Abstract
We studied the value of an IgG Western blot (WB) with Paragonimus kellicotti (Pk) antigen for diagnosis of North American paragonimiasis. The test was evaluated with sera from patients with Pk and Paragonimus westermani infections, with control sera from patients with other helminth infections, and sera from healthy Americans. All 11 proven Pk infection sera and two samples from suspected cases that were negative by P. westermani WB at the Centers for Disease Control and Prevention (CDC) contained antibodies to antigens at 34 kDa and at 21/23 kDa. Seven of 7 P. westermani sera contained antibodies to the 34 kDa antigen, but only 2 recognized the 21/23 kDa doublet. No control samples were reactive with these antigens. Antibody reactivity declined after praziquantel treatment. Thus, the P. kellicotti WB appears to be superior to P. westermani WB for diagnosing Pk infections, and it may be useful for assessing responses to treatment.
Introduction
Most human Paragonimus infections occur in the Far East, but Paragonimus species are also present in other parts of Asia, in sub-Saharan Africa, and in the Americas. North American paragonimiasis (NAP) caused by Paragonimus kellicotti, is a rare human infection, but the zoonosis appears to be emerging in the United States. Only six cases of endemic paragonimiasis were reported from Colorado, Iowa, Michigan, Missouri, and Oklahoma between 1965 and 2007. Since then nine additional cases have been published from Missouri alone, all of them after the consumption of raw or undercooked crayfish during canoeing or camping trips.1,2
Laboratory diagnosis of NAP by parasitology is difficult, because most infected individuals do not have eggs detected in stool, sputum, bronchoalveolar lavage fluid, pleural effusions, or lung biopsies. Paragonimus kellicotti parasites require about 6 to 8 weeks in the mammalian host before they begin producing eggs. Previous case reports showed that months or years may pass between the onset of the illness and parasitological diagnosis.3,4 Diagnosis was often delayed for many months after the onset of symptoms, and patients often failed therapeutic trials of antibiotics and/or steroids before proper diagnosis and treatment.
Serologic testing is an important tool for diagnosis of infections with Paragonimus westermani and related Old World flukes, however experience with its use in P. kellicotti infection is limited. Although serologic tests using P. westermani antigens can be used to diagnose P. kellicotti infection,2,4 an assay using the infecting parasite species might be more sensitive than one using an antigen extract prepared from a heterologous species.
The primary purpose of this study was to test the value of an immunoglobulin G (IgG) Western blot with P. kellicotti antigen as a diagnostic tool for NAP. We also examined the timing of antibody responses to P. kellicotti antigen in experimentally infected gerbils and assessed the persistence of antibodies in patients with NAP after praziquantel treatment.
Material and Methods
Patient sera.
The study protocol was approved by the Human Research Protection Office at Washington University School of Medicine. De-identified patient samples were obtained from Barnes Jewish Hospital in St. Louis, Missouri, the Centers of Diseases Control and Prevention (CDC) in Atlanta, and Heartland Regional Medical Center in St. Joseph, MO. The serum samples were classified according to infection status as shown in Table 1. The panel of sera included samples from individuals with proven P. kellicotti infection, samples from suspected P. kellicotti cases, samples from patients with P. westermani infection, samples from individuals with other helminth infections, and samples from healthy Americans. In addition, sera of Americans with a rheumatoid factor were tested, because it is possible that this could lead to false positive results. Paragonimus kellicotti infection cases were considered to be proven if they had a history of crayfish ingestion with an illness consistent with NAP plus a positive P. westermani serology at CDC and/or P. kellicotti eggs or DNA in their sputum, lung tissue biopsies, or stool. In addition, these patients had no recent history of international travel and their symptoms improved promptly after praziquantel treatment. Suspected NAP cases had compatible case histories with negative P. westermani serology at CDC and no DNA or parasitological evidence of infection.
Table 1.
Serum samples used to evaluate the Western blot assay based on Paragonimus kellicotti adult worm antigen in this study
| Serum group | No. of samples | Characterization |
|---|---|---|
| Pk cases | 11 | Sera from patients with clinical signs of paragonimiasis, positive IgG Western blots with P. westermani antigens at CDC6; positive history of crayfish consumption, no travel outside the United States. |
| Suspected Pk cases | 2 | Sera from North American patients with clinical signs of paragonimiasis who had negative IgG Western blots with P. westermani antigens at CDC6; positive history of crayfish consumption, no travel outside the United States. |
| Potential Pk exposure | 11 | Sera from individuals from Missouri without clinical signs of paragonimiasis who may have eaten raw crayfish. |
| P. westermani cases | 7 | Sera from patients from the Philippines with proven P. westermani infection; eggs were detected in stool, positive Western blots with P. westermani antigens at CDC.6 |
| P. kellicotti treated cases | 7 | Sera from patients with P. kellicotti infections (see above) collected 4–28 weeks post treatment with praziquantel. |
| Healthy Americans | 14 | Sera from Americans with no reason to suspect paragonimiasis (WUSM). |
| Rheumatoid factor | 4 | Sera from Americans with rheumatoid factor (titers ≥ 1:64, WUSM). |
| Schistosoma infection | 21 | Sera from patients with proven Schistosoma mansoni, S. haematobium, or S. japonicum infection (CDC, Uganda). |
| Fasciola hepatica infection | 10 | Sera from patients with proven fasciolasis (CDC). |
| Echinococcus granulosus | 2 | Sera from patients with clinical echinococcosis (CDC) |
| Strongyloides stercoralis | 6 | Sera from African patients with parasitologically proven strongyloidiasis (Uganda). |
Animal sera.
