Abstract
Objectives
Determine if ipsilesional primary motor cortex (M1) in stroke patients processes online visuomotor discordance in gain between finger movement and observed feedback in virtual-reality (VR).
Materials and Methods
Chronic stroke patients flexed (n=7) or extended (n=1) their finger with real-time feedback of a virtual hand presented in VR. Virtual finger excursion was scaled by applying a low-gain (G0.25), high-gain (G1.75), or veridical (G1.00) scaling factor to real-time data streaming from a sensor glove. Effects of visuomotor discordance was assessed through analysis of movement kinematics (joint excursion, movement smoothness, angular velocity) and amplitude of motor evoked potentials (MEPs) elicited with transcranial magnetic stimulation (TMS) applied to ipsilesional M1. Data were analyzed with a repeated measures ANOVA (significance set at 0.05).
Results
G0.25 discordance (relative to veridical) lead to significantly larger joint excursion, online visuomotor correction evidenced by decreased trajectory smoothness, and significantly facilitated agonist MEPs. This effect could not be explained by potential differences in motor drive (background EMG) or by possible differences related to joint angle or angular velocity, as these variables remained invariant across conditions at the time of MEP assessment. M1 was not significantly facilitated in the G1.75 condition. MEPs recorded in an adjacent muscle that was not involved in the task were unaffected by visual feedback in either discordance condition. These data suggest that the neuromodulatory effects of visuomotor discordance on M1 were relatively selective.
Conclusions
Visuomotor discordance may be used to alter movement performance and augment M1 excitability in patients following stroke. Our data illustrate that visual feedback may be a robust way to selectively modulate M1 activity. These data may have important clinical implications for the development of future VR training protocols.
Keywords: Virtual Reality, Transcranial Magnetic Stimulation, Sensorimotor, Plasticity, Rehabilitation
INTRODUCTION
Stroke is the leading cause of physical disability in the United States. Recovery of upper extremity and hand function is often underserved (1-5) and as a result, recent neurorehabilitation studies have focused on the development and synergy of numerous technologies, including noninvasive brain stimulation, virtual reality (VR), and robotic assistance devices (6-8) to improve outcomes of hand function. Notably, studies have shown that performing hand movements with augmented visual feedback in a VR environment may enhance motor recovery and potentiate neural reorganization in patients with stroke (9-14). The mechanism for beneficial effects of VR-based therapy is unkown. One possibility, based on electrophysiological recordings in nonhuman primates during and after adaptation to visuomotor discordance (15-18), suggests that training in VR may facilitate formation of de novo visuomotor mappings and enhance activation in sensorimotor circuits that may be important for functional recovery after stroke. Studies in healthy humans and individuals with chronic stroke too suggest that visuomotor discordance may expedite the learning process (19-21). A unique case of visuomotor learning relates adaptation to gain discordance because this capacity is more easily generalizable than rotation discordance (22), is intact in individuals with stroke (23), and is thought to be mediated at least in part by the motor and premotor areas (24). What is unknown however, is whether the motor system is involved in forming novel visuomotor mappings after adaptation, or if it is involved in detecting discordances between intended actions and feedback within a discrete movement (online feedback). The overarching focus of the current study was to test specifically if the ipsilesional corticospinal system in stroke patients is involved in processing online feedback related to gain discordance.
We tested this in chronic stroke patients with mild-moderate hand impairment by having patients perform a targeted finger flexion movement with the affected hand. To keep subjects in a perpetually de-adapted state, we pseudo-randomly varied (on a trial-by-trial basis) the type of feedback discordance. Feedback was presented in real-time by interfacing kinematic data acquired with a sensor glove using a virtual reality model of the human hand viewed by the subject in first person perspective. Feedback was presented as either veridical or was scaled-down or scaled up by 75%. The excitability of the motor cortex was assayed by measuring motor evoked potentials (MEP) in the agonist and in a control muscle at constant joint-angle during the movement. This was done to control for possible spindle-length dependent effects on the MEP. We hypothesized that if motor cortex is involved in processing online visuomotor discordance, then MEPs should be increased in the altered, relative to the veridical, feedback condition.
