Abstract
Anti-malaria interventions that rely on insecticides can be compromised by insecticide-resistance alleles among malaria vectors. We examined frequency changes of resistance alleles at two loci, knockdown resistance (kdr) and acetylcholinesterase-1 (ace-1), which confer resistance to pyrethroids and DDT, and carbamates, respectively. A total of 7,059 Anopheles gambiae sensu stricto mosquitoes were analyzed from multiple sites across continental Equatorial Guinea. A subset of sites included samples collected pre-intervention (2007) and post-intervention (2009–2011). Both L1014S and L1014F resistance alleles were observed in almost all pre-intervention collections. In particular, L1014F was already at substantial frequencies in M form populations (17.6–74.6%), and at high frequencies (> 50%) in all but two S form populations. Comparison before and throughout anti-vector interventions showed drastic increases in L1014F, presumably caused by intensified selection pressure imposed by pyrethroids used in vector control efforts. In light of these findings, inclusion of other insecticide classes in any anti-vector intervention can be considered prudent.
Introduction
Insecticide resistance poses a significant threat to the success of anti-malaria interventions that rely on indoor residual spraying (IRS) and insecticide-treated nets (ITNs). Resistance in the major Afrotropical malaria vector Anopheles gambiae to pyrethroids, DDT, and carbamate class insecticides includes target site resistance in specific genes that changes the sensitivity of the carrier to insecticides.1–3 DDT and pyrethroids insecticides target the voltage-gated sodium channel, and molecular characterizations have shown that various mutations in the S1–S6 trans-membrane segments of domain II of this gene provides resistance to these insecticides in a number of insect species while preserving voltage-gated sodium channel function in the presence of pyrethroid-class and DDT insecticides.4,5 In An. gambiae sensu stricto, two point mutations are present at amino acid position 1014 of the gene, both of which result in an amino acid substitution conferring reduced susceptibility to pyrethroids and DDT.
The L1014F mutation was first documented in An. gambiae sensu stricto mosquitoes from Côte d'Ivoire in 1998.1 Soon after, the L1014S mutation, was discovered in An. gambiae collected in Kenya.2 Subsequently, these two alleles became nominally associated with the regions where they were first detected and were called knockdown resistance-w (kdr-w) (L1014F) and kdr-e (L1014S), respectively. The L1014F allele results from a substitution of the leucine residue at that position with phenylalanine, whereas the L1014S allele is the result of a replacement with serine.1,2 Subsequent studies have shown that these mutations are not geographically restricted, but can be found within An. gambiae, as well as An. arabiensis populations.6–9 The L1014F allele is present in An. gambiae sensu lato mosquitoes as far east as Uganda and Ethiopia and conversely, the L1014S allele has been detected throughout west and central Africa.7,9–15
As reliance on pyrethroids and DDT for vector control operations has increased, kdr allele frequencies have risen across Africa, often to high levels. For example, Stump and others reported significant increases in kdr allele frequencies among An. gambiae collected from villages in western Kenya that received trial permethrin-treated bed net interventions.16 L1014S frequencies doubled from approximately 3–4% in An. gambiae mosquitoes collected in 1987 to approximately 8% in 2001 and 2002 among villages that received treated nets, whereas frequencies of the allele remained the same in non-intervention sites. In another study, Mathias and others evaluated spatio-temporal variations in kdr frequency in An. gambiae in the same two villages during 1996–2010, during which pyrethroid ITN interventions were dramatically scaled up. They reported “a sharp increase in homozygous frequencies from complete absence in both locations initially to 80.5% in Seme in 2008 and 91.7% for Asembo in 2010.”17 In a recent study on the impact of a large scale IRS and ITN campaign in southern Benin, kdr frequencies were also found to have increased, although this was also true for areas in which no planned interventions were implemented, underscoring the effect of the agricultural and household insecticide use on resistance.18
In east Africa, a similar increase has been observed. For example, in Uganda where DDT is used for IRS and deltamethrin-impregnated ITNs are the cornerstone of vector suppression efforts, a significant increase in L1014S frequencies was observed in An. gambiae sensu stricto in three out five sites during 2001–2002 and 2004–2006.19 In Ethiopia, L1014F allele frequencies in excess of 98% were found in An. arabiensis exposed to DDT, which was used for IRS and ITN control measures.20 In a follow-up study after DDT was discontinued in favor of deltamethrin, > 96% of An. arabiensis vectors were determined to be homozygous and 3.6% were found to be heterozygous for the L1014F allele.20 These studies demonstrate that where pyrethroids and DDT have been applied intensively as part of anti-vector interventions, selection of kdr alleles soon follows. As such, vigilant monitoring of resistance is requisite to inform operational decisions regarding pyrethroid and DDT use as anti-malaria efforts continue to scale up across Africa.
Anopheles gambiae sensu stricto is subdivided into two molecular forms, the M and the S form, that are widely considered to be incipient species.21 The kdr allele originated in the S molecular form of An. gambiae, and is at lower frequency in the M molecular form of An. gambiae in most locations. Nonetheless, an increase in kdr allele frequencies was also observed in An. gambiae M molecular forms in which kdr frequencies increased from 0.5% before initiation of a nationwide ITN distribution program in Niger to 7.2% two seasons later.22
To date, resistance mutations at the same location in the sodium channel gene have not been reported in An. funestus, another important malaria vector distributed throughout sub-Saharan Africa.23 However, a recent report of pyrethroid resistance in field-caught An. funestus mosquitoes from Uganda, concluded that although neither kdr allele was detected, a “correlation between haplotypes and resistance phenotype was observed indicating that mutations in other exons may be conferring the knockdown resistance in this species.”24 Knockdown alleles have been documented beyond Africa in species such An. stephensi and An. culicifacies, important malaria vectors throughout southern Asia and the Middle East,25–27
As kdr allele frequencies in An. gambiae populations have increased throughout Africa, IRS programs have sometimes switched to alternative insecticide classes such as carbamates and organophosphates. As such, the targets of carbamates and other insecticides have also been under heavy selection.28 One such target is the acetylcholinesterase-1 (ace-1R), which serves an essential role in neurotransmission in many arthropods and is targeted by several classes of insecticides, including carbamates and organophosphates.29,30 Target site resistance in this gene, the ace-1R allele in An. gambiae, is conferred through a single amino acid substitution from glycine to serine at residue 119.3 The ace-1R alleles are becoming increasingly prevalent in An. gambiae populations in west Africa.28,31 Reduced susceptibility to carbamate insecticides has been described through the use of bioassays and molecular detection of the ace-1R allele in An. gambiae M and S populations in Côte d'Ivoire, southern Benin, and Burkina Faso.32–36 Similar to the distribution of kdr alleles in continental west Africa, the ace-1R allele was far more prevalent in the S (32%) than the M (3.6%) molecular form.36 In Côte d'Ivoire, ace-1R allele frequencies have reached levels as high as 30.9% and 35.2% in M and S molecular forms, respectively.34 The geographic distribution of ace-1R alleles appears to be closely correlated with areas in which carbamate insecticides are applied as part of agricultural pest management, particularly in cotton and vegetable-growing areas in west Africa.35,36
In 2007, the Equatorial Guinea Malaria Control Initiative (EGMCI), a comprehensive anti-malaria program with a strong emphasis on anti-vector interventions, was initiated in continental Equatorial Guinea. Such interventions included indoor residual spraying of pyrethoids and carbamates in two provinces (Litoral and Kie N'tem) and distribution of ITNs containing deltamethrin in two other provinces (Centro Sur and Wele Nzas). The EGMCI also included monitoring of vector abundance, sporozoite rates, and insecticide-resistance alleles at multiple sites across continental Equatorial Guinea to inform operational decisions on vector suppression efforts.
A study performed in 2004–2005 provided the first report of the presence of the two resistance alleles (L1014S and L1014F) in An. gambiae sensu stricto populations in continental Equatorial Guinea.13 This study surveyed two sites, Miyobo located near the center of the country, where only the S molecular form was present, and a coastal site, Ngonamanga, where the M molecular form comprised nearly 90% of the collections. In Miyobo, the susceptible allele (L1014L) frequency was 75%, and L1014F and L1014S allele frequencies were 9% and 16% respectively. In Ngonamanga, the L1014L allele was present in 97% of all An. gambiae mosquitoes, with L1014F constituting the remaining 3%. No L1014S alleles were detected among the M molecular forms in this site. Among S forms, the L1014S and L1014F alleles were detected in 40% and 32% of mosquitoes collected, respectively, whereas the L1014L allele was detected in only 32% of S molecular form samples.13
In 2007, an extensive pre-intervention survey of vector abundance and insecticide resistance alleles was performed in anticipation of the launch of the EGMCI. Window-mounted exit-traps were used to collect > 4,800 An. gambiae sensu lato mosquitoes in > 30 sites throughout continental Equatorial Guinea.12 The L1014F and L1014S allele frequencies were as high as 59% and 19%, respectively, for S forms, but these allele frequencies were substantially lower for M molecular forms (9.7% for L1014F and 1.8% for L1014S). No ace-1R alleles were detected among any of the mosquitoes collected in these surveys.12 The results from this pre-intervention study provided an important baseline and guidance for the sampling strategy for this study.
We report a comparison of levels of kdr and ace-1R resistance alleles before and after the start of a large-scale pyrethroid and carbamate based anti-vector intervention in continental Equatorial Guinea. We analyzed 8,843 mosquitoes collected throughout continental Equatorial Guinea as part of the entomologic monitoring effort of the EGMCI. This analysis was conducted to evaluate whether levels of target site resistance at the kdr and ace-1R loci increased in response to the programmatic use of pyrethroids and carbamates. Studies of this nature provide important insights into the rate of increase in target site resistance under malaria control operations. Furthermore, we discuss the potential epidemiologic impacts of such resistance mechanisms in the current context as efforts to reduce the global burden of malaria intensify.
