Abstract
Background
Risk for substance use disorder is frequently transmitted across generations due to significant heritability.
Objective
This longitudinal study tests the hypothesis that initial exposure to cannabis in youths having high transmissible risk is a signal event promoting development of cannabis use disorder (CUD).
Methods
At age 22, 412 men were classified into three groups: (1) lifetime CUD, (2) cannabis use without CUD, and (3) no lifetime cannabis use. Transmissible risk, quantified on a continuous scale using the previously validated transmissible liability index (TLI), along with cannabis use and CUD were documented at 10–12, 12–14, 16, 19, and 22 years of age.
Results
The CUD group scored higher on the TLI before they began cannabis use compared to the other two groups. In addition, a progressive increase in TLI severity was evinced by the CUD group beginning at the time of initiation of cannabis use whereas cannabis users who did not subsequently develop CUD exhibited a decline in transmissible risk following first exposure.
Conclusion
Initial use of cannabis potentiates development of CUD in youths who are at high transmissible risk but is inconsequential in youths having low risk. The practical ramifications of these results for prevention are discussed.
Keywords: Transmissible Liability, Addiction, Cannabis use disorder, Longitudinal modeling
INTRODUCTION
Substance use disorder (SUD), like other complex (polygenic, multifactorial) disorders, can be conceptualized as a phenotype that is located on a continuous latent trait termed liability (1–5). Drug-specific metabolic and pharmacological mechanisms notwithstanding, it is notable that up to 100% of genetic variance and 80% of phenotypic variance associated with SUD liability are congenerous to all SUD categories (6–8). Moreover, research conducted on a longitudinally tracked cohort under aegis of the Center for Education and Drug Abuse Research (CEDAR) has shown that the various disorders are indicators of a unidimensional latent trait (9). Complementing these results documenting shared liability and syndrome unity, it has been recently shown that the psychological indicators of transmissible (intergenerational) risk for SUD in the CEDAR cohort also comprise a continuous unidimensional trait (1). Termed the Transmissible Liability Index (TLI), 75% of variance in a Minnesota sample of twins and 85% on a sample accrued at the annual Twinsburg Festival on this trait is genetic (1,10).
In contrast to scales measuring temperament, personality and motivation borrowed from other fields of research that have been utilized in virtually all studies to investigate the psychological antecedents of SUD, the TLI was specifically derived according to theory regarding SUD etiology (3). Employing a multistage procedure to identify the most salient characteristics comprising transmissible risk, including innovative application of item response theory methodology (4), each item in the TLI (see www.pitt.edu/~cedar/tlidocument.html) captures a feature of intergenerational risk that is common to all SUDs; that is, has continuity between parents and offspring. Accordingly, the spectrum of indicators employed in the TLI is wider than in scales measuring particular traits like externalizing behavior, the most ubiquitous childhood disturbance presaging SUD. Furthermore, internalizing disturbances such as anxiety and depression which have been reported to amplify SUD risk (11–15) are also measured in the TLI along with other numerous indicators of biobehavioral self-regulation (16). Notably, the TLI differentiates freshmen entering the University of Maryland from peers who subsequently develop SUD (17), and in the CEDAR sample distinguishes biological children of fathers with and without SUD (1), as well as predicts SUD better than the order of initiation of different substances (18) specified in the gateway hypothesis (19). Significantly, the TLI in the CEDAR sample predicts SUD between childhood and adulthood (20) and in the NESARC sample all SUD categories in adults (21). Recently, it has been shown in CEDAR that the TLI measured in childhood covaries negatively with age of first alcohol and cannabis consumption presaging diagnosis of alcohol and cannabis disorders by early adulthood (22). These findings in conjunction with results of genetic research (1,10) indicate that the TLI accurately measures transmissible risk for SUD.