METHODS
Subjects
Eight subjects (see Table 1, mean age ± 1 SD: 63.0 ± 9.5 years old) who had a single cerebrovascular accident at least six months prior participated. All subjects provided institutionally-approved informed consent. Subjects were included only if they did not have a history of other neurological, cognitive, or orthopedic pathology that could interfere with their performance on the task, if they had spasticity that was ‘2’ or less on the Modified Ashworth Scale, and if they had at least 20 degrees of active finger flexion or extension. Stroke type (hemorrhagic vs. non-hemorrhagic), lesion location (cortical vs. subcortical, left vs. right hemisphere), and hand dominance were not grounds for inclusion/exclusion.
Table 1.
Stroke subject demographics and clinical characteristics.
| ID | Gender | Age | Prestroke Hand Dominance | Months Since Stroke | Stroke Type | Stroke Location | Hemiparetic Side |
|---|---|---|---|---|---|---|---|
| S1 | Male | 58 | Right | 132 | Ischemic | Middle Cerebral | Left |
| S2 | Female | 63 | Right | 53 | Ischemic | Occipital & Parietal | Right |
| S3 | Male | 49 | Right | 144 | Ischemic | Basal Ganglia | Right |
| S4 | Male | 74 | Right | 9 | Ischemic | Frontal | Left |
| S5 | Male | 67 | Right | 78 | Ischemic | Occipital | Left |
| S6 | Female | 68 | Right | 18 | Ischemic | Pons | Left |
| S7 | Male | 53 | Right | 24 | Ischemic | Pons | Left |
| S8 | Female | 75 | Right | 154 | Ischemic | Corona Radiata | Left |
Setup
Subjects were seated with hands hidden from direct line-of-sight under a 1.5 inch thick LED monitor (Fig. 1). Virtual hand models (Virtools software, Dassault Systems) were displayed on the monitor and actuated, in real-time, by joint angle data streaming from sensor gloves (Fifth Dimension Technologies’ 5DT-16MRI Data Gloves, sampling rate: 50 Hz) worn by the subjects. The virtual hands in the display were sized and positioned in 1st person view such that subjects felt a sense of ownership of the virtual hands on the screen.
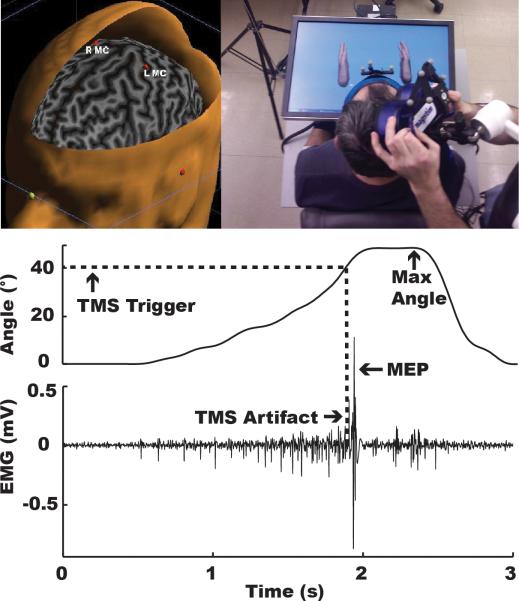
Figure 1.
Virtual reality setup (top) and raw kinematic and EMG data (bottom) acquired from a typical subject. Dashed line shows that TMS stimulation was automatically triggered when the subject's metacarpophalangeal (MCP) joint reached 40°, regardless of visual feedback.
Task
Depending on the subjects’ functional ability, they were instructed to either flex (Subjects 1,2,4,5,6,7,8) or extend (S3) the index finger metacarpophalangeal (MCP) joint of their paretic hand to a 45° angle and return to the fully-extended or fully-flexed position, respectively. A visual cue (the virtual finger turned the color red) was provided when subjects reached the target angle. Subjects had 3.5 seconds to complete the task, with a 2.5 second inter-trial interval.