Methods
Mosquito sampling.
In 2007, before the start of the intervention activities, mosquitoes were collected in window traps affixed to the outside of homes in nine villages (Table 1 and Figure 1). During 2009–2011, mosquitoes were collected by using ultraviolet frequency–modified CDC light traps and human landing collections in residences from 18 sites located throughout continental Equatorial Guinea, including the nine pre-intervention sites (Table 1 and Figure 1).37
Table 1.
Entomologic monitoring sampling sites, geo-coordinates, and sample size of 2007 and 2009–2011 Anopheles gambiae molecular form (M and S) mosquito collections in continental Equatorial Guinea
| Location | Pre-intervention | Post-intervention | Total (2009–2011) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2007 | Total (2007) | 2009 | 2010 | 2011 | |||||||||
| Sentinel site | Province | Latitude (N) | Longitude (E) | M | S | M | S | M | S | M | S | ||
| Aconibe | Wele Nzas | 1°16.979′ | 10°54.072′ | – | 7 | 7 | – | 207 | – | 77 | 3 | 78 | 365 |
| Akurenam | Centro Sur | 1°2.819′ | 10°37.900′ | 1 | 230 | 231 | 21 | 235 | – | 109 | 7 | 208 | 580 |
| Anisok | Wele Nzas | 1°52.141′ | 10°45.007′ | 63 | 93 | 156 | 1 | 111 | – | 84 | 8 | 24 | 228 |
| Cogo | Litoral | 1°5.826′ | 9°43.745′ | 11 | 109 | 120 | 123 | 348 | 1 | 6 | 24 | – | 502 |
| Ebebiyin | Kie N'tem | 2°2.703′ | 11°18.757′ | 1 | 365 | 366 | 2 | 310 | – | 268 | 6 | 120 | 706 |
| Evinayong | Centro Sur | 1°20.973′ | 10°34.627′ | – | – | – | 10 | 123 | – | 14 | – | 58 | 205 |
| Mongomo | Wele Nzas | 1°38.673′ | 11°14.760″ | 1 | 10 | 11 | 3 | 99 | 1 | 363 | – | 476 | 942 |
| Ngolo | Litoral | 1°51.679′ | 9°47.441′ | 9 | 42 | 51 | 280 | 316 | 136 | 8 | 55 | 44 | 839 |
| Ukomba | Litoral | 1°50.604′ | 9°44.730′ | 142 | 40 | 182 | 60 | 69 | 353 | 11 | 50 | 19 | 562 |
| Yengue | Litoral | 2°12.630′ | 9°52.489′ | 298 | 209 | 507 | 19 | 202 | 32 | 42 | 35 | 169 | 499 |
| Total | 526 | 1,105 | 1,631 | 519 | 2,020 | 523 | 982 | 188 | 1,196 | 5,428 | |||
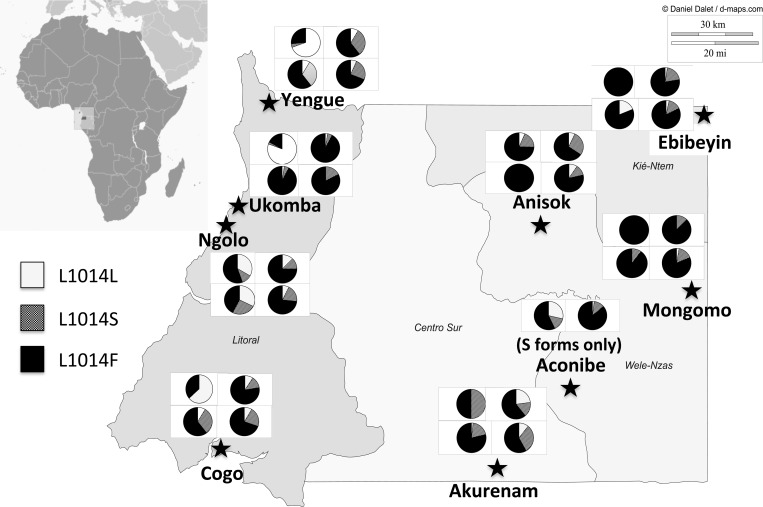
Figure 1.
Continental Equatorial Guinea showing sampling locations. Stars indicate sites for which pre-intervention and post-intervention sampling was conducted. White squares summarize knockdown resistance (kdr) allele frequencies as four pie charts for the M (top) and S (bottom) molecular forms for 2007 (left) and 2009–2011 (right) samples. Circles indicate sampling sites where only post-intervention samples were available.
The average pre- and post-intervention sample sizes were n = 91 and n = 401, respectively. Data from the village of Ayene was combined with a nearby village, Anisok (10–20 km) to increase the sample size. Sites without pre-intervention data were included in the study to provide a comprehensive picture of the spatial variation of kdr and ace-1R allele frequencies in the region.
Intervention activities.
Indoor residual spraying included eight and seven spray rounds in Litoral and Kie N'tem Provinces, respectively. Alpha-cypermethrin (a pyrethroid-class insecticide) was used during rounds 1–4 in Litoral Province and during rounds 1–3 in Kie N'tem Province. Bendiocarb (a carbamate-class insecticide) was applied in round 5 in Litoral Province and rounds 4 and 5 in Kie N'tem Province. Deltamethrin (a pyrethroid-class insecticide) was used during rounds 6–8 in Litoral Province and rounds 6 and 7 in Kie N'tem Province. Deltamethrin-impregnated ITNs were distributed in the two other provinces (Centro Sur and Wele Nzas). The IRS activities were supplemented with distribution of ITNs in two sites in Litoral province (Mbini, and Cogo), although bed net coverage was low in these sites.
Molecular genetic analyses.
Anopheline mosquitoes were initially identified to species complex based on morphology and stored in 80% ethanol before transport to the laboratory for molecular analyses. Heads and thoraces were dissected and subjected to DNA extraction by using the Nucleospin® 96 Tissue Core extraction kit (Macherey-Nagel and Company, Bethlehem, PA). Anopheles gambiae complex mosquitoes were identified to species and molecular form by using polymerase chain reaction (PCR) and restriction fragment length polymorphism assays.38,39 To determine the presence of kdr and ace-1R alleles, allelic discrimination assays described by Bass and others were performed with an ABI 7500 Fast quantitative PCR instrument (Applied Biosystems, Foster City, CA).40,41 The Sensimix II™ (Bioline USA Inc., Taunton, MA) master mixture was used for kdr and ace-1 allele detection assays. TaqMan® MGB™ was obtained from Applied Biosystems. Template DNA volume per reaction was 5 μL.
On some occasions, results from this assay were difficult to interpret, and to validate our genotype assignments, PCR amplicons were sequenced from a subset of these samples (n = 99). The kdr locus was amplified according to conditions described by Pinto and others with slight modifications.6 Amplicons were purified by using ExoSap-IT (New England BioLabs, Ipswich, MA) and sequenced in both directions by using PCR primers with an ABI3730 instrument (Applied Biosystems). Sequence data were analyzed by using Sequencher™ version 4.2.2 (Gene Codes Corporation, Ann Arbor, MI). The kdr sequences from four strains (GK45, R83, NG05, and GK05) were used as references (GenBank Accession nos. EU078895–U078898).6
The ace-1R allelic discrimination assay described by Bass and others was used to screen for susceptible (ace-1S) or resistant (ace-1R) alleles, with minor modifications (5 μL of DNA template was used per reaction).41 Homozygous susceptible and resistant ace-1 plasmid controls were used as controls. Heterozygous controls were created by mixing equimolar amounts of homozygous resistant and susceptible plasmid DNA.
Data analyses.
Field-derived geo-coordinates were plotted to create a collection site map by using d-maps.com.42 Allele and genotype frequencies for the kdr locus were calculated by using GENEPOP.43 Chi-square analyses were performed to determine differences in genotype frequencies between years. Hardy-Weinberg (HW) equilibrium analyses were performed on populations for which > 10 mosquitoes were sampled by using Arlequin (10,000 permutations).44 A Z-test of proportions was performed to determine relative changes in allele frequency proportions pre-intervention and post- intervention by using the In-Silico™ online statistical calculator.45
To explore a possible explanation for HW disequilibria in the kdr locus in some populations, microsatellite data for 16 loci for the M form (Ukomba, n = 93) and the S form (Yengue, n = 62) collected in 2007 were used to perform a Bayesian clustering analysis implemented in STRUCTURE version 2.3.3.46,47 Each STRUCTURE analysis was run for 500,000 generations, with a burn-in period of 100,000 generations by using correlated allele frequencies with no a priori population information. The number of potential distinct genetic clusters (K) examined ranged from 1 to 5 and 5 replicates were run for each K value. STRUCTURE Harvester was used to examine the STRUCTURE output.48
Results
A total of 7,059 An. gambiae mosquitoes were identified from our collections and analyzed for kdr and ace-1R alleles. The 2007 window trap collections provided 1,631 An. gambiae, and the 2009–2011 human landing catches and light trap collections provided 5,428 An. gambiae. In none of the An. gambiae mosquitoes collected before or following the intervention were any ace-1R alleles detected. Quantitative PCR calls were determined to be in agreement with sequence data in 96 (97.0%) of 99 samples.
