Abstract
In June of 2007, West Nile virus (WNV) was detected in sentinel chickens and blood donors in Puerto Rico, where dengue virus (DENV) is hyperendemic. Enhanced human surveillance for acute febrile illness (AFI) began in eastern Puerto Rico on July 1, 2007. Healthcare providers submitted specimens from AFI cases for WNV and DENV virology and serology testing. Over 6 months, 385 specimens were received from 282 cases; 115 (41%) specimens were DENV laboratory-positive, 86 (31%) specimens were laboratory-indeterminate, and 32 (11%) specimens were laboratory-negative for WNV and DENV. One WNV infection was detected by anti-WNV immunoglobulin M (IgM) antibody and confirmed by a plaque reduction neutralization test. DENV and WNV infections could not be differentiated in 27 cases (10%). During a period of active WNV transmission, enhanced human surveillance identified one case of symptomatic WNV infection. Improved diagnostic methods are needed to allow differentiation of WNV and DENV in dengue-endemic regions.
Introduction
West Nile virus (WNV) is a mosquito-borne arbovirus that is amplified in an enzootic cycle involving birds; humans, horses, and other mammals are thought to be dead-end incidental hosts. In the United States, WNV was first detected in humans during an encephalitis outbreak in New York City in 1999.1 The recent emergence of WNV throughout the Americas is thought to be a result of bird migration patterns.2 As of 2007, WNV had been reported in 16 countries in Latin America and the Caribbean3,4; however, few cases of human WNV disease have been reported.5
Surveillance for human WNV disease in Puerto Rico began in late 2002, when the Puerto Rico Department of Health (PRDH) and Centers for Disease Control and Prevention (CDC) Dengue Branch established a passive surveillance system for neuroinvasive WNV disease defined initially as febrile patients hospitalized with encephalitis, meningoencephalitis, acute flaccid paralysis, or Guillain–Barré syndrome as well as all cases of aseptic meningitis in adults 18 years old or older. Reporting criteria were expanded to include pediatric aseptic meningitis cases in June of 2004 after an aseptic meningitis outbreak. To report a suspected case, healthcare providers submit a WNV case report form (WCRF) and a serum and/or cerebrospinal fluid (CSF) specimen to the Dengue Branch for free diagnostic testing, including reverse transcriptase-polymerase chain reaction (RT-PCR) for WNV and dengue virus (DENV) for all acute specimens and DENV and WNV immunoglobulin M (IgM) antibody capture enzyme-linked immunosorbent assay (MAC-ELISA) for all convalescent specimens. From January 1, 2003 to December 31, 2006, no laboratory-positive human cases were detected among the 548 suspected cases reported.
WNV transmission in animals was first identified in Puerto Rico in 2004, when WNV-specific IgG antibody was detected in a free-ranging resident bird6 and three asymptomatic, unvaccinated horses (CDC, unpublished data). In July of 2006, the CDC implemented a sentinel chicken surveillance in the municipalities of Ceiba and Naguabo (US county equivalent) in eastern Puerto Rico to detect and monitor WNV transmission.7,8 In June of 2007, a plaque reduction neutralization test (PRNT) showed the presence of specific WNV neutralizing antibodies in the sentinel chickens, indicating active WNV transmission in Puerto Rico.7 Simultaneously, WNV nucleic acid was detected by RT-PCR in mosquitoes in the same area.7 In September of 2007, WNV was identified by RT-PCR in post-mortem brain tissue taken from an encephalitic horse and viral isolation from a dead falcon, which confirmed enzootic WNV transmission in Puerto Rico.3,8
On July 19, 2007, the American Red Cross in Puerto Rico notified the PRDH of three blood donations that had tested positive in a screening WNV nucleic acid amplification test.3 A letter was sent by PRDH to all healthcare providers in Puerto Rico informing them about the positive donations and sentinel chickens. The letter encouraged reporting and submission of diagnostic specimens from all suspected human cases of WNV disease. However, because passive surveillance efforts had not detected any cases, PRDH and CDC began an enhanced active surveillance for WNV disease in eastern Puerto Rico.
