Abstract
Background
Genetic and environmental factors shape life-long vulnerability to depression, but most gene–environment interaction (G×E) research has focused on cross-sectional assessments rather than life-course phenotypes. This study tests the hypothesis that the G×E involving the length polymorphism in the serotonin-transporter-gene-linked-promoter-region (5-HTTLPR) and childhood maltreatment is specific to depression that runs a persistent course in adulthood.
Methods
The hypothesis is tested in two cohorts. Men and women in the Dunedin Study (N=847), New Zealand, followed to age 32 years with 96% retention and women in the E-Risk Study (N=930), England, followed to age 40 years with 96% retention. Diagnoses of past-year major depressive episode were established at four separate assessments. Depression diagnosed on two or more occasions was considered persistent.
Results
In both cohorts, statistical tests of gene–environment interactions showed positive results for persistent depression but not single-episode depression. Individuals with two short 5-HTTLPR alleles and childhood maltreatment had elevated risk of persistent but not single-episode depression.
Limitations
Some cases of recurrent depression may have been misclassified as single-episode due to non-contiguous assessment windows, but this would have a conservative effect on the findings. Chronic and recurrent depression could not be reliably distinguished due to non-contiguous periods of assessment. Therefore, the term persistent depression is used to describe either chronic or recurrent course.
Conclusions
The specific effect on persistent depression increases the significance of this G×E for public health. Research that does not distinguish persistent course may underestimate G×E effects and account for some replication failures in G×E research.
Keywords: Depression, Persistent course, Gene–environment interactions, Serotonin transporter, Childhood maltreatment
1. Introduction
There is growing appreciation that chronic diseases in adulthood have their roots in early experiences during childhood (Ben-Shlomo and Kuh, 2002; Hertzman, 1999). This is also true for depression, one of the most disabling diseases globally (Danese et al., 2008; Murray and Lopez, 1997). Although adversity at every stage of life contributes to the risk of depression, the effects of childhood experiences are most profound and enduring (Brown et al., 2008; Cicchetti and Rogosch, 2009). Childhood maltreatment, in the form of abuse or neglect, is unfortunately a common experience (Hussey et al., 2006; May-Chahal and Cawson, 2005), and is associated with a 50% increase in the odds of depression in adult life (Widom et al., 2007; Wise et al., 2001). Although childhood maltreatment is a potent risk factor, there are substantial individual differences in outcome and many individuals who experienced maltreatment during childhood remain healthy (Caspi et al., 2010; Cicchetti and Rogosch, 2009).
It has been proposed that individual differences in vulnerability to maltreatment are partially genetically mediated by a common functional length polymorphism in the promoter sequence of the serotonin transporter gene (5-HTTLPR) (Caspi et al., 2003). This hypothesis was supported in a number of studies focusing on threatening events in which physical, sexual, or relational harm against children was carried out or intended (Aguilera et al., 2009; Aslund et al., 2009; Benjet et al., 2010; Cicchetti et al., 2007; Kaufman et al., 2004; Kumsta et al., 2010; Sugden et al., 2010), but inconsistent results have also been reported, especially in studies of childhood adversities other than maltreatment (Araya et al., 2009; Ritchie et al., 2009; Surtees et al., 2006; Wichers et al., 2008). Although the most recent and complete meta-analysis has confirmed that the 5-HTTLPR significantly moderates the depressogenic effects of childhood maltreatment (Karg et al., 2011), these inconsistent results require explanation.
One source of discrepant results may be cross-study inconsistency in the measurement of depression. While the liability to developing depression resulting from childhood maltreatment is life-long, the manifestations of depression are often episodic and their duration and course vary between individuals (Eaton et al., 2008; Judd, 1997; Rhebergen et al., 2009; Solomon et al., 1997). Chronic or recurrent depression is more heritable (Foley et al., 1998; McGuffin et al., 1996) and is more strongly associated with childhood maltreatment (Wiersma et al., 2009) than depression diagnosed at a single time point. Moreover, the influence of childhood maltreatment on depression persistence is direct and cannot be accounted for by more proximal circumstances (Brown et al., 2008). These observations led Brown and Harris (2008) to hypothesize that genetic sensitivity to childhood maltreatment should be specific to depression that is chronic or recurrent.
The present article reports the first test of this hypothesis. Because retrospective assessment of depression is unreliable (Moffitt et al., 2010; Schraedley et al., 2002), a valid test requires longitudinal datasets with repeated assessments. We use data from two population-based cohorts, where depression was assessed on four separate occasions in adulthood with the same diagnostic instrument. The first cohort provides analyses of new data from the Dunedin longitudinal study and the second cohort offers an opportunity to replicate findings in a new dataset. We examine genetic sensitivity to childhood maltreatment in relation to (a) persistent depression, (b) single-episode depression and (c) past-year depression diagnosed at each of the four separate assessment occasions. Comparisons of G×E results for single-episode versus persistent depression test the hypothesis proposed by Brown and Harris (2008). Analyses of past-year depression are comparable to G×E studies that have focused on cross-sectional measurements rather than longitudinal phenotypes. Comparisons of G×E results for depression diagnosed on any given occasion versus persistent depression allow us to gauge the possible contribution of cross-sectional measurement to inconsistencies in the literature.
2. Methods
2.1. Cohorts
Participants in the first sample were members of the Dunedin Multidisciplinary Health and Development Study. Of infants born in Dunedin, New Zealand, between April 1972 and March 1973, 1037 (91% of eligible births; 52% male) participated in the first assessment at age 3. Assessments were undertaken at ages 3, 5, 7, 9, 11, 13, 15, 18, 21, 26 and most recently at age 32 when we assessed 96% of the 1015 Study members still alive in 2004–2005. Genetic data were available for 97% participants. To minimize the risk of artefacts due to population stratification, only 847 Caucasians were included. Study members gave written informed consent before participating. The Otago Ethics Committee approved each phase of the study.
Participants in the second sample were mothers of twins in the Environmental Risk (E-Risk) Longitudinal Twin Study. The E-Risk sample was formed in 1999–2000, when 1116 mothers with 5-year-old twins (93% of eligible families) underwent home-visit assessments (Moffitt, 2002). The women were, on average, 33 years old (SD=5.8; range 20–48) at the initial assessment. Three additional assessments were undertaken when they were, on average, 35, 38, and 40 years old. Attrition was minimal and the last assessment in 2007–2008 included 96% of the women. Genetic data were available for 94% participants. To minimize population stratification, only 930 Caucasian women were included. Women gave written informed consent before participating. The Maudsley Hospital Ethics Committee approved each phase of the study.
