Abstract
We investigated a cluster of five cases of severe pneumonia from one village in Yunnan Province, China. We searched for severe pneumonia in the village and hospitals. We interviewed patients and family members about exposures. We tested acute and convalescent sera for antigen and antibody of severe acute respiratory syndrome, avian influenza, and plague. The only common exposure of the five patients was riding together in the enclosed cab of a truck for 1.5 hours while taking the first patient to the hospital. Seroconversion to plague F1 antigen confirmed plague in three survivors. Unfamiliarity of clinicians with plague and lack of sputum examination, blood culture, or postmortem examination delayed the diagnosis. No plague cases occurred among family and village contacts and health care workers. High infectivity in this cluster was limited to a crowded, poorly ventilated truck.
Introduction
From 1900 to 1949, China had seven major pneumonic plague epidemics with 1.1 million cases of pneumonic plague in 20 provinces and municipalities.1,2 Since 1950, 12 provinces reported plague including seven with reports of bubonic, septicemic, and pneumonic plague in humans.1–3 In many previously enzootic areas of China, clinicians are not familiar with pneumonic plague and may not recognize individual cases or small clusters. Moreover, pneumonia treatment in China is frequently determined without the support of sputum culture or smear making detection of plague unlikely during diagnostic workup.
In a period of 1 week in late October, 2005, a cluster of five cases of severe pneumonia with two deaths was reported in residents of the same administrative village unit, Nanxi, Yulong County, Yunnan Province, China. Because this cluster occurred soon after the severe acute respiratory syndrome (SARS) outbreak in China and during a period of concern about highly pathogenic avian influenza (HPAI) H5N1, determining if this cluster was caused by these or another virulent and highly infectious agent was critical. The county Center for Disease Control and Prevention (CDC) initiated an investigation but did not determine the etiology, and a team from the national Chinese CDC joined the investigation. Initial tests for SARS, highly pathogenic H5N1 avian influenza and plague F1 antibody were all negative.
Materials and Methods
Setting.
Yulong County lies in a mountainous area in northwestern Yunnan Province. The population of 208,000 is dispersed throughout 97 administrative villages (each composed of smaller hamlets), and 16 towns. One secondary care county hospital, 16 primary care town hospitals, and over 100 village clinics provide medical care. The secondary and primary hospitals all report infectious diseases through an electronic surveillance system. A tertiary care referral hospital in the prefecture capital provides more specialized medical care for Yulong and neighboring counties. Previous surveys of hosts and vectors had identified only one rat seropositive for plague antibodies in a different administrative village than Nanxi but nowhere else in Yulong County. In the past 100 years, human plague has not been identified in either Yulong County or Lijiang Prefecture. A qualified plague diagnostic laboratory in the prefecture capital, Lijiang, has the capacity to assist with plague diagnosis.
The Nanxi administrative village is in Huangshan township, Yulong County. It has one central area and seven satellite hamlets. The village lies in a mountain range between 2,900 and 3,300 m of altitude. The vegetation there is mixed pine and shrubs. Villagers are mainly farmers who raise potatoes and rutabagas. Tourists visit Nanxi occasionally for hunting or sightseeing.
Case finding.
We defined a case of severe pneumonia as the acute onset of fever (> 38.5°C) with radiographic evidence of consolidation of more than two pulmonary segments within 3 days after onset of fever in a resident or visitor to Yulong County from October 1 to November 30, 2005. We searched house to house in Nanxi Village for any resident who had recent severe acute respiratory illness or severe infection. We investigated deaths that had occurred in 2005. We interviewed the village doctors and family members from Nanxi to determine travel histories, hospital admissions, treatments, and outcomes. We visited the county hospitals in Yulong County and the neighboring Guchen County, Huangshan township hospital, and the tertiary care hospital in the Lijiang Prefecture. We reviewed the registers in the internal medicine clinics and in emergency departments to identify records of pneumonia cases. We asked doctors in these two departments plus pediatricians if they had diagnosed severe pneumonia cases, their treatments, and outcomes. We searched for all medical records with a diagnosis of pneumonia, “pulmonary infection,” or “respiratory failure” in the inpatient wards of internal medicine and record rooms since September 2004.