The kdr allele frequencies for populations for which pre-intervention and post-intervention data were available are shown in Table 2 and Supplemental Table 1. The kdr alleles were already at considerable frequencies in An. gambiae populations in continental Equatorial Guinea before the intervention. The L1014S allele was detected in all but a single M form population (Cogo) (Table 2) and all S form populations (Table 2). Frequencies of this allele were much lower in the M form than in the S form, ranging from 0% to 19.0% in the M form and from 10.0% to 42.9% in the S form. Although L1014F frequencies were generally high, S and M form populations showed remarkable within-form variation in the frequency of this allele. This variation ranged in frequency from 17.6% to 74.6% in the M form, and from 27.1% to as high as 90.0% in S form. Consequently, the frequency of L1014L, also showed considerable variation before the intervention, as in 2007, ranged from 6.3% to 80.3% and from 0% to 61.5% in the M and S forms, respectively (Table 2).
Table 2.
Frequencies of kdr alleles in Anopheles gambiae M form (A) and S form (B) populations in continental Equatorial Guinea for which pre-intervention (2007) and post-intervention (2009–2011) data were available*
| Site (A) | Intervention | 2007 | 2009–2011 | 2007 | 2009–2011 | Trend | 2007 | 2009–2011 | Trend | 2007 | 2009–2011 | Trend |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2N | 2N | L1014L | L1014L | L1014L | L1014S | L1014S | L1014S | L1014F | L1014F | L1014F | ||
| Anisok | ITN | 126 | 18 | 6.3% | 11.1% | – | 19.0% | 5.6% | – | 74.6% | 83.3% | – |
| Cogo | IRS | 22 | 296 | 63.6% | 9.1% | Decrease | 0.0% | 13.2% | – | 36.4% | 77.7% | Increase |
| Ngolo | IRS | 18 | 942 | 33.3% | 12.4% | Decrease | 11.1% | 12.6% | – | 55.6% | 74.9% | – |
| Ukomba | IRS | 284 | 926 | 80.3% | 2.5% | Decrease | 2.1% | 5.2% | Increase | 17.6% | 92.3% | Increase |
| Yengue | IRS | 596 | 172 | 69.5% | 19.8% | Decrease | 4.0% | 7.0% | – | 26.5% | 73.3% | Increase |
| Average | 50.6% | 11.0% | 7.3% | 8.7% | 42.1% | 80.3% | ||||||
| Site (B) | Intervention | 2007 | 2009–2011 | 2007 | 2009–2011 | Trend | 2007 | 2009–2011 | Trend | 2007 | 2009–2011 | Trend |
| 2N | 2N | L1014L | L1014L | L1014L | L1014S | L1014S | L1014S | L1014F | L1014F | L1014F | ||
| Aconibe | ITN | 14 | 724 | 28.6% | 1.9% | Decrease | 14.3% | 11.7% | – | 57.1% | 86.3% | Increase |
| Akurenam | ITN | 460 | 1,104 | 23.0% | 10.4% | Decrease | 15.9% | 30.3% | Increase | 61.1% | 59.2% | – |
| Anisok | ITN | 176 | 438 | 6.8% | 8.9% | – | 27.8% | 12.1% | Decrease | 65.3% | 79.0% | Increase |
| Mongomo | ITN | 20 | 1,876 | 0.0% | 2.8% | – | 10.0% | 15.9% | – | 90.0% | 81.3% | – |
| Cogo | IRS | 218 | 1,068 | 61.5% | 8.0% | Decrease | 11.5% | 20.0% | Increase | 27.1% | 72.0% | Increase |
| Ebebiyin | IRS | 730 | 1,396 | 2.5% | 3.5% | – | 19.9% | 14.1% | Decrease | 77.7% | 82.4% | Increase |
| Ngolo | IRS | 84 | 736 | 2.4% | 3.1% | – | 42.9% | 15.1% | Decrease | 54.8% | 81.8% | Increase |
| Ukomba | IRS | 80 | 218 | 32.5% | 0.0% | Decrease | 25.0% | 25.7% | – | 42.5% | 74.3% | Increase |
| Yengue | IRS | 418 | 826 | 8.6% | 7.0% | – | 30.9% | 24.5% | Decrease | 60.5% | 68.5% | Increase |
| Average | 18.4% | 5.1% | 22.0% | 18.8% | 59.6% | 76.1% |
kdr = knockdown resistance; ITN = insecticide-treated net; IRS = indoor residual spraying. Significant differences between years for each of the three alleles (L1014L, L1014S, and L1014F) are indicated by increase or decrease in the Trend column. P values are shown in Supplemental Table 1.
The L1014S allele showed a higher frequency in the S form in all five locations for which data from both forms were available. However, this difference was significant only for Ngolo, Ukomba, and Yengue (P < 0.011, by Z-test). Such a pattern was not observed for the L1014F allele, for which only two of the five locations showed a significantly higher frequency for the S form (Ukomba and Yengue; P < 0.0001, by Z-test). In the other three populations, M form samples had a higher frequency of L1014F, although these differences were not significant.
After several years of pyrethroid application in the region, the frequency of L1014F increased in all five M form populations for which pre-intervention and post-intervention data were available (73.3% and 100%, respectively, in 2009–2011) (Table 2 and Supplemental Figure 3). This increase was significant in three of the five populations. The two populations (Anisok and Ngolo) for which the increase was not significant had low sample sizes for one of the collection years. In only one M form population (Yengue), a barely significant increase in L1014S frequency was observed.
An increase in combined kdr allele frequencies and a corresponding reduction in the susceptible allele L1014L was also observed in all S form populations in which L1014L was still present at a frequency > 10% in 2007 (Aconibe, Akurenam, Cogo. and Ukomba; P < 0.001, by Z-test) (Table 2 and Supplemental Figure 4). In the S form in Cogo, the most extreme example, the frequency of L1014F and L1014S increased from 27.1% to 72.0%, and from 11.5% to 20.0%, respectively. However, if L1014L was already at low frequency in pre-intervention samples, no significant decreases in L1014L were observed. Populations that were subjected to IRS and ITNs showed increases in L1014S and/or L1014F, and no obvious difference between the two control methods on the increase of kdr alleles was evident in our data.
To determine whether changes in kdr allele frequencies are best explained by the action of genetic drift rather than selection, we examined changes in allele frequencies for 16 microsatellite loci in the Mongomo, Yengue, and Ukomba populations during 2007–2010 (Supplemental Table 2).46 For this dataset, sample sizes ranged from 36 to 93, with averages of 57, 50, and 82, respectively. The average change in frequency for all microsatellite alleles was 0.040, 0.055, and 0.038, respectively. In contrast, the average change in the frequency of L1014S between 2007 and 2009–2011 was 0.282. We excluded populations that had a sample size < 50 for one of the time points in calculating the average increase in L1014F to avoid large sampling effects. In addition, in eight of the nine populations, the frequency of L1014F increased. It therefore is unlikely that the observed increase in L1014F is explained by genetic drift.
The kdr allele frequency data for populations for which only post-intervention data were available are shown in Table 3. In all thirteen M form populations sampled during 2009–2011, the combined kdr frequencies ranged between 81.2% and 100%, averaging 93.0% (Tables 2 and 3 and Supplemental Figure 3). This high frequency was mostly caused by L1014F, which averaged 81.8%, and ranged from 56.3% and 100% in 2009–2011. In the eighteen S form populations examined in 2009–2011, combined kdr frequencies ranged between 77.9% and 100%, averaging 94.6% (Tables 2 and 3 and Supplemental Figure 4). Similar to the M form, although to a lesser extent, this finding was also caused by high frequencies of L1014F, which ranged from 57.4% to 86.1%, averaging 72.2%.
Table 3.
Frequencies of kdr alleles in Anopheles gambiae M molecular form (A) and S molecular form (B) populations in continental Equatorial Guinea, 2009–2011, following several years of vector control*
| Site (A) | Intervention | 2N | L1014L | 95% CI | L1014S | 95% CI | L1014F | 95% CI |
|---|---|---|---|---|---|---|---|---|
| Akurenam | ITN | 56 | 1.8% | 3.5 | 19.6% | 10.4 | 78.6% | 10.7 |
| Bicurga | ITN | 40 | 0.0% | – | 20.0% | 12.4 | 80.0% | 12.4 |
| Evinayong | ITN | 20 | 0.0% | – | 20.0% | 17.5 | 80.0% | 17.5 |
| Mongomo | ITN | 8 | 0.0% | – | 12.5% | 22.9 | 87.5% | 22.9 |
| Niefang | ITN | 16 | 18.8% | 19.1 | 25.0% | 21.1 | 56.3% | 24.3 |
| Ayamiken | IRS | 54 | 0.0% | – | 18.5% | 10.4 | 81.5% | 10.4 |
| Ebebiyin | IRS | 16 | 18.8% | 19.1 | 0.0% | – | 81.3% | 19.1 |
| Etofili | IRS | 40 | 0.0% | – | 22.5% | 12.9 | 77.5% | 12.9 |
| Mbini | IRS | 196 | 8.2% | 3.8 | 6.1% | 3.4 | 85.7% | 4.9 |
| Site (B) | Intervention | 2N | L1014L | 95% CI | L1014S | 95% CI | L1014F | 95% CI |
| Bicurga | ITN | 332 | 0.3% | 0.6 | 28.6% | 4.9 | 71.1% | 4.9 |
| Evinayong | ITN | 390 | 4.9% | 2.1 | 26.4% | 4.4 | 68.7% | 4.6 |
| Niefang | ITN | 634 | 9.3% | 2.3 | 32.6% | 3.7 | 58.0% | 3.8 |
| Nsork | ITN | 244 | 22.1% | 5.2 | 11.1% | 3.9 | 66.8% | 5.9 |
| Ayamiken | IRS | 476 | 0.8% | 0.8 | 39.1% | 4.4 | 60.1% | 4.4 |
| Etofili | IRS | 62 | 0.0% | – | 32.3% | 11.6 | 67.7% | 11.6 |
| Mbini | IRS | 484 | 4.1% | 1.8 | 13.8% | 3.1 | 82.0% | 3.4 |
| Micomeseng | IRS | 148 | 4.7% | 3.4 | 37.8% | 7.8 | 57.4% | 7.8 |
| Nsok Nsomo | IRS | 484 | 1.7% | 1.2 | 15.5% | 3.2 | 82.9% | 3.4 |
| Average | 5.3% | 26.4% | 68.3% |
kdr = knockdown resistance; CI = confidence interval; ITN = insecticide-treated net; IRS = indoor residual spraying.
