Abstract
Intraocular pressure (IOP) elevation is considered as a major risk factor causing the progression of vision deterioration in glaucoma. Although it is known that the IOP level changes widely throughout the day and night, how the dark or light phase IOP elevation contributes to retinal ganglion cell (RGC) degeneration is still largely unclear. To examine the profile of IOP, modified laser photocoagulation was applied to the trabecular meshwork of Brown Norway rats and both light and dark phase IOPs were monitored approximately 1–2 times a week. The relationship between IOP elevation and RGC degeneration was investigated while RGC body loss was analyzed with Rbpms immunolabeling on retinal wholemount and axonal injury in the optic nerve was semi-quantified. The baseline awake dark and light IOPs were 30.4 ± 2.7 and 20.2 ± 2.1 mmHg respectively. The average dark IOP was increased to 38.2 ± 3.2 mmHg for five weeks after the laser treatment on 270° trabecular meshwork. However, there was no significant loss of RGC body and axonal injury. After laser treatment on 330° trabecular meshwork, the dark and light IOPs were significantly increased to 43.8 ± 4.6 and 23 ± 3.7 mmHg respectively for 5 weeks. The cumulative dark and light IOP elevations were 277 ± 86 and 113 ± 50 mmHg days respectively while the cumulative total (light and dark) IOP elevation was 213 ± 114 mmHg days. After 5 weeks, regional RGC body loss of 29.5 ± 15.5% and moderate axonal injury were observed. Axonal injury and loss of RGC body had a high correlation with the cumulative total IOP elevation (R2 = 0.60 and 0.65 respectively). There was an association between the cumulative dark IOP elevation and RGC body loss (R2 = 0.37) and axonal injury (R2 = 0.51) whereas the associations between neuronal damages and the cumulative light IOP elevation were weak (for RGC body loss, R2 = 0.01; for axonal injury, R2 = 0.26). Simple linear regression model analysis showed statistical significance for the relationships between the total cumulative IOP elevation and RGC body loss (P = 0.009), and axonal injury (P = 0.016). To examine the role of light and dark IOP elevation in RGC body loss and axonal injury, analyses for the association between different light/dark IOP factors and percentage of RGC body loss/axonal injury grading were performed and only the association between the cumulative dark IOP elevation and axonal injury showed statistical significance (P = 0.033). The findings demonstrated that the cumulative total (light and dark) IOP elevation is a risk factor to RGC degeneration in a rat model of experimental glaucoma using modified partial laser photocoagulation at 330° trabecular meshwork. Further investigations are required to understand the role of longer term light and dark phase IOP elevation contributing to the progression of degeneration in different compartments of RGCs.
Keywords: glaucoma, intraocular pressure, retinal ganglion cell, optic nerve, axonal injury, photocoagulation, rat
1. Introduction
The intraocular pressure (IOP) in an individual is extremely dynamic and can be influenced by various physiological factors including blood flow, heart rate (Schmidl et al., 2011), respiration, exercise (Yip et al., 2011), fluid intake (Goldberg and Clement, 2010), systemic medication (Boland and Quigley, 2007), topical drugs (Susanna et al., 2004) and body posture (Liu et al., 1998). The IOP value changes from day to night showing a circadian pattern. The IOP recorded at nighttime is usually higher than that of daytime in healthy subjects who maintain a supine posture at night and a sitting position during the daytime (Liu et al., 1998, 1999). Elevated IOP is a major risk factor for glaucoma and there is a growing body of evidence suggesting that IOP fluctuation contributes to disease progression (Nouri-Mahdavi et al., 2004; Caprioli and Coleman, 2008). Since dark phase IOP is not routinely recorded in patients, it is still unknown how dark phase IOP elevation is associated with glaucomatous process.
Glaucoma is characterized by the progressive loss of RGCs. Degeneration of RGCs involves apoptotic cascades in RGC bodies in the retina and degradation of axons in the optic nerve. The molecular mechanisms of these processes are different, indicating that these processes are somewhat compartmentalized (Whitmore et al., 2005). Although the optic nerve head is generally believed to be the early injury site in glaucoma, the mechanisms about how IOP elevation or other risk factors lead to axonal degeneration are not yet fully understood. The use of animal models of experimental glaucoma provide opportunities to understand the causes of axonal degeneration, the temporal profiles of the degeneration processes within different cellular compartments, and the risk factors associated with the progression of glaucomatous optic neuropathy. Our recent study demonstrated that RGC body loss evaluated by RNA binding protein with multiple splicing (Rbpms) immunohistochemistry is strongly associated with the cumulative IOP elevation in a rat model of experimental glaucoma (Kwong et al., 2011). The experiment utilized laser photocoagulation applied to 360° of the trabecular meshwork and led to relatively high levels of IOP elevation during both dark and light phase and consequently to extensive degeneration of RGC somas and their axons within 5 week. Since the common forms of chronic human glaucoma are characterized by much slower rates of damage, we modified the parameters of photocoagulation in our model by reducing the area of trabecular meshwork treated to achieve a moderate IOP elevation and obtain various IOP elevation level. We found that partial treatment of trabecular meshwork produces a highly reproducible, moderate increase of the IOP with significantly different cumulative dark and light phase IOP values. This study evaluated the differential contribution of the levels of both dark and light IOP elevation to RGC soma loss and axonal injury.