We interviewed case-patients and their relatives, other close associates, and medical staff about exposures and course of illness of all five case-patients. Interviews included contact with persons with respiratory disease, sick or dying livestock, poultry, domestic, and wild animals. We also determined the source of drinking water and food of the cases within 3 days before onset. We asked about epizootics among animals in the area.
We collected sera and throat swabs from the three survivors at different times during the course of illness and convalescence. We tested these for SARS-Coronavirus (immunoglobulin M [IgM], IgG, N protein, and virus nucleic acid) and HPAI H5N1 (reverse transcription-polymerase chain reaction [RT-PCR] for nucleic acid), and for IgM and IgG using a commercial enzyme-linked immunosorbent assay (ELISA) test against a battery of respiratory infectious agents including Mycoplasma pneumonia, Chlamydia pneumoniae, Legionnella, and respiratory syncitial virus. We tested for plague F1 antibody in subacute and convalescent sera using ELISA and indirect hemagglutination inhibition (kit from China Plague and Brucellosis Institute, batch no.: 200501, within the expiration date).
Results
Case finding.
Our house-to-house search of Nanxi Village found no additional cases of severe pneumonia or a person who had recently died of or survived a severe respiratory disease or a severe infection. We also detected no other severe pneumonia cases and no other pneumonia deaths between September 2004 and October 2005 in Yulong County. There were 315 (129/100,000 population per year) total pneumonia cases from departments of three hospitals in Yulong County, the neighboring county, and Lijiang Prefecture from September 2004 to November 2005. We found no unusual increases above the monthly average for Yulong and Guchen Counties or Lijiang Prefecture. During the month when the cluster of severe pneumonia occurred, October 2005, the number of pneumonia diagnoses was actually low compared with historical monthly averages. With the exception of the five patients in our cluster, none of the identified pneumonia cases met our definition of severe pneumonia and none of the patients died. Medical services at all levels in the prefecture reported 69 deaths in 2005. With the exception of the cluster under investigation, none of these deaths were attributed to pneumonia.
Clinical findings.
The first case of pneumonia in the cluster, patient A, was a 26-year-old male farmer. He was otherwise in good health when on October 25 at 4:00 pm, while tending his crops, he felt uncomfortable and dizzy. On October 26, he developed fever, cough, fatigue, dyspnea, and expectorated yellow, blood-tinged sputum. The next morning, he was admitted to a county hospital. At that time his body temperature was 39.5°C. He had no lymphadenopathy. He was treated with cefoperazone and levofloxacin, and was admitted to the medicine department; by November 2 respiratory distress had worsened and he was transferred to the intensive care unit. During his hospitalization his chest radiographs showed initial consolidation of several segments of the right lower lung (RLL) followed by extension to other segments of the right and left lung. His pulmonary function and general condition improved slowly, and he was discharged on November 30.