In five S form populations (Anisok, Mongomo, Ebebiyin, Ngolo, and Yengue) and one M form population (Anisok), the L1014L allele frequency was low (< 8.6%) before the start of the intervention. In these six populations, L1014S and L1014F were present, but L1014F showed a much higher frequency (68.5–83.3%). Interestingly, in four of these six populations the L1014S frequency decreased significantly (P < 0.0006, by Z-test) after the start of the intervention, and the L1014F frequency increased significantly (P < 0.0001, by Z-test). In the two exceptions, Anisok (M form) and Mongomo (S form), few samples were available for 2007 or 2009–2011, and no significant differences between time points were observed for either kdr allele. Only two other populations showed significant differences in L1014S frequency between years (Ukomba, M form and Akurenam, S form), and in both these populations the frequency of this allele increased. However, frequencies of L1014L were relatively high in these two populations in 2007 (80.3% and 23.0%, respectively).
The kdr genotype frequencies for M form populations are shown in Table 4. Almost all M form samples did not show HW equilibrium. The 2007 samples showed an excess of L1014L and L1014F homozygotes. In 2009, the pattern was somewhat different. For example, Ukomba in 2009 had a deficit of L1014S homozygotes, whereas Ngolo had an excess of L1014S and L1014F homozygotes. However, in 2010, Ngolo had a slight deficit of L1014F homozygotes. In 2011, Ngolo again did not show HW equilibrium (although not after Bonferroni correction), with an excess of L1014L and L1014F homozygotes. Thus, although most samples did not show HW disequilibrium, there is no consistent pattern regarding what could be considered missing genotypes.
Table 4.
Genotype frequencies and P values for chi-square tests for deviation from Hardy-Weinberg equilibrium for Anopheles gambiae M molecular forms collected in continental Equatorial Guinea, in 2007 and 2009–2011*
| 2007 Pre-intervention | Genotype | P | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | L/L | L/S | S/S | L/F | S/F | F/F | N | HO | HE$ | FIS$ | |
| Anisok | 4 | 0 | 0 | 0 | 24 | 35 | 63 | 0.38 | 0.41 | 0.06 | < 0.001 |
| Cogo | 7 | 0 | 0 | 0 | 0 | 4 | 11 | 0.00 | 0.48 | 1.00 | 0.001 |
| Ukomba | 114 | 0 | 0 | 0 | 6 | 22 | 142 | 0.04 | 0.31 | 0.86 | < 0.001 |
| Yengue | 201 | 1 | 3 | 11 | 17 | 65 | 298 | 0.10 | 0.44 | 0.78 | < 0.001 |
| Total | 326 | 1 | 3 | 11 | 47 | 126 | 514 | ||||
| 2009 Post-intervention | Genotype | P | |||||||||
| Site | L/L | L/S | S/S | L/F | S/F | F/F | N | HO | HE$ | FIS$ | |
| Akurenam | 0 | 0 | 0 | 0 | 6 | 15 | 21 | 0.28 | 0.25 | −0.14 | 1.000 |
| Cogo | 5 | 0 | 2 | 5 | 31 | 80 | 123 | 0.29 | 0.34 | 0.15 | < 0.001 |
| Ngolo | 0 | 3 | 17 | 7 | 40 | 213 | 280 | 0.18 | 0.27 | 0.33 | < 0.001 |
| Ukomba | 0 | 0 | 0 | 0 | 43 | 17 | 60 | 0.72 | 0.46 | −0.55 | < 0.001 |
| Yengue | 1 | 4 | 0 | 0 | 5 | 9 | 19 | 0.47 | 0.57 | 0.17 | 0.005 |
| Total | 6 | 7 | 19 | 12 | 125 | 334 | 503 | ||||
| 2010 Post-intervention | Genotype | P | |||||||||
| Site | L/L | L/S | S/S | L/F | S/F | F/F | N | HO | HE$ | FIS$ | |
| Ngolo | 2 | 2 | 17 | 81 | 6 | 28 | 136 | 0.65 | 0.60 | −0.09 | < 0.001 |
| Ukomba | 1 | 0 | 0 | 15 | 5 | 332 | 353 | 0.06 | 0.06 | 0.07 | 0.286 |
| Yengue | 0 | 0 | 0 | 0 | 3 | 29 | 32 | 0.09 | 0.09 | −0.03 | 1.000 |
| Total | 3 | 2 | 17 | 96 | 14 | 389 | 521 | ||||
| 2011 Post-intervention | Genotype | P | |||||||||
| Site | L/L | L/S | S/S | L/F | S/F | F/F | N | HO | HE$ | FIS$ | |
| Cogo | 6 | 0 | 0 | 0 | 4 | 14 | 24 | 0.17 | 0.50 | 0.67 | < 0.001 |
| Ngolo | 10 | 0 | 0 | 0 | 0 | 45 | 55 | 0.00 | 0.30 | 1.00 | < 0.001 |
| Ukomba | 3 | 0 | 0 | 0 | 1 | 46 | 50 | 0.02 | 0.13 | 0.85 | < 0.001 |
| Yengue | 8 | 0 | 0 | 12 | 0 | 15 | 35 | 0.34 | 0.49 | 0.30 | 0.845 |
| Total | 27 | 0 | 0 | 12 | 5 | 120 | 164 | ||||
Significant P values are indicated in bold.
The kdr genotype frequencies for S form populations are shown in Table 5. Again, almost all populations were not in HW equilibrium. Frequently, this finding was caused by a deficiency of L1014S homozygotes (e.g., Akurenam in 2009 or Cogo in 2009), but in other cases an excess of L1014S homozygotes was observed (e.g., Ngolo in 2009). Again, no consistent pattern regarding missing genotypes emerged.
Table 5.