In this study, we examine the role of transmissible risk in relation to cannabis use onset and rate of development of cannabis use disorder (CUD) between late childhood and young adulthood. Prior research has focused on any type of SUD as the outcome. However, considering that cannabis is the most frequently consumed illegal drug in the world and the drug at the center of ongoing controversy pertaining to legalization and its distribution as a medicine, it is important to delineate the etiologic trajectory to CUD. Furthermore, whereas 51.5% of 8th–12th grade students admit to lifetime experience with alcohol, tobacco, or illicit drugs (23), (Table 1) and lifetime prevalence of any type of SUD is 16% (24), it is important to accurately identify the subset of youths who are most likely to develop a disorder. Based on findings showing that age at the time of substance initiation mediates the association between transmissible risk in childhood and CUD in adulthood (22) and results showing that SUD manifest by early adulthood is strongly heritable (25,26), it is hypothesized that first use of cannabis is a signal event among youths having high transmissible risk leading rapidly to development of CUD. Demonstrating that initial exposure to cannabis is an especially salient event in high but not low risk youths thus underscores the necessity of targeting prevention to vulnerable youths prior to their first consumption experience.
TABLE 1.
Comparison of the sample at baseline (N = 412) according to attrition or retention status at ages 19 and 22.
| Age 19 | Age 22 | |||
|---|---|---|---|---|
| Retained (N = 297) Mean (SD) |
Attrited (N = 115) Mean (SD) |
Retained (N = 256) Mean (SD) |
Attrited (N = 156) Mean (SD) |
|
| Age | 11.44 (.93) | 11.37 (.93) | 11.43 (.92) | 11.43 (.96) |
| Family SES | 41.94 (14.27) | 37.90 (14.74) | 42.62 (14.31) | 37.76 (14.18) |
| Full Scale IQ* | 109.14 (15.87) | 103.50 (15.62) | 113.70 (14.32) | 107.02 (15.10) |
| Grades in school | 4.55 (1.07) | 4.5 (1.13) | 4.6 (1.15) | 4.5 (1.02) |
| TLI (z-score) | .23 (.97) | .28 (1.03) | .20 (.97) | .31 (1.01) |
| % | % | % | % | |
| African-American | 24.4 | 23.9 | 23.8 | 25.3 |
| European-American | 75.6 | 76.2 | 76.2 | 74.7 |
| Paternal SUD | 50.3 | 52.8 | 38.4 | 41.9 |
| Maternal SUD | 25.4 | 21.1 | 23.1 | 17.1 |
p < .05.
METHODS
Participants
The participants were 22 year old men who have been prospectively tracked since they were 10–12 year old boys. Following baseline assessment (age 10–12) subsequent evaluations were conducted when they attained 12–14, 16, 19, and 22 years of age. To qualify for this study, the boys were required to be in good health determined by history and physical examination, have no lifetime psychosis, an IQ of at least 80 and speak English as their primary language. A detailed description of the sample can be found in prior reports (20,27). The list of publications based on this longitudinally tracked cohort is located at www.pitt.edu/~cedar/publications.html.
The boys were assigned to one of three groups when they attained 22 years of age: (1) lifetime diagnosis of CUD (N = 64); (2) cannabis use without qualifying for CUD diagnosis (N = 178); and (3) no lifetime history of cannabis use (N = 170). Partitioning the sample in this fashion is required to demonstrate gradations in severity of transmissible risk and particularly to determine whether risk severity differs between cannabis users who do not develop CUD and abstainers. Table 1 presents the characteristics of the boys who remained in the study or attrited by ages 19 and 22. No group differences were observed on the TLI, the dependent variable. Moreover, family socioeconomic status, ethnicity, and rate of paternal and maternal SUD were not different between the retained and attrited segments of the sample. Full scale WISC-III IQ was lower in boys who attrited at 19 years of age (F=19.83, p <.001) or 22 (F=10.75, p < .001); however, the mean score of both groups is in the normal range.
Instrumentation
Transmissible Liability Index
Previous reports detailed the rationale (3) and method (4) of deriving and validating the TLI. Transmissible liability, comprising the component of SUD risk that is correlated between generations, was measured in the three groups at ages 10–12, 12–14, 16, 19, and 22 years of age. The items constituting each version of the TLI can be found at www.pitt.edu/~cedar/tlidocument.html. The five age-specific versions of the TLI have internal reliability of .90, .93, .91, .95, and .93. The items measure anxiety (e.g., “I bite my fingernails”), behavior control (e.g., “I often act without thinking”), daily rhythms (e.g., “I get hungry about the same time each day”), sleep quality (e.g., “I move a great deal in my sleep”), disruptive behavior (e.g., “I disturb other children”), adaptability to new situations (e.g., “changes in plans makes me restless”), attention (e.g., “easily distracted”), emotion regulation (e.g., “mood changes quickly and drastically”), oppositional behavior (e.g., “I disobey at school”), and eating behavior (e.g., “my appetite seems the same day after day”). These items (and others not cited here) are indicators of a unidimensional trait (1,10) reflecting psychological self-regulation (18). Furthermore, the TLI score in childhood predicts SUD in adulthood (1,20), and in adults, all categories of SUD (21).