2.2. DNA extraction and genotyping
In Dunedin, DNA was obtained from blood (93%) or buccal swabs (7%). In E-Risk, DNA was obtained from buccal swabs. Genotyping was carried out in the same laboratory, by a technician blind to data on maltreatment and depression. Genotyping of 5-HTTLPR was carried out following a standard protocol(Gelernter et al., 1997) with forward primer sequence 5′-ATGCCAGCACCTAACCCCTAATGT-3′ and reverse primer sequence 5′-GGACCGCAAGGTGGGCGGGA-3′. This amplifies a 419 base pair product for the 16 repeat (‘long’) allele and a 375 base pair product for the 14 repeat (‘short’) allele. PCR was carried out on a PTC-225 DNA engine (MJ Research). Products were separated on a 2.5% agarose gel (MultiABgarose, ABgene) supplemented with Ethidium bromide (0.03%, BDH) and visualised by ultraviolet transillumination. Genotypes were double called. Genotype frequencies for both samples were in Hardy–Weinberg equilibrium (Dunedin χ2(1)=1.913; p=0.1666; E-Risk χ2(1)=0.075; p=0.7835).
2.3. Childhood maltreatment
In Dunedin, we used the maltreatment measure described previously (Caspi et al., 2003). Evidence of childhood maltreatment between ages 3 and 11 years was ascertained from five sources. Three sources were prospective: (a) observations by researchers of rejecting mother–child interactions at age 3; (b) parental reports of harsh discipline at ages 7 and 9; (c) multiple changes of the child’s primary caregiver during the first decade of life. Two sources were retrospective: study members’ reports in adulthood of (d) physical and (e) sexual abuse. A cumulative exposure index was derived from the number of maltreatment experiences during the first decade of life; 64% of the cohort experienced no maltreatment, 27% experienced probable maltreatment (1 indicator), and 9% experienced definite maltreatment (2 or more indicators). There was no association between the 5-HTTLPR and childhood maltreatment in Dunedin (χ2(2)=1.67, p=0.80).
In E-Risk, women completed the Childhood Trauma Questionnaire (CTQ) (Bernstein and Fink, 1998) when they were on average 40 years old. The CTQ inquires about five domains of childhood maltreatment: emotional, physical, and sexual abuse, and emotional and physical neglect. The validity of the CTQ has been established and scoring has been derived for each of the five domains with 0 = no maltreatment, 1 = mild maltreatment, 2 = moderate maltreatment, and 3 = severe maltreatment (Bernstein and Fink, 1998). Since our hypothesis was not specific to a particular category of maltreatment, we used the average of the five domain scores as a continuous indicator of maltreatment, comparable to earlier G×E investigations involving the CTQ (Aguilera et al., 2009). There was no association between the 5-HTTLPR and childhood maltreatment in E-Risk (χ2=0.81, p=0.37).
2.4. Measuring adult depression
Past-year diagnosis of major depression (MDD) was assessed at four separate time-points in each study, providing four non-overlapping one-year-long assessment windows. In Dunedin, MDD was evaluated when Study members were ages 18, 21, 26, and 32 years. In E-Risk, MDD was evaluated when study members were, on average, ages 33, 35, 38, and 40 years. In both studies, MDD diagnosis was established with the Diagnostic Interview Schedule (DIS; Robins et al., 1989; Robins et al., 1995) to obtain DSM-IV diagnosis of MDD (American Psychiatric Association, 1994). Functional impairment was required for diagnosis. We created six outcome variables. Persistent depression, the primary outcome variable, was operationally defined as the presence of past-year MDD at two or more of the four assessments. Single-episode depression (diagnosed on only one of the four occasions) was used for comparison to establish the specificity of the persistence hypothesis. Time 1 depression was a diagnosis of past-year MDD at the first assessment (age 18 in Dunedin and mean age 33 in E-Risk). Time 2 depression was past-year MDD at the second assessment (age 21 in Dunedin and mean age 35 in E-Risk). Time 3 depression was past-year MDD at the third assessment (age 26 in Dunedin and mean age 38 in E-Risk). Time 4 depression was past-year MDD at the fourth assessment (age 32 in Dunedin and mean age 40 in E-Risk).
2.5. Statistical analyses
It has been proposed that gene–environment interactions (G×E) should be conceptualized as departures from additivity of risks between genetic and environmental factors, as such departures most likely correspond to biological causal mechanisms involving both genetic and environmental factors (Rothman et al., 2008; Schwartz, 2006). To follow this recommendation, we tested G×E in a generalized linear model from the binomial family with identity link estimating risk differences for binary outcomes (Wacholder, 1986). Effects of first-order predictors and interaction terms were quantified as risk differences (RD) with 95% confidence intervals (95%CI). RD reflects the absolute increase in probability of depression for each unit of the predictor. For the test of 5-HTTLPR, RD is the absolute increase in the probability of depression with each short allele (e.g. an RD of 0.05 would mean that each short allele increases the risk of depression by 5%). For the test of childhood maltreatment, the RD is the absolute increase in the probability of depression with each grade of maltreatment (e.g. an RD of 0.1 in the Dunedin study would mean that the probability of depression increases by 10% with probable as opposed to no maltreatment and with definite as opposed to probable maltreatment). For the test of the 5-HTTLPR-maltreatment interaction, RD is the increase in the probability of depression per each short allele and unit level of childhood maltreatment over and above the summation of risks of genotype and maltreatment. Tests of the same hypothesis in the two samples were combined with the Fisher’s trend method, taking into account the direction of effect (Fisher, 1932).
An important decision in the analysis of G×E is the selection of a genetic model. Each individual carries two copies of the serotonin transporter gene, and each of these two copies may contain either the more common long variant or the less common short variant, resulting in three possible genotypes: long–long (LL), long–short (LS), and short–short (SS). Although there is agreement that the short variant confers sensitivity to the depressogenic effects of adversity, studies differ in whether one or two short alleles are needed (i.e. whether the “sensitivity” effect is dominant or recessive) or whether the sensitivity increases gradually with the number of short alleles (i.e., an additive genetic model). A synthesis of current evidence does not support any of the genetic models above the others (Caspi et al., 2010; Uher and McGuffin, 2008) and it has been suggested that all three genetic models should be tested and reported in studies of G×E in order to build a transparent, cumulative database (Uher and McGuffin, 2008). Therefore, we test and report the results of additive, recessive and dominant genetic models.
3. Results
3.1. Evaluating the interaction between the 5-HTTLPR short allele and childhood maltreatment in predicting persistent adult depression
Persistent depression was operationally defined as meeting diagnostic criteria for major depression on two or more of the four assessment occasions in adulthood. In the Dunedin cohort, 138 individuals (16.3%) fulfilled criteria for persistent depression. In the E-Risk cohort, 117 (12.6%) fulfilled criteria for persistent depression. Fig. 1 shows the association between childhood maltreatment and persistent depression by 5-HTTLPR genotype in Dunedin (Panel A) and E-risk (Panel B).
