gaunt MW, de Arias AR, Miles MA. Origins of Chagas disease: Didelphis species are natural hosts of Trypanosoma cruzi I and armadillos hosts of Trypanosoma cruzi II, including hybrids. Int J Parasitol. 2005;35:225–233. doi: 10.1016/j.ijpara.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 8.Noireau F, Diosque P, Jansen AM. Trypanosoma cruzi: adaptation to its vectors and its hosts. Vet Res. 2009;40:26. doi: 10.1051/vetres/2009009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llewellyn MS, Lewis MD, Acosta N, Yeo M, Carrasco HJ, Segovia M, Vargas J, Torrico F, Miles MA, Gaunt MW. Trypanosoma cruzi IIc: phylogenetic and phylogeographic insights from sequence and microsatellite analysis and potential impact on emergent Chagas disease. PLoS Negl Trop Dis. 2009;3:e510. doi: 10.1371/journal.pntd.0000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Nature Conservancy (TNC), Fundación Vida Silvestre Argentina (FVSA), Fundación para el Desarrollo Sustentable del Chaco (DeSdel Chaco) y Wildife Conservation Society Bolivia (WCS) Buenos Aires: Fundación Vida Silvestre Argentina; 2005. Evaluación Ecorregional del Gran Chaco Americano/Gran Chaco Americano Ecoregional Assessment.http://www.vidasilvestre.org.ar/sala_redaccion/opublicaciones/publicaciones_bosques_y_selvas/?2980/Evaluacin-Ecorregional-del-Gran-Chaco-Americano Available at. Accessed January 15, 2012. [Google Scholar]
- 11.Gürtler RE, Kitron U, Cecere MC, Segura EL, Cohen JE. Sustainable vector control and management of Chagas disease in the Gran Chaco, Argentina. Proc Natl Acad Sci USA. 2007;104:16194–16199. doi: 10.1073/pnas.0700863104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bucher EH, Huszar PC. Sustainable management of the Gran Chaco of South America. J Environ Manage. 1999;57:99–108. [Google Scholar]
- 13.Mazza S, Romaña C, Schurman K. New observations on the spontaneous infection of armadillos of the country by Trypanosoma cruzi. Prensa Med Argent. 1930;3:3–20. [Google Scholar]
- 14.Carcavallo RU, Martínez A. Entomoepidemiology of the Argentine Republic. Investigaciones Científicas de las Fuerzas Armadas Argentinas. 1968;1:76–92. [Google Scholar]
- 15.Barretto MP. Reservoirs of Trypanosoma (Schizotrypanum) cruzi (Chagas 1909) In: Carcavallo RU, Rabinovich JE, Tonn RJ, editors. Biological and ecological factors in Chagas disease. Buenos Aires, Argentina: Organización Panamericana de la Salud/Servicio Nacional de Chagas; 1985. pp. 275–288. [Google Scholar]
- 16.Wisnivesky-Colli C, Schweigmann NJ, Alberti A, Pietrokovsky SM, Conti O, Montoya S, Riarte A, Rivas C. Sylvatic American trypanosomiasis in Argentina. Trypanosoma cruzi infection in mammals from the Chaco forest in Santiago del Estero. Trans R Soc Trop Med Hyg. 1992;86:38–41. doi: 10.1016/0035-9203(92)90433-d. [DOI] [PubMed] [Google Scholar]
- 17.Schweigmann NJ, Pietrokovsky S, Bottazzi V, Conti O, Bujas MA, Wisnivesky-Colli C. Prevalence of Trypanosoma cruzi infection in opossum (Didelphis albiventris) in Santiago del Estero, Argentina. Rev Panam Salud Publica. 1999;6:371–377. doi: 10.1590/s1020-49891999001100001. [DOI] [PubMed] [Google Scholar]
- 18.Diosque P, Padilla AM, Cimino RO, Cardozo RM, Negrette OS, Marco JD, Zacca R, Meza C, Juarez A, Rojo H, Rey R, Corrales RM, Nasser JR, Basombrio MA. Chagas disease in rural areas of Chaco Province, Argentina: epidemiologic survey in humans, reservoirs, and vectors. Am J Trop Med Hyg. 2004;71:590–593. [PubMed] [Google Scholar]