The animal study protocol was approved by the Animal Studies Committee at Washington University School of Medicine. Mongolian gerbils were infected with P. kellicotti metacercariae as previously described.5 Venous blood was collected from infected Mongolian gerbils at various time points after infection (p.i.). Plasma was separated and preserved at −20°C until use.
Paragonimus antigens.
Adult P. kellicotti worms were obtained from experimentally infected Mongolian gerbils, and soluble total worm antigen was prepared as described previously.5 A P. westermani Chafee extract of adult worms was provided by CDC.6,7 Excretory/secretory (e/s) antigens of P. kellicotti were obtained by culturing two adult flukes (65 days p.i.) overnight in 500 μL phosphate buffered saline (PBS) at room temperature. After removal of the living flukes, the eggs were removed (∼2,000 eggs) by sedimentation for 3 h at 4°C. The supernatant was centrifuged at 19,000 × g for 15 min, and the protein concentration of the supernatant was determined using the Pierce BCA method (Thermo Fisher Scientific, Rockford, IL). The e/s antigen was aliquoted and stored at −70°C until use.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
Parasite antigen was electrophoresed using 4–12% NuPAGE Bis-Tris minigels (Invitrogen, Carlsbad, CA). Gels were fixed, washed, and stained using FASTSilver (G-Biosciences, St. Louis, MO) according to the manufacturer's instructions.
Western blot.
Western blot was performed with three types of antigen (P. kellicotti total worm antigen, P. kellicotti e/s antigen, and P. westermani total worm antigen) as described previously for P. kellicotti.5 Briefly, 10 μg of antigen per cm was separated on a 4–12% reducing gel, blotted onto a nitrocellulose membrane, blocked at room temperature for 30 min using 5% non-fat dry milk (BioRad, Hercules, CA) in PBS, and incubated with patient or animal serum or plasma at a dilution of 1:100 or 1:50, respectively. Serum and secondary antibody were diluted in PBS-tween and incubated at 37°C for 1 and 2 h, respectively. After incubation with a secondary antibody (alkaline phosphatase conjugated anti-human IgG [H+L], for human samples or anti-mouse IgG [H+L] for gerbil samples, Promega, Sunnyvale, CA), blots were washed with PBS-tween, and antibody binding was detected using nitro-blue tetrazolium/5-bromo-4-chloro-3′-indolyphosphate substrate (Sigma, St. Louis, MO). The detection of IgG4 subclass antibodies to helminth antigens frequently increases the specificity of serological assays. Therefore, human IgG4 subclass antibodies were detected as previously described with horseradish peroxidase conjugated monoclonal antibody to human IgG4 (HP6023) and substrate.8
Results
Identification of diagnostic proteins.
We used 11 sera from individuals with proven P. kellicotti or P. westermani infection to identify immunoreactive proteins in the soluble P. kellicotti adult worm antigen extract with diagnostic potential. Fourteen proteins with molecular mass values between 4 and 62 kDa were recognized by antibodies in at least one serum sample from patients with P. kellicotti or P. westermani infection (Table 2). Three of these proteins at 21, 23, and 34 kDa were recognized by all 11 sera from proven P. kellicotti cases (Figure 1, lanes 1, 8, and 9). Therefore, we considered patient sera from North Americans with antibody reactivity to the 21/23 kDa doublet or the 34 kDa band by Western blot to be positive for antibodies to P. kellicotti. Interestingly, the two serum samples from individuals with suspected P. kellicotti infection also contained antibodies to these three antigen bands. All seven sera tested from patients with P. westermani infection had antibodies to the 34 kDa protein, but only two reacted with the 21/23 kDa doublet proteins.
Table 2.