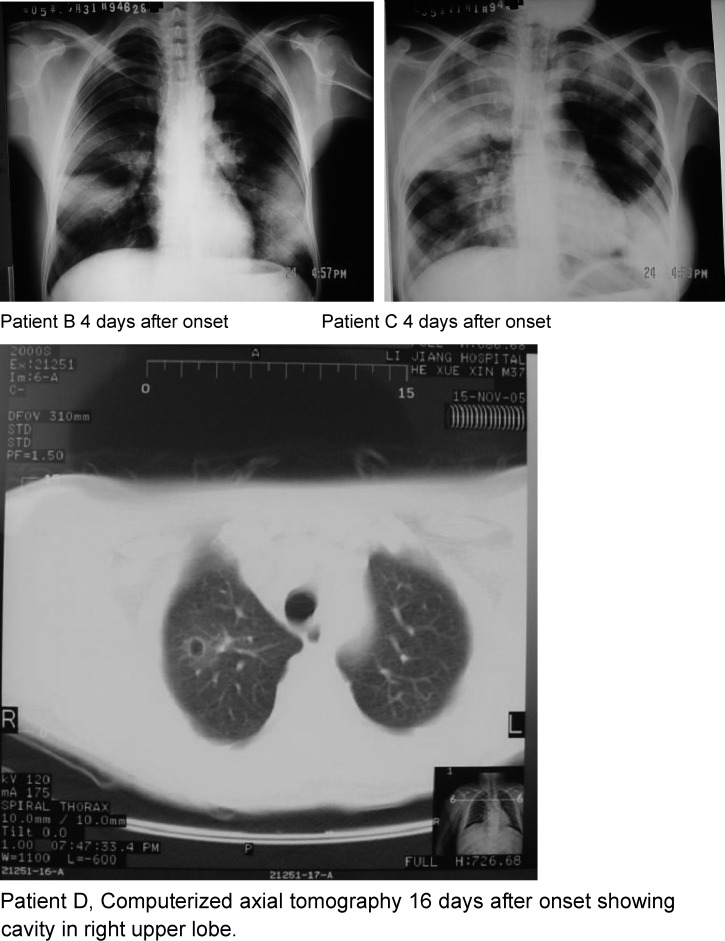
The five cases were characterized by fever lasting 3 (in fatal cases) to 12 days, multisegmental and multilobar consolidation (4), blood streaked or pink frothy sputum (4), and hypotension (3) (Table 1). Computerized axial tomography showed development of a cavity in the right upper lobe of one patient (Figure 1). One patient had an enlarged cervical lymph node with inflammation of the overlying skin. Four patients were treated with antibiotics, beginning on the second to the fourth day of illness. Three received quinolones but none received streptomycin, chloramphenicol, or a tetracycline. The remaining patient did not receive antibiotics and died on the fourth day of illness.
Table 1.
Characteristics of five primary pneumonic plague cases, Yulong County, Yunnan Province, China, October 25–30, 2005*
| ID | Sex | Age | Date of onset | Source of exposure | Initial symptoms | Chest radiograph | Leukocyte count (109/L) | Antibiotics | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| A | M | 26 | 10/25 | Unknown | Dizziness, fever, and fatigue | Bilateral multisegmental consolidation | 3.78 | Cefoperazone Levofloxacin | Recovered discharged on 30/11 |
| B | F | 52 | 10/29 | Exposure to patient A | Fever, cough, myalgias | Bilateral multisegmental consolidation | 8.55 | No record | Died on 01/11 |
| C | F | 22 | 10/30 | Exposure to patient A | Fever, fatigue, sore feet | Bilateral multisegmental consolidation | 7.0–9.12 | Levofloxacin Rocephin | Died on 02/11 |
| D | M | 37 | 10/30 | Exposure to patient A | Fever, sore knee | Bilateral multisegmental consolidation RUL cavity | 15.12 | Levofloxacin Rocephin | Recovered, discharged on 30/11 |
| E | M | 33 | 10/29 | Exposure to patient A | Fever, chills | RUL consolidation | 8.9–13.0 | Amoxicillin | Recovered, discharged on 25/11 |
RUL = right upper lobe.
Figure 1.
Chest radiographs and computerized axial tomography of three primary pneumonic plague patients in a time-space cluster, Yulong County, Yunnan Province, China, October–November 2005.
Testing for SARS indicators (nucleic acid, antigen, IgM/IgG), and HPAI H5N1 (nucleic acid, hemagglutinin HA antigen, HA antibody, and IA) were all negative. Sera taken on November 7 (8 to 13 days after onset) from three surviving patients were non-reactive to F1 antigen of Yersinia pestis by IHA tests at the Lijiang Prefecture CDC Laboratory. Serologic tests for M. pneumoniae, C. pneumonia, Legionella pneumophila, and respiratory syncytial virus on the surviving three patients did not indicate recent infection but showed inconclusive results on different patients for each pathogen. The IgM tests for 16 other respiratory agents were all negative.