Conditions
On a given trial, subjects were provided with either veridical (G1.00, control condition), scaled-down (G0.25), or scaled-up (G1.75) visual feedback, in which the motion of the virtual hand was 100%, 25%, or 175%, respectively, of the actual hand movement. Thus, for every 1° of physical movement, the virtual finger would move 1°, 0.25°, or 1.75°, respectively. Twenty-two trials of each condition (66 total trials) were pseudo-randomly interleaved in one 6.6-minute block. This event-related design assured that any modulatory changes in M1 would reflect online effects of altered feedback rather than effects related to formation of new visuomotor mappings.
Electrophysiology
Electromyographic (EMG) data from the first dorsal interosseous (FDI) and flexor digiti minimi (FDM) (for subjects performing flexion), or from the long forearm extensor (S3) and flexor muscles of the paretic hand were acquired with a 4-Channel Bagnoli EMG System (Delsys, Inc.). Raw analog EMG signal was amplified (×10), streamed to a data acquisition device (NI USB-6221, National Instruments Corp., 2 kHz sampling frequency), and analyzed offline with custom-written MATLAB software.
Neuronavigated Transcranial Magnetic Stimulation
Prior to the experiment, a T1-weighted high-resolution anatomical magnetic resonance image (mprage, 3T Siemens Allegra) was acquired and used to render a 3-dimensional cortical surface. Fiducial markers on the MRI were co-registered with the subject's head to allow frameless neuronavigation (Visor, Advanced Neuro Technology). The cortical ‘hotspot’ for the agonist muscle was identified in the ipsilesional primary motor cortex (M1) as the region with the maximal motor evoked potential (MEP) in five of ten consecutive trials. MEP amplitude was measured as the peak-to-peak amplitude of the EMG signal 20-50 ms after the TMS pulse. MEPs were evoked with transcranial magnetic stimulation (TMS, Magstim Rapid2, 70mm double AFC coil) with the coil positioned tangential to the scalp and the coil handle pointing 45 degrees posteriorly and down. Once a hotspot was identified, the resting motor threshold (RMT) was defined as the minimum intensity required to elicit MEPs greater than 50μV in the agonist muscle on five of ten consecutive trials. For the experiment, TMS was applied to the hotspot at 110% RMT during each movement and on each trial. The TMS pulse was triggered automatically when the subject's MCP joint angle reached 40° (i.e. immediately prior to the target angle). This allowed us to keep joint angle (at the time of TMS stimulation) constant across all conditions, assuring that between-condition MEP differences would not be confounded by discrepancies in joint angle or muscle length.
Outcome Variables
Kinematics
Joint angle data was filtered (10 Hz low-pass 2nd order Butterworth) and analyzed offline with custom-written MATLAB software (The Mathworks, Inc.). For each trial, movement onset and offset were defined as the time when the angular velocity exceeded and fell below 5% of peak angular velocity for greater than 60ms. Kinematic outcome variables included: (1) peak excursion angle, defined as the angle of the MCP joint at the time of movement offset, (2) movement smoothness, defined as the peak value of the third derivative of joint angle (jerk) between movement onset and offset, and (3) instantaneous angular velocity, at the time just prior to TMS stimulation.
Electrophysiology
Outcome electrophysiological variables included (1) MEP of the agonist and control (inactive) muscle, and (2) background EMG of the agonist muscle, calculated as the filtered (5-250 Hz band-pass 2nd order Butterworth filter), rectified, and enveloped (20 Hz low-pass) EMG signal immediately preceding TMS stimulation. Evidence suggests that MEP amplitude correlates with background EMG activity for dynamic, low-force contractions (25-27), such as those used in this experiment. Thus, we included background EMG as an outcome measure to assure that any between-condition differences in MEP amplitude were not a result of differences in background EMG levels, but rather a result of visual feedback (28, 29).
Statistics
All outcome variables were averaged across trials for each condition for each subject. Each subject's mean was submitted to a one-way repeated measures analysis of variance (rmANOVA) with factor “Condition” and levels “G1.00”, “G0.25”, and “G1.75”. Additionally, a linear regression model was generated to determine the amount of variance explained by all of the electrophysiological and kinematic variables. For this, each trial of each condition and subject was submitted for analysis, with MEP as the dependent variable and visual feedback, background EMG, and instantaneous angular velocity as independent variables. Data was analyzed using PASW Statistics 18 (SPSS Inc.). Significance threshold for all analyses was set at 0.05.