2. Materials and methods
2.1. Animals
The use of animals was approved by the Animal Research Committee of the University of California, Los Angeles. The procedures were performed in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Animals were housed with standard food and water provided ad libitum. Lighting was turned on at 3 AM and off at 3 PM. The animals were kept at least 1 week in this environment before surgical procedures and IOP measurement. Surgical procedures were performed on one eye of each rat while the contralateral eye served as an untreated control. Topical ophthalmic ointment (tobramycin, Tobrex; Alcon, Fort Worth, TX) was applied immediately after the laser procedures.
2.2. IOP measurement and partial trabecular laser photocoagulation
The TonoLab tonometer (TonoLab; Colonial Medical Supply, Franconia, NH) was calibrated with hydrostatic pressure ranging from 0 to 70 mmHg in the eyes of anesthetized adult Brown Norway rats as measured manometrically. The linear regression equation of the measured and actual IOP was y = 1.06x + 0.58 with R2 = 0.98 (y = actual IOP; x = measured IOP). After 1 week of accommodation, both light and dark phase IOPs were measured with TonoLab approximately 1–2 times a week at 10 AM and 5 PM, respectively, on 6-month-old male Brown Norway rats in the awake state. After topical anesthesia, 5 successful readings were recorded for each eye and IOP measurement for each animal usually required less than 5 min. In general, the IOP readings became more stable and consistent after 1 week of IOP measurement.
Prior to experimental procedures, the 24-h IOP was monitored to confirm whether the biphasic IOP profile in the rats which were kept in our housing facility was consistent with previous studies (Moore et al., 1996). The IOP readings of both eyes were recorded every 2 h (n = 6) resulting in 12 recordings for one entire day. Each recording was performed and finished within one hour. For example, the IOP reading recorded at 10 AM represented a measurement between 10 AM and 11 AM.
A previous method of trabecular laser photocoagulation was modified (Kwong et al., 2011). Approximately 200 laser burns were delivered ab externo to the 270° or 330° trabecular meshwork at laser settings of 200 μm diameter, 100 mW power, and 50 m sec durations while 90° or 30° trabecular meshwork at the superior region received no laser respectively (Diode laser at 635 nm wavelength, Iridex OcuLight GL; Iridex Corp., Mountain View, CA, USA). Approximately 2–3 weeks after the first laser treatment, a second laser treatment was performed on experimental eyes with no IOP elevation compared to the contralateral control eye.
For IOP analysis, all IOP readings were corrected to the actual IOP and presented as mmHg in this study. The IOP reading in the eye with experimental glaucoma was compared to the contralateral control eye in the same animal. Cumulative IOP elevation, mean IOP, peak IOP and fluctuation were analyzed. Cumulative IOP elevation was calculated by performing separate integrations of the IOP over the days of exposure for the experimental and contralateral control eye (mmHg days) (Kwong et al., 2011). The control eye integral value was subtracted from the experimental eye integral. The IOP readings recorded during the light phase were used for the calculation of cumulative light IOP elevation while the dark IOP readings were used to calculate the cumulative dark IOP elevation. The standard deviation of IOP readings was considered as IOP fluctuation.
2.3. Rbpms immunohistochemistry on retinal wholemounts
Animals were deeply anesthetized with intramuscular injections of 80 mg/kg sodium pentobarbital and then transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffer. The eyes were enucleated and postfixed for 1 h. According to a published protocol (Kwong et al., 2010), entire retinas were incubated with 10% fetal bovine serum for 1 h to block nonspecific staining, and then immersed in the custom-made primary antibody against Rbpms in PBS containing 1% triton, 0.5% BSA, and 0.9% sodium chloride (PBS-T-BSA) overnight at 4 °C. Anti-Rbpms antibodies were generated against N-terminal GGKAEKENTPSEANLQEEEVR polypeptide (Pro-Sci, Poway, CA) in our laboratory as described previously (Kwong et al., 2010). After washing in PBS-T-BSA, the retinas were incubated with secondary Alexa Fluor 488 goat anti-rabbit IgG antibody (1/1000) overnight at 4 °C. They were mounted flat with several radial cuts on glass slides. After air drying overnight, the retinas were counterstained with DAPI and mounted.