None of the patients had sputum smears, sputum cultures, or blood cultures done during their acute illness. Autopsies were not done or postmortem tissue or blood samples taken on the two fatal cases, and their bodies were cremated. At the time the etiology remained undetermined.
However, during a review of the data one team member pointed out that the clinical and epidemiologic features were highly compatible with primary pneumonic plague (PPP). Accordingly, follow-up sera from the three surviving patients were obtained; test results showed rising plague F1 antibody titers 2 and 4 weeks after onset (Table 2). On this basis, we determined the five cases to be PPP.
Table 2.
Serial antibody titers to plague F1 antigen in three survivors of a cluster of five cases of severe pneumonia, Yulong County, China, November 2005*
| Patient A | Patient D | Patient E | ||||||
|---|---|---|---|---|---|---|---|---|
| Days after onset | ELISA | IHI | Days after onset | ELISA | IHI | Days after onset | ELISA | IHI |
| 13 | < 1:20 | 9 | < 1:20 | 8 | < 1:20 | |||
| 16 | 1:2560 | 1:40 | 12 | 1:40000 | 1:80 | 11 | 1:2560 | 1:160 |
| 18 | > 1:40000 | 1:640 | ||||||
| 34 | > 1:40000 | 1:320 | 29 | > 1:40000 | 1:640 | |||
ELISA = enzyme-linked immunosorbent assay; IHI = indirect hemagglutination inhibition.
Epidemiologic features.
The five patients with PPP were adults from 22 to 52 years of age. Three were men and two women. Three PPP patients lived in Luzi hamlet (64 households, 224 residents) and two were drivers who lived in a cluster of rental houses (80 residents) near the Nanxi Village administrative center. All belonged to the Naxi ethnic group.
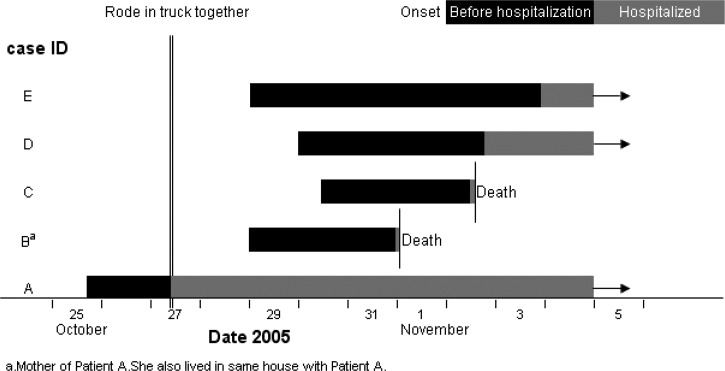
Patient A and his mother (patient B) and father (not ill) lived in the same house in Luxi hamlet. The other three patients had no relationship or common activities with each other or with patients A and B, with one exception. All five rode in the cab of the same light truck on October 27 from 7:00 am to 8:30 am when the mother took her acutely sick son from Luzi to Yulong County hospital (Figure 2). For the entire trip they closed the windows of the truck except for a narrow gap. Patient A was actively coughing during the trip. Nobody else occupied the cab of the truck during this trip. After leaving the truck the mother took patient A to the hospital and the other three persons went their separate ways. All four healthy occupants of the truck cab, including the mother of patient A, developed PPP from 1.5 to 3 days after they shared the truck ride with patient A (Figure 2).
Figure 2.
Time relationships of five primary pneumonic plague cases in a time-space cluster, Yulong County, Yunnan Province, China, October–November 2005.
We found no other PPP cases among 234 other family and village contacts and 153 health care workers (Table 3). None of the health care workers used any precautions (masks, gloves, or gowns) to avoid infection while attending the five patients.
Table 3.
Secondary attack rate among contacts of primary pneumonic plague cases, Yulong County, China, October 2005
| Contact with PPP* patients | Total | Cases | Attack rate (%) |
|---|---|---|---|
| Driver and passengers in truck with PPP patient on Oct 27 | 4 | 4 | 100 |
| Family members† | 14 | 1 | 7.1 |
| Other villagers with known contact | 34 | 0 | 0 |
| Medical staff who attended the PPP patients | 153 | 0 | 0 |
| Other villagers with no recent contact | 186 | 0 | 0 |
PPP = primary pneumonic plague.