IgG reactivity by Western blot with selected Paragonimus kellicotti adult worm antigens for sera from individuals with paragonimiasis and various types of control sera*
| Serum group | Molecular mass, kDa | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 8 | 12 | 14 | 21 | 22 | 23 | 29 | 34 | 44 | 47 | 49 | 55 | 62 | |
| Pk cases (N = 11) | 3 | 6 | 6 | 7 | 11 | 0 | 11 | 1 | 11 | 4 | 4 | 7 | 2 | 7 |
| Suspected Pk cases (N = 2) | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 0 | 0 | 2 |
| Potential Pk exposure (N = 11) | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 6 | 0 | 6 | 0 | 0 |
| P. westermani (N = 7) | 5 | 6 | 5 | 4 | 2 | 1 | 2 | 0 | 7 | 3 | 2 | 3 | 2 | 2 |
| Healthy North Americans (N = 14) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rheumatoid factor (N = 4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Schistosoma infections (N = 21) | 0 | 0 | 0 | 0 | 0 | 11 | 0 | 0 | 0 | 4 | 0 | 9 | 0 | 0 |
| Fasciola hepatica (N = 10) | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Echinococcus granulosus (N = 2) | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 1 | 2 | 0 | 0 |
| Strongyloides stercoralis (N = 6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Numbers refer to the number of sera reactive with various antigens.
Figure 1.
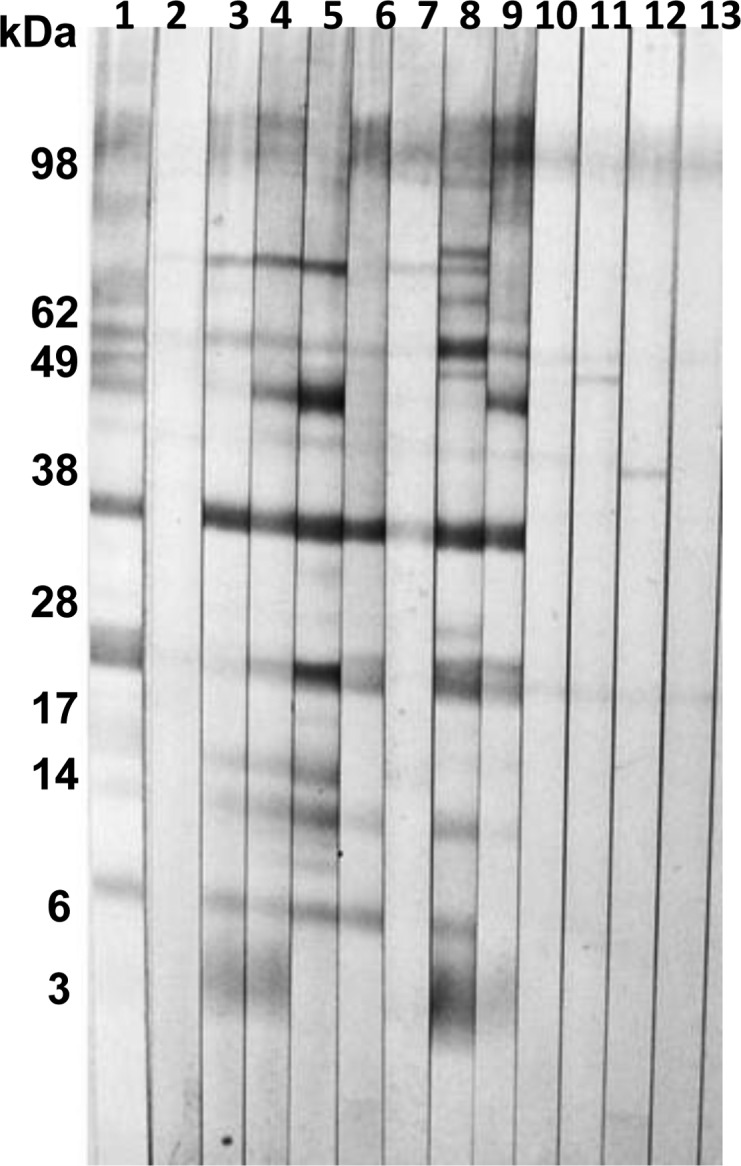
Total IgG Western blot using soluble Paragonimus kellicotti adult worm antigen tested with sera from individuals with or without proven Paragonimus infection. Lane 1, positive control serum from a patient from St. Joseph, Missouri with proven P. kellicotti infection; lane 2, negative control serum from a healthy American without exposure to Paragonimus; lanes 3–7, sera from patients from the Philippines with proven Paragonimus westermani infection; lanes 8 and 9, sera from patients with proven P. kellicotti infection from Missouri; lanes 10 and 11, sera from patients with Echinococcus granulosus infection; lanes 12 and 13, sera from patients with schistosomiasis.
Specificity of the P. kellicotti diagnostic proteins.
None of the 57 sera we tested from parasite-free individuals or from individuals with other helminth infections reacted with the 21/23 or 34 kDa proteins in the soluble P. kellicotti adult worm antigen extract (Table 1). Some of the sera reacted with other proteins (for example, see lanes 11 and 12 in Figure 1). However, these patterns were easily differentiated from those produced by sera from paragonimiasis patients. The use of anti-IgG4 as a secondary antibody did not significantly reduce nonspecific reactivity with other antigens in control samples or enhance reactivity of positive samples with the diagnostic antigen bands (data not shown).