2.4. Retrograde labeling
Procedures for retrograde labeling with Fluorogold (FG, Fluorochrome, Denver, CO) were performed as previously described (Kwong et al., 2011; Piri et al., 2007). The procedure of applying FG at the transected optic nerve was selected because it ensured that all RGC axons were exposed to FG, including the RGCs projecting to areas other than the superior colliculus. Briefly, the optic nerve was exposed through a lateral conjunctival incision. A cross section of the optic nerve was made with the needle knife through the opening of the optic nerve sheath, carefully, not to damage the adjacent blood supply. A gelfoam gauze soaked with 6% FG was applied to the surface of the transected optic nerve. The conjunctival incision was suture and antibiotic ointment was applied. The procedures were only performed on one eye to avoid bilateral blindness. The animals were euthanized 1 day after retrograde labeling.
2.5. RGC quantification
Topographical analysis of immunolabeled cells was performed with a fluorescence microscope (LSM410; Carl Zeiss, Oberkochen, Germany) (Kwong et al., 2011, 2010). Each retina was divided into four quadrants: superior, inferior, nasal, and temporal. Three sampling fields (0.32 × 0.24 mm each) were imaged at each region 1, 2, 3, and 4 mm from the center of the optic nerve in each retina quadrant and averaged for analysis. In total, 48 images were captured on each retina. In each sampling field, the number of Rbpms positive cells was counted in a masked manner. To examine the relationship with IOP factors, the average of cell counts on each retina was used for analysis. For statistical analysis, the density of immuno-positive cells (number of cells per mm2) was compared. The percentage of cell loss was defined as the percentage of the decreased number of cells in the experimental eye against the density in the contralateral control eye of the same animal.
2.6. Optic nerve injury analysis
To semi-quantify the axonal injury, a reliable method of grading optic nerve injury was adopted (Ishii et al., 2003; Jia et al., 2000). The optic nerve segments 1–2 mm behind the globe were dissected from the animals after perfusion with 4% paraformaldehyde, post-fixed with 2% glutaraldehyde, and 1% paraformaldehyde for 24 h at 4 °C. The tissues were dehydrated, processed, and embedded in acrylic resin. One-micrometer-thick sections were cut and stained with 1% toluidine blue. Optic nerve cross sections were examined under light microscopy and assessed by two independent masked observers. A graded scale of optic nerve injury ranging from 1 (normal) to 5 (total degeneration) was used.
2.7. Statistical analysis
The data was averaged and presented as the mean ± SD. Differences in IOP elevation and RGC degeneration between experimental and contralateral eyes were analyzed by paired T-test. To evaluate the relationship between IOP elevation and RGC degeneration, the data was fitted into a simple linear regression model and R2 and P-value were obtained. P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 16 (IBM Corporation, Armonk, NY).
3. Results
3.1. Twenty-four-hour IOP cycle in the rats
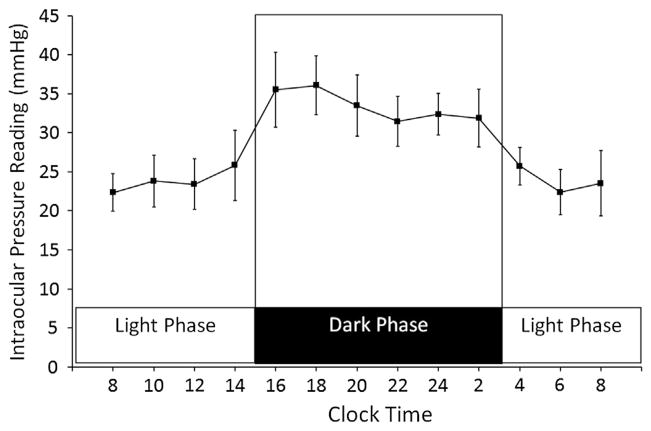
The IOP readings recorded with the TonoLab tonometer from awake Brown Norway rats were averaged and corrected based on a linear equation obtained from the IOP calibration with the pressure transducer. The IOP readings for 12-h light (3 AM–3 PM; 3:00– 15:00) and 12-h dark (3 PM–3 AM; 15:00–3:00) cycle in adult Brown Norway rats were shown in Fig. 1 (n = 6). All the IOP readings in dark phase were significantly higher than all the IOP readings in the light phase (P < 0.05).
Fig. 1.
IOP profile of Brown Norway rats in 12-h light and 12-h dark cycle. Points represent the average of IOP reading recorded from 12 rat eyes. All dark IOP readings were significantly higher than the light IOP readings (P < 0.05).
In this experiment, the average light phase IOP reading was 23.9 ± 1.4 mmHg and the IOP readings appeared to be stable. The light phase IOP readings recorded at 10 AM were consistent and showed no statistical difference when compared to all other time points in the light cycle (all P-values > 0.05).