- 19.Ceballos LA, Cardinal MV, Vazquez-Prokopec GM, Lauricella MA, Orozco MM, Cortinas R, Schijman AG, Levin MJ, Kitron U, Gürtler RE. Long-term reduction of Trypanosoma cruzi infection in sylvatic mammals following deforestation and sustained vector surveillance in northwestern Argentina. Acta Trop. 2006;98:286–296. doi: 10.1016/j.actatropica.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvarado-Otegui JA, Ceballos LA, Orozco MM, Enriquez GF, Cardinal MV, Cura C, Schijman AG, Kitron U, Gürtler RE. The sylvatic transmission cycle of Trypanosoma cruzi in a rural area in the humid Chaco of Argentina. Acta Trop. 2012;124:79–86. doi: 10.1016/j.actatropica.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daszak P, Cunningham AA, Hyatt AD. Emerging infectious diseases of wildlife–threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- 22.Herrera HM, Rademaker V, Abreu UG, D'Andrea PS, Jansen AM. Variables that modulate the spatial distribution of Trypanosoma cruzi and Trypanosoma evansi in the Brazilian Pantanal. Acta Trop. 2007;102:55–62. doi: 10.1016/j.actatropica.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Vaz VC, D'Andrea PS, Jansen AM. Effects of habitat fragmentation on wild mammal infection by Trypanosoma cruzi. Parasitology. 2007;134:1785–1793. doi: 10.1017/S003118200700323X. [DOI] [PubMed] [Google Scholar]
- 24.Cardinal MV, Lauricella MA, Ceballos LA, Lanati L, Marcet PL, Levin MJ, Kitron U, Gürtler RE, Schijman AG. Molecular epidemiology of domestic and sylvatic Trypanosoma cruzi infection in rural northwestern Argentina. Int J Parasitol. 2008;38:1533–1543. doi: 10.1016/j.ijpara.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenière SF, Aliaga C, Waleckx E, Buitrago R, Salas R, Barnabé C, Tibayrenc M, Noireau F. Genetic characterization of Trypanosoma cruzi DTUs in wild Triatoma infestans from Bolivia: predominance of TcI. PLoS Negl Trop Dis. 2012;6:e1650. doi: 10.1371/journal.pntd.0001650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurevitz JM, Ceballos LA, Gaspe MS, Alvarado-Otegui JA, Enriquez GF, Kitron U, Gürtler RE. Factors affecting infestation by Triatoma infestans in a rural area of the humid Chaco in Argentina: a multi-model inference approach. PLoS Negl Trop Dis. 2011;5:e1349. doi: 10.1371/journal.pntd.0001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kreeger TJ, Arnemo JM. Handbook of Wildlife Chemical Immobilization. Vancouver, British Columbia, Canada: Sunquest; 2007. [Google Scholar]
- 28.Gürtler RE, Cecere MC, Lauricella MA, Cardinal MV, Kitron U, Cohen JE. Domestic dogs and cats as sources of Trypanosoma cruzi infection in rural northwestern Argentina. Parasitology. 2007;134:69–82. doi: 10.1017/S0031182006001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burgos JM, Altcheh J, Bisio M, Duffy T, Valadares HM, Seidenstein ME, Piccinali R, Freitas JM, Levin MJ, Macchi L, Macedo AM, Freilij H, Schijman AG. Direct molecular profiling of minicircle signatures and lineages of Trypanosoma cruzi bloodstream populations causing congenital Chagas disease. Int J Parasitol. 2007;37:1319–1327. doi: 10.1016/j.ijpara.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Wiegand T, Moloney KA. Rings, circles, and null-models for point pattern analysis in ecology. Oikos. 2004;104:209–229. [Google Scholar]