Included mother of the first PPP patient who also rode in the truck with him.
Patient A routinely tended the family farm. During the month before onset of PPP, he reported no deviation from this routine, no contact with wild animals, no hunting, no sighting of dead or dying rodents, and no contact with a person with a similar or a grave illness, and did not leave his village. In early October (> 15 days before his PPP onset) a previously healthy domestic dog in his home died. In August 2005, a previously healthy domestic cat died. The three other affected families reported no unexpected deaths or illnesses in their domestic animals including dogs or cats. Other villagers also did not report recent deaths in dogs or cats. The village veterinarian reported that he did not observe any evidence of an epizootic during the months preceding this PPP cluster.
As a response to a cluster of severe pneumonia of unknown cause, several actions were taken beginning on November 2. A cordon sanitaire was setup around Nanxi Village. Special isolation and treatment areas were established in hospitals of the city. Personal protection measures for medical personnel were strengthened. Contacts of cases and the medical personnel who diagnosed or treated the cases or performed the field investigation were quarantined. Among these 408 persons placed under quarantine observation none contracted PPP. No new severe pneumonia, PPP, or other plague infections were found over 2 months of follow-up.
Discussion
This investigation led us to the conclusion that we were dealing with a cluster of PPP. The clinical illnesses including rapid incubation, fulminant progression, bloody sputum, progressive segmental consolidation, cavitation (in one case), and hypotension are all typical of PPP. Plague infection was finally confirmed with a 4-fold rise in anti-F1 in convalescent sera taken 3 weeks after onset in the three survivors.
During the investigation, initial negative F1 antibody tests for plague were misleading as to cause of the illnesses. However, tests for F1 antibody are often negative in patients who are treated with effective antibiotics, if sera are taken within 2 weeks of onset, as were done in these cases.4 Other misleading findings included no characteristic plague exposure for the primary case, no human cases of bubonic plague in the county, and historical information that plague was not enzootic in Yulong County despite a strong plague surveillance system in the province.
Opportunities to establish a diagnosis of PPP were missed early in the hospitalization of these patients. A sputum smear showing sheets of gram-negative coccobacilli or bipolar staining with Geimsa or Waylon stain should immediately raise suspicion of PPP. Although sputum smears are not uniformly helpful with all pneumonias, they may be with some, and should be included in the initial diagnostic workup of all severe pneumonia.5 Direct fluorescent antibody stain of sputum, sputum culture, and blood culture, should confirm the diagnosis of pneumonic plague within a few days. As in these five cases, sputum smears and cultures and blood cultures are rarely done as part of a diagnostic workup of severe pneumonia in China. Similarly, microbiologic testing of postmortem specimens on fatal cases also would have led to a more rapid determination of the etiology.
The epidemiologic pattern also exhibited features of PPP. Other infectious agents that cause pneumonia will result in a substantial proportion of asymptomatic or mild infections relative to each case of severe pneumonia. For these other agents, with the possible exception of SARS, one would rarely expect more than one case of secondary pneumonia in a small group. Under the conditions of close exposure in an enclosed space, plague is among a very small number of organisms that can yield high secondary attack rates of severe pneumonias.
In this cluster, person-to-person transmission of PPP caused infection of three persons and probably a fourth (the mother) within the confines of a truck cab. Despite many more person-hours of contact, family members of secondary cases were not infected nor were medical staff who attended all the patients without any special protective measures. This pattern of transmission is highly characteristic of PPP. Classical studies from the early decades of the last century in China by Wu Lien-Teh, Strong, and Teague indicated that PPP was mainly spread from face-to-face exposure within 1.5 m.6–9 Exposure in small, enclosed, poorly ventilated spaces such as underground inns and crowded train carriages in Manchuria was also an important factor.8 Although highly communicable under these special circumstances, PPP is otherwise poorly transmitted.6,10–12 As demonstrated by this outbreak, both extremes can be true. In the conditions of the enclosed truck cab, the communicability was extremely high, whereas in the open community and among exposed medical staff, the communicability was very low.