RESULTS
Online Changes in Movement Kinematics in Response to Gain Discordance
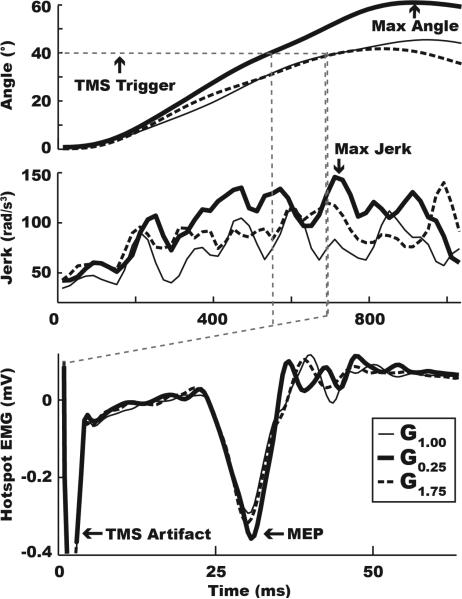
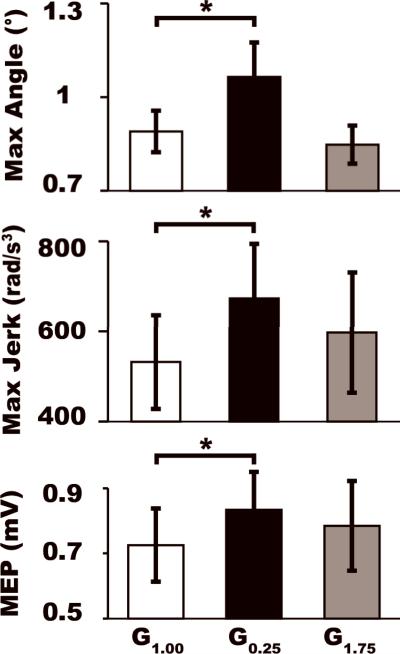
Figure 2 (top and middle) shows the mean joint angle and jerk time traces in each condition for a typical stroke subject. Repeated measures ANOVA revealed that the peak excursion angle (F2,14=6.397, p=0.011) and movement smoothness (F2,14=4.252, p=0.036) were significantly different between the feedback conditions. Post-hoc pairwise comparisons revealed that these between-condition differences were driven by a 19.6% increase in joint angle excursion (t7=-2.535, p=0.039) and 26.6% decrease in smoothness (t7=-2.693, p=0.031) in the G0.25 relative to the veridical condition (see Table 2 and Fig. 3). No differences were noted between the G1.75 and the veridical condition (excursion: t7=0.787, p=0.457; smoothness: t7=-1.200, p=0.269). Additionally, instantaneous velocity at the time of TMS stimulation (F2,14 =1.144, p=0.339) was similar across all three feedback conditions. These data suggest that scaled-down feedback altered subjects’ online control of movement.
Figure 2.
Kinematic and electrophysiological data for a typical subject. Mean angle (°), jerk (rad/s3), and MEP (mV) traces for the G1.00 (thin line), G0.25 (thick line), and G1.75 (dashed line) visual feedback conditions, aligned in time to movement onset. The figure demonstrates that maximum excursion, peak jerk, and M1 facilitation were all greater in the G0.25 condition, but not the G1.75 condition, relative to G1.00 (control condition). Also shown is the TMS artifact (dashed grey line), set to trigger at 40° for every trial of every condition.
Table 2.
Group mean (±1SEM) kinematic and electrophysiological data.
| G1.00 | G0.25 | G1.75 | |
|---|---|---|---|
| Max Angle | 0.890 (0.067) | 1.065 (0.110) | 0.848 (0.061) |
| Max Jerk | 532.0 (104.2) | 673.6 (121.1) | 597.4 (133.7) |
| Instantaneous Velocity | 2.366 (0.394) | 2.598 (0.423) | 2.327 (0.545) |
| Contracting Muscle MEP | 0.725 (0.112) | 0.834 (0.116) | 0.785 (0.138) |
| Contracting Muscle EMG | 0.023 (0.005) | 0.025 (0.005) | 0.024 (0.006) |
| Adjacent Muscle MEP | 0.465 (0.118) | 0.486 (0.129) | 0.484 (0.142) |
Figure 3.
