Abstract
Clostridium difficile (Cd) is a Gram-positive obligate anaerobic pathogen that causes pseudomembranous colitis in antibiotic-treated individuals. Commensal bacteria are known to have a significant role in the intestinal accumulation of Cd after antibiotic treatment, but little is known about how they affect host immunity during Cd infection. Here we report that Cd infection results in translocation of commensals across the intestinal epithelial barrier that is critical for neutrophil recruitment through the induction of an IL-1β-mediated positive feedback loop. Mice lacking ASC, an essential mediator of IL-1β and IL-18 processing and secretion, were highly susceptible to Cd infection. ASC−/− mice exhibited enhanced translocation of commensals to multiple organs after Cd infection. Notably, ASC−/− mice exhibited impaired CXCL1 production and neutrophil influx into intestinal tissues in response to Cd infection. The impairment in neutrophil recruitment resulted in reduced production of IL-1β and CXCL1, but not IL-18. Importantly, translocated commensals were required for ASC/Nlrp3-dependent IL-1β secretion by neutrophils. Mice lacking IL-1β were deficient in inducing CXCL1 secretion, suggesting that IL-1β is the dominant inducer of ASC-mediated CXCL1 production during Cd infection. These results indicate that translocated commensals play a crucial role in CXCL1-dependent recruitment of neutrophils to the intestine through an IL-1β/NLRP3/ASC-mediated positive feedback mechanism that is important for host survival and clearance of translocated commensals during Cd infection.
Introduction
Clostridium difficile (Cd) is a Gram-positive, spore-forming, anaerobic bacterium that causes infectious diarrhea and pseudomembranous colitis in hospitalized patients receiving treatment with antibiotics (1). The diagnosis rate of Cd-associated disease is increasing and reaching as many as 500,000 cases per year in the United States (1). Treatment of patients with broad spectrum antibiotics results in alteration of the normal intestinal microbiota and overgrowth of pathogenic Cd which is normally prevented by commensals (1). Elimination of Cd with vancomycin and/or metronidazole is the primary treatment for Cd-infected patients, but even with antibiotic therapy 15,000 to 20,000 patients die from Cd infection every year (1). The intestinal pathology associated with Cd infection is primarily triggered by the Cd toxins A and B (TcdA and TcdB) that damage the intestinal epithelium (1, 2). Host production of antibodies (Abs) against Cd toxins inhibits disease development (4, 5), but immunocompromised and elderly individuals are more susceptible and exhibit increased morbidity and mortality from Cd infection (1).
Recent in vivo studies using a mouse model of Cd infection revealed protective mechanisms mediated by host innate immune receptors. Nod1, a member of the intracellular Nod-like receptor family, was shown to induce the recruitment of neutrophils to the Cd-damaged intestine (5). Nod1 senses peptidoglycan-related small molecules containing the di-amino acid motif iE-DAP (6), and mediates production of the neutrophil-recruiting chemokine CXCL1 upon activation by Nod1-stimulatory molecules released by Cd (5). Nod1−/− mice showed high mortality during Cd-induced colitis and increased translocation of commensals from damaged intestines to multiple organs (5). Interestingly, neutrophil recruitment induced by TcdA/B is impaired in mice lacking ASC, which is a mediator of IL-1β and IL-18 secretion through caspase-1 activation (7). Anakinra, a blocker of IL-1 signaling, inhibits TcdA/B-induced neutrophil recruitment, suggesting an important role for IL-1 in Cd-induced neutrophil recruitment. Additionally, Cd-infected Nod1−/− mice show high levels of circulating bacterial endotoxin, but impaired IL-1β secretion in the intestine (5). Collectively, these findings suggest that Nod1 plays a role in neutrophil recruitment upstream of IL-1β production to regulate host survival after Cd infection. However, the mechanism by which IL-1β regulates neutrophil recruitment and host survival in response to Cd infection remains unclear.
Here we report that ASC-mediated IL-1β production acts in a positive feedback loop to enhance the recruitment of neutrophils to the Cd-infected intestine. IL-1β was found to be secreted by neutrophils in response to Cd in the presence of translocated commensals, and was required for CXCL1 production in the Cd-damaged intestine. Notably, impairment in IL-1β secretion led to increased loads of translocated commensals resulting in high lethality of ASC−/− mice. Thus, a positive IL-1β-mediated feedback loop for neutrophil recruitment to the Cd-damaged intestine is critical for host survival after Cd infection.
Materials and Methods
Bacteria strains and synthetic immunostimulatory molecules
Cd strains VPI10463 and VPI11186 were cultured as described (5). Escherichia coli NI1076 and Enterobacter hormaechei NI1077 were isolated from the intestine of Cd infected mice and cultured at 37 °C in brain heart infusion (BHI) medium (BD, Franklin Lakes, NJ) in an aerobic environment, and their 16S rRNA gene phylotypes were determined as described (11). E. coli O55:B5 LPS was purchased from Sigma-Aldrich (St. Louis, MO).