Reactivity to excretory/secretory P. kellicotti antigens.
The e/s antigen was tested by Western blot with sera from proven P. kellicotti cases. The sera strongly recognized a 21/23 kDa doublet and had weak reactivity with a more diffuse band at 34 kDa (Figure 2). These results suggest that the 21/23 kDa doublet antigen and possibly the 34 kDA antigen are excreted or secreted by living adult P. kellicotti worms.
Figure 2.
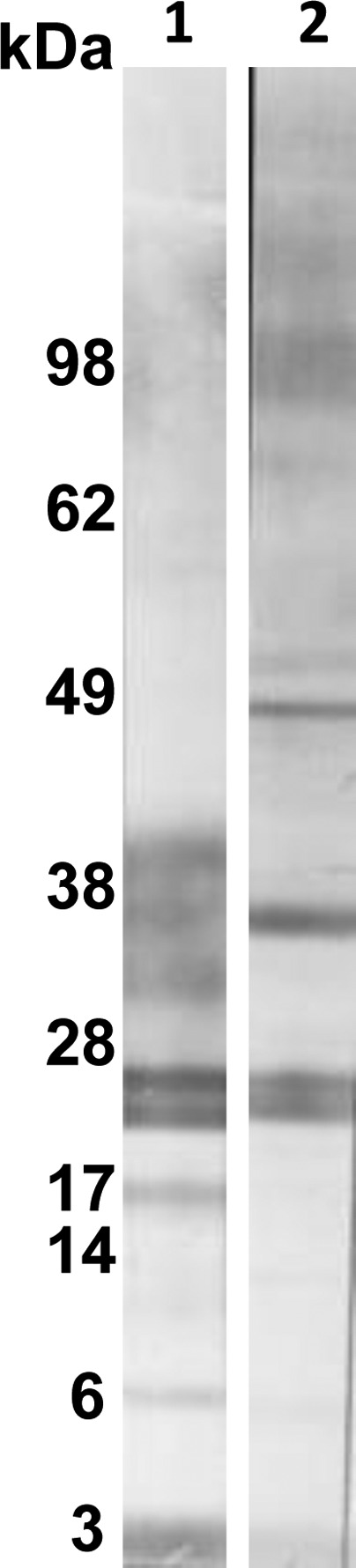
Western blot using Paragonimus kellicotti adult worm excretory/secretory antigen (lane 1) or soluble total worm antigen (lane 2) using a serum specimen from a patient infected with P. kellicotti.
Emergence and persistence of antibodies to P. kellicotti antigens.
Serial serum samples from P. kellicotti-infected gerbils were tested by Western blot to determine the timing of antibody responses to P. kellicotti antigens in the soluble P. kellicotti adult worm antigen extract. Specific IgG antibodies were detected as early as 2 weeks p.i., and all infected gerbils had detectable antibody responses 5 weeks after infection. Sera from infected gerbils did not recognize the same antigens in P. kellicotti extract as human sera; the two most prominent antigens recognized by gerbil sera have molecular masses of ∼28 and 55 kDa (compare with Figure 2B in Reference 5). The reason for the differential antigen recognition of gerbils and humans is not known.
To determine the persistence of P. kellicotti antibodies after successful treatment with praziquantel, we tested sera from five proven P. kellicotti cases before and after treatment. Although all sera were still positive 5 to 9 weeks after treatment, two post-treatment sera produced weaker reactions than pretreatment sera from the same individuals (Figure 3, compare lanes 5, 6, and 7, 8). One serum from a confirmed P. kellicotti case (with positive P. kellicotti and P. westermani Western blots before treatment) was collected 28 weeks after treatment, and this was negative for antibodies to P. kellicotti by Western blot (data not shown). These results suggest that antibodies to P. kellicotti decrease after praziquantel treatment and that they might be useful as a test of cure for the infection.
Figure 3.
