Abstract
A study of West Nile virus (WNV) ecology was conducted in St. Tammany Parish, Louisiana, from 2002 to 2004. Mosquitoes were collected weekly throughout the year using Centers for Disease Control and Prevention (CDC) light traps placed at 1.5 and 6 m above the ground and gravid traps. A total of 379,466 mosquitoes was collected. WNV was identified in 32 pools of mosquitoes comprising four species; 23 positive pools were from Culex nigripalpus collected during 2003. Significantly more positive pools were obtained from Cx. nigripalpus collected in traps placed at 6 m than 1.5 m that year, but abundance did not differ by trap height. In contrast, Cx. nigripalpus abundance was significantly greater in traps placed at 6 m in 2002 and 2004. Annual temporal variation in Cx. nigripalpus peak seasonal abundance has important implications for WNV transmission in Louisiana. One WNV-positive pool, from Cx. erraticus, was collected during the winter of 2004, showing year-round transmission. The potential roles of additional mosquito species in WNV transmission in southeastern Louisiana are discussed.
Introduction
After the introduction of West Nile virus (WNV; Flaviviridae:Flavivirus) into the northeastern United States in 1999, numerous studies were conducted to define vector and host associations in that region. Culex (Culex) mosquitoes, primarily the northern house mosquito Cx. pipiens and Cx. restuans, Cx. salinarius, and Culiseta melanura were implicated as vectors based on virus detection,1–3 vector competence experiments,4–6 and host-feeding preferences.7,8 As WNV spread into the western United States, Cx. tarsalis emerged as an important vector in rural areas.9 Birds, particularly passerine species, are the primary vertebrate amplifying hosts of WNV.10 Cx. pipiens, which feeds predominately on birds, serves as the main enzootic/epidemic vector of WNV in the northeastern United States and urban areas throughout its range.9 Cx. restuans, which also feeds predominantly on birds,7,8 is most abundant during late spring and early summer in the northeast and thus, may be more important in early-season amplification of WNV than transmitting virus to humans.2 Cx. salinarius, which feeds on mammals as well as birds,8 likely serves as a source of infection for humans and equines.2 Prior research indicated that Cx. pipiens and Cx. restuans may be collected more readily from traps placed in the forest canopy than traps placed closer to ground level.11,12 This difference may be because of female mosquitoes selectively seeking avian hosts in the canopy, optimal microenvironmental conditions, or other factors.
In the southern United States, potential WNV vectors include species also incriminated in the north (e.g., Cx. restuans, Cx. salinarius, and Cs. melanura). Additional vectors in this region include the southern house mosquito, Cx. quinquefasciatus, and Cx. nigripalpus, both known vectors of Saint Louis encephalitis virus (SLEV; Flaviviridae:Flavivirus).13–15 Studies in northern Florida conducted during 2001 in response to the initial discovery of WNV in that state identified the virus in each of these species, except Cx. restuans,16 and transmission of the virus by Cx. nigripalpus to a chicken in a baited trap was shown.17 Despite these studies, a number of questions remained concerning the pattern of transmission that WNV would take in the southern United States, such as the precise roles of different mosquito species in enzootic, epizootic, and epidemic transmission, the habitats that would be most favorable to WNV maintenance by enhancing vector–amplifier host contact, and the effect of an extended transmission season because of milder climatic conditions in the Gulf Coast states.
During the summer of 2002, an outbreak of WNV disease occurred in St. Tammany Parish in southeastern Louisiana. Forty cases with four deaths were recorded, with most cases occurring between June and early August.18 Entomologic surveys conducted during this outbreak incriminated Cx. quinquefasciatus as the main vector, with Cx. salinarius possibly acting as a secondary vector.18,19
Concurrent to this outbreak, we undertook a multiyear study of the ecology of potential vectors of WNV in St. Tammany Parish. Our objectives were to (1) describe the composition and relative abundance of mosquito species throughout the year among study sites representing diverse habitat types and within sites by trap elevation and type, (2) test the mosquitoes for the presence of WNV, and (3) analyze these data, along with climate, land cover,20 avian host,21 and other ecological covariates, in relationship to WNV transmission. In this report, we describe the results of mosquito collections and WNV testing in St. Tammany Parish and discuss the potential roles of various species, particularly Cx. nigripalpus, in the transmission cycle of WNV.
Materials and Methods
Study sites.
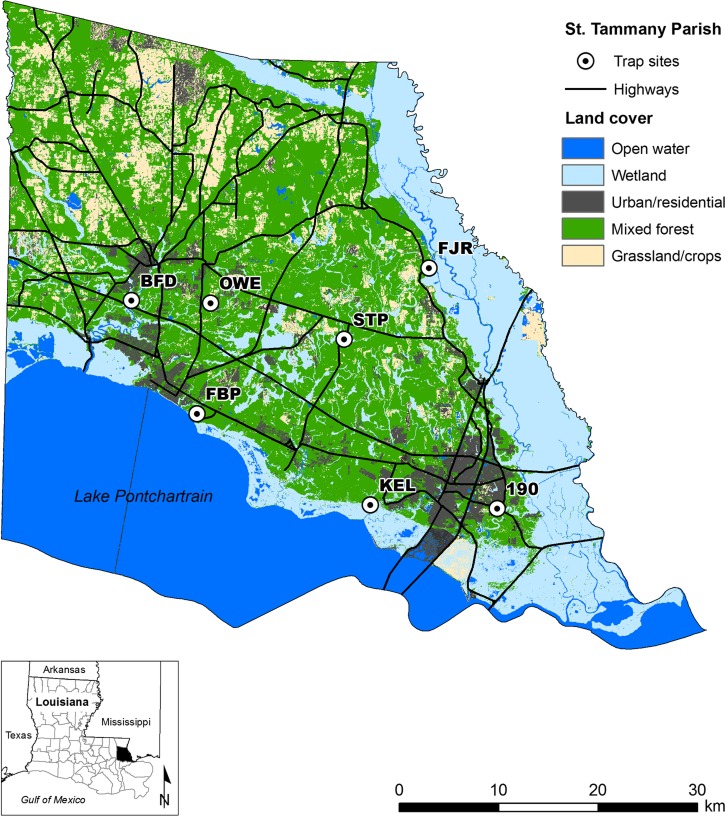
The study was conducted in St. Tammany Parish, located on the north shore of Lake Pontchartrain, in southeastern Louisiana. The parish is 2,220 km2 in size (24% water), with 191,268 residents according to the 2000 census (http://quickfacts.census.gov/qfd/states/22/22103.html). Seven sites were selected to represent a variety of habitats occurring in the parish (Figure 1). These sites were historically used for surveillance by the St. Tammany Parish Mosquito Abatement District (STPMAD). Site 190 is situated in a forest fragment at an elevation of 0.68 m, is frequently flooded, is adjacent to commercial and industrial development, and was characterized by loblolly pine (Pinus taeda), red maple (Acer rubrum), and live oak (Quercus spp.). KEL is a brackish marsh/loblolly pine forest ecotone adjacent to the north shore of Lake Ponchartrain at 0.37 m. FBP is located within a loblolly pine, slash pine (P. elliottii), and water oak (Q. nigra) forested area on the north shore of Lake Ponchartrain at 2.10 m. BFD is a swampy patch dominated by water tupelo (Nyssa aquatica) and water oak located in a low-lying residential area at 1.06 m adjacent to the Tchefuncte River. STP is a slash pine plantation at 10.56 m subject to periodic flooding. OWE is located at 9.06 m in a wooded low-density residential neighborhood characterized by slash pine, swamp bay (Persea palustris), and water tupelo. FJR is an upland site found at 15.40 m with sparse residential development and forest dominated by loblolly pine, Yaupon holly (Ilex vomitoria), and water oak.
Figure 1.
Location of study sites in St. Tammany Parish in southeastern Louisiana.
Mosquito collection and virus testing.