Cannabis Use
Cannabis consumption was recorded as a binary variable (yes/no) at each evaluation using the Lifetime Drug Use Interview (28). This variable enabled testing the hypothesis that first exposure to cannabis is a signal event leading to CUD in youths having high transmissible risk. Lifetime rate of cannabis use in the overall sample was 58.8%.
Cannabis Use Disorder
Abuse and dependence diagnoses were formulated using DSM-III-R criteria because the DSM-IV was introduced 5 years after beginning this longitudinal project. A clinical committee consisting of two psychiatrists and master-level clinical associates who administered the SCID (29) formulated the diagnoses.
Procedure
Written informed consent was provided by the parents and written assent was provided by their sons at ages 10–12, 12–14, and 16. Written informed consent was provided by the boys at ages 19. Additional protection from disclosure was ensured by a Certificate of Confidentiality. Urine drug and breath alcohol tests were conducted at the outset to avoid confounding of the results by substance-induced altered physiological state. None of the participants tested positive to a level of impairment. The protocols were administered in fixed order by research assistants who were blind to diagnostic status of the boys or their parents. At the conclusion of the evaluation, the participants were debriefed and compensated at the rate of $10/hour.
Statistical Analysis
Stability of TLI scores between assessments was determined using product moment correlation. Mixed model analysis with unstructured variance–covariance matrix was conducted to determine growth curve parameters in which the time variable denoted chronological age. Next, a piecewise mixed growth analysis was conducted to fit the TLI growth curves simultaneously before and after cannabis initiation in participants who developed CUD and participants who consumed cannabis without developing CUD (30). Furthermore, a linear trajectory was contrasted to a quadratic trajectory to determine the best fit of the data.
RESULTS
Correlations between TLI Scores across Assessments
At the outset, bivariate correlations were computed between the TLI scores. The correlations between the five assessments are r10–12, 12–14= .52, r10–12, 16= .45, r10–12, 19= .43, r10–12, 22 = .48, r12–14, 16 = .57, r12–14, 19 = .51, r12–14, 22 = .54, r16,19 = .71, r16,22 = .73, and r19,22 = .79. The correlation coefficients are all statistically significant beyond the .001 level. Notably, the magnitude of difference encompassing the period between ages 10–12 and 22 is only .04 smaller than the coefficient between ages 10–12 and 12–14. Moreover, there is amonotonic increase in magnitude of the correlations across timepoints.
Transmissible Risk Severity in Relation to Age and Outcome
Means and standard deviations of the TLI at each assessment for three groups are shown in Table 2. A downward trend in all three groups is observed with respect to chronological age. Simultaneously, considering the scores of the three groups in the mixed model analysis revealed that boys who develop CUD evince a different trajectory than boys who either use cannabis but do not develop CUD (β =.04, t = 3.28, p < .001) and boys who have no lifetime use of cannabis (β =.07, t = 4.67, p < .001). Figure 1 (left panel) depicts the trajectory of each group with the upper and lower 95% confidence interval.
TABLE 2.
Mean and standard deviation of TLI across chronological age in boys who developed cannabis use disorder (CUD), boys who used cannabis (CU) but did not develop CUD, and boys who did not use this drug.
| Age | CUD | CU | Boys with no drug use |
|---|---|---|---|
| 10–12 | .63 (1.05) | .22 (.91) | .01 (.84) |
| 12–14 | .33 (.93) | .05 (.88) | −.33 (.96) |
| 16 | .54 (.94) | .06 (.84) | −.54 (.86) |
| 19 | .46 (.94) | −.15 (.84) | −.81 (.86) |
| 22 | .45 (.95) | −.29 (.86) | −.96 (.88) |
| Average | .48 (.97) | −.02 (.89) | −.53 (.96) |
FIGURE 1.