This report describes the results from the enhanced surveillance conducted from July 1 to December 31, 2007. We discuss the diagnostic challenges of identifying WNV infection in a dengue-endemic region.
Methods
Enhanced surveillance.
Study population.
The objective of the enhanced human surveillance was to determine the proportion of human WNV infection from acute febrile illness (AFI) cases in an area with active WNV enzootic transmission and hyperendemic human dengue transmission. Enhanced human surveillance was implemented during the first week of July in 2007 in the municipalities of Ceiba, Naguabo, Humacao, and Fajardo—the area surrounding the site where the sentinel chickens seroconverted (Figure 1). According to 2000 US Census data, the total population of these four municipalities was 141,504, and the median age of the residents was 31.3 years. The area has four hospitals (total bed capacity of 651) and four outpatient health clinics.
Figure 1.
Site of the enhanced WNV surveillance system—Puerto Rico, July to December of 2007.
Case definition.
A suspect case of WNV was defined as any resident of Ceiba, Humacao, Fajardo, or Naguabo who presented an AFI with symptom onset between July 1 and December 31, 2007. This definition included cases suspected of having WNV neuroinvasive or non-neuroinvasive disease. An AFI was defined by the presence of increased body temperature of at least 37.7°C during the healthcare visit or a history of fever lasting no more than 7 days.
Enhanced surveillance procedures.
Onsite educational seminars were held at the area's hospitals and outpatient facilities to inform providers about WNV and the enhanced surveillance. Surveillance case criteria, WNV fact sheets, and reporting instructions were distributed. Staff members at the healthcare facilities were trained on how to fill out the WCRF and asked to give patients a reminder sheet to return to the hospital for convalescent specimen collection.
Specimens and WCRFs were transported to the CDC Dengue Branch several times per week for testing. The data were entered into a database, and phone calls were made to providers who submitted forms that were missing symptom onset or specimen collection dates. Specimens missing this information could not be classified as acute or convalescent, and therefore, they were not tested.
Laboratory testing.
Acute specimens were tested for DENV and WNV by RT-PCR.9,10 Acute and convalescent specimens were tested for the presence of IgM antibodies to DENV and WNV using MAC-ELISA.11 Specimens with cross-reactivity against DENV and WNV antigen in the MAC-ELISA were tested by PRNT90. When the PRNT90 yielded indeterminate results because of reactivity of more than two viruses (i.e., DENV-1, -2, -3, or -4, St. Louis Encephalitis virus [SLEV], or WNV), a PRNT90 IgG depletion assay was used to determine the infecting virus.12
Laboratory definitions.
A laboratory-positive WNV case was defined as a case with any of the following four findings: detection of WNV nucleic acid in a specimen by RT-PCR, WNV IgM seroconversion from negative to positive by anti-WNV MAC-ELISA in paired specimens, a positive anti-WNV MAC-ELISA in a single specimen with a negative anti-DENV MAC-ELISA, or a PRNT90 (or PRNT90 IgG depletion assay) with a WNV titer at least four times higher than the titer of any of the four DENV types or SLEV.
A laboratory-positive dengue case was defined by any of the following three findings: detection of DENV nucleic acid in a specimen by RT-PCR, DENV IgM seroconversion from negative to positive by anti-DENV MAC-ELISA in paired specimens, or a positive anti-DENV MAC-ELISA in a single specimen with a negative anti-WNV MAC-ELISA.
Laboratory-negative cases were defined as cases in which there was a negative anti-WNV and anti-DENV MAC-ELISA result in the convalescent specimen and either no acute specimen was submitted for diagnostic testing or the acute specimen tested negative by RT-PCR and MAC-ELISA against WNV and DENV.
Laboratory-indeterminate cases were defined as cases in which there was a negative anti-WNV and anti-DENV MAC-ELISA result in the acute specimen and no convalescent specimen was submitted for diagnostic testing.
Undifferentiated flavivirus infection was defined by the presence of all of the following findings: positive anti-WNV and anti-DENV MAC-ELISA in the acute or convalescent specimen with equal reactivity, negative RT-PCR for DENV and WNV in the acute specimen, and a PRNT90 (or PRNT90 IgG depletion assay) with equal reactivity across all five viruses.
Data analysis.