Genotype frequencies and P values for chi-square tests for deviation from Hardy-Weinberg equilibrium for Anopheles gambiae S molecular forms collected in continental Equatorial Guinea, 2007 and 2009–2011*
| 2007 Pre-intervention | Genotype | P | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | L/L | L/S | S/S | L/F | S/F | F/F | N | HO | HE$ | FIS$ | |
| Akurenam | 53 | 0 | 1 | 0 | 71 | 105 | 230 | 0.31 | 0.55 | 0.44 | < 0.001 |
| Anisok | 6 | 0 | 5 | 0 | 39 | 38 | 88 | 0.45 | 0.45 | −0.01 | < 0.001 |
| Cogo | 67 | 0 | 5 | 0 | 15 | 22 | 109 | 0.12 | 0.51 | 0.77 | < 0.001 |
| Ebebiyin | 9 | 0 | 9 | 0 | 127 | 220 | 365 | 0.35 | 0.35 | −0.02 | < 0.001 |
| Mongomo | 0 | 0 | 0 | 0 | 2 | 8 | 10 | 0.29 | 0.26 | −0.09 | 1.000 |
| Ngolo | 1 | 0 | 7 | 0 | 22 | 12 | 42 | 0.52 | 0.52 | 0.00 | 0.019 |
| Ukomba | 13 | 0 | 5 | 0 | 10 | 12 | 40 | 0.21 | 0.66 | 0.68 | < 0.001 |
| Yengue | 14 | 1 | 18 | 7 | 92 | 77 | 209 | 0.48 | 0.53 | 0.10 | < 0.001 |
| Total | 163 | 1 | 50 | 7 | 378 | 494 | 1,093 | ||||
| 2009 Post-intervention | Genotype | P | |||||||||
| Site | L/L | L/S | S/S | L/F | S/F | F/F | N | HO | HE$ | FIS$ | |
| Aconibe | 0 | 0 | 1 | 2 | 46 | 158 | 207 | 0.231 | 0.217 | −0.08 | 0.470 |
| Akurenam | 2 | 0 | 3 | 2 | 141 | 87 | 235 | 0.608 | 0.448 | −0.36 | < 0.001 |
| Anisok | 0 | 0 | 1 | 0 | 18 | 92 | 111 | 0.16 | 0.165 | 0.02 | 1.000 |
| Cogo | 10 | 0 | 5 | 9 | 132 | 192 | 348 | 0.405 | 0.388 | −0.04 | < 0.001 |
| Ebebiyin | 5 | 1 | 5 | 1 | 70 | 228 | 310 | 0.232 | 0.261 | 0.11 | < 0.001 |
| Mongomo | 1 | 1 | 2 | 1 | 53 | 41 | 99 | 0.555 | 0.444 | −0.25 | < 0.001 |
| Ngolo | 1 | 3 | 18 | 9 | 49 | 236 | 316 | 0.193 | 0.277 | 0.30 | < 0.001 |
| Ukomba | 0 | 0 | 0 | 0 | 47 | 22 | 69 | 0.68 | 0.45 | −0.51 | < 0.001 |
| Yengue | 1 | 9 | 1 | 0 | 122 | 69 | 202 | 0.648 | 0.477 | −0.36 | < 0.001 |
| Total | 20 | 14 | 36 | 24 | 678 | 1,125 | 1,897 | ||||
| 2010 Post-intervention | Genotype | P | |||||||||
| Site | L/L | L/S | S/S | L/F | S/F | F/F | N | HO | HE$ | FIS$ | |
| Aconibe | 0 | 1 | 0 | 0 | 5 | 71 | 77 | 0.078 | 0.087 | 0.11 | 0.088 |
| Akurenam | 5 | 19 | 4 | 35 | 41 | 5 | 109 | 0.87 | 0.663 | −0.31 | < 0.001 |
| Anisok | 15 | 0 | 3 | 4 | 20 | 42 | 84 | 0.285 | 0.524 | 0.46 | < 0.001 |
| Ebebiyin | 3 | 1 | 4 | 1 | 82 | 177 | 268 | 0.313 | 0.307 | −0.02 | < 0.001 |
| Mongomo | 3 | 0 | 1 | 3 | 50 | 306 | 363 | 0.146 | 0.155 | 0.06 | < 0.001 |
| Ukomba | 0 | 0 | 0 | 0 | 0 | 11 | 11 | 0 | 0 | – | 1.000 |
| Yengue | 1 | 0 | 0 | 0 | 5 | 36 | 42 | 0.119 | 0.157 | 0.25 | 0.25 |
| Total | 27 | 21 | 12 | 43 | 203 | 648 | 954 | ||||
| 2011 Post-intervention | Genotype | P | |||||||||
| Site | L/L | L/S | S/S | L/F | S/F | F/F | N | HO | HE$ | FIS$ | |
| Aconibe | 3 | 3 | 4 | 2 | 20 | 46 | 78 | 0.32 | 0.42 | 0.25 | < 0.001 |
| Akurenam | 8 | 9 | 14 | 20 | 83 | 74 | 208 | 0.538 | 0.542 | 0.002 | |
| Anisok | 1 | 0 | 0 | 3 | 7 | 13 | 24 | 0.417 | 0.414 | −0.01 | 0.988 |
| Cogo | 21 | 6 | 8 | 6 | 48 | 91 | 180 | 0.333 | 0.511 | 0.35 | < 0.001 |
| Ebebiyin | 13 | 3 | 2 | 0 | 18 | 84 | 120 | 0.175 | 0.375 | 0.53 | < 0.001 |
| Mongomo | 6 | 3 | 15 | 24 | 155 | 273 | 476 | 0.382 | 0.379 | 0.00 | < 0.001 |
| Ngolo | 2 | 4 | 2 | 0 | 6 | 30 | 44 | 0.227 | 0.408 | 0.45 | < 0.001 |
| Ukomba | 0 | 0 | 2 | 0 | 5 | 12 | 19 | 0.263 | 0.371 | 0.30 | 0.235 |
| Yengue | 5 | 3 | 6 | 32 | 49 | 74 | 169 | 0.50 | 0.488 | −0.02 | 0.079 |
| Total | 59 | 31 | 53 | 87 | 391 | 697 | 1,318 | ||||
Significant P values are indicated in bold.
A possible explanation for the observed HW disequilibria could be population subdivision within M and S form populations. We therefore conducted a Bayesian clustering analyses by using microsatellite data from An. gambiae collected in Ukomba (M form) and Yengue (S form) in 2007.46 No subdivision was detected between these two populations (Supplemental Figures 1 and 2), although both exhibited significant deviation from HW equilibrium at the kdr locus in 2007.
Discussion
This study examined target site resistance against two insecticide classes in An. gambiae within an operational context in which IRS and ITN interventions were applied on a nationwide scale. We identified a dramatic increase in the L1014F allele in nearly all sites for which pre-intervention and post-intervention data were available, but no increase in the L1014S allele in the same sites. In sites for which only post-intervention data were available, the frequency of L1014F was high in 2009–2011 for M and S forms. These data are consistent with strong selection imposed on An. gambiae populations by use of pyrethroid insecticides in anti-malarial interventions also reported in other studies.16,18,22
Interestingly, if the combined pre-intervention frequency of L1014S and L1014F was high, L1014S frequencies decreased after the start of the intervention. These observations strongly suggest that L1014S can increase at the expense of the susceptible L1014L allele, but that it tends to decrease when competing primarily with L1014F. That being said, the S form population in Akurenam does not fully match the pattern described above because the frequency of L1014F remained the same while that of L1014S increased at the expense of L1014L. However, our results suggest that although L1014F and L1014S have a higher fitness than the susceptible L1014L in the presence of pyrethroid insecticides, the fitness of L1014F is substantially higher than that of L1014S, at least in the presence of alpha-cypermethrin and deltamethrin. From this result it follows that L1014F should have a larger impact on the efficacy of pyrethroid-based vector control. This idea is consistent with circumstantial evidence suggesting that L1014F provides more protection against pyrethroids.2,6,49,50 Furthermore, we should expect to see a further decrease in L1014S frequency in An. gambiae populations as L1014F gets closer towards fixation. However, we stress that additional data are needed to establish these inferences beyond doubt.
Although no programmatic use of pyrethroids was implemented on continental Equatorial Guinea before control activities, kdr was present at considerable frequencies in An. gambiae populations. Ridl and others reported the presence of L1014F and L1014S alleles in An. gambiae populations across continental Equatorial Guinea sampled in December 2006–July 2007.12 Although samples sizes were low for many of the populations included in that study, L1014F clearly had a much higher frequency than L1014S for both forms. This finding contrasts with results for two An. gambiae S form populations collected in 2004–2005, in which L1014S had a higher frequency than L1014F.13 However, in both these studies L1014F frequencies were much lower in the M form than we observed, ranging only between 0% and 3% and 7.5% and 18.2%, underscoring the impact of pyrethroid use on kdr frequencies. As was observed in our study, Ridl and others also detected considerable heterogeneity of kdr frequencies across continental Equatorial Guinea.12
These high pre-intervention levels of kdr alleles raise the question why these resistance alleles were present at such high frequencies in continental Equatorial Guinea. Unlike neighboring Cameroon, where agricultural uses of insecticides constitute an important proportion of pyrethroid use, Equatorial Guinea relies mostly on subsistence agriculture and has limited agro-industrial capacity.51 However, information on insecticide use for agricultural purposes is not available for Equatorial Guinea.52 Previous anti-vector interventions have been limited to intermittent bed net distributions with little, if any, follow-up monitoring for insecticide resistance conducted by the agencies responsible for their deployment.
The kdr alleles were most likely introduced into Equatorial Guinea through neighboring countries (Cameroon or Gabon). In four villages in southern Cameroon, L1014F and L1014S were present in S form populations in 2007, and L1014F had a considerably higher frequency than L1014S.30 These villages were sampled across a diverse landscape of urban and agro-industrial settings in which pyrethroids and other insecticides are commonly used for agricultural, household nuisance reduction and public health uses, and L1014F increased substantially during 2003–2007. However, L1014S was not found in four M form populations collected during 2003–2007, and only since 2007 was L1014F present in three of these M form populations.30
In Gabon, which borders Equatorial Guinea to the south, Pinto and others observed a much higher frequency of L1014S (63.0%) than L1014F (37.0%) in an S form population only approximately 100 km from the southern border of Equatorial Guinea.8 Therefore, Gabon provides another possible entry route for L1014F and/or L1014S. However, the pre-intervention frequencies of kdr alleles in continental Equatorial Guinea are much higher than can be explained by mosquito migration and several years of genetic drift. That is, a neutral allele that is introduced into a large population is unlikely to reach such a high frequency in a few years through genetic drift. Therefore, it is likely that kdr alleles were already under considerable selection pressure from pyrethroid use, which is readily available for pest control. Nonetheless, use of pyrethroids was greatly increased by initiation of the EGMCI, and L1014F frequencies in S and M form populations continued to increase and have reached high levels in a few years after the start of the campaign.