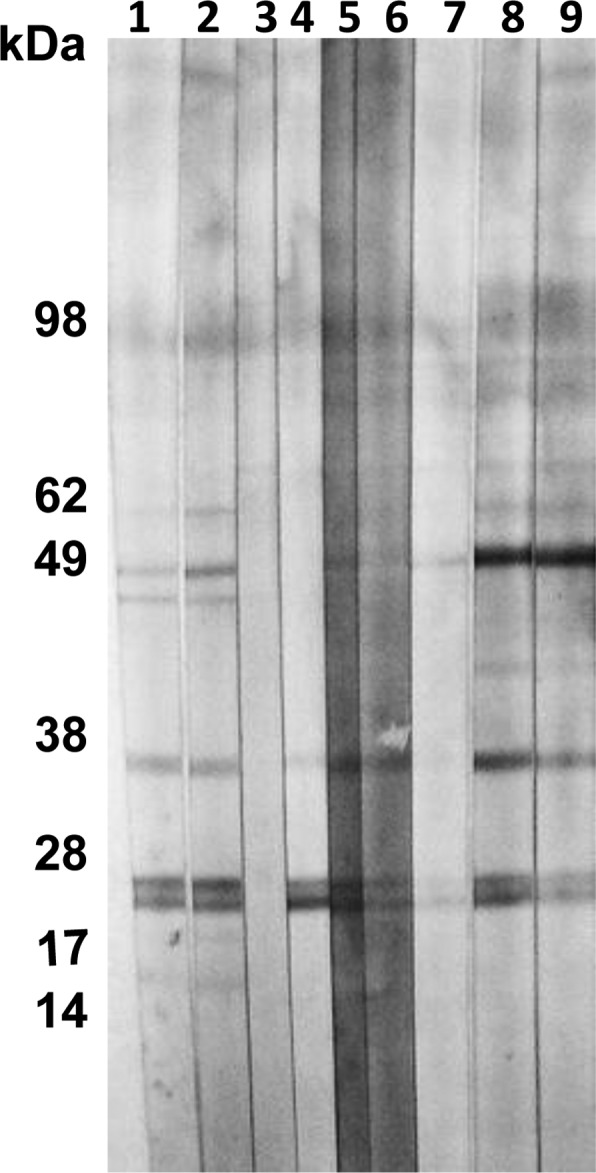
Western blot detection of antibodies to soluble Paragonimus kellicotti adult worm antigen in sera from P. kellicotti patients before or after treatment with praziquantel. Lanes 1, 3, 5, and 7, sera from four patients with proven P. kellicotti infections before treatment; lane 2, serum from the same patient as in lane 1 collected 5 weeks after treatment with praziquantel; lane 4, serum from the same patient as in lane 3 collected 8 weeks after treatment with praziquantel; lane 6, serum from the same patient as in lane 5 collected 9 weeks after treatment with praziquantel; lane 8, serum from the same patient as in lane 7 collected 7 weeks after treatment with praziquantel.
Comparison of Western blot results obtained with P. kellicotti and P. westermani adult worm antigens.
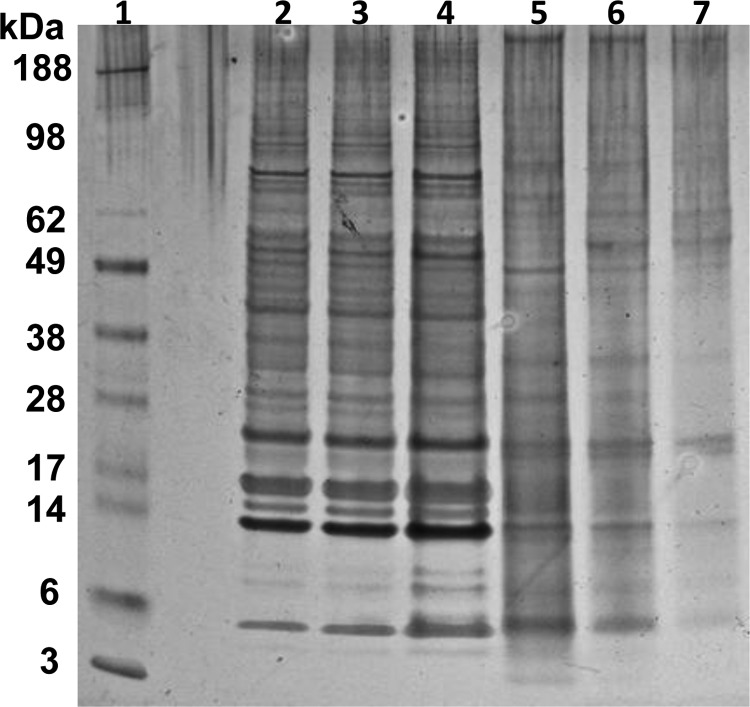
The two antigen preparations were studied by SDS-PAGE (Figure 4). The silver stain showed that both antigen preparations contained a large number of strong, distinct bands. Some of these protein bands were apparently shared between the two antigen preparations (e.g., antigens at ∼25 kDa, ∼13 kDa, and ∼5 kDa). Overall, bands were stronger and more distinct in the P. kellicotti antigen extract. None of the proteins in the P. westermani antigen extract was clearly identifiable as one of the diagnostic proteins in the P. kellicotti Western blot.
Figure 4.
A silver stained SDS page gel shows the major proteins in Paragonimus kellicotti soluble adult worm antigen (lanes 2–4) and in Paragonimus westermani antigen (lanes 5–7). Lane 1, molecular weight markers; lanes 2 and 7, 250 ng protein per lane; lanes 3 and 6, 500 ng protein per lane; lanes 4 and 5, 1 μg protein per lane.