Mice
Wild-type (WT) C57BL/6 (B6) mice were obtained from the Jackson Laboratory (Bar Harbor, ME). IL-1β−/− mice in B6 background were a gift of Y. Iwakura (8). ASC−/− and NLRP3−/−in B6 background were previously described (9, 10). All mice were housed, bred and maintained under specific pathogen-free (SPF) conditions as described (5). The mouse studies were approved by the University of Michigan Committee on Use and Care of Animals.
Cd infection and analyses
Mice were infected 108 CFU of Cd after antibiotic treatment as described (5). Briefly, mice were pre-treated with a cocktail of seven antibiotics before Cd infection as previously described (5). Bacterial number was determined by counting bacterial CFU in feces and tissue homogenates on selective taurocholate-cycloserine-cefoxitin-fructose agar (TCCFA) and BHI plates after 24 h incubation under anaerobic conditions (5). The bacterial population in feces was determined by DGGE analysis as described (11). Tissues harvested from mice 3 day post- infection were fixed and stained with haematoxylin and eosin as described (5). Histological assessment of pathology was performed using a scoring system as described (12). Apoptotic cells were detected by DeadEND Fluorometric TUNEL assay (Promega). Tissues harvested from mice on day 0 or day 1 post-infection were homogenized in T-PER tissue protein extraction reagent (Thermo scientific) and chemokine levels were determined by ELISA. RNA levels of RegIIIβ and RegIIIγ in cecum were determined by quantitative PCR (qPCR) using SYBR Green Master Mix (Applied Biosystems, Foster City, CA) with various cycles at 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min on an ABI Prism 7900HT system (Applied Biosystems). The primers sequence was: ATGGCTCCTACTGCTATGCC and GTGTCCTCCAGGCCTCTTT for RegIIIβ, ATGGCTCCTATTGCTATGCC and GATGTCCTGAGGGCCTCTT for RegIIIγ, TGCGACTTCAACAGCAACTC and GCCTCTCTTGCTCAGTGTCC for GAPDH (13, 14). RegIIIβ or RegIIIγ to GAPDH relative expression was calculated using the 2(–Ct) method and the fold change was calculated by comparing the values to those obtained on day 0 (unstimulated).
Neutrophil depletion
Neutrophils were depleted in vivo utilizing the pan-granulocytic Ab 1A8 (BioXcell, West Lebanon, NH), directed against Ly-6G. 200 μg of the Ab was injected into the peritoneal cavity of WT mice 12 h before Cd infection.
Intestinal permeability and apoptotic cell assay
Mice were given 0.6 mg/g FITC-dextran (4 kDa, Sigma) orally and the serum levels of FITC-dextran was determined as described (5).
Preparation of intestinal immune cells and flow cytometric analysis
Intestinal immune cells were prepared from mice 2 days post-infection as described (5). Intestinal cells were stained with FITC-labeled CD11b mAb (M1/70; eBioscience), and APC-labeled mAb F4/80 (BM8; eBioscience) and PE-labeled Ly-6G mAb (1A8; BD Biosciences). Isotype-matched Abs (BD) were used for control staining. Dead cells were excluded with 7-AAD staining. Fluorescence was assessed using a FACSCalibur analyzer and analyzed using FlowJo software (TreeStar).
Immunostimulation assay
Peritoneal cells were collected from 8-week old mice by lavage 4 h post-injection with 2 ml of 4% (w/v) thioglycollate medium. The peritoneal cells were incubated with LPS or heat-inactivated bacterial samples (MOI 1:1). 4 h post-stimulation, the floating cells were collected and culture in RPMI medium (Invitrogen) with or without Cd (MOI 1:1). The levels of IL-1β and IL-18 in the culture supernatants were determined by ELISA (BD). More than 95% cells were neutrophils as assessed by morphology of the cells stained with a commercial kit (Diff; Dade Behring Inc.).
Limulus amebocyte lysate assay
LPS levels in homogenized tissue samples were determined using the Limulus amebocyte lysate assay kit (Lonza, Walkersville, MD) in accordance with the manufacturer's instructions.
Statistical analysis
Statistical significance between groups was determined by two tailed t-test with unequal variance (Aspin-Welch's t-test). The survival rate of infected mice was analyzed using the log rank test. Differences were considered significant when p values were <0.05.