Mosquitoes were collected for one night per week from July 16, 2002 to November 30, 2004. To sample mosquitoes in the canopy as well as at ground level, two dry ice-baited Centers for Disease Control and Prevention (CDC) miniature light traps were placed at each site, with one trap at 1.5 m (down trap) and one trap at 6 m above the ground (up trap). One gravid trap baited with a fish oil emulsion (Alaska Fish Fertilizer, Alaska Fish Fertilizer Co., Renton, WA; 59 mL in 6.8 L tap water and incubated for 3 days at ambient outdoor temperature)18 was also placed at each site to sample Cx. quinquefasciatus. Traps were set out in the late afternoon and retrieved the next morning. Collections were identified to species and sorted into pools of ≤ 50 specimens by collection site, date, and trap type and height. For logistical reasons, ≤ 500 individuals of each species from each trap and date were pooled for virus testing; the remaining mosquitoes were counted and discarded.
For WNV testing, mosquito pools were placed into 2.0 mL microcentrifuge tubes (Axygen Scientific, Union City, CA) and shipped on dry ice to the CDC Laboratory at Fort Collins. Pools were triturated using a Mixer Mill MM300 (Qiagen, Valencia, CA)22 and tested for virus by Vero cell plaque assay in six-well plates using a double agar overlay as previously described.23 Each pool was also tested by TaqMan reverse-transcriptase polymerase chain reaction (RT-PCR) using WNV-specific primers.22,24
Statistical analysis.
To assess the effects of study factors (year, collection site, and trap height) on mosquito abundances and infection rates, generalized linear models (GLMs) were fit using the function glm in Spotfire S+ (version 8.1; TIBCO Software, Inc., Palo Alto, CA). Because overdispersion was present, collection counts were modeled according to a negative binomial distribution (MASS library), whereas infection rates were assumed to follow a binomial distribution conditional on collection counts. Analysis of deviance was used to determine the significance of main effects and interactions. Confidence intervals (CIs) for the desired comparisons were computed using the multicomp function using Sidak's method to adjust for multiple comparisons. WNV infection rates were calculated as the maximum likelihood estimate (MLE) with 95% CI.25,26
Results
General results.
A total of 379,466 mosquitoes representing ≥ 41 species was collected and identified (Table 1). Cx. salinarius represented 56% of the total collected followed by Cx. nigripalpus (11%), Aedes atlanticus/tormentor (6%), Anopheles crucians s.l. (5%), Cx. erraticus (5%), Ae. vexans (4%), Ae. canadensis canadensis (2%), and other species in lesser numbers. Cx. quinquefasciatus comprised only 1% of the mosquitoes collected. In addition, 40 female Cx. coronator, a species recently recognized in Louisiana,27 were collected by CDC light traps at five sites.
Table 1.
Mosquitoes collected from July 16, 2002 to November 30, 2004 in St. Tammany Parish, LA, and tested for arboviruses
| Species | Total collected | Pools tested | Mosquitoes tested | Mosquitoes collected but not tested |
|---|---|---|---|---|
| Cx. salinarius | 213,378 | 3,195 | 109,043 | 104,335 |
| Cx. nigripalpus | 42,520 | 1,518 | 36,436 | 6,084 |
| Ae. atlanticus/tormentor | 22,050 | 677 | 13,550 | 8,500 |
| An. crucians complex | 20,581 | 1,120 | 16,157 | 4,424 |
| Cx. erraticus | 18,928 | 1,173 | 18,682 | 246 |
| Ae. vexans | 14,430 | 927 | 14,430 | |
| Ae. c. canadensis | 7,173 | 421 | 7,173 | |
| Ae. spp | 6,796 | 419 | 6,713 | 83 |
| Cx. spp. | 6,102 | 467 | 5,742 | 360 |
| Ps. ferox | 5,818 | 545 | 5,818 | |
| Cx. quinquefasciatus | 3,915 | 545 | 3,915 | |
| Ae. sticticus | 2,638 | 112 | 2,014 | 624 |
| Ae. infirmatus | 2,440 | 461 | 2,440 | |
| Ae. fulvus pallens | 1,883 | 284 | 1,883 | |
| Cq. perturbans | 1,760 | 405 | 1,760 | |
| Cx. restuans | 1,622 | 309 | 1,622 | |
| Ae. taeniorhynchus | 1,131 | 112 | 1,131 | |
| Mansonia titillans | 1,097 | 263 | 1,097 | |
| Cs. melanura | 822 | 178 | 822 | |
| Other species* | 4,385 | 1,529 | 4,385 | |
| Total | 379,466 | 14,660 | 254,810 | 124,656 |
Ae. albopictus, Ae. cinereus, Ae. dupreei, Ae. mitchellae, Ae. sollicitans, Ae. thibaulti, Ae. triseriatus/hendersoni, Ae. trivittatus, An. punctipennis, An. quadrimaculatus, An. spp., Cs. inornata, Cx. coronator, Cx. territans, Ma. spp., Orthopodomyia signifera/alba, Ps. ciliata, Ps. columbiae, Ps. horrida, Ps. howardii, Ps. mathesoni, Ps. spp., Ps. varipes, Uranotaenia lowii, Ur. sapphirina, and Ur. spp.
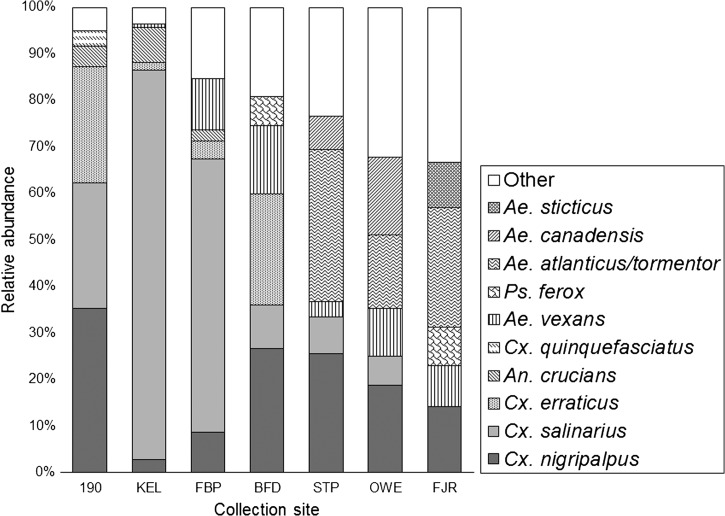
The relative abundance of the five most commonly collected species as a proportion of total mosquitoes collected at each study site is shown in Figure 2. These five species constituted between 67.5% (at FJR) and 96.4% (at KEL) of the total mosquitoes collected at each site. Cx. salinarius was the most abundant species at the two sites closest to Lake Pontchartrain, KEL and FBP, comprising 83.7% (171,835/205,420) and 58.7% (26,010/44,277), respectively, of the total mosquitoes collected at those sites, and it was among the five most abundant species at all other sites except FJR. Cx. nigripalpus was among the three most abundant species at all seven sites, ranging from 2.9% (5,722/205,420) of collections at KEL to 35.4% (12,379/34,925) of collections at 190, and it was the most abundant species at 190, BFD, and OWE. Culex species as a group, including Cx. nigripalpus, Cx. salinarius, Cx. erraticus, and Cx. quinquefasciatus, were the dominant species at sites 190, KEL, FBP, and BFD, comprising 90.6% (31,648/34,925), 88.1% (181,029/205,420), 71.3% (31,566/44,277), and 60.0% (9,271/15,455) of total collections at those sites. At the more upland sites (STP, OWE, and FJR), Ae. vexans, Ae. atlanticus/tormentor, and Ae. canadensis canadensis, Ae. sticticus, and Psorophora ferox were more prominent, comprising 43.2% (15,258/35,290), 42.8% (8,267/19,298), and 52.6% (13,062/24851), respectively, of total collections at those sites.
Figure 2.
Proportional abundance of the five most commonly collected species at each of seven study sites in St. Tammany Parish, Louisiana, 2002–2004.
Of the mosquitoes collected, 254,810 were sorted into 14,660 pools and tested for arboviruses; 32 WNV-infected mosquito pools were identified by TaqMan RT-PCR, and 25 of these pools also yielded isolates in Vero cell plaque assays. WNV was detected in four species, including Cx. nigripalpus (23 pools), Cx. salinarius (3 pools), Cs. melanura (2 pools), Cx. erraticus (1 pool), and Culex spp. (3 pools) (Table 2). Overall point estimates (MLE) for WNV infection rates per 1,000 mosquitoes ranged from 0.03 for Cx. salinarius to 2.47 for Cs. melanura. WNV activity was detected at each site except FBP, which is located on the shore of Lake Pontchartrain. The number of positive pools obtained from a site ranged from three pools from site 190 to nine pools at site OWE. Overall, 30 positive pools were obtained from light traps, and 2 positive pools were obtained from gravid traps (Table 2).