The main issue at hand is the effect of kdr alleles on the efficacy of pyrethroid-based vector control. The selection pressure by pyrethroid use on L1014F in particular is obviously strong, resulting in frequencies close to fixation after only a few years of interventions. A correlation between the kdr genotype and resistance phenotype has also been established.53 However, this correlation does not necessarily mean that the presence of kdr prevents pyrethroid-based vector control from having a large impact. For example, in Burundi, a vector control program based on pyrethroids greatly reduced malaria transmission despite the presence of the L1014S allele at a high frequency.54,55 In Côte d'Ivoire, where a L1014F allele frequency > 80% was observed in An. gambiae, pyrethroid-treated bed nets still provided a protective effect.1 In a recent experimental hut study conducted in Benin, pyrethroid-treated ITNs containing holes were shown to provide a significant reduction in the blood-feeding rate (42%) of highly kdr-resistant (84%) An. gambiae, suggesting that even when ITNs are compromised because of wear and tear, pyrethroids still offer some measure of protection to persons sleeping under them.56
Recently, a modeling study by Kiszewski and others quantified the expected effect of kdr on vector control efficacy (Kiszewski AE and others, unpublished data). That study showed that the presence of kdr during an IRS campaign results in a substantially higher entomologic inoculation rate compared with when the campaign was absent. However, the study also showed that IRS in the presence of kdr still is expected to provide a large reduction in the entomologic inoculation rate. Furthermore, Athrey and others examined the effect of IRS and ITN on the effective population sizes of three An. gambiae populations in Equatorial Guinea, and found that these were reduced approximately 57%, 82%, and 85% around the start of the control, despite the presence of kdr at high frequencies.46 Taken together, these findings indicate that anti-vector interventions based on pyrethroids do not cease to be effective solely because of the presence of kdr alleles, even though kdr is expected to have a negative impact on efficacy of control. That being said, a study with Culex pipiens provided a warning by showing a synergistic effect between kdr and metabolic resistance, resulting in high resistance against pyrethroids.57
The ace-1R gene, which provides resistance against carbamate insecticides, has been reported in An. gambiae populations in west Africa.28,34,36 For example, in Burkina Faso, ace-1R alleles have reached frequencies in excess of 30% in S form populations and 3% in M form populations.34 In Côte d'Ivoire, frequencies of this allele have reached 30.9% and 35.2% among M and S forms, respectively.36 Apparently this finding is caused mostly by agricultural use of carbamate- and organophosphate-class insecticides. Because kdr alleles were found throughout much of the range of An. gambiae shortly after their discovery, the presence of ace-1R was monitored in Equatorial Guinea. Fortunately, no ace-1R alleles were found in any of the pre-intervention and post-intervention samples analyzed. This finding is consistent with the absence of ace-1R in samples from Equatorial Guinea analyzed by Ridl and others,12 and the absence of the allele on Bioko Island, where carbamates have been used as part of an ongoing IRS campaign since 2005.58
The level of resistance provided by ace-1R to various carabamate insecticides is high and therefore has the potential to greatly undermine IRS efficacy.59 In addition, a duplicated allele, ace-1D was recently identified and found to reach frequencies ≤ 65% in Côte d'Ivoire and Burkina Faso, and is likely present in Benin.29 The implication of the ace-1R allele duplication is that it produces a permanent heterozygote, which lessens the fitness deficit attributed to ace-1R.29 Given the current absence of ace-1R, carbamate insecticides remain a suitable choice as part of IRS operations in Equatorial Guinea. However, given the potential of this form of target site resistance to spread and undermine carbamate-based vector control, it is advisable that An. gambiae populations in Equatorial Guinea continue to be monitored for the presence these alleles.
No population structure is evident within the molecular forms of An. gambiae on the geographic scale of this study.60,61 Based on available microsatellite datasets, we found no significant differentiation between the S form in Yengue and Mongomo, two populations 150 km apart.46 This finding could raise the question whether our populations represent independent samples. Considerable gene flow may prevent neutral microsatellite loci from diverging, but given that individual mosquito migration is typically estimated to be at most 2 km, it is difficult to conceive gene flow being high enough that kdr alleles selected for in one population increase correspondingly in a population 150 km apart in the short time span covered in this study.
In most of our collections, populations showed HW disequilibrium for the kdr locus. Several potential explanations could be offered for this observation. The first is possible technical error, which would result in some genotypes consistently not getting scored. Our sequencing validation indicates that this is not a likely explanation because even in genotype calls that we considered difficult, error rates were low. In addition, no clear pattern of specific missing genotypes emerged from our data. Another possible explanation could be previously undetected population subdivision within the M and S forms in Equatorial Guinea, an unlikely explanation that is ruled out by our Bayesian clustering analyses (Supplemental Figures 1 and 2). Finally, selection for specific genotypes in combination with variation in the frequency of alleles throughout the year might explain the observed deviations. It has been reported before that kdr allele frequencies can vary seasonally, and in our data, samples were pooled for an entire year.19,62,63 We also have shown that selection has acted strongly on this locus after the application of pyrethroid insecticides.
In conclusion, our analyses of An. gambiae mosquitoes collected during the vector monitoring component of the EGMCI, indicate that L1014F was already present at substantial frequencies before the start of a large pyrethroid-based IRS and ITN distribution campaign. However, L1014F increased dramatically within the first few years of vector control and is likely to reach fixation soon, if pyrethroid use in this region is continued. These results demonstrate the strong selection pressures exerted on this allele by pyrethroid use. Our data also suggest, although further evidence in needed, that L1014S does not provide the same level of protection against pyrethroids as L1014F because L1014F appears to outcompete L1014S in An. gambiae populations that have been exposed to pyrethroids.49,50 Based on previous studies, the presence of the kdr alleles has almost certainly had a detrimental impact on the efficacy of the vector control, and the incorporation of carbamates in later spray rounds can be considered prudent, given that no ace-1 alleles were detected. Because of reports that kdr in combination with metabolic resistance can provide high levels of resistance, future vector control efforts in the country should include monitoring of metabolic resistance.
Supplementary Material
ACKNOWLEDGMENTS
We thank the late Dr. Brian Sharp (Medical Research Council of South Africa) for his efforts in establishing the window trap collections before the start of intervention activities; the residents at the sampling sites for allowing us into their homes to collect mosquitoes; Moises Atue, Dr. Gloria Nseng, Dr. Luis Segura, Ed Aldrich, Lee Yellott, and Dr. Chris Schwabe for operational support in the field; Medical Care Development International Inc. and Marathon Oil Corporation for providing additional logistical support; Rafah Samir, Vasiliki Pappa, Rob Hallberg, James Shirvell, and Jack Dow for their valuable contributions in the laboratory; Dr. Julia Brown, Dr. Mark Sistrom, Chaz Hyseni, and Ben Evans for analysis support; the Malaria Research and Reference Reagent Resource Center (Manassas, VA) for providing genotyped reference mosquito strain RSP; J. Vulule, M.Q. Benedict, and KISIMU-1 for providing mosquito strain MRA-334; G. Davidson and V. Corbel for providing mosquito strain MRA-762; and Dr. Chris Bass for providing ace-1-susceptible and ace-1-resistant plasmids for use in the ace-1 quantitative PCR.
Footnotes
Author contributions: Michael R. Reddy conceived and planned the study, performed a portion of the pre-intervention collections, supervised the molecular analyses of all specimens, and composed the first draft of the manuscript; Adrian Godoy, performed molecular analyses of collected specimens and contributed to manuscript preparation; Kirstin Dion, supervised and performed molecular analyses and contributed to manuscript preparation; Abrahan Matias, coordinated and supervised the 2009–2011 field collections; Kevin Callender contributed statistical analysis assistance; Anthony E. Kiszewski participated in the study design, provided statistical analysis assistance and editorial input, and contributed to manuscript preparation; Immo Kleinschmidt designed the pre-intervention window trap monitoring system and facilitated the use of archived mosquito samples collected before initiation of intervention activities; Frances C. Ridl processed and archived mosquitoes collected during the pre-intervention period and assisted in the transfer of these specimens to the Yale laboratory for further molecular analysis; Jeffrey R. Powell planned and supervised the study and assisted in analysis of results; Adalgisa Caccone planned and supervised the study, assisted in analysis of results, and contributed to manuscript preparation; and Michel A. Slotman planned and supervised the study, assisted in analysis of results, and co-wrote the manuscript.
Financial support: This study was supported by an operational research grant awarded by the Bioko Island Malaria Control Program to Michael A. Slotman and Adalgisa Caccone. The Bioko Island Malaria Control Program is supported by a consortium led by Marathon Oil Corporation (Houston, TX) and the government of Equatorial Guinea. This study was also supported by EGMCI, which was supported through a grant from the Global Fund to Fight AIDS, Tuberculosis and Malaria.
Authors' addresses: Michael R. Reddy, Department of Epidemiology and Public Health, Yale University, New Haven CT, E-mail: michael.r.reddy@gmail.com. Adrian Godoy, Kirstin Dion, Jeffrey R. Powell, and Adalgisa Caccone, Department of Ecology and Evolutionary Biology, Yale University, New Haven CT, E-mails: adrian.godoy2008@gmail.com, kirstin.dion@yale.edu, jeffrey.powell@yale.edu, and adalgisa.caccone@yale.edu. Abrahan Matias, Medical Care Development International Inc., Silver Spring, MD, E-mail: amatias@mcd.org. Kevin Callender, Department of Psychology, Yale University, New Haven, CT, E-mail: kevin.callender@yale.edu. Anthony E. Kiszewski, Department of Natural and Applied Sciences, Bentley University, Waltham, MA, E-mail: akiszewski@bentley.edu. Immo Kleinschmidt, Medical Research Council Topical Epidemiology Group, London School of Hygiene and Tropical Medicine, London, UK, E-mail: immo.kleinschmidt@lshtm.ac.uk. Frances C. Ridl, Malaria Research Lead Programme, Medical Research Council, Durban, South Africa, E-mail: franridl@gmail.com. Michel A. Slotman, Department of Entomology, Texas A&M University, College Station, TX, E-mail: maslotman@tamu.edu.