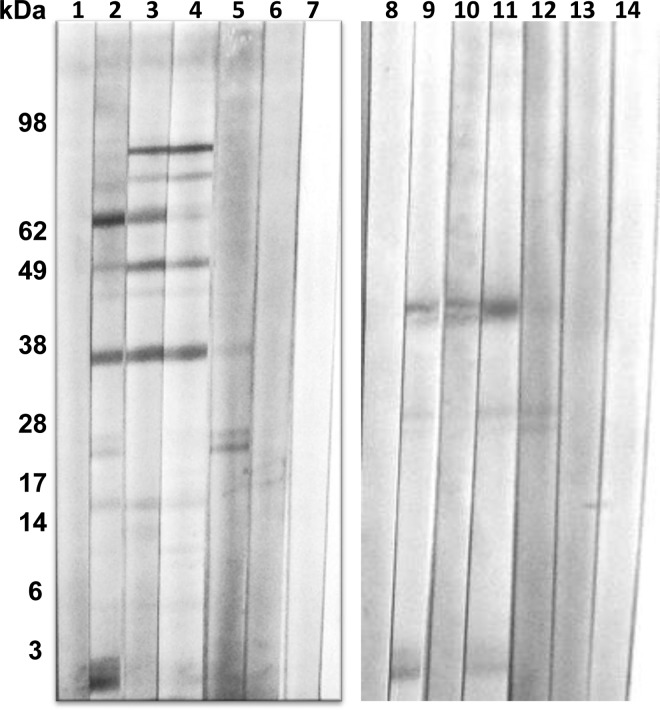
To compare the suitability of the P. kellicotti and P. westermani antigens for the Western blot diagnosis of NAP, we performed blots with both antigens using the same set of patient sera. Both antigens showed almost no background with sera of healthy Americans or other individuals without Paragonimus infection (Figure 5). Sera from confirmed P. kellicotti cases recognized the diagnostic proteins at 34 and 21/23 kDa. Sera from confirmed P. westermani cases reacted strongly with the P. kellicotti antigens at 34 kDa but in 5 of 7 cases not with those at 21/23 kDa. In addition, some of these serum samples strongly reacted with high molecular weight proteins (see lanes 3 and 4 in Figure 5). Sera from P. kellicotti and P. westermani patients tended to react more weakly with P. westermani antigens than with P. kellicotti antigens; they reacted most strongly with P. westermani antigens between 38 and 42 kDa and between 21 and 25 kDa. Analysis of 13 sera from P. kellicotti cases and 7 sera of P. westermani cases using both antigens showed that all 20 sera were positive for antibodies to P. kellicotti antigen, whereas 18 (90%) were positive with the P. westermani antigen. Two sera from suspected P. kellicotti cases were reactive by Western blot with P. kellicotti antigen but not with P. westermani antigen.
Figure 5.
Comparison of Paragonimus kellicotti (lanes 1–7) and Paragonimus westermani antigen (lanes 8–14) for detecting antibodies to Paragonimus antigens by Western blot. Lanes 1 and 8 tested serum from a suspected P. kellicotti patient (later considered to have not been infected) who did not have antibodies to either antigen; lanes 2 and 9 and 5 and 12 were tested with sera from two proven P. kellicotti patients; lanes 3 and 10 and lanes 4 and 11 tested sera from two proven P. westermani patients; lanes 6 and 13 and lanes 7 and 14 tested sera from two healthy Americans without exposure to Paragonimus.
Discussion
To improve the serological diagnosis of NAP, we developed a simple and reliable Western blot assay using soluble P. kellicotti adult worm antigen. All sera from patients with NAP had positive antibody tests, and the assay appears to be highly specific for infection with Paragonimus species.
Three antigens at 21, 23, and 34 kDa in the P. kellicotti adult worm antigen extract were recognized by all 11 proven NAP cases. Although sera from P. westermani patients consistently recognized the 34 kDA protein in P. kellicotti antigen, none of the sera tested from patients without Paragonimus infection contained antibodies that reacted with these three “diagnostic” antigens. These antigens are clearly different from the 8 kDa P. westermani antigen that was previously reported to be specific for paragonimiasis.6 Another study showed that only a 31.5 kDa protein in the Paragonimus heterotremus antigen was recognized by all sera from proven paragonimiasis cases.9 It is possible that this P. heterotremus antigen corresponds to the 34 kDa protein recognized by all NAP and P. westermani cases in this study. Another study reported a non-protein antigen at 35 kDa as a major diagnostic antigen for that infection.10 Unfortunately, most serological studies on human paragonimiasis have used the enzyme-linked immunosorbent assay (ELISA) for antibody detection without providing information about the molecular mass of the antigens recognized by antibodies in sera from infected individuals. Although the ELISA format is more convenient than the Western blot, it is not ideal for use with crude native antigen extracts that may contain cross-reactive antigens together with specific diagnostic antigens. Western blot assays are satisfactory for rare infections like NAP, because the number of sera to be tested is small, and high throughput screening is not required.