Fig. 1.
Proportion of individuals with persistent depression (panels A, B) and single-episode depression (panels C, D) by childhood maltreatment and serotonin transporter genotype in the Dunedin (panels A, C) and E-Risk (panels B, D) cohorts. The y axis shows the probability of being diagnosed with past year major depression at two or more of the four assessments by level of childhood maltreatment and 5-HTTLPR genotype (LL, SL, and SS). In the Dunedin cohort, maltreatment is a composite trichotomous measure based on observer ratings, parent reports and self-reports (0 = None, 1 = Probable, 2 = Definite). In the E-risk study, maltreatment is a rounded average score on the Childhood Trauma Questionnaire (0 = None, 1 = Mild, 2 = Moderate, 3 = Severe). The number of individuals included in each subgroup is given above each bar.
We began by evaluating genetic (5-HTTLPR) and environmental (childhood maltreatment) main effects. There was no consistent association between 5-HTTLPR and persistent depression in either cohort (Table 1). In contrast, childhood maltreatment was strongly associated with persistent depression in both cohorts (Table 2). Individuals who were maltreated in childhood were significantly more likely to experience persistent depression in adulthood, in Dunedin (RD of 8.2% per level of maltreatment, p=0.0002) and in E-Risk (RD of 15.7% per level of maltreatment, p<0.0001).
Table 1.
Effect of 5-HTTLPR on depression in the Dunedin and E-risk cohorts. The outcomes are persistent depression, single-episode depression, and depression in each of the four assessment years.
| Dunedin
|
E-risk
|
|||||||
|---|---|---|---|---|---|---|---|---|
| RD | 95%CI
|
p | RD | 95%CI
|
p | |||
| Lower | Upper | Lower | Upper | |||||
| Additive genetic model | ||||||||
| Persistent depression | 0.0297 | −0.0168 | 0.0761 | 0.2107 | 0.0092 | −0.0276 | 0.0460 | 0.6237 |
| Single-episode depression | 0.0270 | −0.0228 | 0.0768 | 0.2878 | −0.0186 | −0.0589 | 0.0218 | 0.3675 |
| Time 1 depression | 0.0123 | −0.0263 | 0.0510 | 0.5319 | 0.0085 | −0.0219 | 0.0388 | 0.5846 |
| Time 2 depression | 0.0128 | −0.0235 | 0.0491 | 0.4886 | −0.0076 | −0.0381 | 0.0229 | 0.6253 |
| Time 3 depression | 0.0189 | −0.0172 | 0.0549 | 0.3056 | 0.0140 | −0.0153 | 0.0433 | 0.3496 |
| Time 4 depression | 0.0142 | −0.0228 | 0.0511 | 0.4530 | 0.0133 | −0.0183 | 0.0449 | 0.4086 |
| Recessive short allele model | ||||||||
| Persistent depression | 0.0762 | −0.0140 | 0.1665 | 0.0978 | 0.0648 | −0.0089 | 0.1385 | 0.0849 |
| Single-episode depression | 0.0186 | −0.0732 | 0.1104 | 0.6911 | −0.0177 | −0.0918 | 0.0563 | 0.6388 |
| Time 1 depression | 0.0532 | −0.0216 | 0.1279 | 0.1631 | 0.0157 | −0.0421 | 0.0736 | 0.5945 |
| Time 2 depression | 0.0148 | −0.0521 | 0.0817 | 0.6653 | 0.0102 | −0.0479 | 0.0684 | 0.7301 |
| Time 3 depression | 0.0333 | −0.0351 | 0.1016 | 0.3399 | 0.0236 | −0.0339 | 0.0810 | 0.4211 |
| Time 4 depression | 0.0642 | −0.0085 | 0.1369 | 0.0836 | 0.1066 | 0.0383 | 0.1748 | 0.0022 |
| Dominant short allele model | ||||||||
| Persistent depression | 0.0183 | −0.0492 | 0.0859 | 0.5947 | −0.0190 | −0.0740 | 0.0361 | 0.4998 |
| Single-episode depression | 0.0441 | −0.0269 | 0.1150 | 0.2237 | −0.0290 | −0.0889 | 0.0309 | 0.3423 |
| Time 1 depression | −0.0060 | −0.0629 | 0.0508 | 0.8357 | 0.0086 | −0.0354 | 0.0527 | 0.7009 |
| Time 2 depression | 0.0177 | −0.0346 | 0.0700 | 0.5071 | −0.0242 | −0.0703 | 0.0218 | 0.3030 |
| Time 3 depression | 0.0196 | −0.0324 | 0.0715 | 0.4601 | 0.0158 | −0.0259 | 0.0575 | 0.4587 |
| Time 4 depression | −0.0081 | −0.0628 | 0.0465 | 0.7712 | −0.0303 | −0.0778 | 0.0172 | 0.2115 |
Abbreviations: RD risk difference, 95%CI confidence interval of the RDD estimate, p probability of result being due to chance.
Table 2.
Effect of childhood trauma on the risk of depression in the Dunedin and E-Risk cohorts. The outcomes are persistent depression, single-episode depression and depression diagnosed at each of the four assessments.
| Dunedin
|
E-Risk
|
|||||||
|---|---|---|---|---|---|---|---|---|
| RD | 95%CI
|
p | RD | 95%CI
|
p | |||
| Lower | Upper | Lower | Upper | |||||
| Persistent depression | 0.0817 | 0.0393 | 0.1242 | 0.0002 | 0.1568 | 0.1134 | 0.2001 | 0.0000 |
| Single-episode depression | 0.0291 | −0.0264 | 0.0846 | 0.3044 | 0.1026 | 0.0429 | 0.1624 | 0.0008 |
| Time 1 depression | 0.0710 | 0.0268 | 0.1152 | 0.0016 | 0.1022 | 0.0603 | 0.1441 | 0.0000 |
| Time 2 depression | 0.0465 | 0.0051 | 0.0878 | 0.0275 | 0.1435 | 0.1001 | 0.1868 | 0.0000 |
| Time 3 depression | 0.0549 | 0.0139 | 0.0960 | 0.0087 | 0.0997 | 0.0588 | 0.1407 | 0.0000 |
| Time 4 depression | 0.0658 | 0.0231 | 0.1084 | 0.0025 | 0.1415 | 0.0979 | 0.1852 | 0.0000 |
Abbreviations: RD risk difference, 95%CI confidence interval of RD estimate, p probability of result being due to chance.