- 31.Maffey L, Cardinal MV, Ordóñez-Krasnowski PC, Lanati LA, Lauricella MA, Schijman AG, Gürtler RE. Direct molecular identification of Trypanosoma cruzi discrete typing units in domestic and peridomestic Triatoma infestans and Triatoma sordida from the Argentine Chaco. Parasitology. 2012;139:1570–1579. doi: 10.1017/S0031182012000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cleaveland S, Dye C. Maintenance of a microparasite infecting several host species: rabies in the Serengeti. Parasitology. 1995;111((Suppl)):S33–S47. doi: 10.1017/s0031182000075806. [DOI] [PubMed] [Google Scholar]
- 33.Zeledón R. Epidemiology, modes of transmission and reservoir hosts of Chagas' disease. Trypanosomiasis and leishmaniasis with special reference to Chagas disease. CIBA Foundation Symposium. 1974;20((new series)):51–77. [Google Scholar]
- 34.Mazza S, Romaña C, Schürmann K. New observations on the spontaneous infection of armadillos of the country by Trypanosoma cruzi. Finding of this flagellate in Dasypus novemcinctus Lin. in the Chaco of Santa Fe. Prensa Med Argent. 1931;3:20. [Google Scholar]
- 35.Martínez FA, Guana Añasco LG, Resoagli EH. The species of Dasypodidae as reservoirs of Chagas disease in northeastern Argentina. Gac. Vet. (Buenos Aires) 1983;45:376–383. [Google Scholar]
- 36.Bruno N, Cuéllar E. Feeding habits of five armadillos in the Bolivian Chaco. In: Cabrera E, Mercolli C, Resquín R, editors. Manejo de Fauna Silvestre en Amazonía y Latinoamérica. Asunción, Paraguay: CITES Paraguay-Fundación Moisés Bertoni, Universidad de Florida; 2000. pp. 401–441. [Google Scholar]
- 37.Mazza S, Schreiber F. Finding in the Obligado Department, Santa Fe, of another species of mustelid naturally infected by Schizotrypanum cruzi, of infected Triatoma infestans in opossum nests, of infected Triatoma platensis in parrot nests and of Psammolestes coreodes without infestation in dendrocolaptid nests. Misión de Estudios de Patología Regional Argentina. Universidad de Buenos Aires. 1938;34:17–35. [Google Scholar]
- 38.Travi BL, Jaramillo C, Montoya J, Segura I, Zea A, Goncalves A, Velez ID. Didelphis marsupialis, an important reservoir of Trypanosoma (Schizotrypanum) cruzi and Leishmania (Leishmania) chagasi in Colombia. Am J Trop Med Hyg. 1994;50:557–565. doi: 10.4269/ajtmh.1994.50.557. [DOI] [PubMed] [Google Scholar]
- 39.Grisard EC, Carvalho-Pinto CJ, Scholz AF, Toma HK, Schlemper BR, Jr, Steindel M. Trypanosoma cruzi infection in Didelphis marsupialis in Santa Catarina and Arvoredo Islands, southern Brazil. Mem Inst Oswaldo Cruz. 2000;95:795–800. doi: 10.1590/s0074-02762000000600008. [DOI] [PubMed] [Google Scholar]
- 40.Rozas M, Botto-Mahan C, Coronado X, Ortiz S, Cattan PE, Solari A. Short report: Trypanosoma cruzi infection in wild mammals from a chagasic area of Chile. Am J Trop Med Hyg. 2005;73:517–519. [PubMed] [Google Scholar]
- 41.Duffy T, Bisio M, Altcheh J, Burgos JM, Diez M, Levin MJ, Favaloro RR, Freilij H, Schijman AG. Accurate real-time PCR strategy for monitoring bloodstream parasitic loads in chagas disease patients. PLoS Negl Trop Dis. 2009;3:e419. doi: 10.1371/journal.pntd.0000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaunt M, Miles M. The ecotopes and evolution of triatomine bugs (triatominae) and their associated trypanosomes. Mem Inst Oswaldo Cruz. 2000;95:557–565. doi: 10.1590/s0074-02762000000400019. [DOI] [PubMed] [Google Scholar]