Results
ASC is critical for host survival during Cd infection
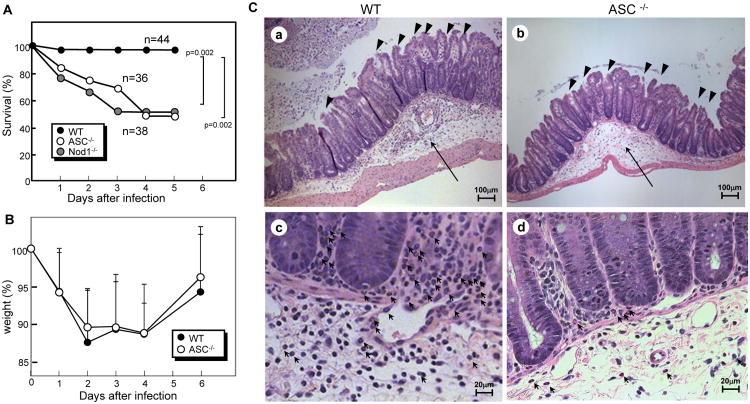
To determine the role of ASC in Cd-associated disease, we infected antibiotic-treated WT and ASC−/− mice with Cd VPI10463 via the oral route. Notably, 97% of the WT mice survived whereas 54% of the ASC−/− mice succumbed by 4 days post-infection (Figure 1A). The survival rate of infected ASC−/− mice was similar to that of Nod1−/− mice (Figure 1A) which are also known to be more susceptible to Cd (5). These results indicate that ASC regulates mouse survival after Cd infection. Mice lost weight after Cd infection, but recovered by day 6 post-infection which was comparable in ASC−/− and WT mice (Figure 1B). Histological analysis revealed that Cd infection induced similar levels of epithelial damage, submucosal edema and severity of inflammation in ASC−/− mice as in WT mice resulting in comparable pathology scores in both groups of mice (Figure 1C, Table 1). Moreover, the level of apoptosis in the intestinal epithelium of ASC−/− mice was comparable to that of WT mice (Supplemental Figure 1A and B). In addition, no significant difference in macrophage and mesothelial cell death was observed between ASC−/− and WT when these cells were infected with Cd in vitro (Supplemental Figure 1C). These results suggest that protection against Cd infection afforded by ASC is not related to alteration in epithelial damage, cell death/survival, or body weight. Remarkably, histological analysis revealed reduced numbers of neutrophils in the intestine of Cd-infected ASC−/− mice when compared to WT mice (Figure 1C). No neutrophils were present in the intestine of both ASC−/− and WT mice before and after antibiotic treatment in the absence of Cd infection (data not shown). These results suggest that ASC regulates the recruitment of neutrophils to the intestine after Cd infection.
Figure 1. ASC−/− mice are more susceptible to Cd.
A to C, Survival (A) and body weight (B) of ASC−/− (n=27) and Nod1−/− (n=36) and WT (n=44) mice infected with Cd were monitored for 14 days. All mice were infected by gastric gavage with 108 CFU of Cd VPI10463 after antibiotic treatment as described in Materials and Methods. No further deaths were observed beyond 5 days after infection. C, Representative histology of ceca from ASC−/− (b and d) and WT mice (a and c) 3 days post-infection stained with haematoxylin and eosin. The arrows and arrow heads show submucosal edema and epithelial damage, respectively (a and b, 200× magnification). Polymorphonuclear neutrophils are indicated by arrows (c and d, 400× magnification).
Table 1. Quantitative analysis of histological damage/inflammation and membrane permeability of Cd-infected mice.
| ASC−/− | WT | ||
|---|---|---|---|
| Histological score (Cd-infected) | Overall | 3.65±1.0 | 3.81±1.9 |
| Inflammation severity | 0.78±0.29 | 1.11±0.70 | |
| Level of involvement | 1.25±0.19 | 1.11±0.62 | |
| Epithelial damage | 1.61±0.77 | 1.59±0.87 | |
| Membrane permeability | non-infected | 31.6 ± 9.3 | 23.6 ±7.9 |
| Cd-infected | 91.4 ±77 | 105 ±86 |
The overall histology, the severity of inflammation, the level of involvement and extent of epithelial damage scores are based on the analysis of 8 WT control and 8 ASC−/− mice on day 3 post-infection. FITC-dextran permeability was analyzed ASC−/− and WT mice (n=10) before and 2 days after Cd infection. Differences in values between ASC−/− and WT mice were not statistically significant.
Reduced neutrophil infiltration in the intestine of ASC-deficient mice infected with Cd
We next performed studies to verify that ASC regulates the intestinal recruitment of neutrophils in response to Cd infection. To assess this, we analyzed immune cell populations in the colon and cecum of infected ASC−/− and WT mice by flow cytometry (Figure 2A and B) Consistent with the histology, the number of CD11b+Ly6G+ F4/80− cells (neutrophils) was reduced in the intestine of ASC−/− mice when compared to WT mice, whereas there were no significant difference in CD11b+F4/80+Ly6G− cells (macrophages) (Figure 2A and B) These observations indicate that ASC regulates the recruitment of neutrophils, but not macrophages, in response to Cd infection in the intestine.
Figure 2. Impaired neutrophil recruitment and CXCL secretion in ASC−/−mice infected with Cd.
A and C,Immune cells isolated from the ceca and colons of ASC−/− and WT mice were analyzed by flow cytometry 2 days post-infection. A, Percentage of cells labeled with anti-CD11b, anti-Ly-6G and anti-F4/80 mAbs are shown. B, Percentage ± SD of CD11b+Ly-6G+F4/80−(neutrophils) or CD11b+Ly-6G−F4/80+ (macrophages) (n=6) are shown. C and D, The levels of CXCL1 (C) and CCL2 (D) in the serum (left axis) or in the indicated tissues (right axis) of ASC−/− and WT mice were determined by ELISA 24 hrs post-infection. SI, small intestine.