Next, we evaluated G×E. As illustrated in Fig. 1, carriers of two 5-HTTLPR short alleles who were exposed to definite/severe maltreatment in childhood had the highest risk of persistent depression, in Dunedin (Panel A) and in E-risk (Panel B). Table 3 shows the results of statistical tests evaluating this G×E. Under the additive genetic model, the G×E was significant in Dunedin (P=.0119) and approached significance in E-Risk (P=.0516). Under the recessive genetic model, the G×E was significant in both Dunedin (P=.0330) and E-Risk (P=.0004). Under the dominant genetic model, the interaction was significant in Dunedin (P=.0403), but not in E-Risk (P=0.6115). When the Dunedin and E-risk results were combined with the Fisher’s trend method, the G×E was significant under additive (P=.003) and recessive (P=.0001) models.
Table 3.
Gene–environment interaction between childhood maltreatment and the 5-HTTLPR genotype in the Dunedin and E-Risk cohorts. The outcomes are persistent depression, single-episode depression and depression at each of the four assessments. G×E is tested as a departure from additivity of risks, with genotype coded under additive, recessive and dominant models, respectively. Combined p values are obtained with the Fisher’s trend method applied to results from the two samples.
| Dunedin
|
E-Risk
|
Combined
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| G×E RD | 95%CI
|
p | G×E RD | 95%CI
|
p | Fisher
|
|||
| Lower | Upper | Lower | Upper | p | |||||
| Additive genetic model | |||||||||
| Persistent depression | 0.0915 | 0.0202 | 0.1628 | 0.0119 | 0.0540 | −0.0004 | 0.1084 | 0.0516 | 0.00300 |
| Single-episode depression | 0.0051 | −0.0773 | 0.0874 | 0.9034 | 0.0672 | −0.0149 | 0.1492 | 0.1087 | 0.23120 |
| Time 1 depression | 0.0789 | 0.0169 | 0.1410 | 0.0126 | 0.0416 | −0.0119 | 0.0951 | 0.1273 | |
| Time 2 depression | 0.0223 | −0.0364 | 0.0811 | 0.4564 | 0.0062 | −0.0496 | 0.0620 | 0.8286 | |
| Time 3 depression | 0.0805 | 0.0247 | 0.1363 | 0.0047 | 0.0030 | −0.0500 | 0.0561 | 0.9109 | |
| Time 4 depression | 0.0177 | −0.0429 | 0.0783 | 0.5662 | 0.0384 | −0.0169 | 0.0937 | 0.1735 | |
| Recessive short allele model | |||||||||
| Persistent depression | 0.1440 | 0.0117 | 0.2763 | 0.0330 | 0.1285 | 0.0673 | 0.1897 | 0.00004 | 0.00001 |
| Single-episode depression | 0.0150 | −0.1418 | 0.1719 | 0.8511 | 0.0665 | −0.0892 | 0.2221 | 0.4028 | 0.59250 |
| Time 1 depression | 0.1274 | 0.0046 | 0.2502 | 0.0420 | 0.0974 | 0.0007 | 0.1942 | 0.0485 | |
| Time 2 depression | 0.0302 | −0.0781 | 0.1384 | 0.5847 | 0.0113 | −0.0877 | 0.1103 | 0.8230 | |
| Time 3 depression | 0.0829 | −0.0274 | 0.1932 | 0.1408 | 0.0189 | −0.0771 | 0.1149 | 0.6994 | |
| Time 4 depression | 0.0910 | −0.0245 | 0.2065 | 0.1226 | 0.1444 | 0.0578 | 0.2309 | 0.0011 | |
| Dominant short allele model | |||||||||
| Persistent depression | 0.1134 | 0.0050 | 0.2218 | 0.0403 | 0.0262 | −0.0749 | 0.1273 | 0.6115 | 0.07500 |
| Single-episode depression | 0.0015 | −0.1143 | 0.1173 | 0.9801 | 0.1020 | −0.0192 | 0.2233 | 0.0990 | 0.22890 |
| Time 1 depression | 0.0999 | 0.0092 | 0.1906 | 0.0309 | 0.0314 | −0.0545 | 0.1173 | 0.4741 | |
| Time 2 depression | 0.0296 | −0.0565 | 0.1157 | 0.5000 | 0.0061 | −0.0852 | 0.0973 | 0.8960 | |
| Time 3 depression | 0.1203 | 0.0410 | 0.1996 | 0.0030 | −0.0037 | −0.0885 | 0.0811 | 0.9317 | |
| Time 4 depression | −0.0180 | −0.1109 | 0.0750 | 0.7048 | −0.0059 | −0.0978 | 0.0859 | 0.8992 | |
Abbreviations: RD risk difference, 95%CI confidence interval of RD estimate, p probability of result being due to chance.
3.2. Comparison with single-episode depression
In order to test the hypothesis that the G×E is specific to persistent depression, we compared results for persistent depression with results for single-episode depression (i.e., depression diagnosed on only one of the four measurement occasions in each cohort) (Fig. 1). In Dunedin, 208 individuals (24.6%) fulfilled criteria for single-episode depression. In E-Risk, 171 (18.4% of the sample) had single-episode depression. Single-episode depression was unrelated to 5-HTTLPR genotype (Table 1). The relationship between single-episode depression and childhood maltreatment was weaker and inconsistent across cohorts (Table 2). None of the G×E tests with single-episode depression as the outcome showed significant results (smallest P=0.099), and there was no significant interaction effect even when the samples were combined with the Fisher’s trend method (Table 3). Examination of estimates and confidence intervals reveals that the differences between results for single-episode and persistent depression are substantial, with estimates for single episode depression falling outside the 95% confidence intervals of estimates for persistent depression in three out of the six comparisons (Table 3).
3.3. Evaluating the interaction between the 5-HTTLPR short allele and childhood maltreatment in predicting cross-sectional snapshots of adult depression
In Dunedin, between 15.6% and 17.1% of individuals were diagnosed with past-year major depression at any given cross-section. In E-risk, between 11.0% and 13.6% of individuals were diagnosed with past-year major depression at any given cross-section. Fig. 2 shows the association between childhood maltreatment and adult depression at each of the 4 assessment periods in adulthood by 5-HTTLPR genotype in Dunedin (Panel A) and E-risk (Panel B).
Fig. 2.