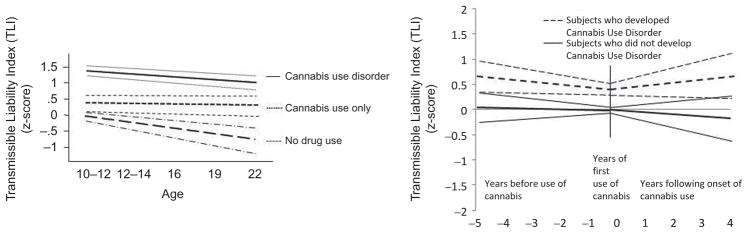
Transmissible risk trajectories and 95% confidence intervals in relation to age and cannabis onset in boys who developed cannabis use disorder or no disorder.
No change in TLI severity is observed across chronological age in the boys who developed CUD (β = −.15, t= −1.57, p=.12) and boys who used cannabis but did not develop CUD (β =.03, t =.41, p =.68). However, boys without lifetime cannabis use exhibited a negative slope (β = −.26, t = −3.03, p =.003); that is, a decline in transmissible risk with increasing chronological age.
Transmissible Risk in Relation to First Use of Cannabis
A different pattern of results emerges when transmissible risk is considered in relation to the timing of cannabis use onset. As shown in the right panel of Figure 1b, negative and positive values on the abscissa indicate, respectively, the years before and after first exposure while the “0” denotes the year cannabis was first consumed. Considering the scores of the two groups simultaneously in piecewise mixed model analysis, transmissible risk was higher before first cannabis use in boys who subsequently developed CUD compared to boys who used cannabis but did not develop this disorder (β =.31, t = 2.19, p =.03). Notably, 95% confidence intervals not only confirm this finding but also do not overlap.
A significant linear decline in TLI scores before (β = −.16, z = −2.02, p =.048) and an increase in TLI scores after first cannabis use (β =.12, z = 3.09, p =.003) was observed in boys who subsequently developed CUD. Participants who used cannabis without developing CUD did not show a linear change in TLI scores before first exposure (β = −.03, t = −.70, p =.49); however, a significant decline in severity of TLI scores was observed after first exposure (β = −.11, t = −2.81, p =.006). In effect, first use presages an increase in severity of biobehavioral characteristics associated with transmissible risk in the boys who developed CUD but boys who did not develop CUD exhibited a decline in risk.
DISCUSSION
Boys who subsequently developed CUD evinced more severe transmissible liability during childhood (prior to initial consumption of cannabis) compared to boys who used cannabis but did not develop to CUD. More importantly, however, it was observed that initiation of cannabis use was followed by a progressive increase in risk that culminated in CUD in participants having high TLI scores but not in boys with low TLI scores. In effect, first exposure to cannabis is a signal event leading to CUD diagnosis by age 22 in youths having high transmissible risk.
These findings inform etiology as well as have practical ramifications for prevention. Notably, once drug use is initiated, social interaction patterns shift to affiliation with peers who similarly do not adhere to societal norms. Thus, habitual consumption is promulgated in vulnerable youths via social interactions with other substance using youths. In other words, deviant socialization mediates the association between transmissible risk in childhood and CUD in adulthood (31). On amore speculative note, in view of theory and findings indicating that propensity for reward dependence is an integral component of SUD risk (32), high transmissible liability may underlie a strong reinforcing effect of drug consumption. Accordingly, cannabis use is more probable and leading, therefore, to habitual consumption and ultimately CUD diagnosis. Evidence pointing to this etiologic pathway is derived from research on animals showing that dopaminergic transmission is persistently enhanced in the ventral tegmentum following a single ingestion of abusable compounds (33,34). Considering that heritability of the liability to CUD is estimated at over 70% (6), and risk for as well as rate of development of cannabis dependence are associated with experienced reinforcing effects (35,36), it would appear important to conjointly take into account transmissible risk and impact of first exposure in research directed at elucidating CUD etiology. From the practical perspective, it would appear that prevention should focus on promoting normative socialization among youths at high risk by emphasizing desistance to non-normative and illegal behaviors, including experimenting with compounds having abuse/dependence potential. Toward this goal, the five age-specific versions of the TLI used in this study have been prepared for administration using a computer adaptive format so that risk can be expeditiously monitored from late childhood to young adulthood (37). Using the Web platform, these versions of the TLI quantify transmissible risk for SUD in less than 5 minutes.