In the dark phase, the average IOP reading was 33.5 ± 1.9 mmHg. Additional IOP readings were recorded at 2:30 PM (30 min before the light was turned off) and the readings were already increased to 30.3 ± 5.8 mmHg (not shown in Fig. 1) suggesting that the IOP in the rats started to increase prior to entering dark cycle. The IOP readings recorded at 4 PM and 6 PM appeared to be the peak in the 24-h cycle. Then, the dark phase IOP declined to a lower level but remained significantly higher than all light phase IOP readings.
3.2. IOP profiles after partial trabecular laser photocoagulation
In this experiment, the baseline awake IOP in the dark and light phases were 30.4 ± 2.7 and 20.2 ± 2.1 mmHg, respectively (n = 20).
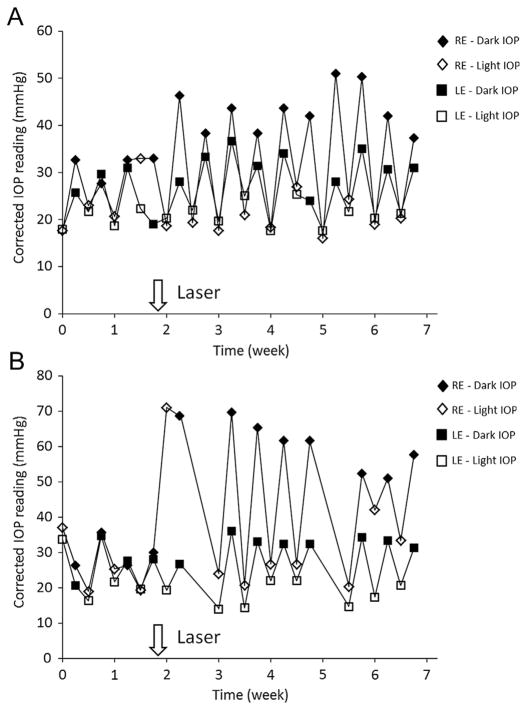
Similar to 360° trabecular laser photocoagulation, an early and dramatic increase in IOP was observed in both dark and light phases in the first week after 270° and 330° photocoagulation. Within 1 week, the light phase IOP measured in the experimental eye dropped to levels similar to that of contralateral control eye. The IOP profiles after 270° and 330° trabecular laser photocoagulation in the representative animals are shown in Fig. 2. There were large circadian IOP fluctuations with high IOP during the dark phase and low IOP during the light phase right after partial 270° laser treatment. After 330° photocoagulation, the dark IOP was increased to a higher level than after 270° photocoagulation. This fluctuation persisted throughout the entire experiment.
Fig. 2.
Representative IOP profile after partial trabecular laser photocoagulation. (A) Laser photocoagulation was applied at 270° trabecular meshwork. The dark phase IOP was significantly elevated after laser treatment for 5 weeks but there was no significant increase in light phase IOP compared to contralateral control eye. The IOP profile of animal #1712 is shown. (B) Laser photocoagulation was applied at 330° trabecular meshwork. The light phase IOP was dramatically increased in the first week after laser photocoagulation and returned to the IOP level comparable to the contralateral untreated control eye. The dark phase IOP was markedly increased. The IOP profile of animal #1730 is shown. Consistent circadian pattern of light and dark phase IOP was noted in untreated control eye. RE = right eye with laser treatment; LE = left eye as contralateral control.
For the period of 5 weeks after laser photocoagulation applied to 330° of the trabecular meshwork, the average IOP significantly increased by 39% to 43.8 ± 4.6 mmHg (P < 0.001) in the dark phase and by 23.7% to 23.0 ± 3.7 mmHg (P = 0.002) in the light phase (N = 10). The cumulative dark phase IOP elevation was 277 ± 86 mmHg days while the cumulative light phase IOP elevation was 113 ± 50 mmHg days. Nevertheless, laser photocoagulation applied to 270° trabecular meshwork induced a significant increase to 38.2 ± 3.2 mmHg in dark phase IOP (P < 0.001) but no statistical significant elevation in the light phase IOP (P = 0.08). For 270° trabecular laser photocoagulation, the cumulative dark and light phase IOP elevation was 203 ± 50 and 18 ± 41 mmHg days respectively. The IOP data is summarized in Table 1.
Table 1.
Analysis of IOP for 5 weeks after partial trabecular laser photocoagulation.
| 270° laser
|
330° laser
|
||||
|---|---|---|---|---|---|
| Contralateral control N = 10 | Experimental N = 10 | Contralateral control N = 10 | Experimental N = 10 | ||
| Mean IOP (mmHg) | Light phase | 21.8 ± 1.6 | 20.4 ± 2.6 (P = 0.08) | 18.6 ± 2.5 | 23 ± 3.7 (*P = 0.002) |
|
| |||||
| Dark phase | 29.2 ± 2.4 | 38.2 ± 3.2 (*P < 0.001) | 31.5 ± 2.9 | 43.8 ± 4.6 (*P < 0.001) | |
|
| |||||
| Cumulative IOP elevation (mmHg days) | Light phase | – | 18 ± 41 | – | 113 ± 50 |
|
| |||||
| Dark phase | – | 203 ± 50 | – | 277 ± 86 | |
P value < 0.05.