A descriptive analysis was performed by calculating the frequencies of the clinical, demographic, and laboratory features of all reported cases. Statistical differences were determined with the χ2 and Fischer's exact tests when applicable. Furthermore, the Wilcoxon rank sum test was used to assess statistical differences in median values of non-parametric variables between groups. All data analyses were conducted using STATA 10.1 (StataCorp, College Station, TX) and SAS 9.2 software (SAS Institute Inc., Cary, NC).
Results
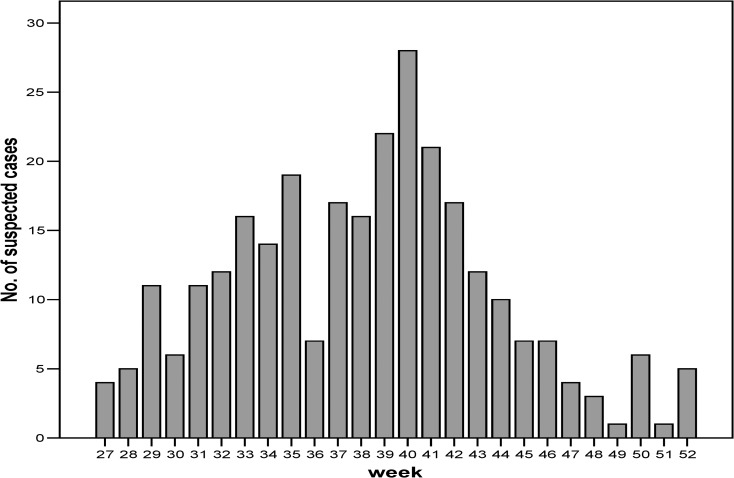
A total of 282 cases was reported over 6 months (Figure 2 ), with a peak during the first week of October (28 cases). The median age was 22 years, and 50% were female (Table 1). Laboratory-negative patients were more likely to be older and female, whereas undifferentiated case-patients tended to be younger and male. Most patients were residents of Fajardo (125 cases, 30.7 cases per 10,000 residents), although Humacao (42 cases, 7.1 cases per 10,000 residents) had the largest population.
Figure 2.
Number of cases reported to the enhanced WNV surveillance system by week of onset.
Table 1.
Characteristics of suspected WNV cases categorized by final laboratory results
| Characteristics | All reported cases (N = 282) | Laboratory-positive dengue cases (N = 115) | Laboratory-negative cases (N = 32) | Indeterminate cases (N = 86) | Undifferentiated cases (N = 27) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| Median age* (years) | 22 | 22 | 42 | 22 | 13 | |||||
| Age range | 4 months to 79 years | 4 months to 72 years | 5–72 years | 10–79 years | 4 months to 68 years | |||||
| Female | 141 | 50.0 | 55 | 47.8 | 18 | 56.2 | 42 | 48.8 | 12 | 44.4 |
| Residence | ||||||||||
| Ceiba | 30 | 10.6 | 7 | 6.2 | 1 | 3.1 | 15 | 17.4 | 1 | 3.7 |
| Fajardo | 125 | 44.3 | 48 | 42.4 | 11 | 34.4 | 43 | 50.0 | 7 | 25.9 |
| Humacao | 42 | 15.3 | 23 | 20.4 | 3 | 9.4 | 9 | 10.5 | 7 | 25.9 |
| Naguabo | 85 | 30.1 | 35 | 31.0 | 17 | 53.1 | 19 | 22.1 | 12 | 44.4 |
Statistically significant difference between laboratory-positive dengue cases and undifferentiated cases (χ2 test, P < 0.01).
The most common symptoms reported were headache, body ache, and joint pain (Table 2). Convulsions were observed in two cases: one laboratory-negative and one DENV infection confirmed by PRNT90. No other neurological manifestation was reported. In contrast, nearly one-third (86, 31%) of all patients reported a hemorrhagic manifestation, most commonly petechiae (46, 16%). Most patients (213, 76%) met the World Health Organization case criteria13 for dengue fever, and one patient met criteria for dengue hemorrhagic fever. More than one-third of the patients (105, 37%) were hospitalized; there were no reported fatalities.
Table 2.