References
- 1.Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Bergé JB, Devonshire AL, Guillet P, Pasteur N, Pauron D. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol. 1998;7:179–184. doi: 10.1046/j.1365-2583.1998.72062.x. [DOI] [PubMed] [Google Scholar]
- 2.Ranson H, Jensen B, Vulule JM, Wang X, Hemingway J, Collins FH. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol Biol. 2000;9:491–497. doi: 10.1046/j.1365-2583.2000.00209.x. [DOI] [PubMed] [Google Scholar]
- 3.Weill M, Malcolm C, Chandre F, Mogensen K, Berthomieu A, Marquine M, Raymond M. The unique mutation in ace-1 giving high insecticide resistance is easily detectable in mosquito vectors. Insect Mol Biol. 2004;13:1–7. doi: 10.1111/j.1365-2583.2004.00452.x. [DOI] [PubMed] [Google Scholar]
- 4.Zlotkin E. The insect voltage-gated sodium channel as target of insecticides. Annu Rev Entomol. 1999;44:429–455. doi: 10.1146/annurev.ento.44.1.429. [DOI] [PubMed] [Google Scholar]
- 5.Soderlund DM, Knipple DC. The molecular biology of knockdown resistance to pyrethroid insecticides. Insect Biochem Mol Biol. 2003;33:563–577. doi: 10.1016/s0965-1748(03)00023-7. [DOI] [PubMed] [Google Scholar]
- 6.Pinto J, Lynd A, Vicente JL, Santolamazza F, Randle NP, Gentile G, Moreno M, Simard F, Charlwood JD, do Rosário VE, Caccone A, Della Torre A, Donnelly MJ. Multiple origins of knockdown resistance mutations in the Afrotropical mosquito vector Anopheles gambiae. PLoS ONE. 2007;2:e1243. doi: 10.1371/journal.pone.0001243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Etang J, Fondjo E, Chandre F, Morlais I, Brengues C, Nwane P, Chouaibou M, Ndjemai H, Simard F. First report of knockdown mutations in the malaria vector Anopheles gambiae from Cameroon. Am J Trop Med Hyg. 2006;74:795–797. [PubMed] [Google Scholar]
- 8.Pinto J, Lynd A, Elissa N, Donnelly MJ, Costa C, Gentile G, Caccone A, do Rosário VE. Co-occurrence of east and West African kdr mutations suggests high levels of resistance to pyrethroid insecticides in Anopheles gambiae from Libreville, Gabon. Med Vet Entomol. 2006;20:27–32. doi: 10.1111/j.1365-2915.2006.00611.x. [DOI] [PubMed] [Google Scholar]
- 9.Verhaeghen K, Van Bortel W, Roelants P, Backeljau T, Coosemans M. Detection of the East and West African kdr mutation in Anopheles gambiae and Anopheles arabiensis from Uganda using a new assay based on FRET/Melt Curve analysis. Malar J. 2006;5:16–24. doi: 10.1186/1475-2875-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yewhalaw D, Bortel WV, Denis L, Coosemans M, Duchateau L, Speybroeck N. First evidence of high knockdown resistance frequency in Anopheles arabiensis (Diptera: Culicidae) from Ethiopia. Am J Trop Med Hyg. 2010;83:122–125. doi: 10.4269/ajtmh.2010.09-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santolamazza F, Calzetta M, Etang J, Barrese E, Dia I, Caccone A, Donnelly MJ, Petrarca V, Simard F, Pinto J, della Torre A. Distribution of knock-down resistance mutations in Anopheles gambiae molecular forms in west and west-central Africa. Malar J. 2008;7:74. doi: 10.1186/1475-2875-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridl FC, Bass C, Torrez M, Govender D, Ramdeen V, Yellot L, Edu AE, Schwabe C, Mohloai P, Maharaj R, Kleinschmidt I. A pre-intervention study of malaria vector abundance in Rio Muni, Equatorial Guinea: their role in malaria transmission and the incidence of insecticide resistance alleles. Malar J. 2008;7:194–203. doi: 10.1186/1475-2875-7-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreno M, Vicente JL, Cano J, Berzosa PJ, de Lucio A, Nzambo S, Bobuakasi L, Buatiche JN, Ondo M, Micha F, Do Rosario VE, Pinto J, Benito A. Knockdown resistance mutations (kdr) and insecticide susceptibility to DDT and pyrethroids in Anopheles gambiae from Equatorial Guinea. Trop Med Int Health. 2008;13:430–433. doi: 10.1111/j.1365-3156.2008.02010.x. [DOI] [PubMed] [Google Scholar]
- 14.Sharp BL, Ridl FC, Govender D, Kuklinski J, Kleinschmidt I. Malaria vector control by indoor residual insecticide spraying on the tropical island of Bioko, Equatorial Guinea. Malar J. 2007;6:52–59. doi: 10.1186/1475-2875-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Djègbè I, Boussari O, Sidick A, Martin T, Ranson H, Chandre F, Akogbéto M, Corbel V. Dynamics of insecticide resistance in malaria vectors in Benin: first evidence of the presence of L1014S kdr mutation in Anopheles gambiae from West Africa. Malar J. 2011;10:261. doi: 10.1186/1475-2875-10-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stump AD, Atieli FK, Vulule JM, Besansky NJ. Dynamics of the pyrethroid knockdown resistance allele in western Kenyan populations of Anopheles gambiae in response to insecticide-treated bed net trials. Am J Trop Med Hyg. 2004;70:591–596. [PubMed] [Google Scholar]
- 17.Mathias DK, Ochomo E, Atieli F, Ombok M, Bayoh MN, Olang G, Muhia D, Kamau L, Vulule JM, Hamel MJ, Hawley WA, Walker ED, Gimnig JE. Spatial and temporal variation in the kdr allele L1014S in Anopheles gambiae s.s. and phenotypic variability in susceptibility to insecticides in western Kenya. Malar J. 2011;10:10. doi: 10.1186/1475-2875-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Padonou GG, Sezonlin M, Ossé R, Aizoun N, Oké-Agbo F, Oussou O, Gbédjissi G, Akogbéto M. Impact of three years of large scale indoor residual spraying (IRS) and insecticide treated nets (ITNs) interventions on insecticide resistance in Anopheles gambiae s.l. in Benin. Parasit Vectors. 2012;5:72–82. doi: 10.1186/1756-3305-5-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verhaeghen K, Bortel WV, Roelants P, Okello PE, Talisuna A, Coosemans M. Spatio-temporal patterns in kdr frequency in permethrin and DDT resistant Anopheles gambiae s.s. from Uganda. Am J Trop Med Hyg. 2010;82:566–573. doi: 10.4269/ajtmh.2010.08-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.della Torre A, Fanello C, Akogbeto M, Dossou-Yovo J, Favia G, Petrarca V, Coluzzi M. Molecular evidence of incipient speciation within Anopheles gambiae s.s. in West Africa. Insect Mol Biol. 2001;20:9–18. doi: 10.1046/j.1365-2583.2001.00235.x. [DOI] [PubMed] [Google Scholar]
- 21.Yewhalaw D, Wassie F, Steurbaut W, Spanoghe P, Van Bortel W, Denis L, Tessema DA, Getachew Y, Coosemans M, Duchateau L, Speybroeck N. Multiple insecticide resistance: an impediment to insecticide-based malaria vector control program. PLoS ONE. 2011;6:e16066. doi: 10.1371/journal.pone.0016066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Czeher C, Labbo R, Arzika I, Duchemin JB. Evidence of increasing Leu-Phe knockdown resistance mutation in Anopheles gambiae from Niger following a nationwide long-lasting insecticide-treated nets implementation. Malar J. 2008;7:189–196. doi: 10.1186/1475-2875-7-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okoye PN, Brooke BD, Koekemoer LL, Hunt RH, Coetzee M. Characterisation of DDT, pyrethroid and carbamate resistance in Anopheles funestus from Obuasi, Ghana. Trans R Soc Trop Med Hyg. 2008;102:591–598. doi: 10.1016/j.trstmh.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 24.Morgan JC, Irving H, Okedi LM, Steven A, Wondji CS. Pyrethroid resistance in an Anopheles funestus population from Uganda. PLoS ONE. 2010;5:e11872. doi: 10.1371/journal.pone.0011872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Enayati AA, Vatandoost H, Ladonni H, Townson H, Hemingway J. Molecular evidence for a kdr-like pyrethroid resistance mechanism in the malaria vector mosquito Anopheles stephensi. Med Vet Entomol. 2003;17:138–144. doi: 10.1046/j.1365-2915.2003.00418.x. [DOI] [PubMed] [Google Scholar]
- 26.Singh OP, Bali P, Hemingway J, Subbarao SK, Dash AP, Adak T. PCR-based methods for the detection of L1014 kdr mutation in Anopheles culicifacies sensu lato. Malar J. 2009;8:154. doi: 10.1186/1475-2875-8-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh OP, Dykes CL, Das MK, Pradhan S, Bhatt RM, Agrawal OP, Adak T. Presence of two alternative kdr-like mutations, L1014F and L1014S, and a novel mutation, V1010L, in the voltage gated Na+ channel of Anopheles culicifacies from Orissa, India. Malar J. 2010;9:146. doi: 10.1186/1475-2875-9-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Djogbénou L, Dabiré R, Diabaté A, Kengne P, Akogbéto M, Hougard JM, Chandre F. Identification and geographic distribution of the ACE-1R mutation in the malaria vector Anopheles gambiae in south-western Burkina Faso, West Africa. Am J Trop Med Hyg. 2008;78:298–302. [PubMed] [Google Scholar]