- 43.Marcili A, Lima L, Valente VC, Valente SA, Batista JS, Junqueira AC, Souza AI, da Rosa JA, Campaner M, Lewis MD, Llewellyn MS, Miles MA, Teixeira MM. Comparative phylogeography of Trypanosoma cruzi TCIIc: new hosts, association with terrestrial ecotopes, and spatial clustering. Infect Genet Evol. 2009;9:1265–1274. doi: 10.1016/j.meegid.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Abba AM, Cassini M, Vizcaíno S. Effects of land use on the distribution of three species of armadillos (Mammalia, Dasypodidae) in the pampas, Argentina. J Mammal. 2007;88:502–507. [Google Scholar]
- 45.Noss A, Cuéllar E, Cuéllar R. Hunter self-monitoring as a basis for biological research: data from the Bolivian Chaco. Mastozool Neotrop. 2003;10:49–67. [Google Scholar]
- 46.Enríquez GF, Cardinal MV, Orozco MM, Lanati L, Schijman AG, Gürtler RE. Discrete typing units of Trypanosoma cruzi identified in rural dogs and cats in the humid Argentinean Chaco. Parasitology. 2012;140:303–308. doi: 10.1017/S003118201200159X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chapman MD, Baggaley RC, Godfrey-Fausset PF, Malpas TJ, White G, Canese J, Miles MA. Trypanosoma cruzi from the Paraguayan Chaco: isoenzyme profiles of strains isolated at Makthlawaiya. J Protozool. 1984;31:482–486. doi: 10.1111/j.1550-7408.1984.tb02999.x. [DOI] [PubMed] [Google Scholar]
- 48.Diotaiuti L, Pereira AS, Loiola CF, Fernandes AJ, Schofield JC, Dujardin JP, Dias JC, Chiari E. Inter-relation of sylvatic and domestic transmission of Trypanosoma cruzi in areas with and without domestic vectorial transmission in Minas Gerais, Brazil. Mem Inst Oswaldo Cruz. 1995;90:443–448. doi: 10.1590/s0074-02761995000400002. [DOI] [PubMed] [Google Scholar]
- 49.Noireau F, Cortez MG, Monteiro FA, Jansen AM, Torrico F. Can wild Triatoma infestans foci in Bolivia jeopardize Chagas disease control efforts? Trends Parasitol. 2005;21:7–10. doi: 10.1016/j.pt.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 50.Ceballos LA, Piccinali RV, Marcet PL, Vazquez-Prokopec GM, Cardinal MV, Schachter-Broide J, Dujardin JP, Dotson EM, Kitron U, Gürtler RE. Hidden sylvatic foci of the main vector of Chagas disease Triatoma infestans: threats to the vector elimination campaign? PLoS Negl Trop Dis. 2011;5:e1365. doi: 10.1371/journal.pntd.0001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rolon M, Vega MC, Roman F, Gomez A, Rojas de Arias A. First report of colonies of sylvatic Triatoma infestans (Hemiptera: Reduviidae) in the Paraguayan Chaco, using a trained dog. PLoS Negl Trop Dis. 2011;5:e1026. doi: 10.1371/journal.pntd.0001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yeo M, Lewis MD, Carrasco HJ, Acosta N, Llewellyn M, da Silva Valente SA, de Costa Valente V, de Arias AR, Miles MA. Resolution of multiclonal infections of Trypanosoma cruzi from naturally infected triatomine bugs and from experimentally infected mice by direct plating on a sensitive solid medium. Int J Parasitol. 2007;37:111–120. doi: 10.1016/j.ijpara.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 53.Burgos JM, Begher SB, Freitas JM, Bisio M, Duffy T, Altcheh J, Teijeiro R, Lopez Alcoba H, Deccarlini F, Freilij H, Levin MJ, Levalle J, Macedo AM, Schijman AG. Molecular diagnosis and typing of Trypanosoma cruzi populations and lineages in cerebral Chagas disease in a patient with AIDS. Am J Trop Med Hyg. 2005;73:1016–1018. [PubMed] [Google Scholar]
- 54.Xavier SC, Roque AL, Lima Vdos S, Monteiro KJ, Otaviano JC, Ferreira da Silva LF, Jansen AM. Lower richness of small wild mammal species and chagas disease risk. PLoS Negl Trop Dis. 2012;6:e1647. doi: 10.1371/journal.pntd.0001647. [DOI] [PMC free article] [PubMed] [Google Scholar]