Abstract
Ciguatera fish poisoning is the most common marine food poisoning worldwide. It has been hypothesized that increasing seawater temperature will result in increasing ciguatera incidence. In St. Thomas, US Virgin Islands, we performed an island-wide telephone survey (N = 807) and a medical record review of diagnosed ciguatera cases at the emergency department of the sole hospital and compared these data with comparable data sources collected in 1980. Annual incidence from both recent data sources remained high (12 per 1,000 among adults in the telephone survey). However, the combined data sources suggest that incidence has declined by 20% or more or remained stable over 30 years, whereas seawater temperatures were increasing. Illness was associated with lower education levels, higher levels of fish consumption, and having previous episodes of ciguatera; population shifts from 1980 to 2010 in these factors could explain an incidence decline of approximately 3 per 1,000, obscuring effects from rising seawater temperature.
Introduction
Ciguatera fish poisoning is the most frequently reported illness associated with harmful algal blooms (HABs) and the most common marine food poisoning worldwide. There are an estimated 50,000 to 500,000 cases per year worldwide,1 making it an important public health concern. It has a global distribution and is endemic in regions where consumption of reef fish is common, particularly in the south Pacific and Caribbean. Affected individuals are classically described as presenting with initial gastrointestinal symptoms followed by neurologic symptoms and (in severe cases) cardiac manifestations. Chronic neurologic symptoms are often reported and may be ongoing or reappear after a period of presumed recovery.2 To prevent cases of ciguatera, it is critical to understand the environmental influences on ciguatera incidence as well as demographic and behavioral risk factors.
Ciguatera is caused by the ingestion of tropical reef fish that have accumulated potent neurotoxins (ciguatoxins) in their flesh and viscera. Ciguatoxins have their origins in precursor compounds called gambiertoxins produced by Gambierdiscus genus dinoflagellates, a type of microalgae. Gambiertoxins undergo biotransformation to ciguatoxins as they move through coral reef trophic levels from herbivorous to large carnivorous fish, where they are eventually consumed by humans.3 Because temperature directly impacts algae growth rates, it is commonly hypothesized that the incidence and geographical range of ciguatera and other HABs will be affected by climate change. In keeping with this hypothesis, positive associations have been found between seawater temperature and ciguatera incidence.4–7 One study also suggested that there is an upper temperature threshold that would limit Gambierdiscus growth and ciguatera incidence.6 However, studies have been concentrated in the South Pacific and largely dependent on public health reporting systems for estimates of incidence.
Members of our group were responsible for an island-wide incidence survey in St. Thomas, US Virgin Islands, in 1980, with survey data correlated with reported cases from the Emergency Department of the St. Thomas hospital (the only hospital on the island).8 In the intervening 30 years, seawater temperatures at St. Thomas have shown a steady increase. In this setting, we hypothesized that ciguatera incidence would show a similar increase across this 30-year period. To test this hypothesis, we undertook island population surveys and reviews of Emergency Department records to assess incidence and explore possible changes in risk factors for illness.
Materials and Methods
Telephone survey.
We performed two telephone surveys in St. Thomas in November of 2010 and October of 2011. We used random digit dialing to select a sample of listed and unlisted residential landline and cellular telephone numbers on the island. The sampling design was single stage, with each telephone number within the sample frame having an equal probability of selection. The household member aged 18 years or older with the next birthday was selected for interview. Households were screened to ensure that they were located on St. Thomas. Phone calls with no response were recontacted at least five times over the duration of the survey.
The survey was designed to take approximately 10 minutes to complete. We developed the questionnaire based on prior research in St. Thomas.8,9 It included questions on demographic characteristics, recent fish consumption (frequency, type of fish, and how obtained), history of ciguatera episodes in the participant and their household members, and ciguatera awareness. History of ciguatera was assessed with the questions: “Have you ever been poisoned by fish? If yes, how many times have you had fish poisoning in your lifetime?” In St. Thomas, the term fish poisoning is considered synonymous with ciguatera. The questionnaire was modified in 2011 to include the number of times that the participant had experienced fish poisoning in the past 5 years, which was the primary measure of incidence as described below.