It is also noteworthy that TLI is the only measure developed which specifically quantifies transmissible risk for SUD. Consistent with findings from many studies showing that externalizing disorders-mediate the association between genetic risk and SUD (38), it is not surprising that a portion of the TLI consists of such items. However, the TLI additionally encompasses features that do not comprise features of externalizing disorder. Thus while externalizing disorders are ubiquitous in youths who are at risk for SUD, the TLI is more comprehensive (3,4). Further indication that the TLI is not simply another scale measuring externalizing behavior, it is noteworthy that the TLI and CBCL externalizing scale score obtained by the boys in this sample at age 10–12 correlate .64, indicating that only 40.1% of variance is shared (Tarter, unpublished).
Lastly, while previous studies have shown that the TLI is informative for explicating a variety of aspects of SUD etiology (1,18,22,31), this is the first study to show that initial drug (cannabis) experience is a signal event only in youths having high transmissible risk. Moreover, from the practical perspective, it is notable that this is the first investigation to demonstrate that severity of transmissible risk predicts the interval between first cannabis use and CUD diagnosis.
Several limitations of this study are noted. First, generalizability of the results to girls cannot be assumed, especially considering that the psychological antecedents of SUD in females are featured by less severe externalizing disturbances and more severe internalizing disturbances (39). Second, because the boys were recruited using the family high-risk paradigm, it is also possible that the sample was not representative of the general population. However, it should be noted that the TLI was replicated in a representative sample comprising the National Epidemiological Survey of Alcohol and Related Conditions (NESARC) (21). The TLI was found in this latter study to predict SUD consequent to using alcohol (OR = 2.5), tobacco (OR = 2.3), cannabis (OR = 3.0), hallucinogens (OR = 3.4), cocaine (OR = 3.5), inhalants (OR=3.6), heroin (OR=4.0), stimulants (OR=3.7), and sedatives (OR = 3.8). Furthermore, the lifetime rate of cannabis use in the present sample (58.8%) also maps closely to the prevalence rate in young adults (55.9%) between 18–30 years of age comprising the 2010 Monitoring the Future Survey (40), (volume II). Finally, due to the small number of subjects manifesting SUD consequent to using other drugs, it could not be determined whether the TLI predicts other SUD categories. Results obtained on the NESARC sample reveal, however, that the TLI measures risk for all types of SUD (21).
In summary, this longitudinal study demonstrates that transmissible risk is elevated before first cannabis use in boys who develop CUD and linearly increases after first cannabis use. Youths who consume cannabis but do not develop CUD exhibit significantly lower transmissible liability before beginning consumption and a linear decrease in risk after first exposure. These results underscore the importance of objectively monitoring youths during development to inform in timely fashion the need for prevention intervention.
Acknowledgments
This work is supported by grants from the National Institute on Drug Abuse P50-DA05605, K05-DA031248, and K02-DA017822.
Footnotes
Declaration of Interest
No conflict of interest are reported by the authors. The authors alone are responsible for the content and writing of the paper.