To begin to understand the mechanism by which ASC regulates the influx of neutrophils to the intestine, we determined the levels of chemokines, CXCL1 and CCL2, which recruit neutrophils and macrophages, respectively (15). We found that the production of CXCL1, but not CCL2, was impaired in the cecum and serum of infected ASC−/− mice when compared to WT mice (Figure 2C to D) In contrast to CXCL1, the amounts of IL-10, IFN-γ, XCL1 and TNFα in the intestine of infected ASC−/− mice were comparable to those in WT mice (Supplemental Figure 2). Unlike CXCL1, none of the other chemokines measured in the serum were significantly increased after C. difficile infection (data not shown). These findings suggest that ASC plays a critical role in eliciting neutrophil recruitment to the infected intestine through specific induction of CXCL1 (Figures 1C, 2A and 2B)
ASC prevents translocation of commensal bacteria but does not regulate the clearance of Cd
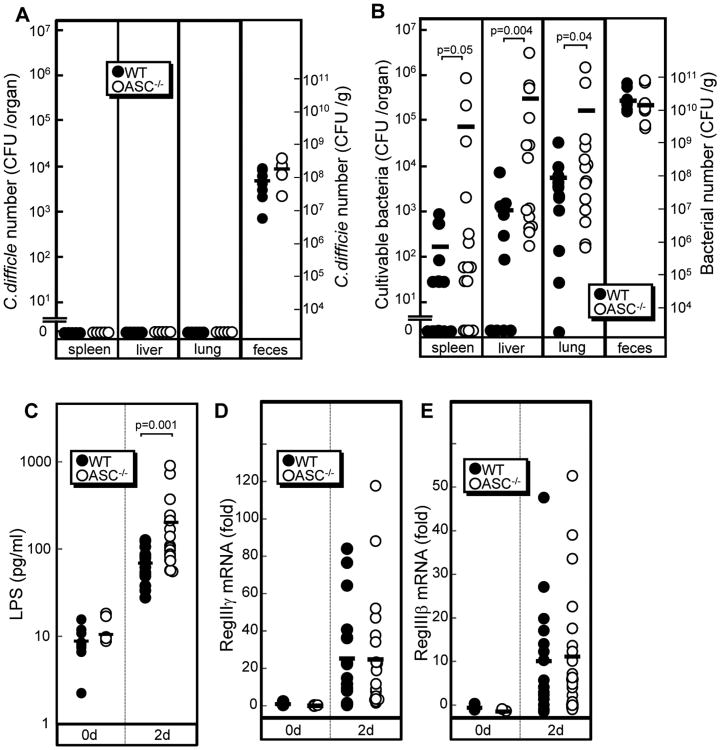
Because neutrophils are important for the elimination of translocated commensals induced by Cd infection (5), we next determined if ASC plays a role in the clearance of Cd and translocation of commensals. There was no significant increase in the number of Cd in the feces of infected ASC−/− mice compared to WT mice (Figure 3A). In contrast, there was a significant increase in the number of commensals in multiple organs of infected ASC−/− mice, when compared to WT mice, whereas the presence of Cd was not detected in these organs (Figure 3A and B) The total number of bacteria and dominant bacterial populations in the feces of ASC−/−mice was comparable to those in WT mice (Figure 3B, Supplemental Figure 3). The latter results suggest that increased bacteria translocation observed in ASC−/− mice is not due to differences in total bacteria or differences in common commensal populations in the intestinal lumen. To characterize the translocated bacteria in infected animals, we cultured bacteria isolated from the organs of ASC−/− mice and identified the bacterial clones by 16S rRNA gene sequencing (11). These isolates included γ-proteobacteria such as Escherichia coli and Enterobacter cloacae/hormaechei group bacteria. Consistently, high levels of endotoxin, a component of proteobacteria, were found in the serum of infected ASC−/− mice, compared with WT mice (Figure 3C). Collectively, these results indicate that ASC−/− mice are defective in the clearance of translocated commensals but not Cd.
Figure 3. Increased translocation of commensals in ASC−/−mice.
A and B, The number of Cd (A) and total culturable bacteria (B) in spleen, liver, lung and feces from ASC−/− (n=11) and WT mice (n=8) on day 2 infection with 108 CFU of Cd VPI10463 were determined by plating on selective TCCFA plates and non-selective BHI media as described in Materials and Methods. C, LPS (endotoxin) levels in serum from WT and Nod1−/− mice before infection and 2 days post-infection was determined by Limulus amebocyte lysate assay (n=19). D and E, The levels of RegIIIγ and RegIIIβ mRNAs in cecum of ASC−/− (n=22) and WT mice (n=17) on day 2 post-infection were determined by quantitative RT-PCR. Bars indicate mean. In all panels, differences with p values are given when p<0.05.