Table 2.
Mosquito pools infected with WNV in St. Tammany Parish, LA, 2002–2004 by trap type and elevation
| Species | Light trap 1.5 m | Light trap 6 m | Gravid trap | Total |
|---|---|---|---|---|
| Cx. nigripalpus | 6 (0.41; 0.17, 0.84)* | 17 (0.86; 0.52, 1.34) | 0 (0.00; 0.00, 2.09) | 23 (0.63; 0.41, 0.93) |
| Cx. salinarius | 1 (0.02; 0.00, 0.07) | 1 (0.03; 0.00, 0.15) | 1 (0.28; 0.02, 1.34) | 3 (0.03; 0.01, 0.08) |
| Cx. erraticus | 1 (0.05; 0.00, 0.26) | 0 (0.00; 0.00, 1.22) | 0† | 1 (0.05; 0.00, 0.26) |
| Cx. spp. | 1 (0.31; 0.02, 1.49) | 1 (0.46; 0.03, 2.24) | 1 (2.55; 0.15, 12.30) | 3 (0.52; 0.14, 1.40) |
| Cs. melanura | 0 (0.00; 0.00, 12.64) | 2 (3.98; 0.71, 13.07) | 0‡ | 2 (2.47; 0.44, 8.09) |
Infection rates are calculated as the MLEs and 95% CIs.
Number of WNV-infected pools (MLE; lower interval, upper interval).
No specimens were collected by this method.
Infection rate was not calculated, because only 10 specimens were collected by this method.
In the following sections, data on a variety of potentially important mosquito species are presented. However, the primary focus is on Cx. nigripalpus because of its high relative abundance at all trap sites and the large number of WNV-positive pools detected in that species.
Season and year effects.
Thirty-one of thirty-two WNV-positive pools were from mosquitoes collected from July to November during 2002 and 2003, including all twenty-three positive pools from Cx. nigripalpus. Twenty-nine of thirty-two positive pools from all species were obtained during 2003. However, one pool, from Cx. erraticus, was from mosquitoes collected on March 2, 2004. This was the only WNV-positive pool obtained during 2004.
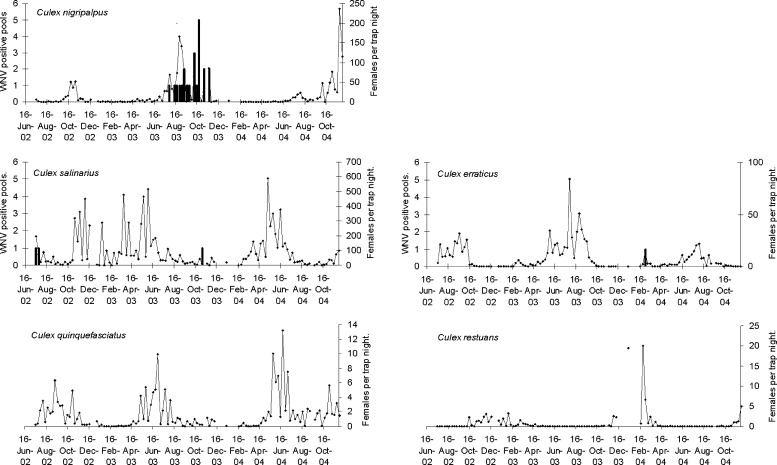
The seasonal abundance of species from which WNV-positive pools were obtained, and of Cx. quinquefasciatus and Cx. restuans is shown in Figure 3.
Figure 3.
Seasonal abundance and West Nile infection in selected mosquito species collected between July 16, 2002 and November 30, 2004. Lines represent mosquitoes per TN, and bars represent WNV-positive pools.
Cx. nigripalpus was most abundant during the late summer and autumn of each year, with a sharp spike in abundance observed in both 2003 and 2004. In 2002 and 2004, population numbers began to increase in early September and peaked on October 22 and November 2 during 2002 and November 23, 2004. During 2003, abundance increased in mid-July, peaked on August 26, and then declined. Additional smaller population spikes occured in early October and mid-November in 2003. Twenty-three WNV-positive pools were obtained from this species, all from females collected between July 29 and November 18, 2003.
Cx. salinarius, the most commonly collected species, was active most months of the year, with a decrease in abundance from July to October. Abundance peaks were observed during 2002 on November 5, throughout 2003, with three approximately equal peaks occurring on March 25, May 20, and June 3, and in 2004 on May 11. Three WNV-positive pools were obtained from this species on July 16 and 23, 2002 and November 4, 2003.
Cs. melanura was collected in low numbers, with a maximum abundance over all sites combined of 2.1/trap night (TN), obtained on June 10, 2003. Thus, seasonal peaks were not distinguished, and this species was not included in Figure 3. This species was detected in most seasons except winter. Two WNV-positive pools were obtained from Cs. melanura collected on August 5 and 19 2003.
Cx. erraticus was primarily a late spring and summer species. Peak populations were obtained on September 17, 2002, July 29, 2003, and August 3, 2004. A single WNV-positive pool was obtained from this species during a small spike in Cx. erraticus abundance on March 2, 2004.
Although we did not obtain WNV-positive pools from either Cx. quinquefasciatus or Cx. restuans, these species are likely important enzootic and in the case of Cx. quinquefasciatus, epidemic vectors in St. Tammany Parish.18,19 Cx. quinquefasciatus was most abundant during the spring and summer months, with peak numbers seen in mid-June to early July in 2003 and 2004. Cx. restuans was collected in small numbers (< 5/TN) from October to March or April. However, in January and February, 2004, a spike in Cx. restuans abundance was observed. On January 13, 19.5/TN were collected, and on February 24, counts were 20/TN. The spike in Cx. restuans abundance occurred just prior to the appearance of a WNV-positive pool of Cx. erraticus. No increases in abundance were seen during this period in other Culex species incriminated as WNV vectors.
Trap and site effects.
Overall, 97% of mosquitoes were collected in light traps, although 65% (249/383) of Ae. albopictus and 83% (3236/3915) of Cx. quinquefasciatus were collected in gravid traps. Cx. nigripalpus was collected significantly more often in up traps than down traps when all sites were combined during 2002 and 2004 but not 2003 (Table 3). Among individual sites, only FJR has significantly more Cx. nigripalpus in up traps than down traps during all 3 years of the study. Non-significant differences in up versus down trap abundance were consistently seen at 190, FBP, and OWE, whereas the remaining sites had significant differences in abundance during 1 year of the study. Only 1 site, KEL, had significantly more Cx. nigripalpus in down traps and that occurred only in 2003.
Table 3.
Abundance of Cx. nigripalpus in CDC light traps placed at 6 (up) and 1.5 m (down) above the ground at seven sites in St. Tammany Parish, LA, during 2002–2004
| Site | 2002 | 2003 | 2004 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean u/TN | Mean d/TN | Ratio u/d* | Difference (P)† | Mean u/TN | Mean d/TN | Ratio u/d | Difference (P) | Mean u/TN | Mean d/TN | Ratio u/d | Difference (P) | |
| 190 | 17.3 | 11.0 | 1.6 | NS | 94.6 | 89.7 | 1.1 | NS | 50.0 | 47.7 | 1.1 | NS |
| KEL | 16.7 | 21.3 | 0.8 | NS | 17.3 | 36.2 | 0.5 | < 0.001 | 29.8 | 22.6 | 1.3 | NS |
| FBP | 4.6 | 7.2 | 0.6 | NS | 17.8 | 17.8 | 1.0 | NS | 32.7 | 9.9 | 3.3 | NS |
| BFD | 9.1 | 3.2 | 2.8 | NS | 19.8 | 11.5 | 1.7 | NS | 31.0 | 19.6 | 1.6 | < 0.001 |
| STP | 40.3 | 17.7 | 2.3 | NS | 44.7 | 27.8 | 1.6 | NS | 76.6 | 25.2 | 3.0 | < 0.001 |
| OWE | 4.2 | 5.4 | 0.8 | NS | 19.7 | 23.4 | 0.8 | NS | 18.5 | 10.5 | 1.8 | NS |
| FJR | 23.2 | 8.5 | 2.7 | < 0.001 | 14.9 | 7.6 | 2.0 | < 0.001 | 32.0 | 4.8 | 6.7 | < 0.001 |
| All sites | 115.4 | 74.3 | 1.6 | < 0.001 | 228.8 | 214 | 1.1 | NS | 270.6 | 140.3 | 1.9 | < 0.001 |
d = down; u = up.