Emergency department visits.
We also performed a medical record review to determine the number of patients diagnosed with ciguatera in the emergency department at Roy Schneider Hospital, the sole hospital on St. Thomas. All available records with a discharge diagnosis of ciguatera were identified from a database of emergency visits and reviewed by the study coordinator. Data on emergency department visits for pre-1980 were obtained from past research on the island.8–10 We were able to obtain annual counts of visits for 1971–1979 and 1995–2011, with gaps from 2000 to 2001 and 2006 because of unavailable records.
Incidence calculations.
Descriptive statistics were examined for all variables obtained in the telephone survey. We used two approaches to calculate incidence on St. Thomas.
Incidence from telephone survey.
We calculated the annual incidence rate among adults using the average number of episodes per year over the previous 5 years as obtained from the 2011 survey. The estimate was weighted for the age, education, and sex distributions in the 2000 Census,11 the most recent year for which data were available in US Virgin Islands.
Incidence from emergency department visits.
We also indirectly calculated the annual incidence using the counts of emergency department visits for ciguatera obtained from the medical record review divided by the proportion of telephone survey participants who visited the emergency department for their most recent ciguatera episode; 2000 Census data were used as the denominator of this incidence estimate. The same calculation was used for 1971–1979 using a weighted average of 1970 and 1980 Census data as the denominator.
Risk factor analysis.
To identify demographic and behavioral risk factors associated with increased odds of ciguatera illness in the telephone survey, we used univariable and multivariable logistic regression with lifetime history of at least one ciguatera episode as the outcome. Variables significant at the P ≤ 0.05 level were retained in the final model, and participants with missing values for these variables were excluded from the logistic analysis. We created a second logistic model for any ciguatera illness in the previous 5 years to assess age and history of past ciguatera illness as risk factors, because these variables could not be assessed in a lifetime model. Older individuals have a longer period of risk and therefore, would be more likely to experience ciguatera in their lifetime, regardless of whether age is a risk factor. Odds ratios and 95% confidence intervals were obtained for both models. Among those individuals with ciguatera in the telephone survey, we also used McNemar's test to assess differences between their most recent fish meal and the fish that was associated with their most recent ciguatera episode.
Behavioral and demographic changes from 1980 to 2010.
Finally, to determine whether the identified risk factors could be associated with the change in incidence over time, we compared the 1980 with 2010–2011 populations based on demographic information from the US Census and behavioral data from this survey and surveys performed in 1980.8,12 Separately for age, education, and fish consumption, we calculated strata-specific rates from the 2011 survey and applied them to the US Virgin Islands population in 1980 and 2000 to obtain a hypothetical total incidence for each time period. We then subtracted the estimate for 1980 from 2000 to estimate the hypothetical risk difference based on the observed changes in the population for the variables of interest. The purpose of this analysis was to preliminarily assess whether, if strata-specific rates remained the same, changes in the population demographics (age and education) and behavior (fish consumption) could explain the change in incidence over time. All analyses were performed with SAS version 9.2. Human subjects research for the telephone survey and medical record review was approved by the University of Florida and University of Maryland School of Medicine Institutional Review Boards.
Seawater temperature.
Sea surface temperature (SST) data were obtained from the Extended Reconstructed Sea Surface Temperature V3b dataset maintained by the National Ocean and Atmospheric Administration's Earth System Research Laboratory in the Physical Sciences Division.13 This dataset was chosen, because it provides continuous measurement back to 1971; however, the data source nearest the US Virgin Islands is located at the southeast coast of Puerto Rico. The temperature curve followed a similar pattern to temperature readings from 1990 to 2011 taken in the US Virgin Islands.
Results
Telephone survey.
