Abstract
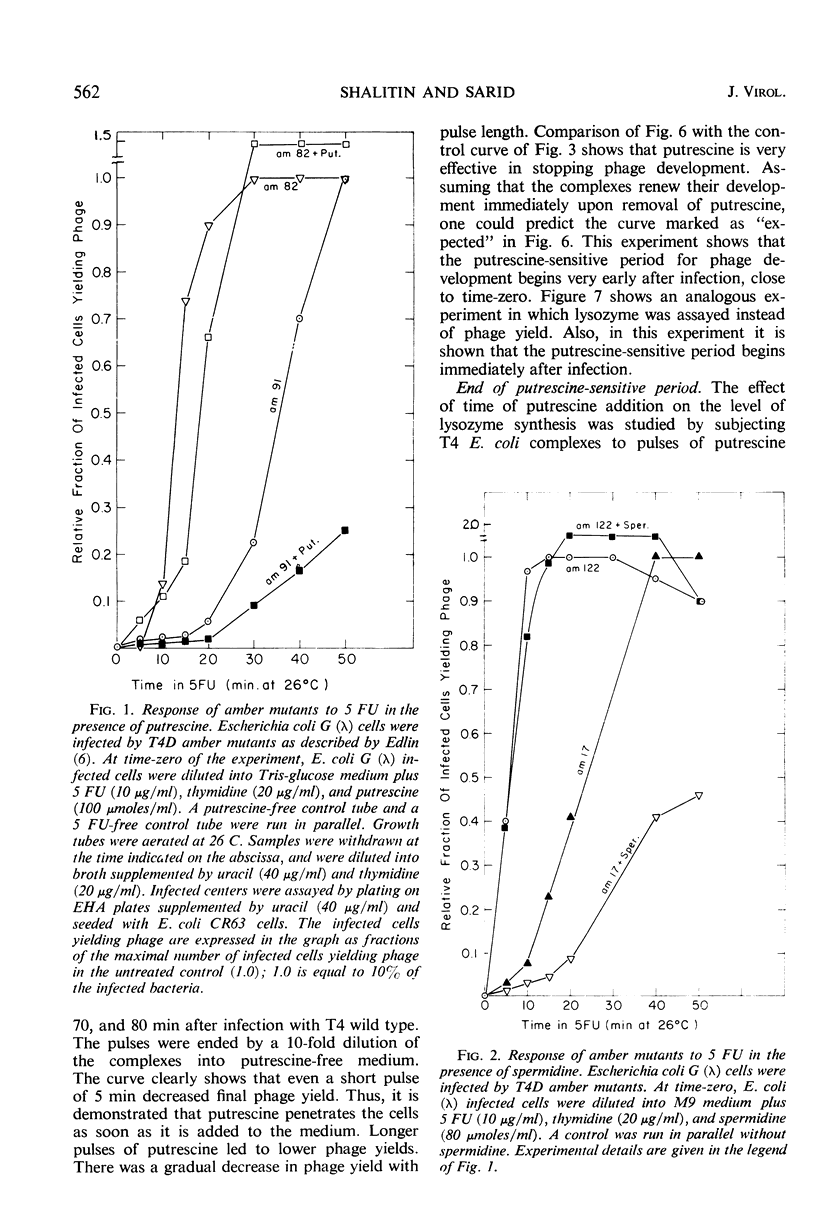
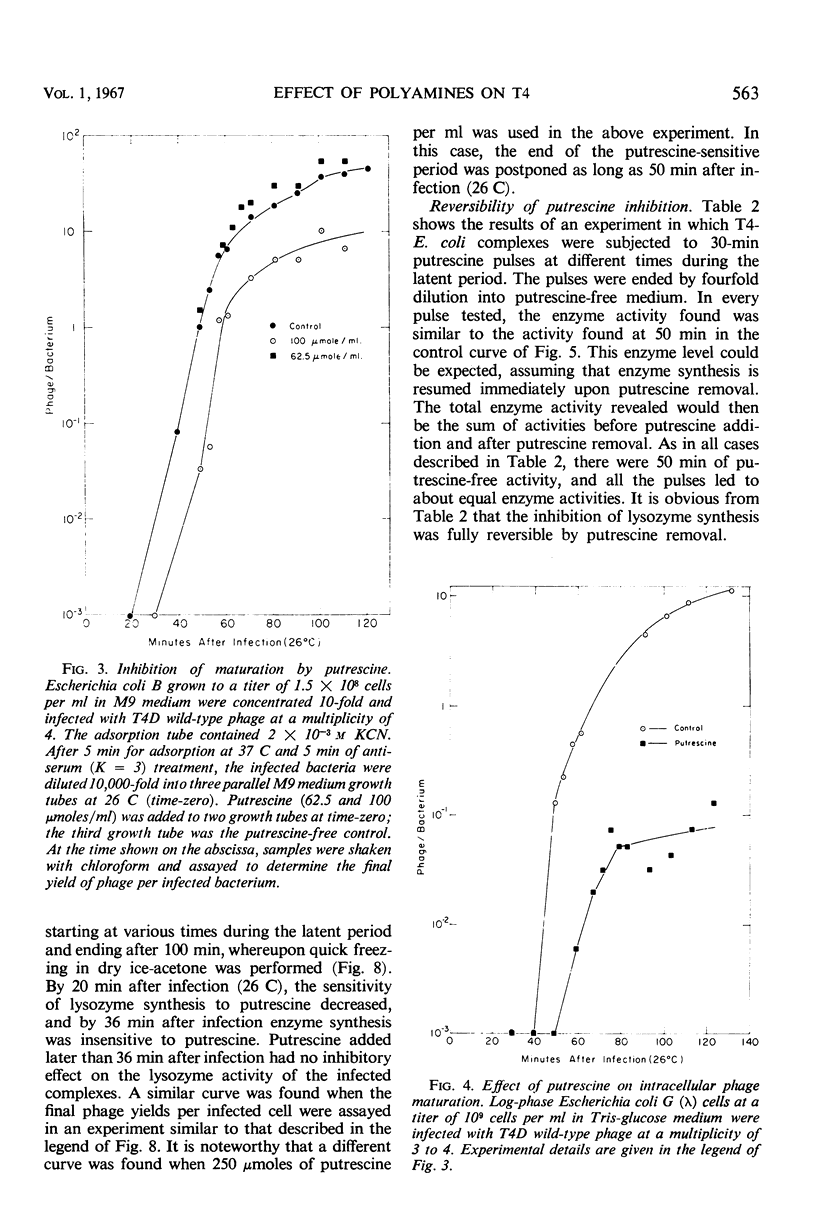
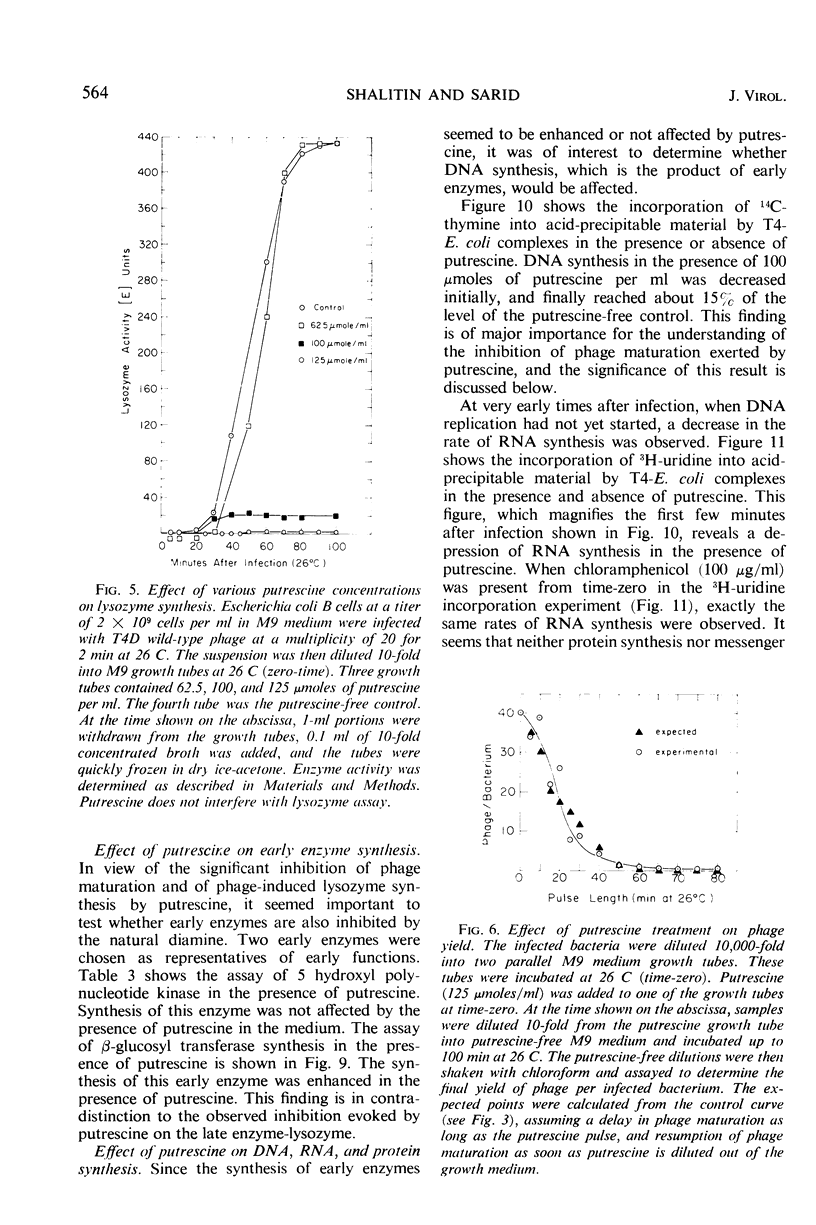
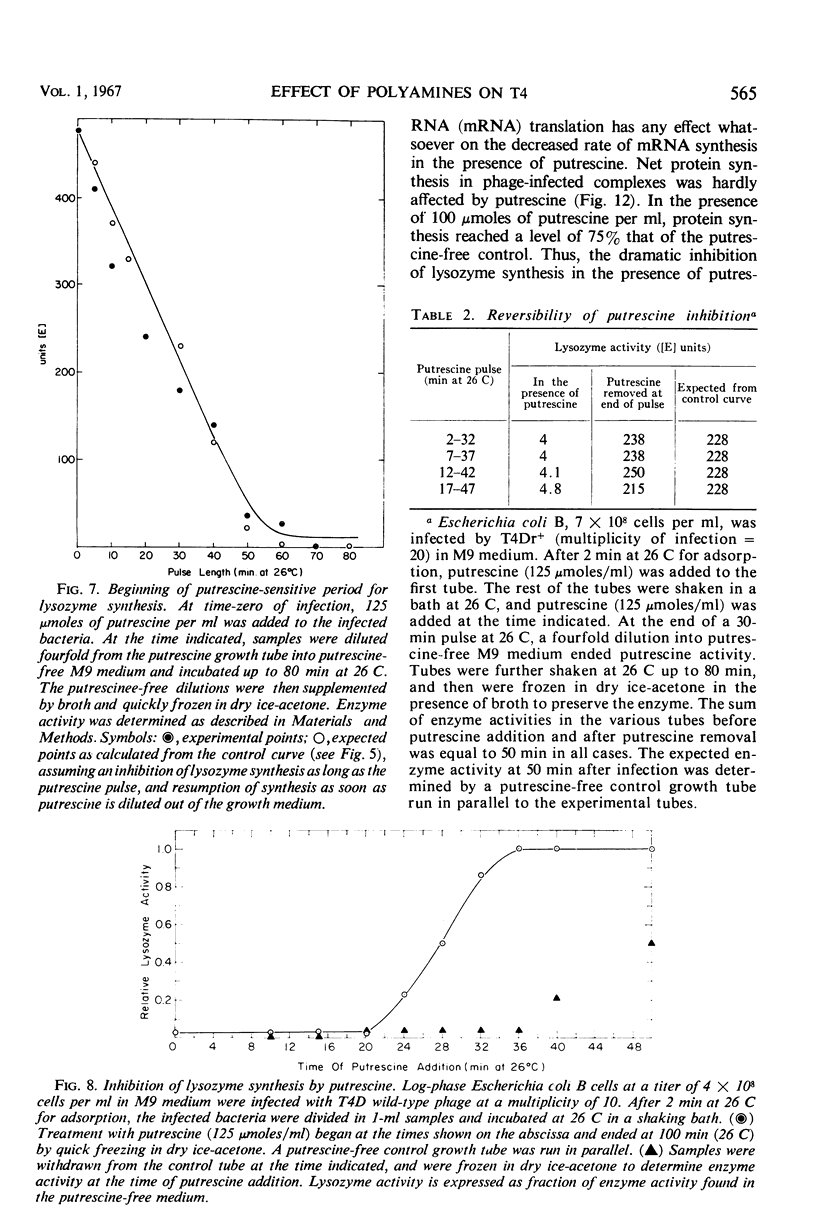
The 5-fluorouracil (5 FU) technique for the phenotypic reversion of amber mutants was used to demonstrate that under certain circumstances, in the presence of putrescine or spermidine, early mutants have an enhanced response to 5 FU, whereas late mutants have a delayed response. Bacteria infected by T4D wild-type bacteriophage did not produce phage in the presence of high putrescine concentrations. Pulse treatments with putrescine showed that the production of lysozyme depends on a putrescine-sensitive process that begins immediately after infection at 26 C and ends at 36 min or even later. The addition of putrescine at any time during the critical period between 0 and 36 min led to