To determine whether the increased bacterial translocation in ASC−/− mice is due to greater intestinal permeability, WT and ASC−/− mice were given FITC-dextran orally before and after Cd infection. No significant difference in FITC-dextran permeability was found between WT and ASC−/− mice (Table 1). Beside neutrophils, RegIII proteins are known to control bacterial populations in the intestine (17,18). We detected high expression levels of RegIIIβ and RegIIIγ mRNAs after Cd infection (Figure 3D and E). However, the levels of both RegIIIβ and RegIIIγ mRNAs in the ceca of ASC−/− mice were same as those in WT mice (Figure 3D and E). These results are consistent with the observation that the numbers of commensals and Cd in the feces is comparable in ASC−/− and WT mice. Thus, ASC is unlikely to control bacterial commensals through the regulation of RegIII protein expression
Translocated commensals are essential for secretion of IL-1β and IL-18 induced by Cd infection in vivo
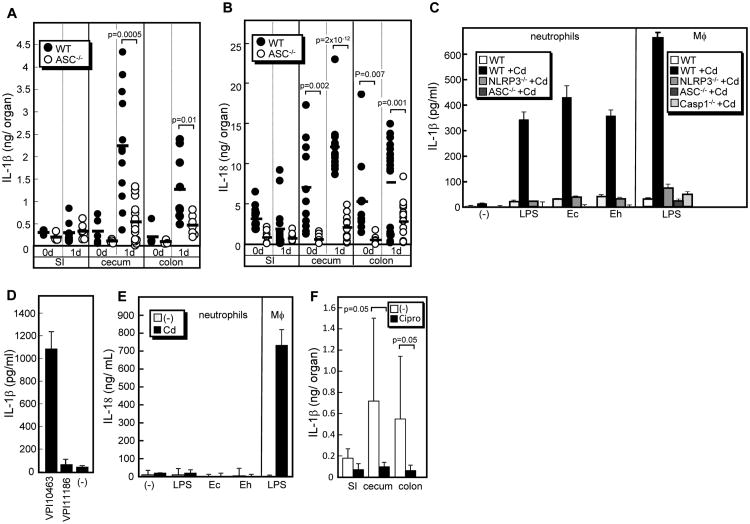
Because ASC is an essential mediator of Cd toxin-mediated IL-1β and IL-18 secretion (7) and these molecules are known to induce CXCL chemokines from intestinal epithelial cells and other cell types (18, 19), we next determined the levels of IL-1β and IL-18 in intestine of ASC−/−and WT mice. After infection, there was an increase in the production of IL-1β and IL-18 in the cecum and colon of WT mice and this increase was impaired in ASC−/− mice (Figure 4A, B and Supplemental Figure 2E). These results indicate that ASC is critical for the intestinal induction of both IL-1β and IL-18 in response to Cd infection. Previous studies showed that IL-1β secretion requires two steps, a proinflammatory stimulus such as LPS to induce pro-IL-1β and a triggering signal such as stimulation with a membrane damaging toxin that activates the inflammasome (20). Because increased levels of translocated γ-proteobacteria and circulating LPS were found in ASC−/− mice, we hypothesized that translocated γ-proteobacteria commensals and their component LPS might be important for IL-1β secretion. To test this, we stimulated neutrophils with Cd in the absence and presence of γ-proteobacterial commensals isolated from the organs of Cd-infected mice. Notably, neutrophils produced IL-1β upon stimulation with Cd, but only in the presence of commensals or LPS (Figure 4C). Production of IL-1β by neutrophils was induced by infection with Cd VPI10463, but not toxin-free VPI11186, in the presence of LPS, suggesting that Cd toxins are critical for IL-1β secretion (Figure 4D). Notably, neutrophils isolated from ASC−/− and Nlrp3−/− mice were greatly impaired in the secretion of IL-1β induced by exposure to Cd and commensals (Figure. 4C). The amount of IL-1β released from neutrophils were comparable to that from LPS-stimulated macrophages, which was caspase-1-dependent (Figure 4C). These results indicate that ASC and Nlrp3, a member of the NLR family that forms an inflammasome, are required for IL-1β secretion by neutrophils in response to stimulation with Cd and commensals. In contrast, IL-18 was not secreted from neutrophils co-stimulated with Cd and commensals, although stimulation of macrophages induced significant levels of IL-18 (Figure 4E). To determine if IL-1β secretion requires γ-proteobacteria in the intestine of Cd-infected mice, we treated mice with ciprofloxacin after Cd infection. Ciprofloxacin effectively killed isolated γ-proteobacterial clones, but not Cd (data not shown). Notably, IL-1β secretion was greatly reduced after ciprofloxacin treatment, although the amount of IL-1β produced varied widely among mice not treated with ciprofloxacin, which may be due mouse-to-mouse variations in the composition of the intestinal microbiota (Figure 4F). Collectively, these results suggest that IL-1β secretion in the intestine of Cd-infected mice requires translocated γ-proteobacteria.
Figure 4. Translocated commensals mediate IL-1β and IL-18 secretion induced by Cd infection.
