Abstract
Toxoplasmosis in humans and other animals is caused by the protozoan parasite Toxoplasma gondii. During the process of host cell invasion and parasitophorous vacuole formation by the tachyzoites, the parasite secretes Rhoptry protein 8 (ROP8), an apical secretory organelle. Thus, ROP8 is an important protein for the pathogenesis of T. gondii. The ROP8 DNA was constructed into a pVAX-1 vaccine vector and used for immunizing BALB/c mice. Immunized mice developed immune response characterized by significant antibody responses, antigen-specific proliferation of spleen cells, and production of high levels of IFN-γ (816 ± 26.3 pg/mL). Challenge experiments showed significant levels of increase in the survival period (29 days compared with 9 days in control) in ROP8 DNA vaccinated mice after a lethal challenge with T. gondii. Results presented in this study suggest that ROP8 DNA is a promising and potential vaccine candidate against toxoplasmosis.
Introduction
Toxoplasma gondii is an obligate intracellular parasite that is known to invade and replicate in almost nucleated cells including humans1; in humans, primary infection can lead to mild fever, lymphadenopathy, lethargy, and sore throat. More severe infection is observed in immunocompromised patients leading to myocarditis, hepatitis, splenomegaly, polymyositis, encephalitis, dermatomyositis, chorioretinitis, and cognitive impairment. In expectant women, the transplacental transmission of the parasite leads to abortion, malformations during the birth of the infant, neurological disorder, loss of sight, and sometimes fetal mortality2,3; in animals, the infection results in abortion generating huge economic losses all over the world and forms the major source of transmission to humans.4,5
Treatment of toxoplasmosis is difficult caused by toxic effects of the drugs and possible reinfection.6 Vaccination is considered the most potent and effective means of fighting the infection.7 By far the only existing vaccine for toxoplasmosis is the live, attenuated formulation using T. gondii S48 strain. However, this attenuated vaccine is considered inappropriate for use in livestock because of the risk of reversion of the infection by the most proliferative form of the parasite.8–10
The DNA vaccination is a method that uses the plasmid vector to express the target antigen. They are known to induce cellular and humoral immune response in animal models3,10–13 and considered to be safe, efficient, and cost effective. Immunization studies with plasmid DNA have been known to induce both cellular14,15 and humoral responses in T. gondii.16
The rhoptry proteins are secreted during host cell invasion of the parasite involved in the parasitophorous vacuole formation and associated with the host cell mitochondria.17 The Rhoptry protein 8 (ROP8) is one of the most abundant proteins belonging to the ROP2 family of proteins and contains a conserved Serine/Threonine kinase domain.18–20 However, rhoptry proteins are degenerate in key positions normally invariant in active eukaryotic kinases and catalytically inactive.21 Several rhoptry genes such as ROP1, 2, 16, and 18 have been evaluated for their potential as vaccine candidates against toxoplasmosis. However, the vaccine potential of ROP8 has not been evaluated.22 Therefore, in this study we evaluated the vaccine potential of ROP8 DNA by evaluating the vaccine-induced protective immune response against toxoplasmosis in mouse model.
Materials and Methods
Mice and parasites.
Six- to 8-week-old female BALB/c mice obtained from the University of Malaya, Animal Experimental Center were used in this study. Use of animals was approved by the Animal Care and Use Committee of the University of Malaya, Faculty of Medicine. Highly virulent tachyzoites (RH-strain) of T. gondii were maintained by successive intraperitoneal passage in mice. Parasites were collected from the intraperitoneal fluids, washed with phosphate buffered saline (PBS), and filtered through 3 μm polycarbonate filters. The filtered suspension was then centrifuged at 1,500 rpm for 10 min and resuspended in sterile PBS for further use. The freshly isolated parasites were also subjected to repeated freeze-thaw cycles to extract total lysate antigen (TLA).
Cloning of ROP8 in eukaryotic expression vector pVAX-1.