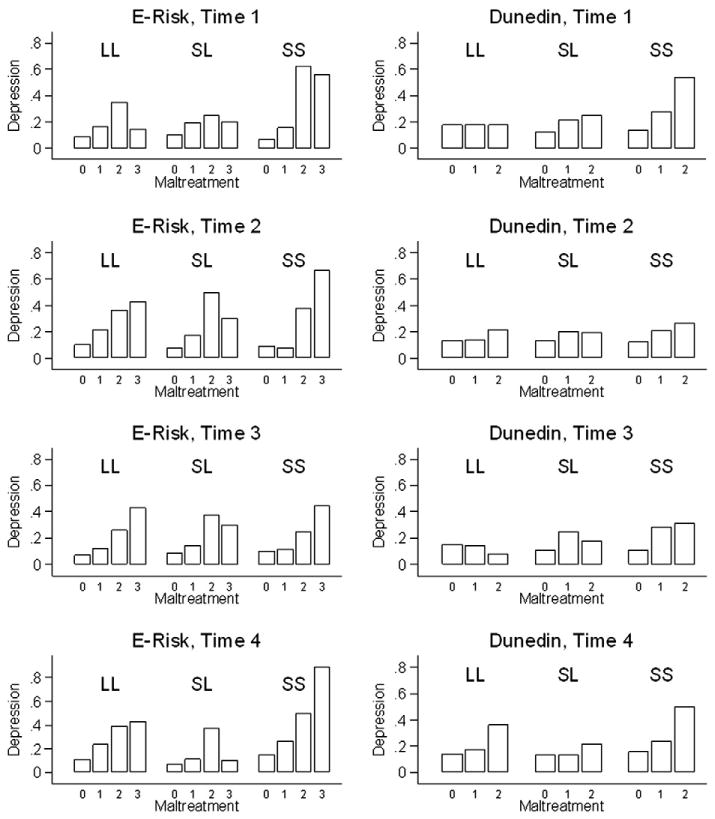
Proportion of individuals with past-year diagnosis of major depression at each assessment in the Dunedin and E-Risk cohorts by level of childhood maltreatment and serotonin transporter genotype. Dunedin: Time 1 = age 18, Time 2 = age 21; Time 3 = age 26, Time 4 = age 32; E-Risk: Time 1 = mean age 33, Time 2 = mean age 35; Time 3 = mean age 38, Time 4 = mean age 40; 5-HTTLPR genotype is marked by “LL”, “LS” and “SS” above each group of bars. In the Dunedin cohort, maltreatment is a composite trichotomous measure based on observer ratings, parent reports and self-reports (0 = None, 1 = Probable, 2 = Definite). In the E-risk study, maltreatment is a rounded average score on the Childhood Trauma Questionnaire (0 = None, 1 = Mild, 2 = Moderate, 3 = Severe). For the number of individuals included in each subgroup, please see Fig. 1.
We first evaluated genetic (5-HTTLPR) and environmental (childhood maltreatment) main effects. There was no consistent association between 5-HTTLPR and cross-sectional depression in either cohort (Table 1). In contrast, childhood maltreatment was significantly associated with adult depression at each of the four occasions in both cohorts (Table 2). Regardless of the measurement occasion in adulthood, individuals who had been maltreated in childhood were significantly more likely to experience depression during a given 12-month period.
Next, we evaluated G×E. In general, carriers of two 5-HTTLPR short alleles who were exposed to definite/severe maltreatment had the highest risk of depression (Fig. 2). However, statistical tests of G×E did not follow a predictable pattern across the four measurement occasions (Table 3). In both Dunedin and E-Risk, there was evidence of a significant G×E on two out of the four measurement occasions in adulthood.
4. Discussion
The present study extends previous knowledge about the interplay between the serotonin transporter gene and childhood maltreatment in the causation of adult depression, in two ways. First, it finds support for the hypothesis that the interaction between the short allele of 5-HTTLPR and childhood maltreatment is specific to depression that runs a persistent course (Brown and Harris, 2008). In two longitudinal cohorts, the interaction between 5-HTTLPR and childhood maltreatment significantly affected the risk of persistent depression but had no effect on time-limited single-episode depression. Second, it suggests that the longitudinal characterization of the clinical course of a disorder may improve the reliability of testing G×E. While analyses of depression phenotypes based on repeated assessments provided consistent evidence of G×E, analyses based on single cross-sectional assessments yielded inconsistent results. These two findings have broader implications for etiological research.
A first implication is that research on environmental risks can be constructively integrated with genetics to illuminate the etiology of depression. Previous studies have reported that childhood maltreatment is strongly and directly related to persistent forms of adult depression (Brown et al., 2008; Wiersma et al., 2009). The present study extends this observation to suggest that 5-HTTLPR confers sensitivity to childhood maltreatment in relation to developing persistent depression. This finding may help to shed light on the problem of “missing heritability” in psychiatric illness (Manolio et al., 2009; Uher, 2009). Twin studies have reported that depression diagnosed on at least two occasions is strongly heritable (Foley et al., 1998; McGuffin et al., 1996), but no genetic polymorphism has been unequivocally linked to this phenotype (Lewis et al., 2010; Muglia et al., 2010; Shi et al., 2010; Sullivan et al., 2009). Since the heritability estimate in twin design includes variation due to interactions between genes and shared environment (Uher, 2008, 2009), and since experiences of maltreatment are often shared by siblings growing up in the same family (Brown et al., 2007; Jaffee et al., 2007), the possibility is raised that G×E of the type reported here could contribute to the observed heritability of persistent or recurrent depression. The present findings also underscore the public-health relevance of G×E, as persistent forms of depression are responsible for most disability, health-care costs and risk of suicide (Greenberg et al., 2003; Young et al., 2008).
A second implication of our results is that the use of longitudinal phenotypes may be essential for advancing etiological research. Concerns have been expressed about the replicability of G×E studies (Risch et al., 2009), but apparent inconsistencies may reflect methodological heterogeneity such as differences in the assessment of environmental exposures (Karg et al., 2011; Uher and McGuffin, 2008, 2010). The present findings suggest that differences between cross-sectional and longitudinal studies also contribute to the heterogeneity of results. We found that detection of a statistically significant G×E from a single assessment may be unreliable (significant G×E was detected two out of four times in each cohort), but becomes more reliable when data from multiple assessments are considered. Focus on first-episode depression is another approach that capitalizes on homogenous course characteristics within a sample and has also brought very promising results (Bukh et al., 2009). These findings suggest that tests of hypotheses about the developmental origins of adult disease (Ben-Shlomo and Kuh, 2002) and about the biological embedding of childhood adversity (Hertzman, 1999) may benefit from adopting a life-course approach to measuring phenotypes. A central challenge for future research is whether reliable assessment of clinical course can be achieved in non-longitudinal studies.