Eight hundred and seven individuals participated in the telephone surveys (four hundred and seven in 2010 and four hundred in 2011). The combined response rate was 25% according to guidelines from the American Association for Public Opinion Research, version 3.114 and was consistent over the 2 years. Characteristics of the survey population are presented in Table 1. Of the participants, 186 (23%) reported that they had ever had ciguatera. Among these affected individuals, there were 339 total episodes. The majority (59%) reported only one episode in their lifetime, with 19% and 12% reporting two and three episodes, respectively; 10% reported 4 or more episodes, with a maximum of 10 episodes. Including illness in household members, 43 households (11%, 95% confidence interval [CI] = 8–14%) reported an episode of ciguatera in the past 5 years.
Table 1.
Characteristics of telephone survey participants in St. Thomas, US Virgin Islands, 2010 and 2011
| Variable | Total (N = 807) | |
|---|---|---|
| n | Percent | |
| Age (years) | ||
| 18–44 | 248 | 31 |
| 45–64 | 338 | 42 |
| 65+ | 154 | 19 |
| Not provided | 67 | 8 |
| Sex (female) | 498 | 62 |
| Education | ||
| Some high school or less | 114 | 14 |
| High school graduate | 240 | 30 |
| Some college or more | 420 | 52 |
| Not provided | 33 | 4 |
When asked about their most recent illness, 56 (30%, 95% CI = 21–39%) individuals reported visiting the emergency department, and 24 (13%, 95% CI = 8–18%) individuals reported seeing another physician. The symptoms caused the affected participants to “take to bed” for a mean of 2.0 days (95% CI = 1.6–2.4, range = 0–14) and miss work for a mean of 2.4 days (95% CI = 1.7–3.0, range = 0–21).
Emergency department visits.
A total of 1,829 cases of ciguatera at Roy Schneider Hospital were recorded from 1971 to 1979 (annual mean = 203), and 1,385 cases were recorded from 1995 to 2011 (with gaps from 2000 to 2001 and from 2005 to 2006, annual mean = 106 for 1995–2011 and 63 for 2007–2011).
Incidence.
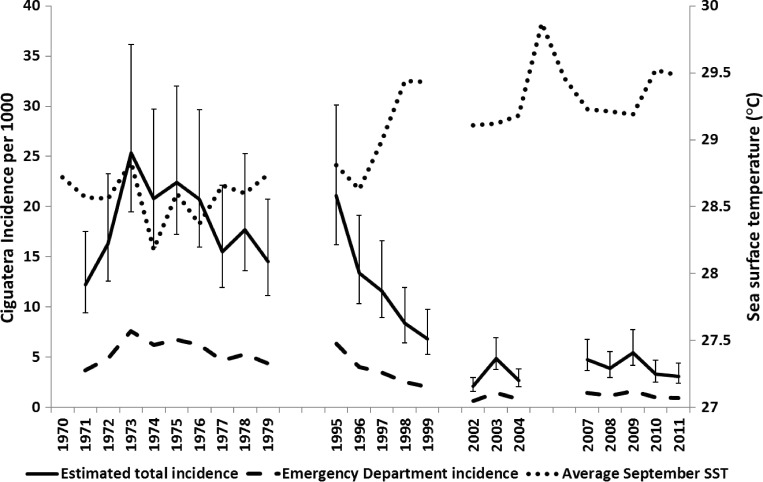
The annual incidence of ciguatera weighted to the 2000 Census was 12 per 1,000 (95% CI = 10–21) based on the 2011 telephone survey. The trend in indirect incidence estimates from the emergency department visits is shown in Figure 1. The average for 2007–2011 was 6 per 1,000 in adults (95% CI = 5–8), down from 18 per 1,000 in the 1970s as collected in 1980.
Figure 1.
Time series of ciguatera incidence in adult residents of St. Thomas based on emergency department (ED) visits* and sea surface temperature. *Total incidence estimates were calculated by dividing the ED incidence by the proportion of persons with ciguatera who visit the ED (30%). Error bars indicate 95% confidence intervals for incidence.
Risk factors.