The genomic DNA of tachyzoites (RH-strain) was isolated using a kit from Qiagen (Valencia, CA). The 1,728 bp ROP8 gene (GenBank ID: AF011377) was amplified using gene-specific primers (forward primer 5′ CCCAAGCTTAGCATGGAATTTTCTGTGTTACG 3′ and reverse primer 5′ CGGAATTCTCATGCCGGTTCTCCATCAG 3′ containing the HindIII and EcoR1 restriction sites as underlined). The polymerase chain reaction (PCR) condition used was 95°C for 10 min for initial denaturation before 30 cycles of amplification, which consisted of denaturation at 94°C for 1 min, annealing at 60°C for 1 min, and elongation at 72°C for 2 min. Final extension of the reaction was carried out at 72°C for 10 min. The ROP8 gene was digested with HindIII and EcoR1 and ligated into the pVAX-1 vector (Invitrogen, USA) digested with the same restriction enzymes. The resulting construct ROP8-pVAX-1 was used for the vaccination studies. The ROP8 gene was also cloned into the pVAX-1-GFP vector and the resulting construct ROP8-pVAX-1-GFP was used for in vitro expression of ROP8 in Chinese hamster ovary (CHO) cells (Figure 1). The pVAX-1 vector meets all the guidelines required for the expression of target genes in animal models and fulfills the requirements by the U.S. Food and Drug Administration (FDA) for formulation of DNA vaccines3; the green fluorescent protein (GFP) gene was incorporated into the pVAX-1 vector to generate pVAX-1-GFP and ROP8 gene is fused to the N-terminus of GFP. Positive recombinant plasmids (ROP8-pVAX-1 and ROP8-pVAX-1-GFP) were extracted using a commercial kit (EndoFree Plasmid free Giga kit, Qiagen). The DNA concentration was quantified using Nano-drop at 260 and 280 nm and sent to a commercial laboratory (GenScript Inc., Piscataway, NJ) for sequencing.
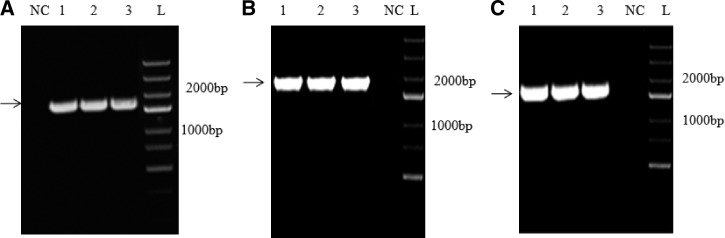
Figure 1.
Agarose gel electrophoresis for (A) polymerase chain reaction (PCR) amplification of ROP8 gene by using genomic DNA of Toxoplasma gondii as template; and (B) colony PCR confirmation for cloning ROP8 in pVAX-1-GFP vector; (C) colony PCR confirmation for cloning ROP8 in pVAX-1 vector. In panels A and B, lanes 1, 2, and 3 represent the amplified product of ROP8 (1191 bp) using gene-specific primers. Lane NC represents the negative control and M represents the DNA ladder.
In vitro expression of ROP8-pVAX-1-GFP in CHO cells.
The CHO cells were transfected with ROP8-pVAX-1-GFP plasmid using TurboFect (Fermentas Inc., Glen Burnie, MA), lipid-based transfection reagent. The transfected cells were observed under an inverted florescence microscope (Olympus, Hicksville, NY) at 24, 48, and 72 h post transfection for the expression of the GFP-tagged recombinant protein. The CHO cells transfected with pVAX-1-GFP vector was used as the control. The cells were harvested and total proteins were extracted and separated on a sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel. The separated proteins were also transferred by electroblotting onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad, USA) under a reducing condition. Blocking of the membranes was done by incubation in Tris-buffered saline (TBS), containing 5% skimmed milk overnight at 4°C. The membranes were washed three times with TBS-t (TBS containing 0.2% Tween-20), and then probed with GFP monoclonal antibody (Invitrogen, diluted 1:1000) or anti-T. gondii positive human sera diluted (1:250) in TBS containing 2.5% skimmed milk for 2 hours with constant shaking at room temperature. After three additional washes, the membrane was treated for 1 hour at room temperature with 1:2500 diluted biotin-labeled anti-mouse immunoglobulin G (IgG) and anti-human IgG (KPL Inc., Gaithersburg, MA), followed by streptavidin-AP (Invitrogen Corp., USA). Finally, the protein band was revealed by using NBT/BCIP (Sigma Chemical CO., USA) as the chromogenic substrate. The color was allowed to develop at room temperature in the dark. The membrane was dried and photographed.
DNA immunization and challenge.