The present study offered several strengths for evaluating G×E. First, we studied two large, representative cohorts with highly complete follow-ups, thereby obviating problems associated with selective attrition which affect the frequency of exposure to severe stress and thereby the evaluation of G×E (Shanahan and Bauldry, 2011). Second, we used the same diagnostic instruments in both cohorts and on multiple occasions, thereby avoiding problems associated with comparing findings across samples that differ in phenotypic ascertainment. Third, we conducted parallel analyses across both cohorts allowing us to evaluate what replicates and what does not. We also note several limitations to our study. First, our study was limited to individuals of European ancestry. Although studies of Asians (Goldman et al., 2010) and African-Americans (Xie et al., 2009) have documented that carriers of 5-HTTLPR short alleles are more likely to develop a variety of stress-related psychiatric conditions, the present findings about the importance of longitudinal phenotypes will need to be evaluated in other ethnic groups. Second, the assessment of depression relied on four noncontiguous one-year assessment windows. Thus some recurrent cases might have been misclassified as single-episode depression, because additional depressive episodes occurred outside the assessment windows. This misclassification may have decreased the effect size of the difference between persistent and single-episode groups. However, cases classified as persistent are unlikely to be misclassified and therefore, this limitation does not invalidate our results on persistent depression. The non-contiguous assessment windows also mean that despite constructing longitudinal phenotypes of depression, we were not able to distinguish between chronic and recurrent depression. Therefore the term ‘persistent depression’ is used in accordance with current terminology (Young et al., 2008). Third, childhood maltreatment was assessed by different instruments in each cohort. Objective indicators and interview measures of adversity have generally produced more consistent results and are less affected by reporting style and recall bias than self-report questionnaire (Karg et al., 2011; Monroe, 2008; Uher and McGuffin, 2010). This may explain why the results in the Dunedin cohort, which used a combination of observer ratings and interview, were statistically somewhat stronger and more consistent across the genetic models than results from the E-Risk study, where childhood maltreatment was assessed with a self-report questionnaire. It is also possible that self-reported maltreatment has larger contribution from emotional memory, which may be moderated by different genotypes (Polanczyk et al., 2009). In summary, the fact that similar results were obtained in two cohorts that used different measures of childhood maltreatment makes the findings more robust and generalizable to various types of childhood maltreatment, but it also means that the results from the two studies cannot be compared in absolute terms.
A related issue concerns the choice of the genetic model in G×E research. The bi-allelic nature of a genetic locus necessitates a choice between three genetic models (additive, recessive, and dominant). Exact replication would require that the same genetic model is detected across different studies. This requirement may be sensible if studies use the same measures (i.e., the phenotype and environmental exposures are ascertained in the same way). In the present study, we measured depression in the same way across the two cohorts but had to rely on different measures of maltreatment in these cohorts. Exposure measurement heterogeneity has implications for matching the genetic model across studies, because the “correct” genetic model could vary depending on the environmental exposure (Uher and McGuffin, 2008). Although the present results favor the hypothesis that the 5-HTTLPR short allele confers risk against a background of childhood maltreatment, we cannot establish whether the genetic effect is additive, recessive, or dominant. Across the two cohorts, we found support for recessive effects, additive effects, and to a lesser extent dominant effects. The literature about the interaction between 5-HTTLPR and adversity is limited by the fact that most studies to date have only reported a test of one genetic model and have not systematically compared different genetic models. As such, extant research does not, at present, support any genetic model above others (Caspi et al., 2010). We hope that researchers will be encouraged by the present report to provide full information about different genetic models, allowing transparent interrogation of accumulating findings.
We have found support for the hypothesis that the interaction between the serotonin transporter gene and childhood maltreatment is specific to persistent forms of depression. Our results recommend attention to matching theory, methods, and measurements in the study of G×E, in the service of advancing knowledge about the origins of disease. We hope that the research reported here will stimulate further studies on this topic, including other genetic markers and novel designs. In particular, investigations focusing on G×E in the first onset of depression and assessing its predictive potential for future clinical course and response to treatment are needed to advance the current knowledge on the determinants of the individual differences in clinical course and outcomes of depression.
Acknowledgments
Role of funding source
This work was supported by grants from the U.K. Medical Research Council (G0100527, G0601483), National Institute on Aging (AG032282), NIMH (MH077874), NICHD (HD061298), the Lady Davis Fellowship from the Hebrew University, and the Jacobs Foundation. Avshalom Caspi is a Royal Society-Wolfson Merit Award holder. The funding bodies had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
The authors thank the participants, families and staff of the Dunedin and E-Risk studies.
Footnotes
Conflict of interest
The authors report that they have no financial or other relevant conflicts of interest.
References
- Aguilera M, Arias B, Wichers M, Barrantes-Vidal N, Moya J, Villa H, van OJ, Ibanez MI, Ruiperez MA, Ortet G, Fananas L. Early adversity and 5-HTT/BDNF genes: new evidence of gene–environment interactions on depressive symptoms in a general population. Psychol Med. 2009;39:1425–1432. doi: 10.1017/S0033291709005248. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Araya R, Hu X, Heron J, Enoch MA, Evans J, Lewis G, Nutt D, Goldman D. Effects of stressful life events, maternal depression and 5-HTTLPR genotype on emotional symptoms in pre-adolescent children. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:670–682. doi: 10.1002/ajmg.b.30888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslund C, Leppert J, Comasco E, Nordquist N, Oreland L, Nilsson KW. Impact of the interaction between the 5HTTLPR polymorphism and maltreatment on adolescent depression. A population-based study. Behav Genet. 2009;39:524–531. doi: 10.1007/s10519-009-9285-9. [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002;31:285–293. [PubMed] [Google Scholar]