Kinematic and electrophysiological group data. Group mean (±1SEM) for the maximum excursion angle, maximum jerk, and MEP are shown for each condition. Asterisk denotes significant differences between the altered feedback and the veridical condition.
M1 Excitability and Gain Discordance
Figure 2 (bottom) illustrates in a typical subject that MEPs recorded in the agonist muscle during the G0.75 condition were increased relative to those recorded in the veridical condition. At the group level, rmANOVA revealed significant between-condition differences in MEP amplitude for the agonist (F2,14=6.083, p=0.013) but not for the control muscle (p>0.05). Figure 3 (see also Table 2) shows that post-hoc pairwise comparisons revealed that the effect was driven by a 14.9% increase in MEP amplitude in the G0.25 relative to the veridical condition (t7=-4.007, p=0.005). No such increases were noted in the G1.75 condition (t7=-1.738, p=0.126). Analysis of background EMG just prior to the MEP measurement revealed no significant between-condition differences (F2,14=0.777, p=0.472), suggesting that motor drive at the time of stimulation was consistent between the feeback conditions and could not account for differences in MEP amplitude. Finally, although S3 performed an extension movement, kinematic performance (angle and angular velocity at the time of stimulation) and electrophysiology (background EMG of the agonist muscle) were comparable to the mean of all other subjects, suggesting that these factors did not skew the group mean MEP effects.
As an additional analysis, we performed a linear regression. Continuous data was z-normalized [Y=(X-μ)/σ)] to each subject's mean and standard deviation. MEP amplitude was specified in the model as the dependent variable. Background EMG, instantaneous angular velocity, and visual feedback condition (G0.25 and G1.75, relative to G1.00) were specified as independent variables. Expectedly, regression analysis revealed that MEP amplitude was significantly positively correlated with background EMG (β=0.129, p=0.032) and the G0.25 condition (β=0.182, p=0.01), but not with instantaneous angular velocity (β=-0.010, p=0.863) or with the G1.75 condition (β=0.077, p=0.272), confirming that M1 excitability changes were driven primarily by scaled-down feedback condition
Specificity of Neuromodulatory Effects in M1
MEPs were recorded from the agonist muscle (FDI: S1, S2, S4, S5, S6, S7, S8; extensor digitorum: S3) and from a secondary adjacent muscle that was not involved in the task (i.e., a control muscle) (FDM: S1, S2, S4, S5, S8; flexor digitorum superficialis: S6). Since the hotspot was centered over the representation of the agonist muscle, MEPs could not be elicited in the control muscle in all participants: S3 and S7 did not have MEPs evoked in the control muscle. In the remaining subjects in whom MEPs were evoked in the control muscle, rmANOVA revealed that control muscle MEPs were similar across visual feedback conditions (F2,14=0.768, p=0.455), suggesting that the effects of feedback on M1 were muscle-specific, localized to the agonist muscle's representation in M1.
DISCUSSION
Recent evidence suggests that activating the somatosensory system, either through haptic assistance (8) or peripheral nerve stimulation (PNS) (30), in a time-locked manner with cortical stimulation and during action execution may facilitate M1 plasticity and enhance performance in stroke patients via a Hebbian-like mechanism. We demonstrate in the current experiment that visual feedback can be used as a similar vehicle to modulate activity in M1. Particularly, we show that online processing of visuomotor gain discordance can increase M1 excitabilty in stroke patients and that this effect was not confounded by background EMG activity or kinematic paramaters (i.e. muscle length or velocity) since these variables remained constant across conditions at the time of TMS stimulation.