Three groups of mice (15 mice per group) were immunized with 100 μg of plasmid DNA suspended in 100 μL sterile PBS by bilateral intramuscular injection into the quadriceps and boosted twice with the same dose at 2-week intervals. Group I was injected with ROP8-pVAX-1, group II with empty pVAX-1 vector as control, and group III with PBS as control. One day before the challenge, blood was collected from the tail vein of each animal and sera were stored at −20°C; twelve mice from each group were challenged intraperitoneally with 1 × 103 tachyzoites. These experiments were repeated twice. The mice sera were tested for presence of ROP8-specific antibodies by western blotting. The TLA proteins separated by SDS-PAGE were transferred by electroblotting as previously described. The membranes were probed with mice sera (diluted 1:250) and later treated with 1:2500 diluted biotin-labeled anti-mouse IgG followed by streptavidin-AP. The protein band was detected using NBT/BCIP substrate.
Cell proliferation assay.
Spleens of three mice from each group were aseptically removed 2 weeks after the last booster injection and single cell suspensions were prepared individually. After lysing the red blood cells with ACK lysis solution, splenocytes were resuspended in RPMI medium supplemented with 10% fetal calf serum, 0.5% gentamicin, and 1% glutamine. Cells were plated in 96-well Costar plates at a density of 2.5 × 105 cells per well and cultured with TLA of tachyzoites (10 μg/mL) and concanavalin A as positive control (5 μg/ml; Sigma) and medium alone as negative control at 37°C with 5% CO2 for 72 h. The proliferative activity was measured using BrdU colorimetric assay (Roche, Indianapolis, IN), according to the manufacturer's instructions. The stimulation index was calculated as the ratio of the average OD570 value of wells containing antigen-stimulated cells to the average OD570 value of wells containing only cells with medium. These experiments were repeated twice.
Cytokine assays.
Culture supernatants from splenocytes stimulated with the tachyzoite lysates were harvested 72 h after stimulation and assayed for levels of interleukin-4 (IL-4) and gamma-interferon (IFN-γ) using a commercial ELISA Kit (BD Science, San Jose, CA). Cytokine concentrations were determined by reference to standard curves constructed with known amounts of mouse recombinant IFN-γ, and IL-4.