Participants with less education were more likely to report a history of ciguatera illness (Table 2). Those participants born in the Caribbean but outside the US Virgin Islands had the highest odds of illness, whereas location of birth outside the Caribbean was associated with lower odds of illness, but the difference between these groups was not significant after adjusting for other factors. After excluding participants who reported never eating fish, because it is a prerequisite for illness, those participants who ate fish at least three times per week had 2.2 times higher odds than those participants who ate fish less than three times a week (95% CI = 1.4–3.5). Among participants reporting a ciguatera illness, the fish meal that caused their ciguatera episode was more likely to have been self-caught (versus purchased) than the fish meal they ate most recently (17% versus 6% of fish meals, P = 0.02).
Table 2.
Crude and adjusted logistic regression models for ciguatera illness in telephone survey participants in St. Thomas, US Virgin Islands
| Variable | Crude | Adjusted* | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Model 1: lifetime history of ciguatera illness (N = 720) | ||||
| Sex (female vs. male) | 0.7 | 0.5–1.1 | – | – |
| Location of birth | ||||
| US Virgin Islands | Ref. | Ref. | ||
| Caribbean, outside US Virgin Islands | 2.0 | 1.3–2.9 | 1.5 | 1.0–2.2 |
| Outside Caribbean | 0.7 | 0.4–1.1 | 0.6 | 0.4–1.1 |
| Education | ||||
| Some high school or less | Ref. | Ref. | ||
| High school graduate | 0.5 | 0.3–0.8 | 0.7 | 0.4–1.2 |
| Some college or more | 0.3 | 0.2–0.5 | 0.6 | 0.3–0.9 |
| Model 2: ciguatera episode in the past 5 years (N = 369) | ||||
| Previous ciguatera episode | 4.2 | 1.9–9.5 | 3.4 | 1.4–8.5 |
| Age (years) | ||||
| 18–44 | Ref. | Ref. | ||
| 45–64 | 2.2 | 0.7–7.0 | 1.6 | 0.5–5.3 |
| 65 or older | 3.1 | 0.9–10.4 | 1.5 | 0.4–5.6 |
Adjusted for location of birth, education, fish consumption, and age. Model 2 is also adjusted for previous ciguatera episode.
From the 2011 survey, having at least one previous episode of ciguatera more than 5 years ago increased the odds of an episode in the 5 years preceding the interview after controlling for age, frequency of fish consumption, education, and location of birth (odds ratio [OR] = 3.4, 95% CI = 1.4–8.5). Age was not significant in this model, particularly after adjusting for previous episodes (Table 2).
Behavioral and demographic changes from 1980 to 2010.
Because frequent fish consumption was identified as the risk factor with the highest odds of illness, we compared fish consumption patterns between the time periods (Table 3). The percentage of participants who said they did not eat fish increased from 8% in 198012 to 17% in 2010/2011. In parallel, the percentage of participants who ate fish at least three times per week decreased from approximately 33%9 to 12% over the same period. The difference in incidence rates from 1980 to 2010 if frequent fish consumption in the population did change by 21% was estimated as −3.1 per 1,000.
Table 3.
Ciguatera related behaviors in the 2010/2011 telephone survey and 1980 in St. Thomas, US Virgin Islands
| Variable | 2010/2011 survey (N = 807) | 1980 surveys | |||
|---|---|---|---|---|---|
| n | Percent | n | Percent | Ref. | |
| Household had at least one episode in past 5 years* | 43/400 | 11 | 22/100 | 22 | McMillan and Granade12 |
| Visited emergency room for most recent episode | 56 | 30 | 5/22; 5/7 | 23; 71 | McMillan and Granade12; Morris and others8 |
| Fish consumption | |||||
| Do not eat fish | 138 | 17 | 8/100 | 8 | McMillan and Granade12 |
| Three or more times per week | 99 | 12 | ∼22/67 | ∼33%† | Morris and others9 |
| Believe certain types of fish are poisonous | 502 | 62 | 39/54 | 72 | Morris and others9 |
| Avoid certain types of fish | 407 | 50 | |||
| Believe they can tell if a fish might be poisonous | 324 | 40 | 14/54 | 26 | Morris and others9 |
For current survey collected only in 2011.
Published for emergency department cases only for 1980 data; survey controls not significantly different.