Ratio of up trap counts to down trap counts.
P values are derived from the results using Sidak's method for multiple comparisons with an overall type I error rate of 0.05.
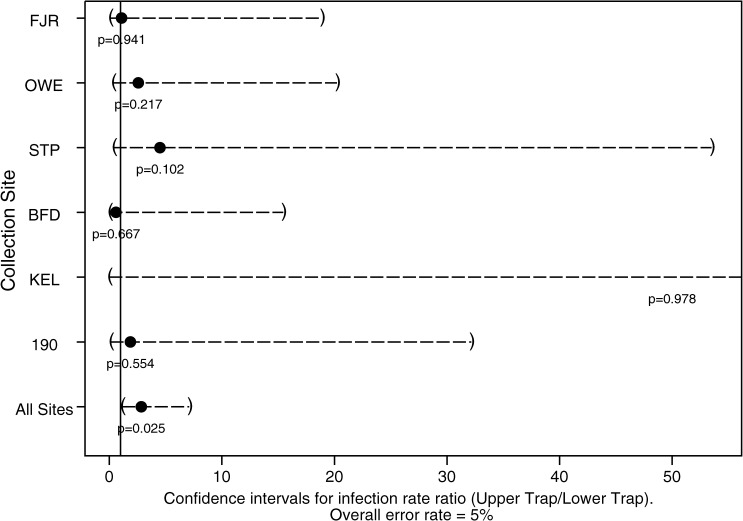
Twenty-one of thirty-two WNV-positive pools from all species came from up traps, including seventeen of twenty-three Cx. nigripalpus-positive pools (Table 2). Two positive pools, Cx. salinarius and Cx. spp., were obtained from gravid traps. Generally, similar numbers of positive pools were obtained from up and down traps at each site, except at STP, where seven of eight (88%) positive pools came from up traps (Table 4). Overall, more than two times as many positive pools were obtained from up traps (21/32, 66%) than down traps (10/32, 31%). When the combined infection rates (IRs) of Cx. nigripalpus collected during 2003 from all down traps were compared with the combined IRs of all up traps, the up trap IR was significantly higher (up/down ratio = 2.9, 95% CI = 1.1–7.1, P = 0.025) (Figure 4 ). Within individual sites, however, there was no difference in IR between up and down traps.
Table 4.
WNV RNA-positive mosquito pools collected at seven sites in St. Tammany Parish, LA, 2002–2004
| Site | WNV-positive pools | |||
|---|---|---|---|---|
| Light trap 1.5 m | Light trap 6 m | Gravid trap | Total | |
| 190 | 1 | 2 | – | 3 |
| KEL | 1 | 2 | 1 | 4 |
| FBP | – | – | – | – |
| BFD | 1 | 1 | – | 2 |
| STP | 1 | 7 | – | 8 |
| OWE | 3 | 5 | 1 | 9 |
| FJR | 2 | 4 | – | 6 |
| Total | 9 | 21 | 2 | 32 |
Figure 4.
Ratios of WNV infection rates in Cx. nigripalpus collected in dry ice-baited CDC light traps set at 1.5 and 6 m above the ground expressed as a point estimate with 95% CIs within parentheses. Where CIs include the value 1.0, infection rate differences between traps heights are not significant.
Discussion
During this study, we detected WNV in 4 of 41 mosquito species tested, including Cx. nigripalpus, Cx. salinarius, Cx. erraticus, and Cs. melanura; 26 of 32 WNV-positive pools identified were from members of the Cx. (Culex) subgenus, Cx. nigripalpus, and Cx. salinarius, but virus was not detected in Cx. quinquefasciatus or Cx. restuans. Virus activity occurred at six of seven study sites, which varied from forest and brackish marsh adjacent to Lake Pontchartrain to upland forest in the east-central portion of the parish. More WNV-positive pools were detected at inland sites than the sites near the lake. Most of the positive pools were from Cx. nigripalpus (23/32) females collected in 2003. Our results are consistent with the results obtained in the northeastern United States showing that Cx. (Culex) mosquitoes are the dominant vectors of WNV.1–3,28 Moreover, the analysis resulted in several important findings pertinent to WNV vector ecology and control in Louisiana: (1) Cx. quinquefasciatus may not be an important WNV vector in rural areas of St. Tammany Parish, (2) Cx. nigripalpus is potentially an important enzootic/epizootic vector, but it varies with its seasonal abundance, and (3) trap elevation is important for detecting WNV-infected mosquitoes.
Cx. quinquefasciatus, the southern member of the Cx. pipiens complex, plays a role in WNV transmission analogous to the role of Cx. pipiens in the north18,19,29,30 and Cx. pipiens-quinquefasciatus hybrids in the middle latitudes of the United States.31–34 In St. Tammany Parish during the 2002 outbreak, Cx. quinquefasciatus was the primary enzootic/epidemic vector, with Cx. salinarius as a possible secondary vector.18,19 Similarly, in East Baton Rouge Parish (EBRP), ∼100 km west of St. Tammany Parish, Cx. quinquefasciatus is the species most frequently found infected with WNV.35,36 In contrast to our finding that Cx. quinquefasciatus represented only 1% of mosquitoes that we collected, this species was the most abundant one collected in the two components of the EBRP study, comprising 41% and 85% of total mosquitoes.36 A likely reason for this difference is that many of the EBRP collection sites were located in urban and suburban areas where WNV activity had previously been detected.
Several studies have indicated that Cx. quinquefasciatus feeds more readily on mammals, particularly in urban settings, than Cx. pipiens, which would enhance its potential as a vector to humans and other mammals.37–39 In EBRP, human DNA was detected in only 7% of identified Cx. quinquefasciatus blood meals, but this result, combined with a high percentage of blood meals taken on known avian amplifier hosts, indicated a primary role for this species as an enzootic and epidemic vector.40
Because Cx. quinquefasciatus was not commonly collected, our results may be most pertinent to understanding enzootic transmission of WNV in rural areas with a paucity of primary vectors such as Cx. quinquefasciatus. The large year-to-year variation in the number of WNV-infected pools detected at our study sites (2 pools in 2002, 29 pools in 2003, and 1 pool in 2004) suggests that focal maintenance of virus endemicity may be unstable in areas where the relative abundance of Cx. quinquefasciatus is low. Support for this hypothesis comes from the results of on-going surveillance by the STPMAD in other suburban and rural locations that identified 11 WNV-infected pools (10 Cx. quinquefasciatus) during June and July, 2002,18 28 positive pools (13 Cx. quinquefasciatus) in 2003, and 42 positive pools (26 Cx. quinquefasciatus) in 2004 (Palmisano C, unpublished data). Apparently, enzootic transmission by Cx. quinquefasciatus was relatively stable, if not increasing, in areas where that species was abundant. The STPMAD surveillance program focuses specifically on monitoring Cx. quinquefasciatus populations in areas of the parish where septic ditches provide ideal larval habitat for this species.18 These ditches, which contain the treated effluent from residential septic tanks and filter beds, are the main producers of Cx. quinquefasciatus in the parish. Although these ditches are located in both suburban and rural areas of the parish, none were located near our study sites. The fact that we did not obtain any WNV-positive pools from this species may reflect the relatively small numbers tested or reduced levels of virus amplification in areas where Cx. quinquefasciatus populations are small. The detection by STPMAD in 2003 and 2004 of WNV in a number of species in addition to Cx. quinquefasciatus mirrors results found in EBRP during those years.36
Cx. nigripalpus was the second most commonly collected species, and it was among the three most abundant species collected at all seven sites. This species is the primary vector for SLEV in southern Florida,14,15 and WNV was isolated from this species in northern Florida in 2001, the first year that the virus was detected in that state.16,17 Eight isolates of WNV were obtained during 2005 from Cx. nigripalpus collected in chicken-baited lard can traps placed at three widely dispersed sites in Florida.41 This study also noted a low rate of WNV transmission to the chickens restrained within the traps, despite seven of eight virus-positive pools containing only blooded females, a finding that corroborates previous work.17 Vitek and others41 speculate that the lack of virus dissemination within the infected mosquitoes may be responsible for the low transmission rate, but their experimental protocol did not allow them to test this idea.41 In southern Florida, onset of human cases and the appearance of anti-WNV antibodies in sentinel chickens are temporally and spatially associated with prior cycles of drought and moisture in a manner similar to that seen with SLEV, thus providing strong evidence that Cx. nigripalpus transmits WNV in that area.42 However, the involvement of Cx. nigripalpus in the WNV transmission cycle in other areas across its geographic range is unclear.