A and B, The levels of IL-1β (A) and IL-18 (B) in indicated organs prepared from WT and ASC−/− mice 24 hr post-infection were determined by ELISA. SI, small intestine. C to E, IL-1β levels produced by cultured cells was determined 4 hr post-stimulation. C, Neutrophils isolated from WT, NLRP3−/− and ASC−/− mice were stimulated with Cd VPI10463 (MOI 1:1) or left alone in the absence or presence of 1 μg/ml E. coli O55B5 LPS or isolated commensal clones possessing phylotypes identical to E. coli (Ec) and E. hormaechei (Eh) (MOI 1:1). D, Neutrophils isolated from WT mice were stimulated with Cd VPI10463, toxin-less VPI11186 or left alone in the presence of E. coli O55B5 LPS. E, IL-18 produced by neutrophils was determined 4 hr post-stimulation. Bone-marrow derived macrophages stimulated with LPS were used as control cells. F, WT mice were treated with or without 417 mg/kg ciprofloxacin in the drinking water after Cd infection. The levels of IL-1β in cecum 24 hr post-Cd infection were determined by ELISA. The p values <0.05 are shown.
Neutrophils are major source of IL-1β production in response to Cd infection
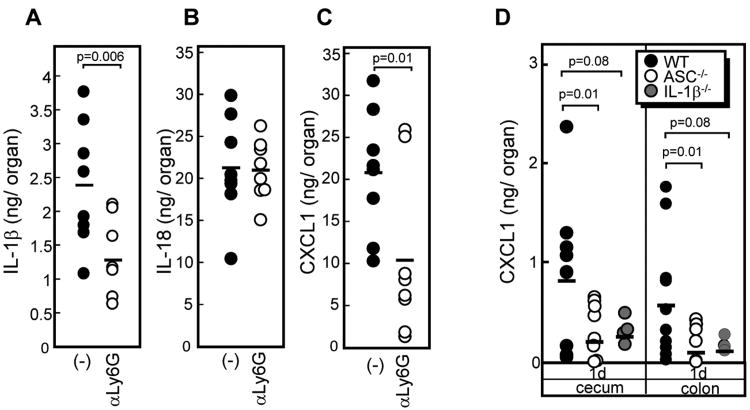
The high amounts of IL-1β secreted by isolated neutrophils upon exposure to Cd infection and commensals suggest that these cells might be a major source of IL-1β in the cecum of Cd-infected mice. To test this, we assessed the production of IL-1β in the cecum of mice depleted of neutrophils (>99% neutrophil depletion) with anti-Ly6G, an antibody specific to neutrophils (supplemental Figure 4). Neutrophil depletion resulted in a significant decrease in IL-1β in the ceca of Cd-infected mice, whereas the IL-18 levels were not affected (Figure 5A and B) Remarkably, neutrophil depletion resulted in reduced levels of CXCL1 in the ceca of Cd-infected mice (Figure. 5C). This suggests that IL-1β mediates a positive feedback loop of neutrophil recruitment by inducing CXCL1 in the intestine during Cd infection. To directly demonstrate that IL-1β is responsible for Cd-induced CXCL1 production in intestine of Cd-infected mice, we infected WT, IL-1β−/− and ASC−/− mice with Cd. Importantly, the CXCL1 levels in the ceca of infected IL-1β−/− mice were greatly reduced and comparable to that found in ASC−/−mice (Figure 5D). Collectively, these results suggest that IL-1β is important for Cd-induced CXCL1 production in the intestine of infected mice.
Figure 5. Neutrophils are a major source of IL-1β and CXCL1 but not IL-18 during Cd infection.
Mice were depleted of neutrophils by injection of 200 μg of the anti-Ly-6G Ab into the peritoneal cavity of WT mice 12 h before Cd infection. IL-1β (A), IL-18 (B), and CXCL1 (C) levels in ceca from neutrophil-depleted and control mice 24 hr post-infection were determined by ELISA. D, IL-1β−/− mice showed impaired CXCL chemokine production. The CXCL1 levels in indicated samples isolated from Cd-infected ASC−/−, IL1β−/− and WT mice were determined by ELISA.
Discussion
Here we show that ASC is critical for host survival during Cd infection. However, intestinal damage and alteration in body weight was comparable in ASC−/− and WT mice. Although no difference in fecal Cd numbers and RegIII induction levels were found between ASC−/− and WT mice, ASC−/− mice were defective in the clearance of translocated commensals. Administration of Cd toxins to ileal loops showed that ASC mediates Cd toxin-dependent intestinal inflammation and pyroptosis of macrophages, and ASC deficiency and IL-1 blockage reduced the disease pathology score (7). However, we found comparable intestinal damage and cell death levels in Cd-infected ASC−/− and WT mice. Although there is not a clear explanation for the difference in results, it may be explained by the use of different experimental approaches. While the other authors studied the response to ileal administration of exogenous toxins, we assessed the role of ASC and IL-1 during natural infection with Cd in mice. In the case of infection with Cd, for example, immunostimulatory bacterial molecules present in Cd (e.g. Nod1 and TLR4 ligands) which are known to activate NF-κB could enhance the survival of host cells and this mechanism would not be present in the model of intestinal inflammation induced by administration of purified Cd toxins.