3.3. Regional RGC degeneration after partial trabecular laser photocoagulation
Immunohistochemistry with antibody against Rbpms, the RGC marker, was used to visualize and topographically quantify RGC bodies in retinal wholemounts. Regional loss of RGC bodies was observed in retinas 5 weeks after 330° trabecular laser photocoagulation. The microphotographs in Fig. 3A demonstrated the remarkable loss of Rbpms positive cell bodies in retinal quadrants. The optic nerve cross section of the same animal is shown in Fig. 3B and demonstrates the localized lesion of degenerated axons with condensed and dilated axonal content, degenerated and irregular myelin sheaths, and reactive glia. The findings indicate that the RGC degeneration is regional at both axonal and cell body levels in this rat model of experimental glaucoma generated by 330° trabecular laser photocoagulation. In contrast, there was no noticeable loss of Rbpms-positive cells in the retina and axonal damage in the optic nerve 5 weeks after 270° laser treatment (Table 2).
Fig. 3.
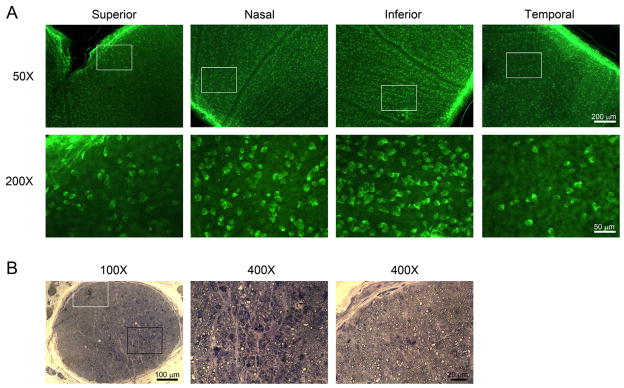
Regional loss of RGC bodies and axonal damage 5 weeks after partial (330°) trabecular laser photocoagulation. (A) Rbpms immunohistochemistry on retinal whole mounts (Animal #1730) revealed decreased density of RGC bodies in superior and temporal retinal quadrants. The insets represented the locations of higher magnification views of the retina shown in the lower panel. (B) Cross section of optic nerve (Animal #1730) stained with toluidine blue demonstrated different morphological features. Higher magnification views of localized optic nerve lesions were shown in the middle panel (black insert) and normal-appearing axons in the right panel (white insert). Localized lesion was characterized by dilated axon, degenerated myelin sheath, condensed axonal content and reactive astrocytes.
Table 2.
Analysis of RGC degeneration at 5 weeks after partial trabecular laser photocoagulation.
| 270° laser N = 10 | 330° laser N = 10 | |
|---|---|---|
| Percentage loss of Rbpms positive cell body | 0.6 ± 7.5 | 29.5 ± 15.5 |
| P = 0.847 | *P = 0.004 | |
| Axonal injury grading (1 = normal–5 = severe) | 1.03 ± 0.08 | 2.3 ± 1.6 |
| P = 0.343 | *P = 0.027 |
P value < 0.05.
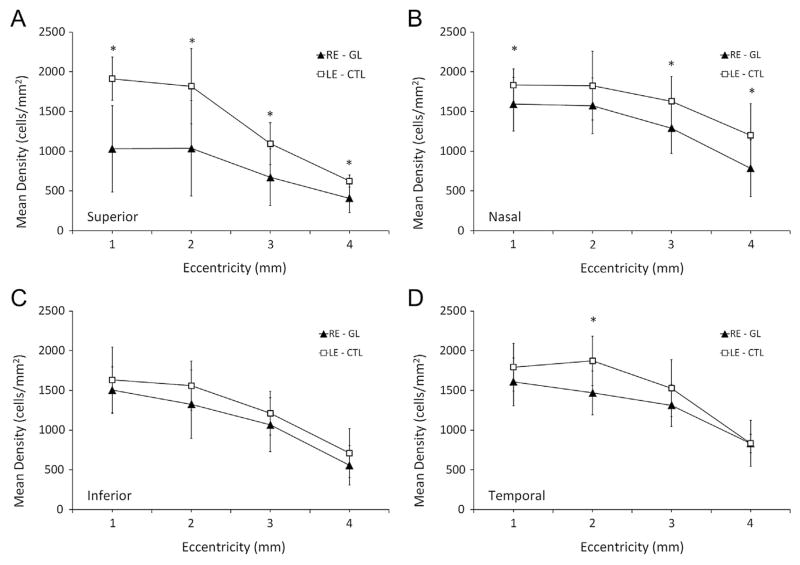
To quantify RGC body loss, the number of Rbpms-positive cells was counted at different eccentricities in 4 retinal quadrants including superior, nasal, inferior, and temporal. Consistently, Fig. 4 showed that the mean densities of RGC body at all distances in superior quadrants were significantly lower than that of contralateral control, whereas none of the locations in the inferior quadrant had significantly reduced density 5 weeks after 330° trabecular laser photocoagulation. No significant decreased density of RGC body in the retinas after 270° trabecular laser photocoagulation was noted (Table 2). This observation indicates that the regional loss of RGC body is taking place when laser photocoagulation is applied to more than 270° of the trabecular meshwork. Overall, 5 weeks after 330° trabecular laser photocoagulation, the RGC body density was significantly reduced by 29.5 ± 15.5% (P = 0.004) compared to contralateral controls. In the optic nerve, the average grading of axonal injury was 2.3 ± 1.6 (P = 0.027; grade 1 represents normal; grade 5 represents severe damage), and indicated moderate axonal injury (Table 2). Approximately, 99.5 ± 1.2% of FG positive cells were Rbpms positive while 99.3 ± 1.1% of Rbpms positive cells were FG positive (N = 8).