Statistical analysis.
Data were analyzed using SPSS software (SPSS, Inc., Chicago, IL) and their statistical significance was determined by the analysis of variance test. A value of P < 0.05 was considered as significant.
Results
Expression of ROP8-pVAX-1-GFP plasmid in CHO cells.
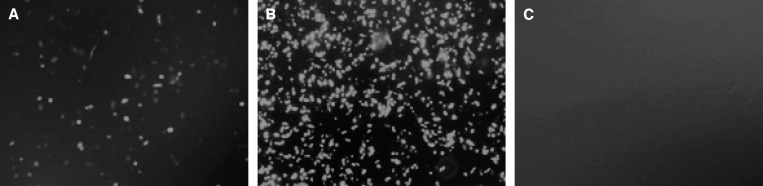
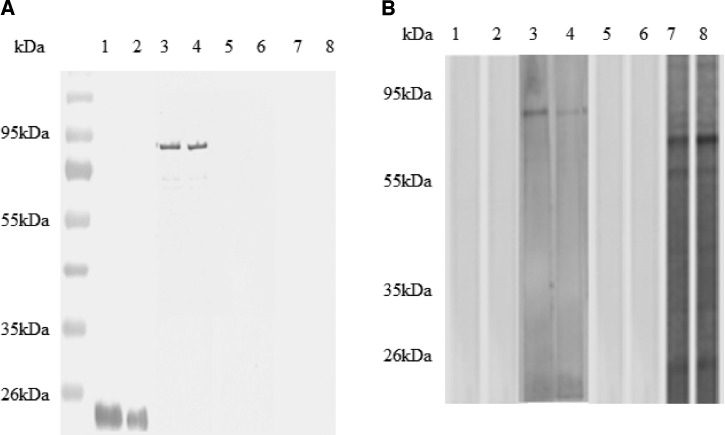
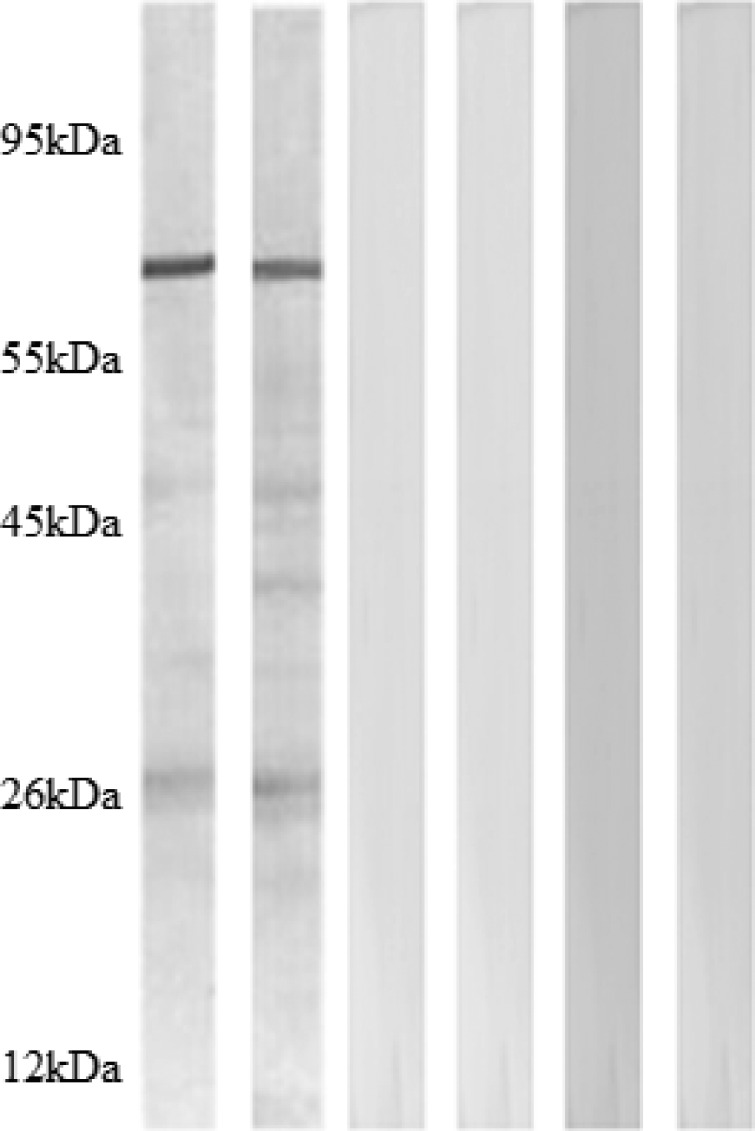
Expression of ROP8-pVAX-1-GFP plasmid was evaluated in CHO cells by fluorescence microscopy at 72 h after transfection (Figure 2). The results showed that CHO cells can be transfected with ROP8-pVAX-1-GFP and pVAX-1-GFP plasmids as evidenced by the green fluorescence. Subsequently, we confirmed the identity of the recombinant ROP8 (rROP8) by western blotting using GFP mAb or the serum from a toxoplasmosis-positive patient. The GFP mAb and patient serum detected the GFP tagged rROP8 at 92 kDa (Figure 3A and B).
Figure 2.
Expression analyses of ROP8 protein in transfected Chinese hamster ovary (CHO) cells at 72 h post-transfection (A) ROP8-pVAX-1-GFP protein expressed in CHO cells; (B) expression of pVAX-1-GFP as positive control; (C) transfection with no DNA as negative control.
Figure 3.
Immunoblot analysis of expression and antigenicity of ROP8 protein. Lysate of Chinese hamster ovary (CHO) cells transfected with pVAX-1-GFP (lanes 1 and 2), ROP8-pVAX-1-GFP (lanes 3 and 4), mock transfection (lanes 5 and 6), ROP8-pVAX-1 (lanes 7 and 8) were loaded into 12% sodium dodecyl sulfate (SDS) polyacrylamide gel, transferred to polyvinylidene difluoride (PVDF) membrane, and reacted with an anti-GFP mAb (A), and Toxoplasma gondii-infected human serum (B). Bound antibodies were detected using alkaline phosphatase conjugated goat anti-mouse IgG (A) and anti-human IgG (B), respectively. Positions of molecular size standards are indicated on the left of the panel.
Humoral immune response of ROP8-pVAX-1 in mice.
The sera obtained from mice (N = 15) of all the groups one day before the challenge were analyzed individually for the presence of specific ROP8 antibody by western blotting using TLA of the tachyzoites. We observed that only the serum from mice vaccinated with ROP8-pVAX-1 reacted with a protein of 65 kDa in the TLA of tachyzoites. The estimated size of the native ROP8 is 65 kDa (Figure 4). Only two strips for each group are presented.