Clinical features of suspected WNV cases by laboratory diagnosis
| Clinical feature* | Number (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Laboratory-positive dengue (N = 115) | Laboratory-negative cases (N = 32) | Laboratory-indeterminate cases (N = 86) | Undifferentiated cases (N = 27) | |||||
| Headache | 95 | 82.6 | 24 | 75.0 | 76 | 88.4 | 20 | 74.1 |
| Body ache†‡ | 92 | 80.0 | 28 | 87.5 | 69 | 80.2 | 15 | 55.5 |
| Joint pain | 76 | 66.1 | 21 | 65.6 | 60 | 69.8 | 16 | 59.3 |
| Eye pain§ | 71 | 61.7 | 15 | 46.9 | 49 | 56.9 | 12 | 44.4 |
| Rash†‡ | 46 | 40.0 | 7 | 21.9 | 25 | 29.1 | 15 | 55.5 |
| Hemorrhage¶ | 43 | 37.4 | 12 | 37.5 | 17 | 19.8 | 10 | 37.0 |
| Diarrhea | 32 | 27.8 | 11 | 34.4 | 25 | 29.1 | 11 | 40.7 |
| Cough | 24 | 20.9 | 9 | 28.1 | 30 | 34.9 | 7 | 25.9 |
| Conjunctivitis | 1 | 0.9 | 1 | 3.1 | 2 | 2.3 | 1 | 3.7 |
| Convulsions | 1 | 0.9 | 1 | 3.1 | 0 | 0.0 | 0 | 0.0 |
| Met WHO criteria | ||||||||
| DF | 94 | 43.7 | 21 | 9.9 | 67 | 31.5 | 20 | 9.4 |
| DHF | 1 | 1.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Hospitalized | 52 | 46.0 | 12 | 37.5 | 18 | 21.0 | 16 | 59.3 |
DF = dengue fever; DHF = dengue hemorrhagic fever; WHO = World Health Organization.
Fever was a requirement to enter the study. There were no cases of encephalitis, aseptic meningitis, or acute paralysis reported.
Statistically significant difference between laboratory-positive dengue cases and undifferentiated cases (χ2 test, P < 0.01).
Statistically significant difference between laboratory-negative dengue cases and undifferentiated cases (χ2 test, P < 0.01).
Statistically significant difference between laboratory-positive and -negative dengue cases (χ2 test, P < 0.01).
Hemorrhage included petechiae, ecchymosis, hematemesis, hematochezia, epistaxis, bleeding gums, hematuria, or vaginal bleeding.
A total of 385 serum specimens was received from 282 cases; of these specimens, 51 (18%) were paired specimens. No CSF specimens were submitted, and most (73%) were acute specimens. The specimens of 21 (7%) cases were not processed because of inadequate volume or missing WCRF information.
Of 282 reported cases, 115 (41%) cases were laboratory-positive for DENV (Table 3). Most (71, 62%) were RT-PCR–positive; 59 (83%) cases were DENV-3, 9 (13%) cases were DENV-2, 2 (3%) cases were DENV-1, and 1 (1%) cases was DENV-4. Of the RT-PCR–positive cases, 17 (24%) cases had anti-DENV IgM antibodies detected. No WNV-positive specimens were identified by RT-PCR; 28 (24%) of 115 laboratory-positive dengue cases had a positive anti-DENV IgM antibody in a single convalescent serum specimen with no anti-WNV IgM antibody detected. Of the remaining 16 laboratory-positive dengue cases, 4 cases had anti-DENV IgM antibody seroconversion in paired specimens, and 12 cases were initially undifferentiated before PRNT90 confirmed a recent DENV infection. Thirty-two (11%) patients were laboratory-negative for WNV and DENV. Eighty-six (31%) patients were laboratory-indeterminate because of a lack of a convalescent specimen. Anti-DENV and anti-WNV IgM antibody had equal reactivity by MAC-ELISA in 39 (14%) specimens; all 39 cases were RT-PCR–negative for DENV and WNV. Of these cases, 12 (31%) cases were diagnosed as having a recent DENV infection by a PRNT90 IgG depletion assay; however, 27 (69%) cases could not be differentiated as DENV or WNV infection by PRNT90 testing and were classified as undifferentiated flavivirus infections.