Fig. 4.
Density of RGC bodies in (A) superior, (B) nasal, (C) inferior, and (D) temporal retinal quadrant after 5 weeks of IOP elevation. Significant losses of Rbpms-positive cells were noted at all distances in superior but not in inferior quadrant. (*P < 0.05; N = 12) GL = experimental glaucoma; CTL = control.
3.4. Correlation between cumulative dark IOP elevation and RGC degeneration
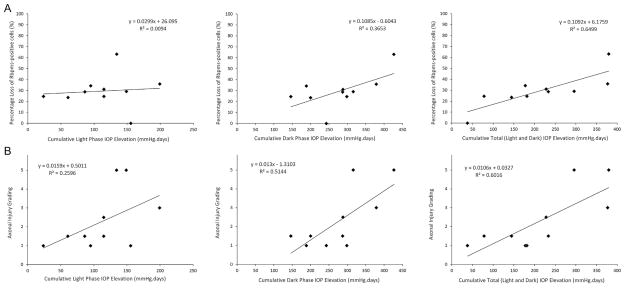
The correlation between the percent loss of Rbpms-positive cells and axonal injury grading with cumulative light, dark, and total IOP elevation by performing simple linear regression is showed in Fig. 5. The increasing cumulative dark IOP elevation was correlated with an increase in the percent loss of Rbpms-positive cells (R2 = 0.37; P = 0.089) and a statistically significant increase in the axonal injury (R2 = 0.51; P = 0.033). Comparatively, the cumulative light IOP elevation showed no correlation with the percent loss of Rbpms-positive cells (R2 = 0.01; P = 0.895) and a weak linear correlation with axonal injury (R2 = 0.26; P = 0.186). However, the cumulative total (light and dark) IOP elevation showed significant and higher correlations with loss of Rbpms-positive cells (R2 = 0.65; P = 0.009) and axonal injury grading (R2 = 0.6; P = 0.016).
Fig. 5.
Relationship between cumulative IOP elevation and RGC degeneration after partial (330°) trabecular laser photocoagulation. (A) Simple linear regression line analyses between RGC body loss and cumulative light (upper left), dark (upper center) and total (upper right) IOP elevation were performed. There was a high correlation between the cumulative total IOP elevation and RGC body loss. (B) Simple linear regression line analyses between axonal injury and cumulative light (lower left), dark (lower center) and total (lower right) IOP elevation were performed. There were high correlations between the cumulative dark IOP elevation and axonal injury, and between the cumulative total IOP elevation and axonal injury.
For the analyses of other IOP factors, there was no correlation between the mean IOP including both dark and light phase and RGC degeneration including Rbpms-positive cells and axonal injury (all R2 < 0.1; P > 0.05). Compared to cumulative dark IOP elevation, the peak of dark IOP elevation and the peak of light IOP showed a weak linear correlation with axonal injury (R2 = 0.314 and 0.156 respectively) but no correlation with loss of Rbpms-positive cell (R2 = 0.076 and 0.113 respectively). IOP fluctuation also showed a weak linear relationship with both axonal injury (R2 = 0.295) and loss of Rbpms-positive cells (R2 = 0.312).
4. Discussion
Consistent with other studies, we observed a strong correlation between RGC loss and cumulative IOP elevation in a rat model of experimental glaucoma after trabecular laser photocoagulation. This further supports the concept that the cumulative IOP elevation as a crucial factor which initiates RGC loss (Kwong et al., 2011). Our study also indicates that measurement of both light and dark phase IOPs may be required to better understand the glaucomatous process of RGC death. Although the dark phase IOP appears to be higher than the light phase IOP in humans (Liu et al., 1998, 1999) and rodents (Moore et al., 1996), the dark phase IOP is rarely monitored and its role in glaucoma remains largely undetermined.