Our data show that Cx. nigripalpus can maintain enzootic or epizootic WNV activity during a transmission season. The 23 WNV-positive pools from Cx. nigripalpus during 2003 represented 72% of the total positive pools detected during the study. In contrast, no positive pools were found in Cx. nigripalpus in either 2002 or 2004. A shift in the seasonal abundance of this species may be responsible. Cx. nigripalpus abundance increased in late September/early October during 2002 and 2004 and peaked in early November in 2002 and late November in 2004. In contrast, Cx. nigripalpus abundance in 2003 increased during mid-July and peaked in late August. The earlier appearance of Cx. nigripalpus in 2003 brought its peak abundance that year more closely aligned temporally with the May–July peak of Cx. quinquefasciatus than occurred in either 2002 or 2004. This result would provide an increased opportunity for host-seeking Cx. nigripalpus to feed on birds recently infected by Cx. quinquefasciatus and establish a new transmission cycle. In 2002, the situation is less clear than 2004. Because our study began on July 16, 2002, we have no data from before this date. Also, during 2002, Cx. quinquefasciatus were captured through October when Cx. nigripalpus populations began increasing. However, 2002 was an epidemic year, and WNV disease case numbers dropped sharply after July.18 The STPMAD did not detect positive Cx. quinquefasciatus pools after July,18 and a separate investigation conducted from August 3 to 1519 did not detect virus-positive mosquito pools after August 10. This finding suggests that virus transmission in 2002 had subsided well before Cx. nigripalpus abundance began to peak, although serologic conversions in sentinel chickens continued at a low level into November. Because WNV-infected birds are viremic for 1 week or less,9 the later emergence of Cx. nigripalpus, as seen in 2002 and 2004, would reduce the likelihood that host-seeking females would take an infectious blood meal. This hypothesis assumes that Cx. quinquefasciatus was the primary species involved in WNV amplification during 2003 and that the seasonal abundance peaks that we observed in this species were representative of areas within the parish where Cx. quinquefasciatus populations are larger and virus transmission by this species occurred.
Studies conducted in EBRP during 2002–2004 reached similar conclusions, suggesting that the role of Cx. nigripalpus in enzootic amplification of WNV may be limited by its seasonality of host-seeking and the high percentage of mammalian blood meals taken by this species.40 In this study, Cx. nigripalpus was not seen in light traps before July, and 66% of identified blood meals were from mammalian sources (2.7% from humans). A shift in Cx. nigripalpus seasonality was also associated with increased SLEV transmission in northern Florida, an area where extensive activity of this virus is not typically seen.43 Additional longer-term studies are needed to more rigorously test the association between Cx. nigripalpus seasonality and vector status.
Infection rates during 2003 were significantly higher in Cx. nigripalpus from up traps than down traps when collections from all sites were combined but not from individual sites. This result is likely because of smaller sample sizes at individual sites yielding broad overlapping CIs in statistical analyses. Moreover, Cx. nigripalpus was as likely to be collected in down traps as up traps at all seven sites combined in 2003 but was collected significantly more often in up traps in 2002 and 2004. The relative proportions of Cx. nigripalpus collected in traps placed at 1.5 and 3.0 m in EBRP during 2002–2004 were similar (49% versus 51%),36 suggesting that this difference in trap height was not significant for host-seeking females. In Connecticut, significantly more Cx. pipiens and WNV isolates were obtained from traps placed in the canopy than near the ground, and significantly more Cx. pipiens and WNV isolates were obtained from traps placed in catch basins than at ground level.44–46 In similar height studies on Cx. pipiens-quinquefasciatus hybrids using chicken-baited can traps in Memphis, Tennessee, all WNV-positive specimens were collected from traps at the highest elevations, 4.6 and 7.6 m, and IR differences between heights were marginally non-significant.33,34 In Connecticut, parity dissections of Cx. pipiens captured in the canopy and catch basins revealed that 61% of females in the canopy were nulliparous and probably host-seeking, whereas 87% of females in the catch basins either contained eggs or were parous, indicating that they had previously fed and oviposited or were seeking a site for oviposition.45 These results and the results of other studies47,48 suggest that collection of the important WNV vector species and detection of WNV may be done more efficiently by trapping in the tree canopy rather than or in addition to placing traps closer to the ground.
Cx. salinarius comprised 56.2% of the mosquitoes that we collected, and 81% of those mosquitoes came from one site (KEL) located adjacent to a brackish marsh at the edge of Lake Pontchartrain. Although Cx. salinarius was the most abundant species collected during our study, we obtained only three WNV-positive pools from this species. This species was most abundant in our study during the winter, spring, and autumn months, with the summer months being the period of lowest abundance. Because Cx. salinarius feeds readily on both birds and mammals,7,49,50 it would seem well-positioned to play a role in both enzootic and epidemic transmission of WNV. In a 5-year study conducted in Connecticut, WNV was isolated from 32 pools of Cx. salinarius collected from 1999 to 2003.28 However, environmental factors may limit contact between Cx. salinarius and infected birds. Cx. salinarius was most abundant at the sites closest to Lake Pontchartrain, KEL and FBP (519.2/TN and 78.3/TN, respectively), but these two sites did not yield as many WNV-positive pools from all mosquito species (KEL = 4, FBP = 0) as the inland sites (STP = 8, OWE = 9, FJR = 6). Analysis of a subset of our 2003 data indicated that infection rates in Culex mosquitoes were inversely associated with the percentage of wetland cover at study sites20 and that this association is likely because of a lower ratio of WNV-competent to -incompetent birds in intact wetland areas, which would dampen virus amplification.21 Sites KEL and FBP are located in large wetland areas and had the highest proportions of WNV-incompetent species.21 In contrast, in EBRP, the proportional abundance of Cx. salinarius was much lower, < 2% of total mosquitoes trapped, but seven WNV-positive pools were detected.36 The likely explanation for this result is that ∼50% of avian blood meals identified in engorged Cx. salinarius were from passerine species,40 two of which, the northern cardinal (Cardinalis cardinalis) and the northern mockingbird (Mimus polyglottos), are competent WNV amplifier hosts.51
Among the potential mechanisms advanced to explain WNV overwintering is continued low-level transmission between mosquitoes and birds.52 Our observations of WNV in host-seeking Cx. nigripalpus collected during November of 2003 and Cx. erraticus trapped in March of 2004 support other studies showing that year-round WNV transmission by mosquitoes occurs in sub-tropical areas of the southern United States.36,53 The WNV-positive pool that we detected in March was associated temporally with a relatively large spike in the abundance of Cx. restuans at four of our study sites. Although we did not detect WNV in Cx. restuans, this species was implicated as an early-season vector in the northeastern United States.2 Thus, it is possible that the spike in Cx. restuans abundance in St. Tammany Parish initiated a brief cycle of WNV amplification in birds that led to a spillover of virus into species that are not generally considered important WNV vectors. Alternate explanations of virus detection in mosquitoes during winter include survival of mosquitoes infected during the fall or vertical transmission of virus. The former would require survival of infected mosquitoes for many months in a metabolically active state, whereas the latter mechanism has not been confirmed in Cx. erraticus.