Table 3.
Final laboratory diagnoses for suspected WNV cases
| Diagnosis | Laboratory test | Number | Percent |
|---|---|---|---|
| Laboratory-positive | |||
| Acute DENV infection | RT-PCR for DENV-positive | 71 | 25.2 |
| Acute DENV infection | MAC-ELISA seroconversion for DENV in paired sera | 4 | 1.4 |
| Recent DENV infection | PRNT90 | 12 | 4.3 |
| Recent flavivirus infection | MAC-ELISA for DENV-positive in single sera | 28 | 9.9 |
| Acute WNV infection | MAC-ELISA for WNV-positive in single sera plus PRNT90 | 1 | 0.4 |
| Subtotal | 116 | 41.2 | |
| Laboratory-negative | MAC-ELISA DENV- and WNV-negative in convalescent sera | 32 | 11.3 |
| Laboratory-indeterminate | RT-PCR DENV- and WNV-negative in acute sera | 86 | 30.5 |
| Undifferentiated | MAC-ELISA for DENV- and WNV-positive | 27 | 9.6 |
| Not processed* | None | 21 | 7.4 |
| Total | 282 | 100.0 | |
Serum specimens were not processed for 21 patients because of missing onset or collection date information or inadequate specimen volume.
Only one patient was given a serologic diagnosis of WNV. The patient was a 37-year-old pregnant woman who presented to her healthcare provider on the first day of illness with fever and complaints of myalgia, nausea, diarrhea, cough, and nasal congestion. Paired serum specimens were obtained on days 1 and 99 after symptom onset. The acute specimen was RT-PCR–negative for DENV and WNV; however, a positive result was obtained for anti-WNV IgM antibodies with a negative anti-DENV IgM antibody. PRNT90 results showed reactivity to WNV at a titer of 1:64 and no reactivity to DENV or SLEV. The convalescent-phase serum specimen was negative for both anti-DENV and anti-WNV IgM antibodies.
Among the laboratory-positive dengue patients, headache was the most commonly reported symptom (95, 83%) followed by body aches (92, 80%) (Table 2). Eye pain was much more commonly reported among laboratory-positive patients than patients with laboratory-negative or undifferentiated diagnoses. Rash was reported by 40% of laboratory-positive dengue patients compared with 22% of laboratory-negative patients, 29% of laboratory-indeterminate patients, and 56% of undifferentiated patients.
Hospitalization was most common among patients with undifferentiated disease (59%) followed by laboratory-positive (46%), -negative (38%), and -indeterminate (21%) patients. Laboratory-positive patients were most likely to fit the case definition for dengue fever.
Discussion
Our results highlight the difficulty in confirming a WNV case in a dengue-endemic area. Additionally, although we were able to confirm that 103 of 282 cases (∼37%) had dengue using standard diagnostic assays, 12 additional cases ultimately diagnosed with dengue were equally reactive to anti-DENV and anti-WNV IgM by MAC-ELISA and required additional testing. Laboratory confirmation of WNV infection often depends on serologic assays, because WNV is often undetectable by RT-PCR while the patient is symptomatic.14,15 Cross-reactivity between flavivirus antigens varies by the infecting flavivirus and the history of prior infection.16 For this reason, CDC testing guidelines for WNV suggest that MAC-ELISA be performed using antigens for WNV and SLEV or WNV and DENV in dengue-endemic areas.17 If MAC-ELISA results against these viruses are similar, PRNT is recommended for confirmatory testing. However, PRNT assays are labor- and resource-intensive, and they are more accurate in determining the infecting virus in patients with a primary flavivirus infection than patients with secondary infections.14,18 Presumably, through original antigenic sin, an acute WNV infection in patients with prior dengue infection may result in higher titers of neutralizing antibody titer against dengue than WNV. Thus, this testing guideline is problematic for dengue-endemic areas with a high proportion of secondary infections. Additionally, as our results illustrate, some cases will remain undifferentiated flavivirus infections even after PRNT90 and PRNT90 IgG depletion assays are performed. Notably, our laboratory-positive WNV patient was serologically diagnosed. However, the patient had no travel history outside Puerto Rico in the 2 weeks before her illness, spent most of her life in the continental United States (except for the previous 2 years), and no evidence of prior dengue infection.