The IOP findings in adult Brown Norway rats maintained in an animal room with 12-h light and 12-h dark are comparable to the data described by Moore et al. (1996). The level of dark IOP is always higher than the level of light IOP in awake animals as measured by TonoPen (Moore et al., 1996) and TonoLab. Based on the results from statistical analyses, the IOP readings recorded at 10 AM are similar to other light phase time points while the IOP readings at both 4 and 6 PM may represent the peak level of dark IOP. Our data supports that our IOP measurement scheme at 10 AM and 5 PM in our housing setting (light cycle = 3 AM–3 PM; dark cycle = 3 PM–3 AM) is valid for studying the association between dark/light IOP and RGC degeneration.
In the present study, we modified the parameters of standard laser treatment and applied laser to 270° and 330° of the trabecular meshwork instead of 360° as described in previous studies (Kwong et al., 2011) in order to mimic a more moderate form of glaucoma as is commonly seen in glaucoma patients. In addition, 270° trabecular laser photocoagulation elevated IOP level in dark phase and 330° trabecular laser photocoagulation elevated IOP levels in both light and dark phases indicate an advantage of inducing various IOP levels and patterns by modifying laser photocoagulation’s parameters (as illustrated in Fig. 4). Unexpectedly, no RGC body loss or axonal degeneration was observed after the exposure to the dark IOP elevation induced by 270° trabecular laser photocoagulation. However, we could not eliminate the possibility that the cumulative dark IOP elevation plays a role in the progression of RGC degeneration in a longer period over 5 weeks in this rat model. Further investigations to define the role of IOP factors including mean, peak and fluctuation contributing to RGC degeneration under different IOP conditions are definitely required. Compared to a recent study using modified laser treatment after draining aqueous humor in Brown Norway rats (Biermann et al., 2012), our procedures appear to be less invasive, however, both studies strongly support that experimental glaucoma rat model using laser photocoagulation can be used to adjust IOP conditions in order to investigate cell death and neuroprotection.
In the rats with 330° trabecular laser photocoagulation, regional RGC loss was evident with approximately 30% of total RGC body loss and moderate axonal injury. In this model of experimental glaucoma, the average level of dark IOP is always higher than the average light IOP. We evaluated the relationship between IOP properties in addition to compartmental RGC degeneration and found a strong correlation between the cumulative dark phase IOP elevation and axonal injury (R2 = 0.51). Linear regression analysis indicates that the increase in axonal injury is related to increasing cumulative dark phase IOP elevation (P = 0.33) rather than other IOP factors such as mean, peak and fluctuation of dark and light IOP elevations. These results suggest that dark IOP should also be measured in order to better monitor the disease progress in experimental glaucoma model.
Similar to episcleral vein injection of hypertonic saline model (Morrison et al., 1997), large diurnal IOP fluctuation was clearly present in Brown Norway rats after performing IOP elevation induction procedures. However, there is a weak correlation between RGC degeneration and IOP fluctuation in the present study (R2 = 0.295) or no correlation in our recent study (R2 = 0.03) using trabecular laser photocoagulation (Kwong et al., 2011). These findings may be specific for this strain of animals; they appear to tolerate significant fluctuation in IOP (more 70% IOP increase from light phase to dark phase in control animal) with little or no damage to RGCs. It is possible that the laminar beam-like structure in the optic nerve head of Brown Norway rat experiences less stress/strain or the retinal neurons are more resistant to IOP-related mechanical stress.
In summary, the present study analyzes RGC degeneration at the cell body and axonal levels and describes the IOP profile in the light and dark phase after partial (270° and 360°) trabecular laser photocoagulation. After 330° trabecular laser treatment, we observed regional loss of RGC somas in the retina and moderate axonal injury in the optic nerve. The results also demonstrated that the cumulative dark IOP elevation but not the light phase IOP, is strongly correlated with axonal injury, and an increase in axonal injury is strongly associated with the increase in the cumulative dark phase IOP elevation. This IOP relationship may only describe a subgroup of glaucomatous degeneration and should be further testified in other IOP conditions. Nevertheless, we believe that monitoring dark phase IOP may help us better understand the glaucoma disease progression, particularly in patients with well controlled awake office IOPs.
Acknowledgments
The authors would like to thank Sam C.-S. Chang for technical support and IOP measurement.
Footnotes
Support: NIH/NEI EY018644 (NP) and Research to Prevent Blindness (JC).
Financial disclosure
None.