The diagnostic 21/23 kDa proteins in the P. kellicotti antigen extract are e/s antigens, and e/s antigens have been previously used for the serological diagnosis of P. heterotremus infection.11 The 21/23 doublet antigens were excreted and/or secreted into PBS after a simple overnight culture of a small number of P. kellicotti adult worms. Additional work will be required to further characterize these antigens.
Our results showed that gerbils produce IgG antibodies to P. kellicotti antigens as early as 2 weeks p.i. Furthermore, some of the NAP sera we tested were obtained shortly after the onset of symptoms and within a few months after crayfish ingestion. Positive antibody tests with these sera contrast with a report that stated that human IgG antibodies are mainly seen in long-standing P. westermani infections, whereas only IgM is detected in early stages of infection.12 Although it is always difficult to directly compare results from naturally occurring infections in humans and experimental infections in animals, our results suggest that humans with NAP generally have positive IgG antibody tests soon after the onset of pulmonary symptoms. It is also important to note that the P. kellicotti Western blot can also be used to assess the outcome of treatment. Antibody reactivity decreased within weeks of treatment in several cases. In another case, a patient's serum became antibody negative 28 weeks after successful treatment. All of the NAP cases who provided sera for this study were successfully treated using praziquantel.2
The P. kellicotti antigen was not only useful for detecting cases of NAP, but sera from patients with P. westermani infections acquired in the Philippines also recognized proteins in the P. kellicotti extract. The SDS-PAGE analysis suggested that the P. kellicotti adult worm antigen extract used in this study contained more proteins that were better resolved than the P. westermani antigen extract. The P. westermani antigen used in this study was produced many years ago using ether to extract undesirable lipids (Chaffee extract) from the adult P. westermani worm preparation.7 Ether extraction is no longer a preferred method for antigen preparation, so the P. kellicotti adult worm antigen extract described in this work may be a useful alternative for diagnosis of paragonimiasis caused by P. kellicotti or P. westermani. Molecular phylogenetic studies using mitochondrial or non-coding DNA markers indicate that P. westermani and P. kellicotti are not closely related within the genus Paragonimus.5,13,14 However, these studies provide little information about cross-reactivity of diagnostic antigens. In the past, worm extracts or e/s antigens from a number of old-world Paragonimus species such as P. westermani, P. heterotremus, and Paragonimus africanus have been used for serological diagnosis of paragonimiasis.6,15–18 Only a few studies identified single antigens of P. westermani with diagnostic potential. Since the P. kellicotti adult worm transcriptome has been sequenced (Mitreva M, McNulty SN, and others, unpublished data), we were able to compare the amino acid sequences of several of the previously described Paragonimus antigens to predicted sequences of P. kellicotti orthologues. For example, a cysteine proteinase (cp2) had only 53% identity with the P. kellicotti orthologue and a major egg antigen had 86% identity.19,20 These results are in line with our observations that show that sera of NAP cases show stronger reactions with homologous P. kellicotti antigens than with P. westermani antigens.
In conclusion, we have developed and evaluated a Western blot assay for the detection of IgG antibodies to P. kellicotti adult worm antigens for the serological diagnosis of NAP. This test appears to be sensitive and specific for human paragonimiasis. Antibodies to P. kellicotti appear within a few weeks after infection in animals and are present when pulmonary symptoms develop in humans. Antibody reactivity wanes over a period of months after successful treatment with praziquantel.
ACKNOWLEDGMENTS
We thank the physicians and clinical laboratories that provided sera for this study.
Footnotes
Financial support: This study was supported by a grant from the Barnes Jewish Hospital Foundation.
Authors' addresses: Peter U. Fischer, Kurt C. Curtis, Luis A. Marcos, and Gary J. Weil, Infectious Diseases Division, Department of Internal Medicine, Washington University School of Medicine, St. Louis, MO, E-mails: Pufische@DOM.wustl.edu, KCurtis@DOM.wustl.edu, LMarcos@DOM.wustl.edu, and GWeil@DOM.wustl.edu. Scott M. Folk, Heartland Regional Medical Center, St. Joseph, MO, E-mail: scott.folk@heartland-health.com. Patricia P. Wilkins, Division of Parasitic Diseases and Malaria, Center for Global Health, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: pma1@cdc.gov.