The clinical diagnosis of non-neuroinvasive WNV disease is difficult in dengue-endemic areas because of the similar clinical presentations of the two viruses.15,19 Both WNV and DENV can cause neuroinvasive disease.17,18,20–24 Some researchers have hypothesized that dengue endemicity might eventually produce sufficient cross-protective immunity to modulate WNV disease, resulting in a less severe clinical syndrome and making WNV difficult to detect in dengue-endemic countries.19,25 These factors may explain why few cases of human WNV disease have been detected in Latin America and the Caribbean.5,19
Dengue has been endemic in Puerto Rico for more than four decades, with large epidemics every 3–5 years26–29 resulting in high seropositivity among adolescents and adults.30 In 2007, WNV was detected in sentinel chickens in the same month as an island-wide dengue epidemic involving all four DENV types.29 Thus, it was not surprising that 41% of cases were laboratory-positive for DENV, because the laboratory-positivity rate for island-wide surveillance ranged from 30% to 40%.29
Limitations.
There are three main limitations to our study. Underreporting is inherent when relying on provider-initiated requests for testing. Additionally, although patients were educated and reminded to return for convalescent specimen collection, few did so, resulting in numerous laboratory-indeterminate cases. Finally, because dengue is endemic in Puerto Rico and there was an island-wide outbreak in 2007, WNV diagnosis may have been more difficult.
Conclusions.
During a period of active epizoonotic WNV transmission in a dengue-endemic area, enhanced surveillance detected only one case of symptomatic WNV infection in a patient without prior DENV infection. Using standard methods, it was not possible to distinguish between WNV and DENV infections in 27 of 282 cases because of serological cross-reactivity. In dengue-endemic areas, WNV disease may be difficult to detect and diagnose because of similar clinical presentations and cross-reactivity on diagnostic tests. Improved diagnostic methods are needed to allow differentiation of WNV and DENV during emergence of WNV in dengue-endemic regions.
ACKNOWLEDGMENTS
From the Puerto Rico Department of Health, we acknowledge Sandra Claudio, Mildred Rivera, Edna Ponce, and María Robles for case investigation support; Tamara Jusino for laboratory support; and Alex Rivera, Kassandra Flores, and Christina Villalba for field support. We recognize the Centers for Disease Control and Prevention Dengue Branch Laboratory staff for performing diagnostic testing. Additionally, the authors thank Luis Manuel Santiago for statistical analysis assistance.
Footnotes
Authors' addresses: Jomil M. Torres-Aponte, Epidemiology and Research Office, Puerto Rico Department of Health, San Juan, Puerto Rico, E-mails: dzq9@cdc.gov or jomtorres@salud.gov.pr. Richard R. Luce, Center for Global Health, Centers for Disease Control and Prevention, Libreville, Gabon, E-mail: dwe5@cdc.gov. Elizabeth Hunsperger, Jorge L. Muñoz-Jordan, Manuela Beltrán, Edgardo Vergne, D. Fermín Argüello, and Kay M. Tomashek, Dengue Branch, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, San Juan, Puerto Rico, E-mails: enh4@cdc.gov, ckq2@cdc.gov, mvb6@cdc.gov, edv1@cdc.gov, dla7@cdc.gov, and kct9@cdc.gov. Enid J. García, School of Medicine, University of Puerto Rico, San Juan, Puerto Rico, E-mail: enid.garcia3@upr.edu. Wellington Sun, Center for Biologics Evaluation and Research, Division of Vaccines and Related Product Applications, Food and Drug Administration, Rockville, MD, E-mail: wellington.sun@fda.hhs.gov.