Figure 4.
Detection of specific anti-ROP8 antibodies in sera of mice immunized with ROP8-pVAX-1, by western blotting using Toxoplasma gondii antigen. Reactivity of sera from mice immunized with, ROP8-pVAX-1 (lanes 1 and 2), empty plasmid pVAX-1 (lanes 3 and 4), and phosphate buffered saline (PBS) (lanes 5 and 6) are shown. Western blot for sera from all 12 mice were performed individually but only two strips for each group are presented. The positions and molecular masses (in kilodaltons) of protein standards are also shown.
Lymphoproliferative immune response.
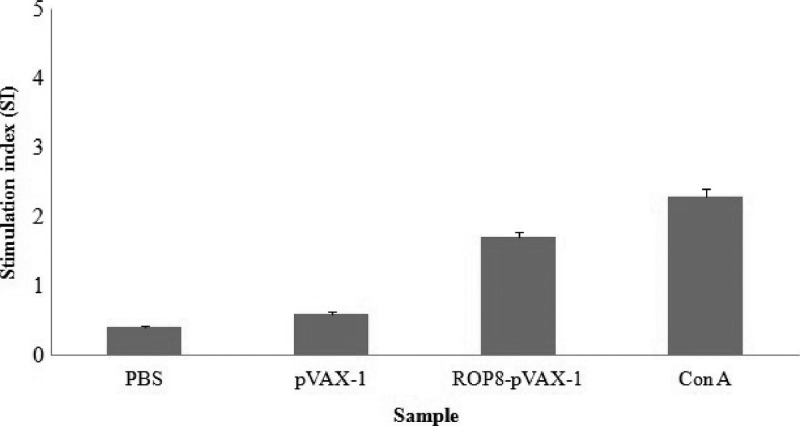
Spleen cells from mice immunized with ROP8-pVAX-1 2 weeks after the final injection showed significant proliferative response to TLA (1.7 ± 0.06) compared with the pVAX-1 control or PBS (P < 0.05) (Figure 5).
Figure 5.
The in vitro proliferation of splenocytes from mice vaccinated with ROP8-pVAX-1 after stimulation with total lysate antigen (TLA). Splenocytes from mice immunized with pVAX-1, PBS, ROP8-pVAX-1 were harvested 2 weeks after the third DNA booster. Following 72 h of stimulation with TLA, BrdU was added and then the stimulation index was calculated as the ratio of the average OD570 value of wells containing antigen-stimulated cells to the average OD570 value of wells containing only cells with medium.
Cytokine production.
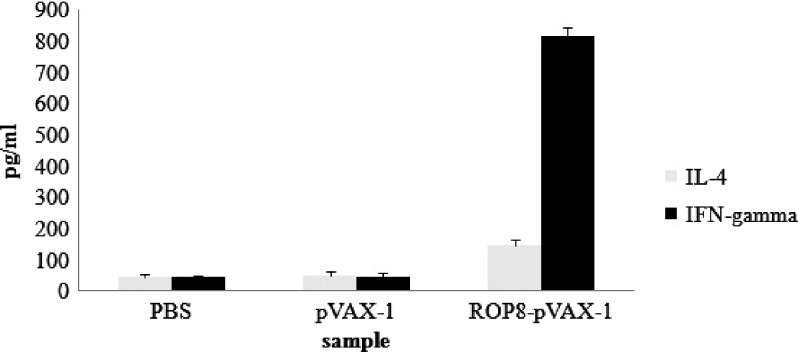
The TLA stimulated splenocyte culture supernatants from mice immunized with ROP8-pVAX-1 or pVAX-1 were evaluated for the production of IL-4 and IFN-γ. The result showed that splenocytes from mice immunized with ROP8-pVAX-1 secreted significantly high levels of IFN-γ (816 ± 26.3 pg/mL) compared with spleen cells cultured from mice immunized with pVAX-1 (48 ± 10.8) or PBS (45 ± 6.6). Only a low level of IL-4 was detected in the culture supernatant of splenocytes from mice immunized with ROP8-pVAX-1 (148 ± 18.3) but was significantly higher compared with mice immunized with pVAX-1 (50 ± 13.6) or PBS (47 ± 6.1) (Figure 6).