References
- Biermann J, van Oterendorp C, Stoykow C, Volz C, Jehle T, Boehringer D, Lagreze WA. Evaluation of intraocular pressure elevation in a modified laser-induced glaucoma rat model. Exp Eye Res. 2012;104:7–14. doi: 10.1016/j.exer.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Boland MV, Quigley HA. Risk factors and open-angle glaucoma: classification and application. J Glaucoma. 2007;16:406–418. doi: 10.1097/IJG.0b013e31806540a1. [DOI] [PubMed] [Google Scholar]
- Caprioli J, Coleman A. Intraocular pressure fluctuation a risk factor for visual field progression at low intraocular pressures in the advanced glaucoma intervention study. Ophthalmology. 2008;115:1123–1129. doi: 10.1016/j.ophtha.2007.10.031. [DOI] [PubMed] [Google Scholar]
- Goldberg I, Clement CI. The water drinking test. Am J Ophthalmol. 2010;150:447–449. doi: 10.1016/j.ajo.2010.06.035. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Kwong JMK, Caprioli J. Retinal ganglion cell protection with geranylgeranylacetone, a heat shock protein inducer, in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2003;44:1982–1992. [PubMed] [Google Scholar]
- Jia L, Cepurna WO, Johnson EC, Morrison JC. Patterns of intraocular pressure elevation after aqueous humor outflow obstruction in rats. Invest Ophthalmol Vis Sci. 2000;41:1380–1385. [PubMed] [Google Scholar]
- Kwong JMK, Caprioli J, Piri N. RNA binding protein with multiple splicing: a new marker for retinal ganglion cells. Invest Ophthalmol Vis Sci. 2010;51:1052–1058. doi: 10.1167/iovs.09-4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong JMK, Quan A, Kyung H, Piri N, Caprioli J. Quantitative analysis of retinal ganglion cell survival with Rbpms immunolabeling in animal models of optic neuropathies. Invest Ophthalmol Vis Sci. 2011;52:9694–9702. doi: 10.1167/iovs.11-7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JH, Kripke DF, Hoffman RE, Twa MD, Loving RT, Rex KM, Gupta N, Weinreb RN. Nocturnal elevation of intraocular pressure in young adults. Invest Ophthalmol Vis Sci. 1998;39:2707–2712. [PubMed] [Google Scholar]
- Liu JH, Kripke DF, Twa MD, Hoffman RE, Mansberger SL, Rex KM, Girkin CA, Weinreb RN. Twenty-four-hour pattern of intraocular pressure in the aging population. Invest Ophthalmol Vis Sci. 1999;40:2912–2917. [PubMed] [Google Scholar]
- Moore CG, Johnson EC, Morrison JC. Circadian rhythm of intraocular pressure in the rat. Curr Eye Res. 1996;15:185–191. doi: 10.3109/02713689608997412. [DOI] [PubMed] [Google Scholar]
- Morrison JC, Moore CG, Deppmeier LM, Gold BG, Meshul CK, Johnson EC. A rat model of chronic pressure-induced optic nerve damage. Exp Eye Res. 1997;64:85–96. doi: 10.1006/exer.1996.0184. [DOI] [PubMed] [Google Scholar]
- Nouri-Mahdavi K, Hoffman D, Coleman AL, Liu G, Li G, Gaasterland D, Caprioli J. Advanced glaucoma intervention study. Predictive factors for glaucomatous visual field progression in the advanced glaucoma intervention study. Ophthalmology. 2004;111:1627–1635. doi: 10.1016/j.ophtha.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Piri N, Song M, Kwong JMK, Caprioli J. Modulation of alpha and beta crystallin expression in rat retinas with ocular hypertension-induced ganglion cell degeneration. Brain Res. 2007;1141:1–9. doi: 10.1016/j.brainres.2006.11.095. [DOI] [PubMed] [Google Scholar]
- Schmidl D, Garhofer G, Schmetterer L. The complex interaction between ocular perfusion pressure and ocular blood flow – relevance for glaucoma. Exp Eye Res. 2011;93:141–155. doi: 10.1016/j.exer.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Susanna R, Sheu WP Latin American Glaucoma Society. Comparison of latanoprost with fixed-combination dorzolamide and timolol in adult patients with elevated intraocular pressure: an eight-week, randomized, open-label, parallel-group, multicenter study in Latin America. Clin Ther. 2004;26:755–768. doi: 10.1016/s0149-2918(04)90075-6. [DOI] [PubMed] [Google Scholar]
- Whitmore AV, Libby RT, John SW. Glaucoma: thinking in new ways – a rôle for autonomous axonal self-destruction and other compartmentalised processes? Prog Retin Eye Res. 2005;24:639–662. doi: 10.1016/j.preteyeres.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Yip JL, Broadway DC, Luben R, Garway-Heath DF, Hayat S, Dalzell N, Lee PS, Bhaniani A, Wareham NJ, Khaw KT, Foster PJ. Physical activity and ocular perfusion pressure: the EPIC-Norfolk eye study. Invest Ophthalmol Vis Sci. 2011;52:8186–8192. doi: 10.1167/iovs.11-8267. [DOI] [PubMed] [Google Scholar]