References
- 1.Nash D, Mostashari F, Fine A, Miller J, O'Leary D, Murray K, Huang A, Rosenberg A, Greenberg A, Sherman M, Wong S, Layton M. West Nile Outbreak Response Working Group, 2001. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 1999;344:1807–1814. doi: 10.1056/NEJM200106143442401. [DOI] [PubMed] [Google Scholar]
- 2.Owen J, Moore F, Panella N, Edwards E, Bru R, Hughes M, Komar N. Migrating birds as dispersal vehicles for West Nile virus. Ecohealth. 2006;3:79–85. [Google Scholar]
- 3.Centers for Disease Control and Prevention Detection of West Nile virus in blood donations—Puerto Rico, 2007. MMWR Morb Mortal Wkly Rep. 2008;57:577–580. [PubMed] [Google Scholar]
- 4.Artsob H, Gubler DJ, Enria D, Morales M, Pupo M, Bunning M, Dudley JP. West Nile virus in the new world: trends in the spread and proliferation of West Nile virus in the Western Hemisphere. Zoonoses Public Health. 2009;56:357–369. doi: 10.1111/j.1863-2378.2008.01207.x. [DOI] [PubMed] [Google Scholar]
- 5.Komar N, Clark GG. West Nile virus activity in Latin America and the Caribbean. Pan Am J Public Health. 2006;19:112–117. doi: 10.1590/s1020-49892006000200006. [DOI] [PubMed] [Google Scholar]
- 6.Dupuis AP II, Marra PP, Reitsma R, Jones MJ, Louie KL, Kramer LD. Short report: serologic evidence for West Nile virus transmission in Puerto Rico and Cuba. Am J Trop Med Hyg. 2005;73:474–476. [PubMed] [Google Scholar]
- 7.Barrera R, Hunsperger E, Muñoz-Jordán JL, Amador M, Diaz A, Smith J, Bessoff K, Beltran M, Vergne E, Verduin M, Lambert A, Sun W. First isolation of West Nile virus in the Caribbean. Am J Trop Med Hyg. 2008;78:666–668. [PubMed] [Google Scholar]
- 8.Hunsperger EA, McElroy KL, Bessoff K, Colon C, Barrera R, Munoz-Jordan JL. West Nile virus from blood donors, vertebrates, and mosquitoes, 2007. Emerg Infect Dis. 2009;15:1298–1300. doi: 10.3201/eid1508.090333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chien LJ, Liao TL, Shu PY, Huang JH, Gubler DJ, Chang GJ. Development of real-time reverse transcriptase PCR assays to detect and serotype dengue viruses. J Clin Microbiol. 2006;44:1295–1304. doi: 10.1128/JCM.44.4.1295-1304.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, Komar N, Panella NA, Allen BC, Volpe KE, Davis BS, Roehrig JT. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burke DS, Nisalak A, Ussery MA. Antibody capture immunoassay detection of Japanese encephalitis virus immunoglobulin m and g antibodies in cerebrospinal fluid. J Clin Microbiol. 1982;6:1034–1042. doi: 10.1128/jcm.16.6.1034-1042.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunsperger E, Beltran M, Acosta LN, Jordan-Munoz J, Torres J, Tomashek KM. Serological evaluation of suspected West Nile virus human cases following its introduction during a dengue outbreak in Puerto Rico in 2007. Clin Vaccine Immunol c. 2011 doi: 10.1128/CVI.00040-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization . Dengue Hemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. 2nd ed. Geneva: World Health Organization; 1997. [Google Scholar]
- 14.Sampathkumar P. West Nile virus: epidemiology, clinical presentation, diagnosis, and prevention. Mayo Clin Proc. 2003;78:1137–1144. doi: 10.4065/78.9.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayes EB, Sejvar JJ, Zaki SR, Lanciotti RS, Bode AV, Campell GL. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg Infect Dis. 2005;11:1174–1179. doi: 10.3201/eid1108.050289b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gubler DJ. The continuing spread of West Nile virus in the Western Hemisphere. Clin Infect Dis. 2007;45:1039–1046. doi: 10.1086/521911. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention West Nile Virus Infection Information for Clinicians. 2008. http://www.cdc.gov/ncidod/dvbid/westnile/resources/fact_sheet_clinician.htm Available at.