It was interesting to note that neuromodulatory effects of visuomotor gain discordance were only noted for the scaled-down feedback condition. Analysis of movement kinematics also revealed that that subjects’ online performance was affected, relative to that of the veridical feedback condition, only by scaled-down discordance. No changes in performance were noted in the scaled-up condition, suggesting that this discordance did not elicit behavioral modifications. A parsimonious explanation therefore is that neuromodulatory effects in M1 may reflect processing of online adaptation to feedback. Since no online adaptation was observed in the scaled-up feedback condition, M1 excitability remained unchanged. This finding is consistent with work demonstrating that M1 processing of visuomotor adaptation to gain may be sensitive to certain featuers of the gain, for example the magnitude of the discordance (24). However, our data build on the literature showing that stroke subjects retain some ability to adapt to gain discordance when moving with their unaffected side, by demonstrating that this may not be the case for movements performed with the affected side (23). Although this issue needs to be further explored in future designs, it may be that just as the aging brain shows decreased ability to adapt and generalize across varying degrees of visuomotor discordance (32), so too might the ipsilesional motor system have a limited capacity to respond to multiple types of visuomotor discordances, when presented in close temporal proximity.
Another interesting result arising from this study is that visuomotor discordance effects on M1 seem to be relatively specific to the muscle involved in the task. Support for this comes from the absence of neuromodulatory effects in the adjacent (control) muscle, which was not involved in the task, and in the case of subject S3 (the only subject to perform extension movements), neuromodulatory effects were elicited in the extensor muscles. This data, paired with other studies using imagery paradigms to show that MEPs are only facilitated in the imagined muscle performing a movement (33), suggest that discordance-induced modulation of M1 is neither direction-specific nor global in nature. Rather, neuromodulatory effects of visuomotor discordance on M1 excitability are likely constrained to an intrinsic, muscle-based, topography.
A limitation of our trial-by-trial paradigm is that the current experiment does not delineate the specific role that M1 plays in learning visuomotor discordance in stroke patients, but rather only implicates M1 as a potential region involved in trial-by-trial feedback-related excitability changes. Recent studies in our laboratory, however, suggest that visuomotor gain leads to both trial-by-trial and learning-related changes in M1 excitabity in healthy individuals (31), although the changes were not as robust as the excitability increases seen in this patient study. Thus, the future direction of our laboratory will be to investigate M1 excitability effects of visuomotor gain adaptation in stroke patients. In these studies, we will include a larger patient population, allowing us to discern which patients and which lesion types (i.e. cortical vs. subcortical, left vs. right, level of impairment, etc.) are most responsive to visuomotor discordance-related changes in M1 excitability. Furthermore, by using the non-paretic hand as an additional control model, we will compare within-subject modulatory effects of visual feedback on M1 excitability in the lesioned vs non-lesioned hemispheres.
CONCLUSION
Our data demonstrate, on the one hand, that M1 may play an important role in processing online visuomotor feedback in patients with chronic stroke, and second that visuomotor discordance through virtual reality may be a viable vehicle for eliciting neuromodulatory effects on the motor system. This may have important implications for framing therapeutic interventions after stroke.
Acknowledgements
This work was supported by the National Institute of Neurological Disorders and Stroke (F30 NS071945) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K01 HD059983 and R01 HD058301).
Footnotes
Authorship Statement
Mr. Hamid Bagce and Drs. Eugene Tunik and Sergei Adamovich conceived, ran, analyzed/interpreted the data and wrote the manuscript. Ms. Soha Saleh assisted with running the experiment. All authors approved the final manuscript.
Conflict of Interest
The authors report no conflict of interest.