An initial assessment of zoonotic plague around Nanxi Village in January 2006 revealed Y. pestis by cultures from rodents and several domestic dogs and cats that were plague F-1 antibody-seropositive.13 A more complete investigation identified Y. pestis isolated from a naturally dead rat (Rattus nitidus), from fleas found on that rat, and from two field mice, Apodemus chevrieri.13 A serosurvey of dogs and cats revealed anti-plague antibodies in ∼25% of dogs and cats around Nanxi Village.14
One perplexing issue remains unresolved and is an important limitation of this investigation. We could not identify the source of the infection of the primary case. Clinically, the patient had PPP and not secondary pneumonic plague. He had not left his village and no other severe pneumonia cases among village residents preceded his case. He had not hunted or killed wildlife or handled dead or dying rodents. Farm cats were found to be seropositive and can become sick with pharyngeal plague and transmit by droplets to humans. However, we found no indication that he had contact with a sick cat during his incubation period. Because dogs, albeit rarely, can develop pneumonic plague and infect humans7,15,16; the unexpected death of the family dog raises the possibility of dog to human transmission. However, the history indicated that the dog died too far outside of the maximum incubation period of PPP to have infected his owner. Our broader search of hospitals in the area and mortality reports also found no evidence of a broader epidemic of severe pneumonia. There is a strict regulation for reporting of a severe pneumonia case because of the SARS epidemic in China. All five PPP case-patients in this outbreak were reported by a clinician from the county hospital to CDC of a different level, including county, prefecture, province, and nation, and finally to the Ministry of Health within 3 days, very timely and without any delay, and not any reluctance to report at all.
In this outbreak, patients were delayed to be diagnosed as plague because they showed atypical symptoms as a result of antibiotic treatment. Although local residents held a viewpoint that a person should die in his own home, two deceased case-patients died in the hospital, and then their relatives took their body back home for burial after receiving thorough disinfection from CDC's professional staff.
We can conclude that PPP was transmitted to three and possibly four persons during a 1.5-hour ride in an enclosed truck cab. To strengthen the response to severe pneumonia in China, we recommend a systematic approach that includes well-established microbiological methods such as sputum smears and culture, blood culture, repeat antibody tests 2–4 weeks after onset, and postmortem specimens in addition to organism-specific tests.
Footnotes
Authors' addresses: Huiming Luo, Furong Li, Xu Xie, and Guang Zeng, Chinese Center for Disease Control and Prevention, Chinese Field Epidemiology Training Program, Beijing, China, E-mails: hmluo@vip.sina.com, lifurong2005@yahoo.com.cn, xiexv@hotmail.com, and zeng4605@vip.sina.com. Xingqi Dong and Zhizhong Song, Yunnan Provincial Endemic Disease Prevention Institute – Office, Dali, China, E-mails: Dongxq99@vip.sina.com and 13808766541@126.com. Zhujun Shao and Zhongjie Li, Chinese Center for Disease Control and Prevention, Control and Prevention for Infectious Disease, Beijing, China, E-mails: shaozhujun@126.com and lizhongjiecdc@163.com. Zhaohui Tong, Capital Medical College Affiliated Beijing Chaoyang Hospital - Internal Medicine, Beijing, China, E-mail: tongzhh@hotmail.com. Guangfa Wang, Peking University First Hospital - Internal Medicine, Beijing, China, E-mail: wangguangfa@hotmail.com. Hongtao Zhang and Tielong Yang, Lijiang Center for Disease Control and Prevention, Epidemiological Department, Beijing, China, E-mails: zhtaozr@126.com and ytl19531205@yahoo.com.cn. Gao He and Zeyuan He, Yulong Center for Disease Control and Prevention, Epidemiological Department, Beijing, China, E-mails: hg13308889009@yahoo.com.cn and ljlizhaoxiu@163.com. Robert E. Fontaine, United States Centers for Disease Control and Prevention, Division of Public Health Systems and Workforce Development, Atlanta, GA, E-mail: ref1@cdc.gov.