The types of fish consumed that were not associated with poisoning displayed considerable overlap between the two time periods. In 1980, olewife/triggerfish (46%), king mackerel (31%), grouper (28%), doctorfish (26%), red hind (24%), yellowtail snapper (19%), parrotfish (19%), and grunt (13%) made up the most commonly eaten fish consumed in the past month.9 In 2010/2011, yellowtail snapper (15%), other or unspecified snapper (14%), olewife/triggerfish (12%), grouper (10%), hardnose (9%), red hind (7%), and king mackerel (6%) were the most commonly eaten for the most recent meal.
Table 4 presents age, income, and education information for the US Virgin Islands in 1980 and 2000. The risk difference in incidence rates based on the changes in the population demographics from 1980 to 2000 was +1.7 per 1,000 for age and −1.3 per 1,000 for education. Age was used despite not being significant in the logistic model, because older individuals were more likely to report previous illnesses. These factors combined with fish consumption have a crude estimated risk difference of −2.7 per 1,000 from 1980 to 2010/2011.
Table 4.
Demographics of US Virgin Islands, 1980 and 2000
| US Virgin Islands 1980 | US Virgin Islands 2000 | St. Thomas 2000 | Observed strata-specific rate per 1,000 in survey | |
|---|---|---|---|---|
| Total population | 96,569 | 108,612 | 51,181 | 12 |
| Age (years) | ||||
| Under 18 | 40,924 (42%)* | 34,289 (32%) | 15,077 (29%) | – |
| 18–44 | 38,022 (39%)* | 38,171 (35%) | 18,884 (37%) | 7 |
| 45–64 | 13,148 (14%) | 27,035 (25%) | 12,900 (25%) | 15 |
| 65 and above | 4,475 (5%) | 9,117 (8%) | 4,320 (8%) | 24 |
| Education | ||||
| Some high school or less | 22,472 (50%) | 25,876 (39%) | 11,915 (37%) | 25 |
| High school graduate | 11,543 (26%) | 17,044 (26%) | 8,483 (27%) | 9 |
| Some college or more | 10,971 (24%) | 22,683 (35%) | 11,511 (36%) | 13 |
Data obtained from US Census Bureau.
Values are approximate, because 1980 Census data are recorded as 15–19; proportional distribution assumed.
Discussion
Ciguatera remains a major public health problem in the US Virgin Islands, affecting approximately 1% of the population each year. Although comparable studies have yet to be conducted in other regions, these data suggest that the current rate of ciguatera in the Virgin Islands is among the highest in the world. The findings are consistent with a recent estimate of 11 per 1,000 for Culebra, Puerto Rico15 and higher than an estimated average for the South Pacific of 2–10 per 1,000.16 Given the potential for chronic, debilitating symptoms, there is a need to understand risk factors for ciguatera. Climate change (i.e., global warming) has been recently introduced as a potential contributor to increased disease rates in some areas.4–7 Therefore, in this study, it was hypothesized that increases in SST from 1980 to the present would be accompanied by an increase in disease rates over the same time period.
Contrary to the original hypothesis, however, our study results indicated a possible decline in ciguatera incidence in St. Thomas from 1980 to 2010/2011. The annual incidence in 1980, based on a household survey, was estimated as 7 per 1,000, but after adjusting to include only adults as in this study, it was 14 per 1,000. In contrast, the rate in adults in the current survey was 12 per 1,000. We found that 11% of households had at least one person with a ciguatera episode compared with 22% in a survey by McMillan and Granade12 in 1980. Where numbers were most striking was the drop in calculated incidence based on number of emergency room cases from an estimated 18 in 1,000 in the 1970s to 6 in 1,000 in 2007–2011, which was statistically significant. Taken together, these studies suggest that rates have declined or at least remained stable.