Figure 6.
Production of IFN-γ and IL-4 by splenocytes from mice vaccinated with ROP8-pVAX-1. Data represent the mean production of IFN-γ (A) and IL-4 (B) as determined by enzyme-linked immunosorbent assay (ELISA) of mice vaccinated with phosphate buffered saline (PBS), ROP8-pVAX-1, and pVAX-1 after 72 h of stimulation with total lysate antigen (TLA).
Analysis of protective immunity against T. gondii challenge.
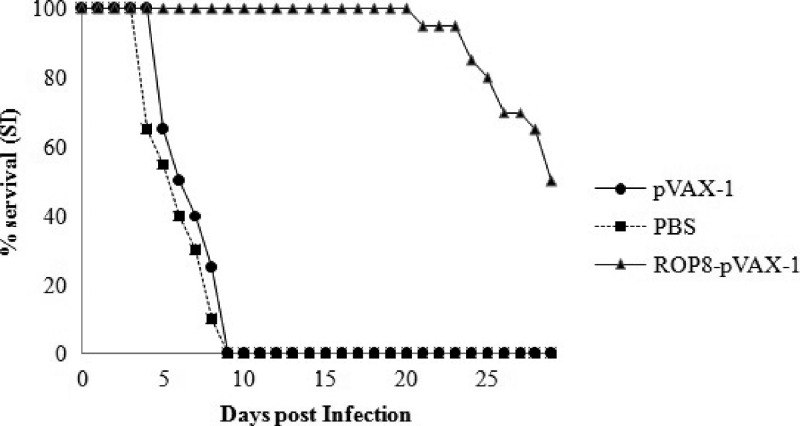
To evaluate the level of protection induced by ROP8-pVAX-1 immunization in mice against T. gondii infection, mice were challenged with live tachyzoites (RH strain). The survival curve for the three groups of mice (Figure 7) revealed that there was no difference in survival between the mice immunized with pVAX-1 and PBS. Average survival time was 9 days from the time of challenge. Mice immunized with ROP8-pVAX-1 DNA had a 100% survival rate until Day 9, whereas all the control mice died. On top of that, mice immunized with ROP8-pVAX-1 DNA showed a significant increase in the survival time (29 days, P < 0.05).
Figure 7.
Survival rate of mice immunized with ROP8-pVAX-1 (▲), pVAX-1 (●), and phosphate buffered saline (PBS) (■) when challenged with 1 × 103 tachyzoites, the number of mice for each group is 12.
Discussion
The data presented in this study show that ROP8 DNA vaccination developed significant humoral and cell-mediated immune response and confers significant protection in mice against a lethal challenge of the parasite.
Before performing the DNA vaccine study, we confirmed that the ROP8 gene can be expressed in eukaryotic cells by transfecting CHO cells. The recombinant rROP8-GFP expressed in CHO cells had an apparent molecular mass of 92 kDa that was 27 kDa greater than that of the native ROP8 protein found in the tachyzoite lysate. The difference in the molecular weight is caused by the fusion of the GFP protein (24 kDa) to the rROP8 along with the post-translational modifications associated with the recombinant protein. The identity of rROP8 in CHO cells was further confirmed using GFP mAb, again showing a single band at the same size. Mice immunized with the ROP8 DNA did not show any signs of adverse reaction. These immunized mice developed significant levels of anti-ROP8 IgG antibodies that recognize native ROP8 protein found in TLA of T. gondii in western blots. Our results thus show that DNA vaccination with ROP8 is immunogenic and generates specific antibodies.
We then asked the question, if this DNA immunization can confer protection against a challenge infection. Various T. gondii proteins such as the micronemal protein, dense granular protein, rhoptry proteins, matrix protein, and surface antigens have been evaluated as potential vaccine candidates for toxoplasmosis. Results presented in this study show that ROP8 is also a potential vaccine candidate. Mice immunized with ROP8-pVAX-1 survived for a prolonged period of time (29 days) after a challenging infection compared with the control groups. Previous studies using ROP16 and ROP18 DNA vaccination trials also gave a similar survival time of 21.6 ± 9.9 and 27.9 ± 15.1 days, respectively, in mice.4,7 Our studies show that compared with ROP16 and ROP18, the ROP8 DNA vaccination confers a higher degree of protection.