In EBRP, Cx. restuans was implicated as an important early-season vector of WNV, because 10 virus-positive pools of this species were detected along with 4 positive pools of Cx. erraticus, all collected during March and April of 2003.36 Interestingly, during the EBRP study, WNV was also isolated from numerous pools of floodwater Aedes species and Ps. ferox, Anopheles species, and Coquillettidia perturbans collected during March and April of 2003. These isolations from presumably non-vector species are, perhaps, indicative of the high level of WNV transmission that occurred during the epidemic of 2002 in southeastern Louisiana and the higher relative abundance of Cx. quinquefasciatus at EBPR study sites compared with our sites. In the mid-southern United States, WNV was most frequently detected during multiple years in Cx. erraticus and Cx. salinarius, including two virus-positive pools of overwintering Cx. erraticus collected in February and March of 2005, indicating that these species may be important vectors in that region.54
Culex erraticus feeds predominantly on mammals, but will also feed on birds.32,49 Studies conducted in Tennessee showed that avian blood meal hosts of Cx. erraticus included American robins (Turdus migratorius), common grackles (Quiscalus quiscula), house finches (Carpodacus mexicanus), and Carolina wrens (Thryothorus ludovicianus).32,55 Robins, grackles, and house finches are effective amplifier hosts of WNV9 and year-round or winter residents in southern Louisiana.55 Serologic evidence of infection with WNV was obtained from Carolina wrens and common grackles captured in St. Tammany Parish in 2002.56
Although we collected only 811 Cs. melanura during our study, we identified WNV in two pools from August of 2003, and this species had the highest overall WNV infection rate. Cs. melanura is the primary enzootic vector of eastern equine encephalitis virus in the United States.57 Studies of Cs. melanura host preferences have shown that ∼90% or more of blood meals are taken from birds,8,58,59 with a small percentage taken from mammals. Thus, this species may play a role in the avian amplification cycle of WNV and potentially transmit the virus to humans or other mammals.
Our investigation has several potential limitations. From mid-December to early January each year, mosquito collections were not performed, and from early January to mid-February of 2004, trapping was intermittent. Also, during all 3 years but especially in 2002, intensive aerial and ground-level adulticiding was conducted throughout the parish. This activity is likely responsible for some of the fluctuations in mosquito abundance seen during the spring to fall time period each year. However, records of adulticiding in the vicinity of our trap sites were not available for analysis. Finally, when > 500 individuals of a species were present in a trap, only 500 were pooled for virus testing. Because there was no obvious selection bias in choosing specimens for virus testing, we feel that the results obtained (the IR) are representative of those specimens not tested.
Despite these possible limitations, our study resulted in several important findings. In areas of the southern United States where Cx. quinquefasciatus populations are small or absent, enzootic WNV transmission may be maintained by Cx. nigripalpus. However, the contribution of Cx. nigripalpus to enzootic maintenance in a given year may fluctuate with variations in its seasonal abundance: more effective in years when populations peak during mid-summer but less effective when populations peak during the fall. Cx. salinarius, although numerous and possessing host preference characteristics that would make it an effective enzootic or epidemic vector, did not contribute significantly to WNV transmission at our study sites. Cs. melanura probably contributes to the amplification of WNV in avian hosts. WNV transmission can occur year-round in southern Louisiana, and Cx. erraticus may assist in virus overwintering. Our findings have implications for effective surveillance and control of WNV vectors in this region. Because Cx. quinquefasciatus is the vector of primary importance in urban and suburban areas of St. Tammany Parish, larval and adult control measures should continue to focus on that species. Monitoring of Cx. nigripalpus abundance in suburban and rural areas may also be useful to detect its early summer appearance and the possibility of enhanced enzootic WNV transmission. In addition, trapping of Cx. nigripalpus in the tree canopy may be a more sensitive indicator of virus activity than trapping closer to the ground. Additional research should be conducted to determine if targeted control against this species is warranted in efforts to prevent WNV transmission.
ACKNOWLEDGMENTS
The authors would like to thank the staff of the St. Tammany Parish Mosquito Abatement District, especially M. Berrios, M. Brody, B. Massery, G. Polk, P. Roy, D. Roy, and C. Smotherman, and N. Lyons and S. Schwamberger of the Department of Tropical Medicine, Tulane University School of Public Health and Tropical Medicine, for their dedication and assistance during this project. We would also like to acknowledge Chet Moore, formerly of Centers for Disease Control and Prevention Fort Collins, for his role in conceiving and supporting this project in its earliest stages and the continuing support of Roger Nasci and Harry Savage, Centers for Disease Control and Prevention Fort Collins. We also thank the anonymous reviewers and Sandra Cooper, US Geological Survey, for improvements to the manuscript.
Disclaimer: The opinions expressed in this article are the opinions of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. This article has been peer reviewed and approved for publication consistent with U.S. Geological Survey Fundamental Science Practices (http//pubs.usgs.gov/circ/1367/). Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Footnotes
Financial support: Funding for this study was provided by the US Geological Survey and the Centers for Disease Control and Prevention.
Authors' addresses: Marvin S. Godsey Jr., Kristen Burkhalter, Mark Delorey, Leah Colton, Dawn Charnetzky, and Genevieve Sutherland, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, Fort Collins, CO, E-mails: mjg9@cdc.gov, ktb3@cdc.gov, esy7@cdc.gov, ant6@cdc.gov, dcharnetzky@yahoo.com, and genevieve_sutherland@yahoo.com. Raymond J. King, Public Health Informatics and Technology Program Office, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: rnk8@cdc.gov. Vanessa O. Ezenwa, U.S. Geological Survey, Eastern Geography Science Center, Reston, VA; Current address: Odum School of Ecology and Department of Infectious Diseases, University of Georgia, Athens, GA, E-mail: vezenwa@uga.edu. Lawrence A. Wilson, Fernbank Science Center, Atlanta, GA, E-mail: larry.wilson@fernbank.edu. Michelle Coffey, Lesley E. Milheim, and Stephen C. Guptill, U.S. Geological Survey, Eastern Geography Science Center, Reston, VA, E-mails: mcoffey@usgs.gov, lmilheim@usgs.gov, and sguptill@guptillgeoscience.com. Viki G. Taylor and Charles Palmisano, St. Tammany Parish Mosquito Abatement District, Slidell, LA, E-mails: bugladyviki@yahoo.com and chuck_palmisano@yahoo.com. Dawn M. Wesson, Department of Tropical Medicine, School of Public Health and Tropical Medicine, Tulane University, New Orleans, LA, E-mail: wesson@tulane.edu.