References
- 1.Zhang G, Liu Z. The results of plague surveillance in China, 2006. Chin J Control Endem Dis. 2007;22:1–6. [Google Scholar]
- 2.Ji S, He J, Teng Y. The discovery and research of plague natural foci in China. Chin J Epidemiol. 1990;11:1–14. [Google Scholar]
- 3.Dai JF, Guan XH. Bio-terrorism and the strategy of prevention and control on plague in China. Chinese J Soc Med. 2008;25:189–191. [Google Scholar]
- 4.Dennis DT, Mead PS. Yersinia species, including plague. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. Philadelphia, PA: Churchill Livingstone; 2009. [Google Scholar]
- 5.Donowitz GR. Mandel, Douglas, and Bennett's Principles and Practice of Infectious Diseases. Philadelphia, PA: Churchill Livingstone; 2009. Acute pneumonia. [Google Scholar]
- 6.Kool JL. Risk of person-to-person transmission of pneumonic plague. Clin Infect Dis. 2005;40:1166–1172. doi: 10.1086/428617. [DOI] [PubMed] [Google Scholar]
- 7.Strong R, Teague O. Studies on pneumonic plague and plague immunization. Philipp J Sci. 1912;8:227. [Google Scholar]
- 8.Wu LT. A Treatise on Pneumonic Plague. Geneva: League of Nations; 1926. [Google Scholar]
- 9.Wu LT. Recent Knowledge on Pneumonic Plague. North Manchurian Plague Service Report; 1928. pp. 55–65. [Google Scholar]
- 10.Gani R, Leach S. Epidemiologic determinants for modeling pneumonic plague outbreaks. Emerg Infect Dis. 2004;10:608–614. doi: 10.3201/eid1004.030509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joshi K, Thakur JS, Kumar R, Singh AJ, Ray P, Jain S, Varma S. Epidemiological features of pneumonic plague outbreak in Himachal Pradesh, India. Trans R Soc Trop Med Hyg. 2009;103:455–460. doi: 10.1016/j.trstmh.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 12.Ratsitorahina M, Chanteau S, Rahalison L, Ratsifasoamanana L, Boisier P. Epidemiological and diagnostic aspects of the outbreak of pneumonic plague in Madagascar. Lancet. 2000;355:111–113. doi: 10.1016/S0140-6736(99)05163-6. [DOI] [PubMed] [Google Scholar]
- 13.Song ZZ, Xia LX, Liang Y, Guo Y, Lu L, Wang GL, Cai WF, Zhang ZF, He YT, Zhang FX, Dong XQ, Yu GL, Wang J, Yu DZ. Confirmation and study of plague natural foci for Yulong County and Guchengqu in Yunnan Province. Endemic Dis Bull. 2008;23:3–7. [Google Scholar]
- 14.Li B, Guo Y, Guo Z, Liang Y, Zhu Z, Zhou Q, Yan Y, Song Z, Yang R. Serologic survey of the sentinel animals for plague surveillance and screening for complementary diagnostic markers to F1 antigen by protein microarray. Am J Trop Med Hyg. 2008;79:799–802. [PubMed] [Google Scholar]
- 15.Pollitzer R. Plague. Geneva: World Health Organization; 1954. p. 305. WHO Monograph Series. [Google Scholar]
- 16.Wang H, Cui Y, Wang Z, Wang X, Guo Z, Yan Y, Li C, Cui B, Xiao X, Yang Y, Qi Z, Wang G, Wei B, Yu S, He D, Chen H, Chen G, Song Y, Yang R. A dog-associated primary pneumonic plague in Qinghai Province, China. Clin Infect Dis. 2011;52:185–190. doi: 10.1093/cid/ciq107. [DOI] [PubMed] [Google Scholar]