References
- 1.Vanyukov M, Kirisci L, Moss H, Tarter R, Reynolds M, Maher B, Kirillova G, Ridenour T, Clark D. Measurement of the risk for substance use disorders. Phenotypic and genetic analysis of an index of common liability. Behav Genet. 2009;39:233–244. doi: 10.1007/s10519-009-9269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falconer DS. The inheritance of liability to certain diseases estimated from the incidence among relatives. Ann Hum Genet. 1965;29:51–76. [Google Scholar]
- 3.Vanyukov M, Tarter R, Kirisci L, Kirillova G, Maher B, Clark D. Liability to substance use disorders. 1. Common mechanisms and manifestations. Neurosci Biobehav Rev. 2003a;27:507–515. doi: 10.1016/j.neubiorev.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Vanyukov MM, Kirisci L, Tarter RE, Simkevitz HF, Kirillova GP, Maher BS, Clark DB. Liability to substance use disorders: 2. A measurement approach. Neurosci Biobehav Rev. 2003b;27:517–526. doi: 10.1016/j.neubiorev.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Vanyukov MM, Tarter RE, Kirillova GP, Kirisci L, Reynolds MD, Kreek MJ, Conway KP, Maher B, Iacono W, Bierut L, Neale MC, Clark D, Ridenour TA. Common liability to addiction and “gateway hypothesis”: Theoretical, empirical and evolutionary perspective. Drug Alcohol Depend. 2012;123 (supplement):S3–S17. doi: 10.1016/j.drugalcdep.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psychiatry. 2003a;160:687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- 7.Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environment risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003b;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 8.Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, True W, Lin N, Toomey R, Eaves L. Cooccurrence of abuse of different drugs in men: The role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry. 1998;55:967–972. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- 9.Kirisci L, Tarter R, Vanyukov M, Martin C, Mezzich A, Brown S. Application of item response theory to quantify substance use disorder severity. Addict Behav. 2006;31:1035–1049. doi: 10.1016/j.addbeh.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 10.Hicks BM, Iacono WG, McGue M. Index of the transmissible common liability to addiction: Heritability and prospective associations with substance abuse and related outcomes. Drug Alcohol Depend. 2012;123:S18–S23. doi: 10.1016/j.drugalcdep.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall W, Degenhardt L, Teeson M. Understanding comorbidity between substance use, anxiety and affective disorders: Broadening the research base. Addict Behav. 2009;34:526–530. doi: 10.1016/j.addbeh.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Lopez B, Turner RJ, Saavedra LM. Anxiety and risk for substance dependence among late adolescents/young adults. J Anxiety Disord. 2005;19:275–294. doi: 10.1016/j.janxdis.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 13.O’Neil KA, Conner BT, Kendall PC. Internalizing disorders and substance use disorders in youth: Comorbidity, risk, temporal order, and implications for intervention. Clin Psychol Rev. 2011;31:104–112. doi: 10.1016/j.cpr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Preuss UW, Schuckit MA, Smith TL, Barnow S, Danko GP. Mood and anxiety symptoms among 140 children from alcoholic and control families. Drug Alcohol Depend. 2002;67:235–242. doi: 10.1016/s0376-8716(02)00076-5. [DOI] [PubMed] [Google Scholar]
- 15.Wu L-T, Gersing K, Burchett B, Woody GE, Blazer DG. Substance use disorders and comorbid Axis I and II psychiatric disorders among young psychiatric patients: Findings from a large electronic health records database. J Psychiatr Res. 2011;45:1453–1462. doi: 10.1016/j.jpsychires.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarter R, Horner M, Ridenour T. Developmental perspective of substance use disorder etiology. In: Shaffer H, editor. Addiction Syndrome Handbook. Washington, DC: American Psychological Press; 2012. [Google Scholar]
- 17.Arria A, Vincent K, Caldeira K. Measuring liability for substance use disorder among college students: Implications for screening and early intervention. Am J Drug Alcohol Abuse. 2009;35:233–241. doi: 10.1080/00952990903005957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarter R, Kirisci L, Mezzich A, Ridenour T, Fishbein D, Vanyukov M. Does the “gateway” sequence increase prediction of CUD beyond deviant socialization? Implications for Prevention and Policy Drug Alcohol Depend. 2012;123(supplement):S72–S78. doi: 10.1016/j.drugalcdep.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kandel D, Yamaguchi K. Developmental stages of involvement in substance use. In: Ott P, Tarter R, Ammerman R, editors. Sourcebook on Substance Abuse: Etiology, Epidemiology, Assessment, and Treatment. Boston, MA: Allyn & Bacon; 1999. pp. 50–74. [Google Scholar]