References
- 1.Nasci RS, White DJ, Stirling H, Oliver J, Daniels TJ, Falco RC, Campbell S, Crans WJ, Savage HM, Lanciotti RS, Moore CG, Godsey MS, Gottfried KL, Mitchell CJ. West Nile virus isolates from mosquitoes in New York and New Jersey, 1999. Emerg Infect Dis. 2001;7:626–630. doi: 10.3201/eid0704.010404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreadis TG, Anderson JF, Vossbrinck CR. Mosquito surveillance for West Nile virus in Connecticut, 2000: isolation from Culex pipiens, Cx. restuans, and Culiseta melanura. Emerg Infect Dis. 2001;7:670–674. doi: 10.3201/eid0704.010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernard KA, Maffei JG, Jones SA, Kauffman EB, Ebel GD, Dupuis AP, Ngo KA, Nicholas DC, Young DM, Shi P-Y, Kulasekera V, Eidson M, White DJ, Stone WB, State West Nile Virus Surveillance Team NY, Kramer LD. West Nile virus infection in birds and mosquitoes, New York State, 2000. Emerg Infect Dis. 2001;7:679–685. doi: 10.3201/eid0704.010415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turell MJ, O'Guinn M, Oliver J. Potential for New York mosquitoes to transmit West Nile virus. Am J Trop Med Hyg. 2000;62:413–414. doi: 10.4269/ajtmh.2000.62.413. [DOI] [PubMed] [Google Scholar]
- 5.Turell MJ, O'Guinn M, Dohm DJ, Jones JW. Vector competence of North American mosquitoes (Diptera:Culicidae) for West Nile virus. J Med Entomol. 2001;38:130–134. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- 6.Sardelis MR, Turell MJ, Dohm DJ, O'Guinn ML. Vector competence of selected North American Culex and Coquillettidia mosquitoes for West Nile virus. Emerg Infect Dis. 2001;7:1018–1022. doi: 10.3201/eid0706.010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apperson CS, Harrison GA, Unnasch TR, Hassan HK, Irby WS, Savage HM, Aspen SE, Watson DW, Rueda LM, Engber BR, Nasci RS. Host-feeding habits of Culex and other mosquitoes (Diptera:Culicidae) in the Borough of Queens in New York City, with characters and techniques for identification of Culex mosquitoes. J Med Entomol. 2002;39:777–785. doi: 10.1603/0022-2585-39.5.777. [DOI] [PubMed] [Google Scholar]
- 8.Magnarelli LA. Host feeding patterns of Connecticut mosquitoes (Diptera:Culicidae) Am J Trop Med Hyg. 1977;26:547–552. doi: 10.4269/ajtmh.1977.26.547. [DOI] [PubMed] [Google Scholar]
- 9.Hayes EB, Komar N, Nasci RS, Montgomery SP, O'Leary DR, Campbell GR. Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis. 2005;11:1167–1173. doi: 10.3201/eid1108.050289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Main AJ, Tonn RJ, Randall EJ, Anderson KS. Mosquito densities at heights of five and twenty-five feet in southeastern Massachussetts. Mosq News. 1966;26:243–248. [Google Scholar]
- 12.Novak RJ, Peloquin J, Rohrer W. Vertical distribution of adult mosquitoes (Diptera:Culicidae) in a northern deciduous forest in Indiana. J Med Entomol. 1981;18:116–122. [Google Scholar]
- 13.Beadle LD, Menzies GC, Hayes GR, Jr, Von Zuben FJ, Jr, Eads RB. St. Louis encephalitis in Hidalgo County, Texas. Vector control and evaluation. Pub Hlth Rep. 1957;72:531–535. [PMC free article] [PubMed] [Google Scholar]
- 14.Chamberlain RW, Sudia WD, Coleman PH, Beadle LD. Vector studies in the St. Louis encephalitis epidemic, Tampa Bay area, Florida. Am J Trop Med Hyg. 1964;13:456–461. doi: 10.4269/ajtmh.1964.13.456. [DOI] [PubMed] [Google Scholar]
- 15.Dow RP, Coleman PH, Meadows KE, Work TH. Isolation of St. Louis encephalitis virus from mosquitoes in the Tampa Bay area of Florida during the epidemic of 1962. Am J Trop Med Hyg. 1964;13:462–474. doi: 10.4269/ajtmh.1964.13.462. [DOI] [PubMed] [Google Scholar]
- 16.Godsey MS, Blackmore MS, Panella NA, Burkhalter K, Gottfried K, Halsey LA, Rutledge R, Langevin SA, Gates R, Lamonte KM, Lambert A, Lanciotti RS, Blackmore CGM, Loyless T, Stark L, Oliveri R, Conti L, Komar N. West Nile virus epizootiology in the southeastern United States, 2001. Vector Borne Zoonotic Dis. 2005;5:82–89. doi: 10.1089/vbz.2005.5.82. [DOI] [PubMed] [Google Scholar]
- 17.Rutledge CR, Day JF, Lord CC, Stark LM, Tabachnick WJ. West Nile virus infection rates in Culex nigripalpus do not reflect transmission rates in Florida. J Med Entomol. 2003;40:253–258. doi: 10.1603/0022-2585-40.3.253. [DOI] [PubMed] [Google Scholar]
- 18.Palmisano CT, Taylor V, Caillouet K, Byrd B, Wesson DM. Impact of West Nile virus outbreak upon St. Tammany Parish mosquito abatement district. J Am Mosq Control Assoc. 2005;21:33–38. doi: 10.2987/8756-971X(2005)21[33:IOWNVO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 19.Godsey MS, Jr, Nasci R, Savage HM, Aspen S, King R, Powers AM, Burkhalter K, Colton L, Charnetzky D, Lasater S, Taylor V, Palmisano CT. West Nile virus-infected mosquitoes, Louisiana, 2002. Emerg Infect Dis. 2005;11:1401–1406. doi: 10.3201/eid1109.040443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ezenwa VO, Milheim LE, Coffey MF, Godsey MS, King RJ, Guptill SC. Land cover variation and West Nile virus prevalence: patterns, processes and implications for disease control. Vector Borne Zoonotic Dis. 2007;7:173–180. doi: 10.1089/vbz.2006.0584. [DOI] [PubMed] [Google Scholar]
- 21.Ezenwa VO, Godsey MS, King RJ, Guptill SC. Avian diversity and West Nile virus: testing associations between biodiversity and infectious disease risk. Proc Biol Sci. 2006;273:109–117. doi: 10.1098/rspb.2005.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nasci RS, Gottfried KL, Burkhalter KL, Kulasekera VL, Lambert AJ, Lanciotti RL, Hunt AR, Ryan JR. Comparison of vero cell plaque assay, TaqMan® reverse transcriptase polymerase chain reaction RNA assay, and Vectest™ antigen assay for detection of West Nile virus in field-collected mosquitoes. J Am Mosq Control Assoc. 2002;18:294–300. [PubMed] [Google Scholar]
- 23.Beaty BJ, Calisher CH, Shope RS. Arboviruses. In: Schmidt NJ, Emmons RW, editors. Diagnostic Procedures for Viral, Rickettsial, and Chlamydial Infections. Washington, DC: American Public Health Association; 1989. pp. 797–856. [Google Scholar]
- 24.Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, Komar N, Panella NA, Allen BC, Volpe KE, Davis BS, Roehrig JT. Rapid detection of West Nile virus from human clinical specimens, field collected mosquitoes and avian samples by a TaqMan RT-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biggerstaff BJ. Confidence intervals for the difference of two proportions estimated from pooled samples. JABES. 2008;13:478–496. [Google Scholar]
- 26.Tebbs JM, McCann MH. Large-sample hypothesis tests for stratified group-testing data. JABES. 2007;12:534–551. [Google Scholar]
- 27.Debboun M, Kuhr DD, Rueda LM, Pecor JE. First record of Culex (Culex) coronator in Louisiana, USA. J Am Mosq Control Assoc. 2005;21:455–457. doi: 10.2987/8756-971X(2006)21[455:FROCCC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Andreadis TG, Anderson JF, Vossbrinck CR, Main AJ. Epidemiology of West Nile virus in Connecticut: a five-year analysis of mosquito data 1999–2003. Vector Borne Zoonotic Dis. 2004;4:360–378. doi: 10.1089/vbz.2004.4.360. [DOI] [PubMed] [Google Scholar]
- 29.Lillibridge KM, Parsons R, Randle Y, Travassos de Rosa APA, Guzman H, Siirin M, Wuithiranyagool T, Hailey C, Higgs S, Bala AA, Pascua R, Meyer T, Vanlandingham DL, Tesh RB. The 2002 introduction of West Nile virus into Harris County, Texas, and area historically endemic for St. Louis encephalitis. Am J Trop Med Hyg. 2004;70:676–681. [PubMed] [Google Scholar]