REFERENCES
- 1.Lang CE, Wagner JM, Bastian AJ, Hu Q, Edwards DF, Sahrmann SA, et al. Deficits in grasp versus reach during acute hemiparesis. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 2005;166(1):126–36. doi: 10.1007/s00221-005-2350-6. [DOI] [PubMed] [Google Scholar]
- 2.Lang CE, Wagner JM, Edwards DF, Sahrmann SA, Dromerick AW. Recovery of grasp versus reach in people with hemiparesis poststroke. Neurorehabilitation and neural repair. 2006;20(4):444–54. doi: 10.1177/1545968306289299. [DOI] [PubMed] [Google Scholar]
- 3.Nakayama H, Jorgensen HS, Raaschou HO, Olson TS. Recovery of upper extremity function in stroke patients: the Copenhagen stroke study. Arch Phys Med Rehabil. 1994;75:394–8. doi: 10.1016/0003-9993(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 4.Wade DT, Langton-Hewer R, Wood VA, Skilbeck CE, Ismail HM. The hemiplegic arm after stroke: measurement and recovery. J Neurol Neurosurg Psychiatry. 1983;46(6):521–4. doi: 10.1136/jnnp.46.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olsen TS. Arm and leg paresis as outcome predictors in stroke rehabilitation. Stroke; a journal of cerebral circulation. 1990;21(2):247–51. doi: 10.1161/01.str.21.2.247. [DOI] [PubMed] [Google Scholar]
- 6.Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011;377(9778):1693–702. doi: 10.1016/S0140-6736(11)60325-5. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi CD, Der-Yeghiaian L, Le V, Motiwala RR, Cramer SC. Robot-based hand motor therapy after stroke. Brain : a journal of neurology. 2008;131(Pt 2):425–37. doi: 10.1093/brain/awm311. [DOI] [PubMed] [Google Scholar]
- 8.Buetefisch C, Heger R, Schicks W, Seitz R, Netz J. Hebbian-type stimulation during robot-assisted training in patients with stroke. Neurorehabilitation and neural repair. 2011;25(7):645–55. doi: 10.1177/1545968311402507. [DOI] [PubMed] [Google Scholar]
- 9.Merians AS, Fluet GG, Qiu Q, Saleh S, Lafond I, Davidow A, et al. Robotically facilitated virtual rehabilitation of arm transport integrated with finger movement in persons with hemiparesis. Journal of neuroengineering and rehabilitation. 2011;8:27. doi: 10.1186/1743-0003-8-27. PMCID: 3113321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saposnik G, Levin M. Virtual reality in stroke rehabilitation: a meta-analysis and implications for clinicians. Stroke; a journal of cerebral circulation. 2011;42(5):1380–6. doi: 10.1161/STROKEAHA.110.605451. [DOI] [PubMed] [Google Scholar]
- 11.Saposnik G, Teasell R, Mamdani M, Hall J, McIlroy W, Cheung D, et al. Effectiveness of virtual reality using Wii gaming technology in stroke rehabilitation: a pilot randomized clinical trial and proof of principle. Stroke; a journal of cerebral circulation. 2010;41(7):1477–84. doi: 10.1161/STROKEAHA.110.584979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merians AS, Tunik E, Adamovich SV. Virtual reality to maximize function for hand and arm rehabilitation: exploration of neural mechanisms. Studies in health technology and informatics. 2009;145:109–25. [PMC free article] [PubMed] [Google Scholar]
- 13.Adamovich SV, Fluet GG, Mathai A, Qiu Q, Lewis J, Merians AS. Design of a complex virtual reality simulation to train finger motion for persons with hemiparesis: a proof of concept study. Journal of neuroengineering and rehabilitation. 2009;6:28. doi: 10.1186/1743-0003-6-28. PMCID: 2729310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adamovich SV, Fluet GG, Tunik E, Merians AS. Sensorimotor training in virtual reality: a review. NeuroRehabilitation. 2009;25(1):29–44. doi: 10.3233/NRE-2009-0497. PMCID: 2819065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arce F, Novick I, Mandelblat-Cerf Y, Vaadia E. Neuronal correlates of memory formation in motor cortex after adaptation to force field. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(27):9189–98. doi: 10.1523/JNEUROSCI.1603-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paz R, Boraud T, Natan C, Bergman H, Vaadia E. Preparatory activity in motor cortex reflects learning of local visuomotor skills. Nature neuroscience. 2003;6(8):882–90. doi: 10.1038/nn1097. [DOI] [PubMed] [Google Scholar]
- 17.Wise SP, Moody SL, Blomstrom KJ, Mitz AR. Changes in motor cortical activity during visuomotor adaptation. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 1998;121(3):285–99. doi: 10.1007/s002210050462. [DOI] [PubMed] [Google Scholar]