Previous studies showed that Nod1-mediated neutrophil recruitment to Cd-damaged intestine is important for host survival (5). Because Nod1 stimulation directly induced CXCL1 from intestinal epithelial cells and other cells in vitro (21, 22), it was surprising that induced production of CXCL1 in response to Cd infection required IL-1β and ASC signaling in vivo. However, Nod1−/− mice are impaired in the production of IL-1β after Cd infection (5), suggesting the possibility that Nod1 stimulation induces IL-1β secretion. Notably, infection of macrophages or neutrophils with Cd alone did not induce the secretion of IL-1β, although Cd is highly Nod1-stimulatory and can induce CXCL1 production in vitro. These observations suggested that Nod1 stimulation promotes IL-1β in the mouse intestine not by direct induction of IL-1β in the intestine, but by an indirect mechanism. Here we showed that neutrophils secrete IL-1β upon co-stimulation with toxin-positive Cd and translocated commensals. IL-1β was required for CXCL1 production in the intestine, suggesting a positive feedback loop of neutrophil recruitment at Cd-damaged sites via IL-1β and CXCL1 (Fig. 6). Because neutrophil depletion results in increased translocation of commensals to multiple organs in Cd-infected mice (5), the results indicate that efficient neutrophil recruitment is important for the removal of translocated commensals. Translocated commensal bacteria and their product LPS, a TLR4-stimulatory molecule, are important to induce IL-1β secretion. Collectively, these results suggest that TLR4 is important for host survival during Cd infection, which is consistent with previous studies (23). No neutrophil infiltration was detected in the intestine of non-infected mice and Nod1 is required for neutrophil recruitment in the intestine of Cd-infected mice (5), suggesting that Nod1 initially triggers low level of neutrophil recruitment and in turn neutrophils themselves mediate effective neutrophil recruitment via IL-1β secretion upon stimulation of translocated commensals in the intestine. Our current and previous studies suggest that the removal of translocated commensals during Cd infection is important for host survival. The current therapeutic approach of Cd-induced pseudomembranous colitis focused on the removal of Cd by treating patients with vancomycin and metronidazole as the primary antibiotics (1). However, antibiotic treatment is not effective in a significant group of patients, some of who die from severe complications (1). Our studies suggest the possibility that elimination of translocated commensals might be a beneficial approach to treat patients with Cd-induced pseudomembranous colitis. Although further studies are required to test this, our findings suggest that a dual approach to eliminate translocated commensals and Cd by a combination of vancomycin and antibiotics that selectively target commensals might be beneficial in patients suffering from severe forms of intestinal disease.
Figure 6. Model for the role of the ASC/IL-1β-mediated positive feedback loop of neutrophil recruitment for the clearance of translocated commensals and host survival.
See text for details.
Supplementary Material
Acknowledgments
We are grateful to Y. Iwakura (University of Tokyo) and G. Chen (University of Michigan) for materials and Aaron Burberry and Zachary Benet for careful review of the manuscript.
3Abbreviations used in this paper
- Ab
antibody
- ASC
apoptosis-associated speck-like protein containing a caspase-recruitment domain
- B6, BHI
brain heart infusion
- C57BL/6
- Cd
Clostridium difficile
- DGGE
denaturing gradient gel electrophoresis
- FITC
fluorescein isothiocyanate
- IL
interleukin
- mAb
monoclonal antibody
- TCCFA
taurocholate cycloserin cefoxitin fructose agar
- WT
wild-type
Footnotes
Disclosures: The authors have no conflicting financial interests.
The work was supported by NIH grants R01 DE018503 (to N Inohara) and R01 DK091191 (to G Nuñez).