The IFN-γ dependent Th1-predominant immune response appears to be critical for protection against T. gondii.4,22 The ROP8 vaccinated animals in our studies showed that antigen-specific spleen cells secreted high levels of IFN-γ. We also found that there is a significant correlation between the presence of high levels of IFN-γ secreting cells and degree of protection conferred. Thus, our findings are in agreement with previous reports that Th1-mediated responses are highly protective in T. gondii infection.14,23,24
This study showed that a DNA vaccine encoding the T. gondii ROP8 protein is a simple and potent vaccine that elicits specific immune responses, and provides significant protection against a virulent strain of T. gondii. Therefore, ROP8 can be considered as a potential vaccine candidate against toxoplasmosis. It may be possible to provide complete protection against T. gondii infection by combining ROP8 with other immunogenic proteins as previously described, such as ROP16, ROP18, MIC3, SAG1, and GRA4.4,7,22,25,26
ACKNOWLEDGMENTS
We thank Diagnostic Laboratory (Para: SEAD), Department of Parasitology, University of Malaya and University Malaya Medical Centre (UMMC) for providing human serum samples.
Footnotes
Financial support: This work was supported by the University of Malaya IPPP grant (PS194/2009C) and UM High Impact Research Grant UM-MOHE (UM.C/HIR/MOHE/MED/02, E000013-20001) from the Ministry of Higher Education Malaysia.
Authors' addresses: Sonaimuthu Parthasarathy, Mun Yik Fong, and Yee Ling Lau, TIDREC, Department of Parasitology, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia, E-mails: parthawillbe@gmail.com, fongmy@um.edu.my, and lauyeeling@um.edu.my. Kalyanasundaram Ramaswamy, Department of Biomedical Sciences, College of Medicine, University of Illinois Rockford, Rockford, IL, E-mail: ramswamy@uic.edu.
References
- 1.Dubey JP. The history of Toxoplasma gondii–the first 100 years. J Eukaryot Microbiol. 2008;55:467–475. doi: 10.1111/j.1550-7408.2008.00345.x. [DOI] [PubMed] [Google Scholar]
- 2.Fatoohi A, Cozon G, Greenland T, Ferrandiz J, Bienvenu J, Picot S, Peyron F. Cellular immune responses to recombinant antigens in pregnant women chronically infected with Toxoplasma gondii. Clin Diagn Lab Immunol. 2002;9:704–707. doi: 10.1128/CDLI.9.3.704-707.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLeod R, Kieffer F, Sautter M, Hosten T, Pelloux H. Why prevent, diagnose and treat congenital toxoplasmosis? Mem Inst Oswaldo Cruz. 2009;104:320–344. doi: 10.1590/s0074-02762009000200029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuan ZG, Zhang XX, He XH, Petersen E, Zhou DH, He Y, Lin RQ, Li XZ, Chen XL, Shi XR. Protective immunity induced by Toxoplasma gondii rhoptry protein 16 against toxoplasmosis in mice. Clin Vaccine Immunol. 2011;18:119–124. doi: 10.1128/CVI.00312-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill D, Dubey J. Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microbiol Infect. 2002;8:634–640. doi: 10.1046/j.1469-0691.2002.00485.x. [DOI] [PubMed] [Google Scholar]
- 6.Bhopale GM. Development of a vaccine for toxoplasmosis: current status. Microbes Infect. 2003;5:457–462. doi: 10.1016/s1286-4579(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 7.Yuan ZG, Zhang XX, Lin RQ, Petersen E, He S, Yu M, He XH, Zhou DH, He Y, Li HX. Protective effect against toxoplasmosis in mice induced by DNA immunization with gene encoding Toxoplasma gondii ROP18. Vaccine. 2011;29:6614–6619. doi: 10.1016/j.vaccine.2011.06.110. [DOI] [PubMed] [Google Scholar]
- 8.Buxton D. Toxoplasmosis: the first commercial vaccine. Parasitol Today. 1993;9:335–337. doi: 10.1016/0169-4758(93)90236-9. [DOI] [PubMed] [Google Scholar]
- 9.Buxton D, Innes E. A commercial vaccine for ovine toxoplasmosis. Parasitology-Cambridge. 1995;110:11. doi: 10.1017/s003118200000144x. [DOI] [PubMed] [Google Scholar]