- Benjet C, Thompson RJ, Gotlib IH. 5-HTTLPR moderates the effect of relational peer victimization on depressive symptoms in adolescent girls. J Child Psychol Psychiatry. 2010;51:173–179. doi: 10.1111/j.1469-7610.2009.02149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein D, Fink L. Childhood Trauma Questionnaire Manual. The Psychological Corp; San Antonio, TX: 1998. [Google Scholar]
- Brown GW, Craig TK, Harris TO. Parental maltreatment and proximal risk factors using the Childhood Experience of Care & Abuse (CECA) instrument: a life-course study of adult chronic depression — 5. J Affect Disord. 2008;110:222–233. doi: 10.1016/j.jad.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Brown GW, Craig TK, Harris TO, Handley RV, Harvey AL, Serido J. Child-specific and family-wide risk factors using the retrospective Childhood Experience of Care & Abuse (CECA) instrument: a life-course study of adult chronic depression — 3. J Affect Disord. 2007;103:225–236. doi: 10.1016/j.jad.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris TO. Depression and the serotonin transporter 5-HTTLPR polymorphism: a review and a hypothesis concerning gene–environment interaction. J Affect Disord. 2008;111:1–12. doi: 10.1016/j.jad.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Bukh JD, Bock C, Vinberg M, Werge T, Gether U, Vedel KL. Interaction between genetic polymorphisms and stressful life events in first episode depression. J Affect Disord. 2009;119:107–115. doi: 10.1016/j.jad.2009.02.023. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Adaptive coping under conditions of extreme stress: multilevel influences on the determinants of resilience in maltreated children. New Dir Child Adolesc Dev. 2009;2009:47–59. doi: 10.1002/cd.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Sturge-Apple ML. Interactions of child maltreatment and serotonin transporter and monoamine oxidase A polymorphisms: depressive symptomatology among adolescents from low socioeconomic status backgrounds. Dev Psychopathol. 2007;19:1161–1180. doi: 10.1017/S0954579407000600. [DOI] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65:409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton WW, Shao H, Nestadt G, Lee HB, Bienvenu OJ, Zandi P. Population-based study of first onset and chronicity in major depressive disorder. Arch Gen Psychiatry. 2008;65:513–520. doi: 10.1001/archpsyc.65.5.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. Statistical Methods for Research Workers. Oliver & Boyd; London: 1932. [Google Scholar]
- Foley DL, Neale MC, Kendler KS. Reliability of a lifetime history of major depression: implications for heritability and co-morbidity. Psychol Med. 1998;28:857–870. doi: 10.1017/s0033291798006977. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kanzler H, Cubells JF. Serotonin transporter protein (SCL6A4) allele and haplotype frequencies and lincage disequilibrium in African and European-American and Japanese populations and in alcohol dependent subjects. Hum Genet. 1997;101:243–246. doi: 10.1007/s004390050624. [DOI] [PubMed] [Google Scholar]
- Goldman N, Glei DA, Lin YH, Weinstein M. The serotonin transporter polymorphism (5-HTTLPR): allelic variation and links with depressive symptoms. Depress Anxiety. 2010;27:260–269. doi: 10.1002/da.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, Corey-Lisle PK. The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry. 2003;64:1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- Hertzman C. The biological embedding of early experience and its effects on health in adulthood. Ann NY Acad Sci. 1999;896:85–95. doi: 10.1111/j.1749-6632.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- Hussey JM, Chang JJ, Kotch JB. Child maltreatment in the United States: prevalence, risk factors, and adolescent health consequences. Pediatrics. 2006;118:933–942. doi: 10.1542/peds.2005-2452. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Caspi A, Moffitt TE, Polo-Tomas M, Taylor A. Individual, family, and neighborhood factors distinguish resilient from non-resilient maltreated children: a cumulative stressors model. Child Abuse Negl. 2007;31:231–253. doi: 10.1016/j.chiabu.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd LL. The clinical course of unipolar major depressive disorders. Arch Gen Psychiatry. 1997;54:989–991. doi: 10.1001/archpsyc.1997.01830230015002. [DOI] [PubMed] [Google Scholar]
- Karg K, Shedden K, Burmeister M, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011 doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, Gelernter J. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci U S A. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumsta R, Stevens S, Brookes K, Schlotz W, Castle J, Beckett C, Kreppner J, Rutter M, Sonuga-Barke E. 5HTT genotype moderates the influence of early institutional deprivation on emotional problems in adolescence: evidence from the English and Romanian Adoptee (ERA) study. J Child Psychol Psychiatry. 2010;51:755–762. doi: 10.1111/j.1469-7610.2010.02249.x. [DOI] [PubMed] [Google Scholar]
- Lewis CM, Ng MY, Butler AW, Cohen-Woods S, Uher R, Pirlo K, Weale ME, Schosser A, Paredes UM, Rivera M, Craddock N, Owen MJ, Jones L, Jones I, Korszun A, Aitchison KJ, Shi J, Quinn JP, Mackenzie A, Vollenweider P, Waeber G, Heath S, Lathrop M, Muglia P, Barnes MR, Whittaker JC, Tozzi F, Holsboer F, Preisig M, Farmer AE, Breen G, Craig IW, McGuffin P. Genome-wide association study of major recurrent depression in the U.K. population. Am J Psychiatry. 2010;167:949–957. doi: 10.1176/appi.ajp.2010.09091380. [DOI] [PubMed] [Google Scholar]
- Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, Cho JH, Guttmacher AE, Kong A, Kruglyak L, Mardis E, Rotimi CN, Slatkin M, Valle D, Whittemore AS, Boehnke M, Clark AG, Eichler EE, Gibson G, Haines JL, Mackay TF, McCarroll SA, Visscher PM. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May-Chahal C, Cawson P. Measuring child maltreatment in the United Kingdom: a study of the prevalence of child abuse and neglect. Child Abuse Negl. 2005;29:969–984. doi: 10.1016/j.chiabu.2004.05.009. [DOI] [PubMed] [Google Scholar]
- McGuffin P, Katz R, Watkins S, Rutherford J. A hospital-based twin register of the heritability of DSM-IV unipolar depression. Arch Gen Psychiatry. 1996;53:129–136. doi: 10.1001/archpsyc.1996.01830020047006. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. Teen-aged mothers in contemporary Britain. J Child Psychol Psychiatry. 2002;43:727–742. doi: 10.1111/1469-7610.00082. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Taylor A, Kokaua J, Milne BJ, Polanczyk G, Poulton R. How common are common mental disorders? Evidence that lifetime prevalence rates are doubled by prospective versus retrospective ascertainment. Psychol Med. 2010;40:899–909. doi: 10.1017/S0033291709991036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe SM. Modern approaches to conceptualizing and measuring human life stress. Annu Rev Clin Psychol. 2008;4:33–52. doi: 10.1146/annurev.clinpsy.4.022007.141207. [DOI] [PubMed] [Google Scholar]