References
- 1.Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526–536. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 2.Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, Minton NP. The role of toxin A and toxin B in Clostridium difficile infection. Nature. 2010;467:711–713. doi: 10.1038/nature09397. [DOI] [PubMed] [Google Scholar]
- 3.Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med. 2000;342:390–397. doi: 10.1056/NEJM200002103420604. [DOI] [PubMed] [Google Scholar]
- 4.Kelly CP, Kyne L. The host immune response to Clostridium difficile. J Med Microbiol. 2011;60:1070–1079. doi: 10.1099/jmm.0.030015-0. [DOI] [PubMed] [Google Scholar]
- 5.Hasegawa MT, Yamazaki N, Kamada K, Tawaratsumida YG, Kim G, Núñez N, Inohara Nucleotide-binding oligomerization domain 1 mediates recognition of Clostridium difficile and induces neutrophil recruitment and protection against the pathogen. J Immunol. 2011;186:4872–4880. doi: 10.4049/jimmunol.1003761. [DOI] [PubMed] [Google Scholar]
- 6.Chamaillard M, Hashimoto M, Horie Y, Masumoto J, Qiu S, Saab L, Ogura Y, Kawasaki A, Fukase K, Kusumoto S, Valvano MA, Foster SJ, Mak TW, Núñez G, Inohara N. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003;4:702–707. doi: 10.1038/ni945. [DOI] [PubMed] [Google Scholar]
- 7.Ng J, Hirota SA, Gross O, Li Y, Ulke-Lemee A, Potentier MS, Schenck LP, Vilaysane A, Seamone ME, Feng H, Armstrong GD, Tschopp J, Macdonald JA, Muruve DA, Beck PL. Clostridium difficile toxin-induced inflammation and intestinal injury are mediated by the inflammasome. Gastroenterology. 2010;139:542–552. doi: 10.1053/j.gastro.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Zheng H, Fletcher D, Kozak W, Jiang M, Hofmann KJ, Conn CA, Soszynski D, Grabiec C, Trumbauer ME, Shaw A. Resistance to fever induction and impaired acute-phase response in interleukin-1 beta-deficient mice. Immunity. 1995;3:9–19. doi: 10.1016/1074-7613(95)90154-x. [DOI] [PubMed] [Google Scholar]
- 9.Ozören N, Masumoto J, Franchi L, Kanneganti TD, Body-Malapel M, Ertürk I, Jagirdar R, Zhu L, Inohara N, Bertin J, Coyle A, Grant EP, Núñez G. Distinct roles of TLR2 and the adaptor ASC in IL-1beta/IL-18 secretion in response to Listeria monocytogenes. J Immunol. 2006;176:4337–4342. doi: 10.4049/jimmunol.176.7.4337. [DOI] [PubMed] [Google Scholar]
- 10.Kanneganti TD, Ozören N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, Bertin J, Coyle A, Grant EP, Akira S, Núñez G. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 11.Hasegawa M, Osaka T, Tawaratsumida K, Yamazaki T, Tada H, Chen GY, Tsuneda S, Núñez G, Inohara N. Transitions in oral and intestinal microflora composition and innate immune receptor-dependent stimulation during mouse development. Infect Immun. 2010;78:639–650. doi: 10.1128/IAI.01043-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 14.Gutierrez O, Pipaon C, Inohara N, Fontalba A, Ogura Y, Prosper F, Nunez G, Fernandez-Luna JL. Induction of Nod2 in myelomonocytic and intestinal epithelial cells via nuclear factor-kappa B activation. J Biol Chem. 2002;277:41701–41705. doi: 10.1074/jbc.M206473200. [DOI] [PubMed] [Google Scholar]
- 15.Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 16.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stelter C, Käppeli R, König C, Krah A, Hardt WD, Stecher B, Bumann D. Salmonella-induced mucosal lectin RegIIIβ kills competing gut microbiota. PLoS One. 2011;6:e20749. doi: 10.1371/journal.pone.0020749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anisowicz A, Zajchowski D, Stenman G, Sager R. Functional diversity of gro gene expression in human fibroblasts and mammary epithelial cells. Proc Natl Acad Sci U S A. 1988;85:9645–9649. doi: 10.1073/pnas.85.24.9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morel JC, Park CC, Kumar P, Koch AE. Interleukin-18 induces rheumatoid arthritis synovial fibroblast CXC chemokine production through NFkappaB activation. Lab Invest. 2001;81:1371–1383. doi: 10.1038/labinvest.3780351. [DOI] [PubMed] [Google Scholar]
- 20.Franchi L, Muñoz-Planillo R, Reimer T, Eigenbrod T, Núñez G. 2010. Inflammasomes as microbial sensors. Eur J Immunol. 2010;40:611–615. doi: 10.1002/eji.200940180. [DOI] [PubMed] [Google Scholar]
- 21.Masumoto J, Yang K, Varambally S, Hasegawa M, Tomlins SA, Qiu S, Fujimoto Y, Kawasaki A, Foster SJ, Horie Y, Mak TW, Núñez G, Chinnaiyan AM, Fukase K, Inohara N. NOD1 acts as an intracellular receptor to stimulate chemokine production and neutrophil recruitment in vivo. J Exp Med. 2006;203:203–213. doi: 10.1084/jem.20051229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishio H, Kanno S, Onoyama S, Ikeda K, Tanaka T, Kusuhara K, Fujimoto Y, Fukase K, Sueishi K, Hara T. Nod1 ligands induce site-specific vascular inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1093–1099. doi: 10.1161/ATVBAHA.110.216325. [DOI] [PubMed] [Google Scholar]
- 23.Ryan A, Lynch M, Smith SM, Amu S, Nel HJ, McCoy CE, Dowling JK, Draper E, O'Reilly V, McCarthy C, O'Brien J, Ní Eidhin D, O'Connell MJ, Keogh B, Morton CO, Rogers TR, Fallon PG, O'Neill LA, Kelleher D, Loscher CE. A role for TLR4 in Clostridium difficile infection and the recognition of surface layer proteins. PLoS Pathog. 2011;7:e1002076. doi: 10.1371/journal.ppat.1002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.