One plausible explanation for these results is that St. Thomas could have reached an upper temperature threshold that is limiting Gambierdiscus growth. Multiple studies have shown that Gambierdiscus growth rates are highest at warm temperatures, with the maximum around 30°C, above which growth rates drop off dramatically.7,17–19 A threshold was also suggested by Llewellyn6 based on data in the South Pacific. However, the threshold suggested in the past is approximately 30°C, which St. Thomas does not typically exceed; the average temperature in the warmest month was 29.3°C from 2007 to 2010. Alternatively, a positive association between seawater temperature and ciguatera incidence may exist in St. Thomas, but it is obscured by other environmental or human factors, some of which would act to decrease incidence. The complexity of this relationship is also highlighted in other regions. For instance, although most studies in the Pacific Islands suggest an increase in ciguatera incidence from 1973–1983 to 1998–2008 while seawater warmed, some countries and territories reported declines over that period without explanation.16,20
Our findings support the idea that other factors, including demographic and behavioral, are associated with changes in ciguatera incidence over time, with lower socioeconomic status (particularly education), fish consumption three times a week or more, being born in the Caribbean (outside the US Virgin Islands), and previous ciguatera episodes associated with illness.21–23 Lower socioeconomic status presumably increases risk of ciguatera, because lower income individuals may have few affordable protein alternatives to locally caught fish.21 Persons born on another Caribbean Islands may not have had as much experience with ciguatera (and fish at high risk of being toxic, such as barracuda) as persons born in the Virgin Islands; this finding may also serve as a proxy for differences in socioeconomic status. Lastly, a change in incidence may be driven further by the corresponding change in the number of people at higher risk because of previous ciguatera episodes.
Aside from seawater temperature and the factors explored in this study, several factors that we did not asses may also influence ciguatera incidence and should be considered in future studies. Coral reef health may impact Gambierdiscus growth, because disturbances to coral reef, whether through bleaching, hurricanes, or other physical injury, open surfaces for Gambierdiscus to colonize.24–26 These factors are episodic and may not follow a consistent trend over the past decades. Another factor could be a change in the toxins produced by the dominant Gambierdiscus species present in the US Virgin Islands. M. Richlen and others (unpublished data) have identified at least four Gambierdiscus species in the region that differ in toxicity. Changes in the Gambierdiscus species composition through time, in response to differential environmental tolerances, could then alter the types and amount of toxins produced and vectored into the food chain.
This study has a few important limitations. We relied on self-report in the telephone survey for both case ascertainment and risk factor history. As with most telephone surveys performed in recent years, our response rate was low, primarily because of non-answers. Despite this result, studies have indicated that increasing the response rate of surveys does not drastically change the study findings.27,28 The telephone survey format is known to preferentially sample households with higher income and education levels, because some lower income households may not have a telephone. By weighting the survey sample by education, sex, and age, we attempted to reduce this bias. Selection bias may also have been an issue, with persons interested in ciguatera more likely to participate. Finally, we used 2000 Census data for our weighted estimate. Annual updates are not available for the US Virgin Islands, and 2010 demographic data are not yet available. However, the total population of St. Thomas changed very little from 2000 to 2010 (51,181–51,634).11
Although the above limitations are important to consider when interpreting our results, it is also noteworthy that very few studies on ciguatera have been performed with such detailed data. Most studies thus far have relied on public health reports of illness, which vary in quality by location and are biased by underreporting. We have not been able to identify any other studies that included primary data collection from different time periods. To best manage the potential biases described above, we examined both survey data, which is sensitive but not necessarily specific, and emergency department visits, which are specific but not sensitive to mild cases, and we found the same trend in both sets of data.
In summary, our findings provide important insight into the occurrence of ciguatera fish poisoning in the Caribbean. Despite existing predictions based on increasing seawater temperatures, incidence has not increased since 1980. This result may be partly because of changes in the population makeup, with fewer people in the lowest socioeconomic category, and changes in ciguatera-related behaviors, with less fish consumption overall and lower risk fish types making up a larger proportion of fish consumed. The influence of environmental factors, including seawater temperature and coral reef health, is still not clear. Additional research is needed to identify and characterize the factors associated with ciguatera in this region to improve our understanding of how the human effects of HABs, such as ciguatera, can be prevented. This information could also inform climate change policy that impacts seawater temperature and coral reef health.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the Florida Survey Research Center at the University of Florida for their assistance in the administration of the telephone survey. We also appreciate the work of Sparkle Roberts, Margaret Abbott, Vasu Misra, and the rest of the CaribCATCH team.