- 20.Kirisci L, Tarter R, Mezzich A, Ridenour T, Reynolds M, Vanyukov M. Prediction of cannabis use between childhood and young adulthood: Clarifying the phenotype and environ-type. Am J Addict. 2009;18:36–47. doi: 10.1080/10550490802408829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ridenour TA, Tarter RE, Kirisci L, Vanyukov MM. Could a continuous measure of individual transmissible risk be useful in clinical assessment of substance use disorder? Drug Alcohol Depend. 2011;119:10–17. doi: 10.1016/j.drugalcdep.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirisci L, Tarter R, Ridenour T, Zhai Z-W, Fishbein D, Reynolds M, Vanyukov M. Age of alcohol and cannabis use onset mediates the association of transmissible risk in childhood and development of alcohol and cannabis disorders: Evidence for common liability. Exp Clin Psychopharmacol. 2013;23:38–45. doi: 10.1037/a0030742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnston L, O’Malley P, Bachman J, Schulenberg J. Monitoring the Future National Results on Adolescent Drug Use: Overview of Key Findings, 2011. Ann Arbor: Institute for Social Research, The University of Michigan; 2012. [Google Scholar]
- 24.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y-C, Prescott CA, Walsh D, Patterson DG, Riley BP, Kendler K, Kuo P-H. Different phenotypic and genotypic presentations in alcohol dependence: Age at onset matters. J Stud Alcohol Drugs. 2011;72:752–762. doi: 10.15288/jsad.2011.72.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cloninger C, Bohman M, Sigvardsson S. Inheritance of alcohol abuse: Cross fostering analysis of adopted men. Arch Gen Psychiatry. 1981;36:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- 27.Tarter R, Vanyukov MM. Theoretical and operational framework for research into the etiology of substance use disorder. J Child Adolesc Subst Abuse. 2001;10:1–12. [Google Scholar]
- 28.CEDAR. Lifetime Drug Use Interview (unpublished manuscript) Center for Education and Drug Abuse Research; 711 Salk Hall: University of Pittsburgh; Pittsburgh, PA: 1989. p. 15213. [Google Scholar]
- 29.Spitzer R, Williams J, Miriam G. Instruction Manual for the Structured Clinical Interview for DSM-III-R. New York, NY: New York State Psychiatric Institute; 1987. [Google Scholar]
- 30.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Haboken, NJ: John Wiley and Sons, Inc; 2004. [Google Scholar]
- 31.Tarter R, Fishbein D, Kirisci L, Mezzich A, Ridenour T, Vanyukov M. Deviant socialization mediates transmissible and contextual risk on CUD development: A prospective study. Addiction. 2011;106:1301–1308. doi: 10.1111/j.1360-0443.2011.03401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- 33.Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 34.Ungless MA, Whistle JL, Malenka RC, Bonci A. Single cocaine exposure to vivo induces long term potentiation in dopamine neurons. Nature. 2011;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- 35.Grant JD, Sherrer JF, Lyons MJ, Tsuang M, True WR, Bucholz KK. Subjective reactions to cocaine and marijuana are associated with abuse and dependence. Addict Behav. 2003;30:1574–1586. doi: 10.1016/j.addbeh.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Sherrer J, Grant J, Duncan A, Sartor C, Haber J, Jacob T, Bucholz K. Subjective effects to cannabis are associated with use, abuse and dependence after adjusting for genetic and environmental influences. Drug Alcohol Depend. 2009;105:76–82. doi: 10.1016/j.drugalcdep.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirisci L, Tarter R, Reynolds M, Ridenour T, Stone C, Vanyukov M. Computer adaptive testing of liability to addiction: Identifying individuals at risk. Drug Alcohol Depend. 2012;123(Supplement 1):S79–S86. doi: 10.1016/j.drugalcdep.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vrieze SL, McGue M, Miller MB, Hicks BM, Iacono WG. Three mutually informative ways to understand the genetic relationships among behavioral disinhibition, alcohol use, drug use, nicotine use/dependence, and their co-occurrence: Twin biometry, GCTA, and genome-wide scoring. Behav Genet. 2013;43:97–107. doi: 10.1007/s10519-013-9584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leadbeater BJ, Kuperminc GP, Blatt SJ, Hertzog C. A multivariate model of gender differences in adolescents’ internalizing and externalizing problems. Develop Psychol. 1999;35:1268–1281. doi: 10.1037//0012-1649.35.5.1268. [DOI] [PubMed] [Google Scholar]
- 40.Johnston L, O’Malley P, Bachman J, Schulenberg J. Monitoring the Future National Survey Results on Drug Use, 1975–2010: Volume II, College Students & Adults Ages 19–50. Ann Arbor: Institute for Social Research, The University of Michigan; 2011. [Google Scholar]