- 29.Djogbénou L, Labbé P, Chandre F, Pasteur N, Weill M. Ace-1 duplication in Anopheles gambiae: a challenge for malaria control. Malar J. 2009;18:70–75. doi: 10.1186/1475-2875-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nwane P, Etang J, Chouaibou M, Toto JC, Kerah-Hinzoumbé C, Mimpfoundi R, Awono-Ambene HP, Simard F. Trends in DDT and pyrethroid resistance in Anopheles gambiae s.s. populations from urban and agro-industrial settings in southern Cameroon. BMC Infect Dis. 2009;30:163–171. doi: 10.1186/1471-2334-9-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Djogbénou L, Chandre F, Berthomieu A, Dabiré R, Koffi A, Alout H, Weill M. Evidence of introgression of the ace-1(R) mutation and of the ace-1 duplication in West African Anopheles gambiae s. s. PLoS ONE. 2008;3:e2172. doi: 10.1371/journal.pone.0002172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chandre F, Darriet F, Doannio JM, Rivière F, Pasteur N, Guillet P. Distribution of organophosphate and carbamate resistance in Culex pipiens quinquefasciatus (Diptera: Culicidae) in West Africa. J Med Entomol. 1997;34:664–671. doi: 10.1093/jmedent/34.6.664. [DOI] [PubMed] [Google Scholar]
- 33.N'Guessan R, Darriet F, Guillet P, Carnevale P, Traore-Lamizana M, Corbel V, Koffi AA, Chandre F. Resistance to carbosulfan in Anopheles gambiae from Ivory Coast, based on reduced sensitivity of acetylcholinesterase. Med Vet Entomol. 2003;17:19–25. doi: 10.1046/j.1365-2915.2003.00406.x. [DOI] [PubMed] [Google Scholar]
- 34.Ahoua Alou LP, Koffi AA, Adja MA, Tia E, Kouassi PK, Koné M, Chandre F. Distribution of ace-1R and resistance to carbamates and organophosphates in Anopheles gambiae s.s. populations from Côte d'Ivoire. Malar J. 2010;9:167–173. doi: 10.1186/1475-2875-9-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corbel V, N'Guessan R, Brengues C, Chandre F, Djogbenou L, Martin T, Akogbéto M, Hougard JM, Rowland M. Multiple insecticide resistance mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin, West Africa. Acta Trop. 2007;101:207–216. doi: 10.1016/j.actatropica.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Dabiré KR, Diabaté A, Namontougou M, Djogbenou L, Kengne P, Simard F, Bass C, Baldet T. Distribution of insensitive acetylcholinesterase (ace-1R) in Anopheles gambiae s.l. populations from Burkina Faso (West Africa) Trop Med Int Health. 2009;14:396–403. doi: 10.1111/j.1365-3156.2009.02243.x. [DOI] [PubMed] [Google Scholar]
- 37.Cohnstaedt LW, Gillen JI, Munstermann LE. Light-emitting diode technology improves insect trapping. J Am Mosq Control Assoc. 2008;24:331–334. doi: 10.2987/5619.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott J, Brogdon W, Collins F. Identification of single specimens of the Anopheles gambiae group by polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 39.Fanello C, Santolamazza F, della Torre A. Simultaneous identification of species and molecular forms of the Anopheles gambiae complex by PCR-RFLP. Med Vet Entomol. 2002;16:461–464. doi: 10.1046/j.1365-2915.2002.00393.x. [DOI] [PubMed] [Google Scholar]
- 40.Bass C, Nikou D, Donnelly MJ, Williamson MS, Ranson H, Ball A, Vontas J, Field LM. Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: a comparison of two new high-throughput assays with existing methods. Malar J. 2007;6:111–121. doi: 10.1186/1475-2875-6-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bass C, Nikou D, Vontas J, Donnelly MJ, Williamson MS, Field LM. The vector population monitoring tool (VPMT): high-throughput DNA-based diagnostics for the monitoring of mosquito vector populations. Malar Res Treat. 2010;2010:e190434. doi: 10.4061/2010/190434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dalet D. d-maps.com. http://d-maps.com/m/guineeeq/guineeeq50.pdf/ Available at. Accessed July 16, 2012.
- 43.Raymond M, Rousset F. GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered. 1995;86:248–249. [Google Scholar]
- 44.Excoffier L, Lischer HE. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour. 2010;10:564. doi: 10.1111/j.1755-0998.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 45.Joosse SA. Two-Proportion Z-Test Calculator. 2011. http://in-silico.net/statistics/ztest Available at.
- 46.Athrey G, Hodges TK, Reddy MR, Overgaard HJ, Matias A, Ridl FC, Kleinschmidt I, Caccone A, Slotman MA. The effective population size of malaria mosquitoes: large impact of vector control. PLoS Genet. 2012;8:e1003097. doi: 10.1371/journal.pgen.1003097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Earl DA, von Holdt BM. Structure Harvester: A Website and Program for Visualizing Structure Output and Implementing the Evanno Method. 2011. http://taylor0.biology.ucla.edu/struct_harvest/ Conserv Gene Resource. doi:10.1007/s12686-011-9548-7. Available at. Accessed March 4, 2012.
- 49.Reimer L, Fondjo E, Patchoké S, Diallo B, Lee Y, Ng A, Ndjemai HM, Atangana J, Traore SF, Lanzaro G, Cornel AJ. Relationship between kdr mutation and resistance to pyrethroid and DDT insecticides in natural populations of Anopheles gambiae. J Med Entomol. 2008;45:260–266. doi: 10.1603/0022-2585(2008)45[260:rbkmar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 50.Lynd A, Weetman D, Barbosa S, Egyir Yawson A, Mitchell S, Pinto J, Hastings I, Donnelly MJ. Field, genetic, and modeling approaches show strong positive selection acting upon an insecticide resistance mutation in Anopheles gambiae s.s. Mol Biol Evol. 2010;27:1117–1125. doi: 10.1093/molbev/msq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.CIA World Factbook - Equatorial Guinea. http://www.cia.gov/library/publications/the-world-factbook/geos/ek.html Available at. Accessed April 2, 2012.
- 52.Molina R, Benito A, Roche J, Blanca F, Amela C, Sanchez A, Alvar J. Baseline entomological data for a pilot malaria control program in Equatorial Guinea. J Med Entomol. 1993;30:622–624. doi: 10.1093/jmedent/30.3.622. [DOI] [PubMed] [Google Scholar]
- 53.Donnelly MJ, Corbel V, Weetman D, Wilding CS, Williamson MS, Black WC., IV Does kdr genotype predict insecticide-resistance phenotype in mosquitoes? Trends Parasitol. 2009;25:213–219. doi: 10.1016/j.pt.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 54.Protopopoff N, Verhaeghen K, Van Bortel W, Roelants P, Marcotty T, Baza D, D'Alessandro U, Coosemans M. A significant increase in kdr in Anopheles gambiae is associated with an intensive vector control intervention in Burundi highlands. Trop Med Int Health. 2008;13:1479–1487. doi: 10.1111/j.1365-3156.2008.02164.x. [DOI] [PubMed] [Google Scholar]
- 55.Protopopoff N, Van Bortel W, Marcotty T, Van Herp M, Maes P, Baza D, D'Alessandro U, Coosemans M. Spatial targeted vector control in the highlands of Burundi and its impact on malaria transmission. Malar J. 2007;6:158. doi: 10.1186/1475-2875-6-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ngufor C, N'Guessan R, Boko P, Odjo A, Vigninou E, Asidi A, Akogbeto M, Rowland M. Combining indoor residual spraying with chlorfenapyr and long-lasting insecticidal bed nets for improved control of pyrethroid-resistant Anopheles gambiae: an experimental hut trial in Benin. Malar J. 2011;10:343–349. doi: 10.1186/1475-2875-10-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hardstone MC, Leichter CA, Scott JG. Multiplicative interaction between the two major mechanisms of permethrin resistance, kdr and cytochrome P450-monooxygenase detoxification, in mosquitoes. J Evol Biol. 2009;22:416–423. doi: 10.1111/j.1420-9101.2008.01661.x. [DOI] [PubMed] [Google Scholar]
- 58.Overgaard HJ, Reddy VP, Abaga S, Matias A, Reddy MR, Kulkarni V, Schwabe C, Segura L, Kleinschmidt I, Slotman MA. Malaria transmission after five years of vector control on Bioko Island, Equatorial Guinea. Parasit Vectors. 2012;5:253. doi: 10.1186/1756-3305-5-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Djogbénou L, Weill M, Hougard JM, Raymond M, Akogbéto M, Chandre F. Characterization of insensitive acetylcholinesterase (ace-1R) in Anopheles gambiae (Diptera: Culicidae): resistance levels and dominance. J Med Entomol. 2007;44:805–810. doi: 10.1603/0022-2585(2007)44[805:coiaai]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 60.Moreno M, Salgueiro P, Vicente JL, Cano J, Berzosa PJ, de Lucio A, Simard F, Caccone A, Do Rosario VE, Pinto J, Benito A. Genetic population structure of Anopheles gambiae in Equatorial Guinea. Malar J. 2007;6:137. doi: 10.1186/1475-2875-6-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Slotman MA, Parmakelis A, Marshall JC, Awono-Ambene PH, Antonio-Nkondjo C, Simard F, Caccone A, Powell JR. Patterns of selection in anti-malarial immune genes in malaria vectors: evidence for adaptive evolution in LRIM1 in Anopheles arabiensis. PLoS ONE. 2007;2:e793. doi: 10.1371/journal.pone.0000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ndiath MO, Sarr JB, Gaayeb L, Mazenot C, Sougoufara S, Konate L, Remoue F, Hermann E, Trape JF, Riveau G, Sokhna C. Low and seasonal malaria transmission in the middle Senegal River basin: identification and characteristics of Anopheles vectors. Parasit Vectors. 2012;5:21. doi: 10.1186/1756-3305-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dery DB, Brown C, Asante KP, Adams M, Dosoo D, Amenga-Etego S, Wilson M, Chandramohan D, Greenwood B, Owusu-Agyei S. Patterns and seasonality of malaria transmission in the forest-savannah transitional zones of Ghana. Malar J. 2010;9:314. doi: 10.1186/1475-2875-9-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.