a corresponding delay in lysozyme synthesis after the inhibitor was removed. Intracellular phage maturation was delayed by the addition of 100 μmoles of putrescine per ml. Early enzymes were not affected by the diamine, but the level of phage deoxyribonucleic acid was considerably decreased by the inhibitor. The putrescine-sensitive process that affects the timing of maturation is suggested to be the natural process controlling the T4 “clock.”
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T., ROSENTHAL S. M. Presence of polyamines in certain bacterial viruses. Science. 1958 Apr 11;127(3302):814–815. doi: 10.1126/science.127.3302.814-a. [DOI] [PubMed] [Google Scholar]
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- AVRON M. Photophosphorylation as a tool for the synthesis of specifically labeled nucleotides. Anal Biochem. 1961 Dec;2:535–543. doi: 10.1016/0003-2697(61)90021-5. [DOI] [PubMed] [Google Scholar]
- EDLIN G. GENE REGULATION DURING BACTERIOPHAGE T4 DEVLOPMENT. I. PHENOTYPIC REVERSION OF T4 AMBER MUTANTS BY 5-FLUOROURACIL. J Mol Biol. 1965 Jun;12:363–374. doi: 10.1016/s0022-2836(65)80260-1. [DOI] [PubMed] [Google Scholar]
- Kornberg A., Zimmerman S. B., Kornberg S. R., Josse J. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. INFLUENCE OF BACTERIOPHAGE T2 ON THE SYNTHETIC PATHWAY IN HOST CELLS. Proc Natl Acad Sci U S A. 1959 Jun;45(6):772–785. doi: 10.1073/pnas.45.6.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C. C. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc Natl Acad Sci U S A. 1965 Jul;54(1):158–165. doi: 10.1073/pnas.54.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEKIGUCHI M., COHEN S. S. THE SYNTHESIS OF MESSENGER RNA WITHOUT PROTEIN SYNTHESIS. II. SYNTHESIS OF PHAGE-INDUCED RNA AND SEQUENTIAL ENZYME PRODUCTION. J Mol Biol. 1964 May;8:638–659. doi: 10.1016/s0022-2836(64)80114-5. [DOI] [PubMed] [Google Scholar]
- Shalitin C. Selective gene transcription in bacteriophage T4 by putrescine. J Virol. 1967 Jun;1(3):569–575. doi: 10.1128/jvi.1.3.569-575.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE I. The effect of ultraviolet light on the production of bacterial virus protein. J Gen Physiol. 1957 Mar 20;40(4):521–531. doi: 10.1085/jgp.40.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIBERG J. S., DIRKSEN M. L., EPSTEIN R. H., LURIA S. E., BUCHANAN J. M. Early enzyme synthesis and its control in E. coli infected with some amber mutants of bacteriophage T4. Proc Natl Acad Sci U S A. 1962 Feb;48:293–302. doi: 10.1073/pnas.48.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]