Footnotes
Financial support: Funding for this project was provided by the Centers for Disease Control and Prevention. Partial funding for D.M.A. was also provided by National Oceanic and Atmospheric Administration Grant NA11NOS4780060 through the ECOHAB program.
Authors' addresses: Elizabeth G. Radke, Robert L. Cook, and J. Glenn Morris Jr., Department of Epidemiology and Emerging Pathogens Institute, University of Florida, Gainesville, FL, E-mails: bethradke@epi.ufl.edu, cookrl@phhp.ufl.edu, and jgmorris@epi.ufl.edu. Lynn M. Grattan, University of Maryland School of Medicine, Baltimore, MD, E-mail: LGrattan@som.umaryland.edu. Tyler B. Smith, University of the Virgin Islands, St. Thomas, US Virgin Islands, E-mail: tsmith@live.uvi.edu. Donald M. Anderson, Woods Hole Oceanographic Institution, Woods Hole, MA, E-mail: danderson@whoi.edu.
References
- 1.Fleming LE, Baden DG, Bean JA, Weisman R, Blythe DG. In: Seafood Toxin Diseases: Issues in Epidemiology and Community Outreach. Reguera B, Blanco J, Fernandez ML, Wyatt T, editors. Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO; Galicia: 1998. pp. 245–248. (Harmful Algae). [Google Scholar]
- 2.Lehane L, Lewis RJ. Ciguatera: recent advances but the risk remains. Int J Food Microbiol. 2000;61:91–125. doi: 10.1016/s0168-1605(00)00382-2. [DOI] [PubMed] [Google Scholar]
- 3.Dickey RW, Plakas SM. Ciguatera: a public health perspective. Toxicon. 2010;56:123–136. doi: 10.1016/j.toxicon.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Hales S, Weinstein P, Woodward A. Ciguatera (fish poisoning), El Nino, and Pacific sea surface temperatures. Ecosyst Health. 1999;5:20–25. [Google Scholar]
- 5.Chateau-Degat ML, Chinain M, Cerf N, Gingras S, Hubert B, Dewailly E. Seawater temperature, Gambierdiscus spp. variability and incidence of ciguatera poisoning in French Polynesia. Harmful Algae. 2005;4:1053–1062. [Google Scholar]
- 6.Llewellyn LE. Revisiting the association between sea surface temperature and the epidemiology of fish poisoning in the South Pacific: reassessing the link between ciguatera and climate change. Toxicon. 2010;56:691–697. doi: 10.1016/j.toxicon.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Tester PA, Feldman RL, Nau AW, Kibler SR, Wayne Litaker R. Ciguatera fish poisoning and sea surface temperatures in the Caribbean Sea and the West Indies. Toxicon. 2010;56:698–710. doi: 10.1016/j.toxicon.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 8.Morris JG, Lewin P, Smith CW, Blake PA, Schneider R. Ciguatera fish poisoning epidemiology of the disease on St. Thomas, U.S. Virgin Islands. Am J Trop Med Hyg. 1982;31:574–578. doi: 10.4269/ajtmh.1982.31.574. [DOI] [PubMed] [Google Scholar]
- 9.Morris JG, Blake PA, Feldman RA, Bennett JV. Ciguatera Fish Poisoning, St. Thomas, Virgin Islands. Atlanta, GA: Centers for Disease Control and Prevention; 1980. EPI-80-63-2. [Google Scholar]
- 10.Morril WT, Romansky NM. The Incidence of Ciguatera Poisoning in St. Thomas, V.I. Report under NOAA Contract 28-79 (Task 3) Washington, DC: National Oceanic and Atmospheric Administration; 1980. [Google Scholar]
- 11.US Census Bureau . Census SF1: 100% Data. Washington, DC: US Department of Commerce; 2000. [Google Scholar]