- Muglia P, Tozzi F, Galwey NW, Francks C, Upmanyu R, Kong XQ, Antoniades A, Domenici E, Perry J, Rothen S, Vandeleur CL, Mooser V, Waeber G, Vollenweider P, Preisig M, Lucae S, Muller-Myhsok B, Holsboer F, Middleton LT, Roses AD. Genome-wide association study of recurrent major depressive disorder in two European case–control cohorts. Mol Psychiatry. 2010;15:589–601. doi: 10.1038/mp.2008.131. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–1442. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, Caspi A, Williams B, Price TS, Danese A, Sugden K, Uher R, Poulton R, Moffitt TE. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: replication and extension. Arch Gen Psychiatry. 2009;66:978–985. doi: 10.1001/archgenpsychiatry.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhebergen D, Beekman AT, Graaf R, Nolen WA, Spijker J, Hoogendijk WJ, Penninx BW. The three-year naturalistic course of major depressive disorder, dysthymic disorder and double depression. J Affect Disord. 2009;115:450–459. doi: 10.1016/j.jad.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie K, Jaussent I, Stewart R, Dupuy AM, Courtet P, Ancelin ML, Malafosse A. Association of adverse childhood environment with late-life depression. J Clin Psychiatry. 2009;70:1281–1288. doi: 10.4088/JCP.08m04510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Cottler L, Bucholz KK, Compton W. Diagnostic Interview Schedule for DSM-IV. Washington University School of Medicine; St Louis, MO: 1995. [Google Scholar]
- Robins LN, Helzer JE, Cottler L, Goldring E. Diagnostic Interview Schedule, Version III-R. Washington University School of Medicine; St Louis, MO: 1989. [Google Scholar]
- Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. Wolter Kluwer Helath; Lippincott Williams & Wilkins; Philadephia: 2008. [Google Scholar]
- Schraedley PK, Turner RJ, Gotlib IH. Stability of retrospective reports in depression: traumatic events, past depressive episodes, and parental psychopathology. J Health Soc Behav. 2002;43:307–316. [PubMed] [Google Scholar]
- Schwartz S. Modern epidemiology approaches to interaction: application to the study of genetic interactions. In: Hernandez LM, Blazer DG, editors. Genes : Behavior, and the Social Environment: Moving Beyond the Nature/Nurture Debate. National Academy of Sciences and Institute of Medicine, National Academic Press; Washington, D.C: 2006. pp. 310–354. [Google Scholar]
- Shanahan M, Bauldry S. Improving environmental markers in gene–environment research: insights from life course sociology. In: Rohmer D, Kendler KS, editors. The Dynamic Genome: The Role of Genes and Environments in Youth Development and Mental Health. Oxford University Press; NY: 2011. [Google Scholar]
- Shi J, Potash JB, Knowles JA, Weissman MM, Coryell W, Scheftner WA, Lawson WB, Depaulo JR, Jr, Gejman PV, Sanders AR, Johnson JK, Adams P, Chaudhury S, Jancic D, Evgrafov O, Zvinyatskovskiy A, Ertman N, Gladis M, Neimanas K, Goodell M, Hale N, Ney N, Verma R, Mirel D, Holmans P, Levinson DF. Genome-wide association study of recurrent early-onset major depressive disorder. Mol Psychiatry. 2010;16:193–201. doi: 10.1038/mp.2009.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon DA, Keller MB, Leon AC, Mueller TI, Shea MT, Warshaw M, Maser JD, Coryell W, Endicott J. Recovery from major depression. A 10-year prospective follow-up across multiple episodes. Arch Gen Psychiatry. 1997;54:1001–1006. doi: 10.1001/archpsyc.1997.01830230033005. [DOI] [PubMed] [Google Scholar]
- Sugden K, Arseneault L, Harrington H, Moffitt TE, Williams B, Caspi A. Serotonin transporter gene moderates the development of emotional problems among children following bullying victimization. J Am Acad Child Adolesc Psychiatry. 2010;49:830–840. doi: 10.1016/j.jaac.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, de Geus EJ, Willemsen G, James MR, Smit JH, Zandbelt T, Arolt V, Baune BT, Blackwood D, Cichon S, Coventry WL, Domschke K, Farmer A, Fava M, Gordon SD, He Q, Heath AC, Heutink P, Holsboer F, Hoogendijk WJ, Hottenga JJ, Hu Y, Kohli M, Lin D, Lucae S, MacIntyre DJ, Maier W, McGhee KA, McGuffin P, Montgomery GW, Muir WJ, Nolen WA, Nothen MM, Perlis RH, Pirlo K, Posthuma D, Rietschel M, Rizzu P, Schosser A, Smit AB, Smoller JW, Tzeng JY, van DR, Verhage M, Zitman FG, Martin NG, Wray NR, Boomsma DI, Penninx BW. Genome-wide association for major depressive disorder: a possible role for the presynaptic protein piccolo. Mol Psychiatry. 2009;14:359–375. doi: 10.1038/mp.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surtees PG, Wainwright NW, Willis-Owen SA, Luben R, Day NE, Flint J. Social adversity, the serotonin transporter (5-HTTLPR) polymorphism and major depressive disorder. Biol Psychiatry. 2006;59:224–229. doi: 10.1016/j.biopsych.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Uher R. The role of genetic variation in the causation of mental illness: an evolution-informed framework. Mol Psychiatry. 2009;14:1072–1082. doi: 10.1038/mp.2009.85. [DOI] [PubMed] [Google Scholar]
- Uher R. The implications of gene–environment interactions in depression: will cause inform cure? Mol Psychiatry. 2008;13:1070–1078. doi: 10.1038/mp.2008.92. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Mol Psychiatry. 2010;15:18–22. doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol Psychiatry. 2008;13:131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- Wacholder S. Binomial regression in GLIM: estimating risk ratios and risk differences. Am J Epidemiol. 1986;123:174–184. doi: 10.1093/oxfordjournals.aje.a114212. [DOI] [PubMed] [Google Scholar]
- Wichers M, Kenis G, Jacobs N, Mengelers R, Derom C, Vlietinck R, van OJ. The BDNF Val(66)Met×5-HTTLPR×child adversity interaction and depressive symptoms: an attempt at replication. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:120–123. doi: 10.1002/ajmg.b.30576. [DOI] [PubMed] [Google Scholar]
- Widom CS, DuMont K, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Arch Gen Psychiatry. 2007;64:49–56. doi: 10.1001/archpsyc.64.1.49. [DOI] [PubMed] [Google Scholar]
- Wiersma JE, Hovens JG, van Oppen P, Giltay EJ, van Schaik DJ, Beekman AT, Penninx BW. The importance of childhood trauma and childhood life events for chronicity of depression in adults. J Clin Psychiatry. 2009;70:983–989. doi: 10.4088/jcp.08m04521. [DOI] [PubMed] [Google Scholar]
- Wise LA, Zierler S, Krieger N, Harlow BL. Adult onset of major depressive disorder in relation to early life violent victimisation: a case–control study. Lancet. 2001;358:881–887. doi: 10.1016/S0140-6736(01)06072-X. [DOI] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Brady K, Weiss RD, Farrer L, Gelernter J. Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Arch Gen Psychiatry. 2009;66:1201–1209. doi: 10.1001/archgenpsychiatry.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AS, Klap R, Shoai R, Wells KB. Persistent depression and anxiety in the United States: prevalence and quality of care. Psychiatr Serv. 2008;59:1391–1398. doi: 10.1176/appi.ps.59.12.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]