- 10.Jongert E, Roberts CW, Gargano N, Förster-Waldl E, Petersen E. Vaccines against Toxoplasma gondii: challenges and opportunities. Mem Inst Oswaldo Cruz. 2009;104:252–266. doi: 10.1590/s0074-02762009000200019. [DOI] [PubMed] [Google Scholar]
- 11.Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki V, Gromkowski SH, Deck RR, Dewitt CM, Friedman A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 12.Robinson H. DNA vaccines: basic mechanism and immune responses (Review) Int J Mol Med. 1999;4:549–550. doi: 10.3892/ijmm.4.5.549. [DOI] [PubMed] [Google Scholar]
- 13.Spencer JA, Smith BF, Guarino AJ, Blagburn BL, Baker HJ. The use of CpG as an adjuvant to Toxoplasma gondii vaccination. Parasitol Res. 2004;92:313–316. doi: 10.1007/s00436-003-1039-7. [DOI] [PubMed] [Google Scholar]
- 14.Denkers EY, Gazzinelli RT. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev. 1998;11:569–588. doi: 10.1128/cmr.11.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angus C, Klivington-Evans D, Dubey J, Kovacs JA. Immunization with a DNA plasmid encoding the SAG1 (P30) protein of Toxoplasma gondii is immunogenic and protective in rodents. J Infect Dis. 2000;181:317–324. doi: 10.1086/315186. [DOI] [PubMed] [Google Scholar]
- 16.Jongert E, De Craeye S, Dewit J, Huygen K. GRA7 provides protective immunity in cocktail DNA vaccines against Toxoplasma gondii. Parasite Immunol. 2007;29:445–453. doi: 10.1111/j.1365-3024.2007.00961.x. [DOI] [PubMed] [Google Scholar]
- 17.Carey KL, Jongco AM, Kim K, Ward GE. The Toxoplasma gondii rhoptry protein ROP4 is secreted into the parasitophorous vacuole and becomes phosphorylated in infected cells. Eukaryot Cell. 2004;3:1320–1330. doi: 10.1128/EC.3.5.1320-1330.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beckers C, Dubremetz JF, Mercereau-Puijalon O, Joiner KA. The Toxoplasma gondii rhoptry protein ROP 2 is inserted into the parasitophorous vacuole membrane, surrounding the intracellular parasite, and is exposed to the host cell cytoplasm. J Cell Biol. 1994;127:947–961. doi: 10.1083/jcb.127.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanks SK, Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 1995;9:576–596. [PubMed] [Google Scholar]
- 20.Sinai AP, Joiner KA. The Toxoplasma gondii protein ROP2 mediates host organelle association with the parasitophorous vacuole membrane. J Cell Biol. 2001;154:95–108. doi: 10.1083/jcb.200101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu W, Wernimont A, Tang K, Taylor S, Lunin V, Schapira M, Fentress S, Hui R, Sibley LD. Novel structural and regulatory features of rhoptry secretory kinases in Toxoplasma gondii. EMBO J. 2009;28:969–979. doi: 10.1038/emboj.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ismael AB, Sekkai D, Collin C, Bout D, Mévélec MN. The MIC3 gene of Toxoplasma gondii is a novel potent vaccine candidate against toxoplasmosis. Infect Immun. 2003;71:6222–6228. doi: 10.1128/IAI.71.11.6222-6228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gazzinelli R, Hakim F, Hieny S, Shearer G, Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991;146:286. [PubMed] [Google Scholar]
- 24.Jordan KA, Hunter CA. Regulation of CD8+ T cell responses to infection with parasitic protozoa. Exp Parasitol. 2010;126:318–325. doi: 10.1016/j.exppara.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mévélec MN, Bout D, Desolme B, Marchand H, Magné R, Bruneel O, Buzoni-Gatel D. Evaluation of protective effect of DNA vaccination with genes encoding antigens GRA4 and SAG1 associated with GM-CSF plasmid, against acute, chronical and congenital toxoplasmosis in mice. Vaccine. 2005;23:4489–4499. doi: 10.1016/j.vaccine.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 26.Desolme B, Mévélec MN, Buzoni-Gatel D, Bout D. Induction of protective immunity against toxoplasmosis in mice by DNA immunization with a plasmid encoding Toxoplasma gondii GRA4 gene. Vaccine. 2000;18:2512–2521. doi: 10.1016/s0264-410x(00)00035-9. [DOI] [PubMed] [Google Scholar]