- 18.Mandelblat-Cerf Y, Paz R, Vaadia E. Trial-to-trial variability of single cells in motor cortices is dynamically modified during visuomotor adaptation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(48):15053–62. doi: 10.1523/JNEUROSCI.3011-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei Y, Bajaj P, Scheidt R, Patton J. A real-time haptic/graphic demonstration of how error augmentation can enhance learning.. IEEE international conference on robotics and automation (ICRA); Barcelona. 2005. [Google Scholar]
- 20.Matsuoka Y, Brewer BR, Klatzky RL. Using visual feedback distortion to alter coordinated pinching patterns for robotic rehabilitation. J Neuroeng Rehabil. 2007;4:17. doi: 10.1186/1743-0003-4-17. PMCID: 1904454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patton JL, Stoykov ME, Kovic M, Mussa-Ivaldi FA. Evaluation of robotic training forces that either enhance or reduce error in chronic hemiparetic stroke survivors. Exp Brain Res. 2006;168(3):368–83. doi: 10.1007/s00221-005-0097-8. [DOI] [PubMed] [Google Scholar]
- 22.Krakauer JW, Pine ZM, Ghilardi MF, Ghez C. Learning of visuomotor transformations for vectorial planning of reaching trajectories. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20(23):8916–24. doi: 10.1523/JNEUROSCI.20-23-08916.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palluel-Germain R, Jax SA, Buxbaum LJ. Visuo-motor gain adaptation and generalization following left hemisphere stroke. Neuroscience letters. 2011;498(3):222–6. doi: 10.1016/j.neulet.2011.05.015. PMCID: 3119783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coombes SA, Corcos DM, Sprute L, Vaillancourt DE. Selective regions of the visuomotor system are related to gain-induced changes in force error. Journal of neurophysiology. 2010;103(4):2114–23. doi: 10.1152/jn.00920.2009. PMCID: 2853269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aranyi Z, Mathis J, Hess CW, Rosler KM. Task-dependent facilitation of motor evoked potentials during dynamic and steady muscle contractions. Muscle Nerve. 1998;21(10):1309–16. doi: 10.1002/(sici)1097-4598(199810)21:10<1309::aid-mus10>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 26.Ni Z, Takahashi M, Yamashita T, Liang N, Tanaka Y, Tsuji T, et al. Functional demanded excitability changes of human hand motor area. Exp Brain Res. 2006;170(2):141–8. doi: 10.1007/s00221-005-0201-0. [DOI] [PubMed] [Google Scholar]
- 27.Kasai T, Yahagi S. Motor evoked potentials of the first dorsal interosseous muscle in step and ramp index finger abduction. Muscle & nerve. 1999;22(10):1419–25. doi: 10.1002/(sici)1097-4598(199910)22:10<1419::aid-mus12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 28.Datta AK, Harrison LM, Stephens JA. Task-dependent changes in the size of response to magnetic brain stimulation in human first dorsal interosseous muscle. The Journal of physiology. 1989;418:13–23. doi: 10.1113/jphysiol.1989.sp017826. PMCID: 1189957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flament D, Goldsmith P, Buckley CJ, Lemon RN. Task dependence of responses in first dorsal interosseous muscle to magnetic brain stimulation in man. J Physiol. 1993;464:361–78. doi: 10.1113/jphysiol.1993.sp019639. PMCID: 1175390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Celnik P, Paik NJ, Vandermeeren Y, Dimyan M, Cohen LG. Effects of combined peripheral nerve stimulation and brain polarization on performance of a motor sequence task after chronic stroke. Stroke. 2009;40(5):1764–71. doi: 10.1161/STROKEAHA.108.540500. PMCID: 2692264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bagce HF, Saleh S, Adamovich SV, Krakauer JW, Tunik E. Exaggeration of visual errors during goal-directed movements enhances primary motor cortex excitability in healthy subjects and stroke patients.. Society for Neuroscience Annual Meeting; San Diesgo, CA. Program No. 84.132010. p. Program No. 84.13. [Google Scholar]
- 32.Hegele M, Heuer H. Adaptation to a direction-dependent visuomotor gain in the young and elderly. Psychol Res. 2010;74(1):21–34. doi: 10.1007/s00426-008-0221-z. [DOI] [PubMed] [Google Scholar]
- 33.Facchini S, Muellbacher W, Battaglia F, Boroojerdi B, Hallett M. Focal enhancement of motor cortex excitability during motor imagery: a transcranial magnetic stimulation study. Acta Neurol Scand. 2002;105(3):146–51. doi: 10.1034/j.1600-0404.2002.1o004.x. [DOI] [PubMed] [Google Scholar]