- 30.Reisen WK, Lothrop H, Chiles R, Madon M, Cossen C, Woods L, Husted S, Kramer V, Edman J. West Nile virus in California. Emerg Infect Dis. 2004;10:1369–1378. doi: 10.3201/eid1008.040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savage HM, Anderson M, Gordon E, McMillen L, Colton L, Charnetzky D, Delorey M, Aspen S, Burkhalter K, Biggerstaff BJ, Godsey MS. Oviposition activity patterns and West Nile virus infection rates for members of the Culex pipiens complex at different habitat types within the hybrid zone, Shelby County, TN, 2002 (Diptera:Culicidae) J Med Entomol. 2006;43:1227–1238. doi: 10.1603/0022-2585(2006)43[1227:oapawn]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 32.Savage HM, Aggarwal D, Apperson CS, Katholi CR, Gordon E, Hassan HK, Anderson M, Charnetzky D, McMillen L, Unnasch EA, Unnasch TR. Host choice and West Nile virus infection rates in blood-fed mosquitoes, including members of the Culex pipiens complex, from Memphis and Shelby County, Tennessee, 2002–2003. Vector Borne Zoonotic Dis. 2007;7:365–386. doi: 10.1089/vbz.2006.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Savage HM, Anderson M, Gordon E, McMillen L, Colton L, Delorey M, Sutherland G, Aspen S, Charnetzky D, Burkhalter K, Godsey M. Host seeking heights, host seeking activity patterns, and West Nile virus infection rates for members of the Culex pipiens complex at different habitat types within the hybrid zone, Shelby County, TN, 2002 (Diptera:Culicidae) J Med Entomol. 2008;45:276–288. doi: 10.1603/0022-2585(2008)45[276:hhhapa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 34.Kothera L, Zimmerman EM, Richards CM, Savage HM. Microsatellite characterization of subspecies and their hybrids in Culex pipiens complex (Diptera:Culicidae) mosquitoes along a north-south transect in the central United States. J Med Entomol. 2009;46:236–248. doi: 10.1603/033.046.0208. [DOI] [PubMed] [Google Scholar]
- 35.Gleiser RM, Mackay AJ, Roy A, Yates MM, Vaeth RH, Faget GM, Folsom AE, Augustine WF, Jr, Wells RA, Perich MJ. West Nile virus surveillance in East Baton Rouge Parish, Louisiana. J Am Mosq Control Assoc. 2007;23:29–36. doi: 10.2987/8756-971X(2007)23[29:WNVSIE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 36.Mackay AJ, Roy A, Yates MM, Foil LD. West Nile virus detection in mosquitoes in East Baton Rouge Parish, Louisiana, from November 2002 to October 2004. J Am Mosq Control Assoc. 2008;24:28–35. doi: 10.2987/5681.1. [DOI] [PubMed] [Google Scholar]
- 37.Edman JD. Host-feeding patterns of Florida mosquitoes. III. Culex (Culex) and Culex (Neoculex) J Med Entomol. 1974;11:95–104. doi: 10.1093/jmedent/11.1.95. [DOI] [PubMed] [Google Scholar]
- 38.Niebylski ML, Meek CL. Blood-feeding of Culex mosquitoes in an urban environment. J Am Mosq Control Assoc. 1992;8:173–177. [PubMed] [Google Scholar]
- 39.Molaei G, Andreadis TG, Armstrong PM, Bueno R, Jr, Dennett JA, Real SV, Sargent C, Bala A, Radle Y, Guzman H, Travassos de Rosa A, Wuithiranyagool T, Tesh RB. Host feeding pattern of Culex quinquefasciatus (Diptera:Culicadae) and its role in transmission of West Nile virus in Harris County, Texas. Am J Trop Med Hyg. 2007;77:73–81. [PubMed] [Google Scholar]
- 40.Mackay AJ, Kramer WL, Meece JK, Brumfield RT, Foil LD. Host feeding patterns of Culex mosquitoes (Diptera:Culicidae) in East Baton Rouge Parsh, Louisiana. J Med Entomol. 2010;47:238–248. doi: 10.1603/me09168. [DOI] [PubMed] [Google Scholar]
- 41.Vitek CJ, Richards SL, Mores CN, Day JF, Lord CC. Arbovirus transmission by Culex nigripalpus in Florida, 2005. J Med Entomol. 2008;45:483–493. doi: 10.1603/0022-2585(2008)45[483:atbcni]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaman J, Day JF, Stieglitz M. Drought-induced amplification and epidemic transmission of West Nile virus in southern Florida. J Med Entomol. 2005;42:134–141. doi: 10.1093/jmedent/42.2.134. [DOI] [PubMed] [Google Scholar]
- 43.Zyzak M, Loyless T, Cope S, Wooster M, Day JF. Seasonal abundance of Culex nigripalpus Theobald and Culex salinarius Coquillett in north Florida, USA. J Vector Ecol. 2002;27:155–162. [PubMed] [Google Scholar]
- 44.Anderson JF, Andreadis TG, Main AJ, Kline DL. Prevalence of West Nile virus in tree canopy-inhibiting Culex pipiens and associated mosquitoes. Am J Trop Med Hyg. 2004;71:112–119. [PubMed] [Google Scholar]
- 45.Anderson JF, Andreadis TG, Main AJ, Ferrandino FJ, Vossbrinck CR. West Nile virus from female and male mosquitoes (Diptera:Culicidae) in subterranean, ground, and canopy habitats in Connecticut. J Med Entomol. 2006;43:1010–1019. doi: 10.1603/0022-2585(2006)43[1010:wnvffa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 46.Andreadis TG, Armstrong PM. A two-year evaluation of canopy trapping for Culex mosquitoes and West Nile virus in an operational surveillance program in the northeastern United States. J Am Mosq Control Assoc. 2007;23:137–148. doi: 10.2987/8756-971X(2007)23[137:ATEOEC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 47.Darbro JM, Harrington LC. Bird-baited traps for surveillance of West Nile mosquito vectors: effect of bird species, trap height, and mosquito escape rates. J Med Entomol. 2006;43:83–92. doi: 10.1093/jmedent/43.1.83. [DOI] [PubMed] [Google Scholar]
- 48.Drummond CL, Drobnack J, Backenson PB, Ebel BD, Kramer LD. Impact of trap elevation on estimates of abundance, parity rates, and body size of Culex pipiens and Culex restuans (Diptera:Culicidae) J Med Entomol. 2006;43:177–184. doi: 10.1603/0022-2585(2006)043[0177:ioteoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 49.Apperson CS, Hassan HK, Harrison BA, Savage HM, Aspen SE, Farajollahi A, Crans W, Daniels TJ, Falco RC, Benedict M, Anderson M, McMillen L, Unnasch TR. Host feeding patterns of established and potential mosquito vectors of West Nile virus in the eastern United States. Vector Borne Zoonotic Dis. 2004;4:71–82. doi: 10.1089/153036604773083013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Molaei G, Andreadis TG, Armstrong PM, Anderson JF, Vossbrinck CR. Host feeding patterns of Culex mosquitoes and West Nile virus transmission, northeastern United States. Emerg Infect Dis. 2006;12:468–474. doi: 10.3201/eid1203.051004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Komar N, Panella NA, Langevin SA, Brault AC, Amador M, Edwards E, Owen JC. Avian hosts for West Nile virus in St. Tammany Parish, Louisiana, 2002. Am J Trop Med Hyg. 2005;73:1031–1037. [PubMed] [Google Scholar]
- 52.Reisen WK, Fang Y, Lothrop HD, Martinez VM, Wilson J, O'Connor P, Carney R, Cahoon-Young B, Shafii M, Brault AC. Overwintering of West Nile virus in southern California. J Med Entomol. 2006;43:344–355. doi: 10.1603/0022-2585(2006)043[0344:oownvi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 53.Tesh RB, Parsons R, Siirin M, Randle Y, Sargent C, Guzman H, Wuithranyagool T, Higgs S, Vanlandingham DL, Bala AA, Haas K, Zerinque B. Year-round West Nile virus activity, Gulf coast region, Texas and Louisiana. Emerg Infect Dis. 2004;10:1649–1652. doi: 10.3201/eid1009.040203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cupp EW, Hassan HK, Yue X, Oldland WK, Lilley BM, Unnasch TR. West Nile virus infection in mosquitoes in the Mid-South USA, 2002–2005. J Med Entomol. 2007;44:117–125. doi: 10.1603/0022-2585(2007)44[117:wnviim]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen SB, Lewoczko K, Huddleston DB, Moody E, Mukerjee S, Dunn JR, Jones TF, Wilson R, Moncayo AC. Host feeding patterns of potential vectors of eastern equine encephalitis virus at an epizootic focus in Tennessee. Am J Trop Med Hyg. 2009;81:452–456. [PubMed] [Google Scholar]
- 56.Sibley DA. The Sibley Field Guide to the Birds of Eastern North America. New York, NY: Alfred A. Knopf; 2003. [Google Scholar]
- 57.Morris CD. Eastern equine encephalitis. In: Monath TM, editor. The Arboviruses. Epidemiology and Ecology. Vol. 3. Boca Raton, FL: CRC Press; 1988. pp. 1–20. [Google Scholar]
- 58.Nasci RS, Edman JD. Blood-feeding patterns of Culiseta melanura (Diptera:Culicidae) and associated sylvan mosquitoes in southeastern Massachusetts eastern equine encephalitis enzootic foci. J Med Entomol. 1981;18:493–500. [Google Scholar]
- 59.Molaei G, Oliver J, Andreadis TG, Armstrong PM, Howard JJ. Molecular identification of blood-meal sources in Culiseta melanura and Culiseta morsitans from an endemic focus of eastern equine encephalitis virus in New York. Am J Trop Med Hyg. 2006;75:1140–1147. [PubMed] [Google Scholar]