- 12.McMillan JP, Granade HR. Ciguatera fish poisoning in the United States Virgin Islands: preliminary studies. J College Virgin Islands. 1980;6:84–107. [Google Scholar]
- 13.NOAA/OAR/ESRL/PSD . NOAA Extended Reconstructed Sea Surface Temperature V3b. Washington, DC: National Oceanic and Atmospheric Administration; 2011. [Google Scholar]
- 14.AAPOR . American Association for Public Opinion Research Response Rate Calculator V3.1. Deerfield, IL: American Association for Public Opinion Research; 2010. [Google Scholar]
- 15.Azziz-Baumgartner E, Luber G, Conklin L, Tosteson TR, Granade HR, Dickey RW, Backer LC. Assessing the incidence of ciguatera fish poisoning with two surveys conducted in Culebra, Puerto Rico, during 2005 and 2006. Environ Health Perspect. 2012;120:526–529. doi: 10.1289/ehp.1104003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skinner MP, Brewer TD, Johnstone R, Fleming LE, Lewis RJ. Ciguatera fish poisoning in the Pacific islands (1998 to 2008) PLoS Negl Trop Dis. 2011;5:e1416. doi: 10.1371/journal.pntd.0001416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bomber J, Guillard R, Nelson W. Roles of temperature, salinity, and light in seasonality, growth, and toxicity of ciguatera-causing Gambierdiscus toxicus adachi et fukuyo. J Exp Mar Biol Ecol. 1988;115:53–65. [Google Scholar]
- 18.Morton S, Norris D. Effect of temperature, salinity, and light intensity on the growth and seasonality of toxic dinoflagellates associated with ciguatera. J Exp Mar Biol Ecol. 1992;157:79–90. [Google Scholar]
- 19.Rongo T, van Woesik R. Ciguatera poisoning in Rarotonga, southern Cook islands. Harmful Algae. 2011;10:345–355. doi: 10.1016/j.toxicon.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 20.Lewis ND. Epidemiology and impact of ciguatera in the Pacific: a review. Mar Fish Rev. 1986;48:6–13. [Google Scholar]
- 21.Lewis RJ, Tilman AR. Ciguatera: ecological, clinical, and socioeconomic perspectives. Crit Rev Environ Sci Technol. 1993;23:137–156. [Google Scholar]
- 22.Bagnis R, Kuberski T, Laugier S. Clinical observations on 3009 cases of ciguatera (fish poisoning) in the south-Pacific. Am J Trop Med Hyg. 1979;28:1067–1073. doi: 10.4269/ajtmh.1979.28.1067. [DOI] [PubMed] [Google Scholar]
- 23.Glaziou P, Martin PMV. Study of factors that influence the clinical-response to ciguatera fish poisoning. Toxicon. 1993;31:1151–1154. doi: 10.1016/0041-0101(93)90130-b. [DOI] [PubMed] [Google Scholar]
- 24.Gillespie N. Possible origins of ciguatera. Toxic Plants & Animals; a Guide for Australia. 1987:170–179. [Google Scholar]
- 25.Bagnis R. Natural versus anthropogenic disturbances to coral reefs: Comparison in epidemiological patterns of ciguatera. Memoirs of the Queensland Museum. 1994;34:455–460. [Google Scholar]
- 26.Ruff TA. Ciguatera in the pacific—a link with military activities. Lancet. 1989;1:201–205. doi: 10.1016/s0140-6736(89)91212-9. [DOI] [PubMed] [Google Scholar]
- 27.Keeter S, Kennedy C, Dimock M, Best J, Craighill P. Gauging the impact of growing nonresponse on estimates from a national RDD telephone survey. Public Opin Q. 2006;70:759–779. [Google Scholar]
- 28.Lee S, Brown ER, Grant D, Belin TR, Brick JM. Exploring nonresponse bias in a health survey using neighborhood characteristics. Am J Public Health. 2009;99:1811–1817. doi: 10.2105/AJPH.2008.154161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.