- 18.Kuno G, Gubler D, Oliver A. Use of original antigenic sin theory to determine the serotypes of previous dengue infections. Trans R Soc Trop Med Hyg. 1993;87:103–105. doi: 10.1016/0035-9203(93)90444-u. [DOI] [PubMed] [Google Scholar]
- 19.Garza MDL, Rodriguez DR, Blitvich BJ, Reyes MA, Fernandez-Salas I, Ramos J, Farfan-Ale JA, Cazares R, Martinez C, Tavitas MI, Rivas-Estilla AM. Serologic surveillance for West Nile virus and other flaviviruses in febrile patients, encephalitic patients, and asymptomatic bloods donor in northern Mexico. Vector Borne Zoonotic Dis. 2010;10:1–7. doi: 10.1089/vbz.2008.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madden K. West Nile virus infection and its neurological manifestations. Clin Med Res. 2003;1:145–150. doi: 10.3121/cmr.1.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solomon T, Mallewa M. Dengue and other emerging flaviviruses. J Infect. 2001;42:104–115. doi: 10.1053/jinf.2001.0802. [DOI] [PubMed] [Google Scholar]
- 22.Lum LCS, Lam SK, Choy YS, George R, Harun F. Dengue encephalitis: a true entity? Am J Trop Med Hyg. 1996;54:256–259. doi: 10.4269/ajtmh.1996.54.256. [DOI] [PubMed] [Google Scholar]
- 23.Solomon T, Dung NM, Vaughn DW, Kneen R, Thao LT, Raengsakulrach B, Loan HT, Day NP, Farrar J, Myint KS, Warrell MJ, James WS, Nisalak A, White NJ. Neurological manifestations of the dengue infection. Lancet. 2000;355:1053–1059. doi: 10.1016/S0140-6736(00)02036-5. [DOI] [PubMed] [Google Scholar]
- 24.Cam BV, Fonsmark L, Hue NB, Phuong NT, Poulsen A, Heegaard E. Prospective case-control study of encephalopathy in children with dengue hemorrhagic fever. Am J Trop Med Hyg. 2001;65:848–851. doi: 10.4269/ajtmh.2001.65.848. [DOI] [PubMed] [Google Scholar]
- 25.Mostashari F, Bunning ML, Kitsutani PT, Singer DA, Nash D, Cooper MJ, Katz N, Liljebjelke KA, Biggerstaff BJ, Fine AD, Layton MC, Mullin SM, Johnson AJ, Martin DA, Hayes EB, Campbell GL. Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet. 2001;358:261–264. doi: 10.1016/S0140-6736(01)05480-0. [DOI] [PubMed] [Google Scholar]
- 26.Dietz V, Gubler DJ, Ortiz S, Kuno G, Casta-Velez A, Sather GE. The 1986 dengue and dengue hemorrhagic fever epidemic in Puerto Rico: epidemiologic and clinical observations. P R Health Sci J. 1996;15:201–210. [PubMed] [Google Scholar]
- 27.Rigau-Perez JG, Ayala-Lopez A, Garcia-Rivera EJ, Hudson SM, Vorndam V, Reiter P, Cano MP, Clark GG. The reappearance of dengue-3 and a subsequent dengue-4 and dengue-1 epidemic in Puerto Rico in 1998. Am J Trop Med Hyg. 2002;67:355–362. doi: 10.4269/ajtmh.2002.67.355. [DOI] [PubMed] [Google Scholar]
- 28.Rigau-Perez JG, Vorndam AV, Clark GG. The dengue and dengue hemorrhagic fever epidemic in Puerto Rico, 1994–1995. Am J Trop Med Hyg. 2001;64:67–74. doi: 10.4269/ajtmh.2001.64.67. [DOI] [PubMed] [Google Scholar]
- 29.Tomashek KM, Rivera A, Jordan-Munoz JL, Hunsperger E, Santiago L, Padro O, Garcia E, Sun W. Description of a large island-wide outbreak of dengue in Puerto Rico, 2007. Am J Trop Med Hyg. 2009;81:467–474. [PubMed] [Google Scholar]
- 30.Mohammed H, Tomashek KM, Stramer SL, Hunsperger E. Prevalence of anti-dengue immunoglobulin G antibodies among American Red Cross blood donors in Puerto Rico, 2006. Transfusion. 2012;52:1652–1656. doi: 10.1111/j.1537-2995.2011.03492.x. [DOI] [PubMed] [Google Scholar]