References
- 1.Centers for Disease Control and Prevention Human paragonimiasis after eating raw or undercooked crayfish—Missouri, July 2006–September 2010. MMWR Morb Mortal Wkly Rep. 2010;59:1573–1576. [PubMed] [Google Scholar]
- 2.Lane MA, Marcos LA, Onen NF, Demertzis LM, Hayes EV, Davila SZ, Nurutdinova DR, Bailey TC, Weil GJ. Paragonimus kellicotti flukes in Missouri, USA. Emerg Infect Dis. 2012;18:1263–1267. doi: 10.3201/eid1808.120335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lane MA, Barsanti MC, Santos CA, Yeung M, Lubner SJ, Weil GJ. Human paragonimiasis in North America following ingestion of raw crayfish. Clin Infect Dis. 2009;49:e55–e61. doi: 10.1086/605534. [DOI] [PubMed] [Google Scholar]
- 4.Procop GW. North American paragonimiasis (caused by Paragonimus kellicotti) in the context of global paragonimiasis. Clin Microbiol Rev. 2009;22:415–446. doi: 10.1128/CMR.00005-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer PU, Curtis KC, Marcos LA, Weil GJ. Molecular characterization of the North American lung fluke Paragonimus kellicotti in Missouri and its development in Mongolian gerbils. Am J Trop Med Hyg. 2011;84:1005–1011. doi: 10.4269/ajtmh.2011.11-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slemenda SB, Maddison SE, Jong EC, Moore DD. Diagnosis of paragonimiasis by immunoblot. Am J Trop Med Hyg. 1988;39:469–471. doi: 10.4269/ajtmh.1988.39.469. [DOI] [PubMed] [Google Scholar]
- 7.Chaffee EF, Bauman PM, Shapilo JJ. Diagnosis of schistosomiasis by complement-fixation. Am J Trop Med Hyg. 1954;3:905–913. doi: 10.4269/ajtmh.1954.3.905. [DOI] [PubMed] [Google Scholar]
- 8.Weil GJ, Ogunrinade AF, Chandrashekar R, Kale OO. IgG4 subclass antibody serology for onchocerciasis. J Infect Dis. 1990;161:549–554. doi: 10.1093/infdis/161.3.549. [DOI] [PubMed] [Google Scholar]
- 9.Maleewong W, Wongkham C, Pariyanonda S, Intapan P, Pipitgool V, Daenseegaew W, Morakote N. Antigenic components of Paragonimus heterotremus recognized by infected human serum. Parasite Immunol. 1991;13:89–93. doi: 10.1111/j.1365-3024.1991.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 10.Indrawati I, Chaicumpa W, Setasuban P, Ruangkunaporn Y. Studies on immunodiagnosis of human paragonimiasis and specific antigen of Paragonimus heterotremus. Int J Parasitol. 1991;21:395–401. doi: 10.1016/0020-7519(91)90096-p. [DOI] [PubMed] [Google Scholar]
- 11.Maleewong W, Wongkham C, Intapan P, Pariyanonda S, Morakote N. Excretory-secretory antigenic components of Paragonimus heterotremus recognized by infected human sera. J Clin Microbiol. 1992;30:2077–2079. doi: 10.1128/jcm.30.8.2077-2079.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura-Uchiyama F, Onah DN, Nawa Y. Clinical features of paragonimiasis cases recently found in Japan: parasite-specific immunoglobulin M and G antibody classes. Clin Infect Dis. 2001;32:e151–e153. doi: 10.1086/320750. [DOI] [PubMed] [Google Scholar]
- 13.Blair D, Wu B, Chang ZS, Gong X, Agatsuma T, Zhang YN, Chen SH, Lin JX, Chen MG, Waikagul J, Guevara AG, Feng Z, Davis GM. A molecular perspective on the genera Paragonimus Braun, Euparagonimus Chen and Pagumogonimus Chen. J Helminthol. 1999;73:295–299. [PubMed] [Google Scholar]
- 14.Blair D, Xu ZB, Agatsuma T. Paragonimiasis and the genus Paragonimus. Adv Parasitol. 1999;42:113–222. doi: 10.1016/s0065-308x(08)60149-9. [DOI] [PubMed] [Google Scholar]
- 15.Aka NA, Assoumou A, Adoubrynk D, Domoua K, Kouadio F, Moyou-Somo R, Nakamura-Uchiyama F, Nawa Y, Rondelaud D, Dreyfuss G. First findings on the seroepidemiology of human paragonimosis at the anti-tuberculosis centre of Divo, Repubuc of Ivory Coast (West Africa) Parasite. 2008;15:157–161. doi: 10.1051/parasite/2008152157. [DOI] [PubMed] [Google Scholar]
- 16.Doanh PN, Dung do T, Thach DT, Horii Y, Shinohara A, Nawa Y. Human paragonimiasis in Viet Nam: epidemiological survey and identification of the responsible species by DNA sequencing of eggs in patients' sputum. Parasitol Int. 2011;60:534–537. doi: 10.1016/j.parint.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Nkouawa A, Okamoto M, Mabou AK, Edinga E, Yamasaki H, Sako Y, Nakao M, Nakaya K, Blair D, Agatsuma T, Enyong P, Shibahara T, Moyou-Somo R, Ito A. Paragonimiasis in Cameroon: molecular identification, serodiagnosis and clinical manifestations. Trans R Soc Trop Med Hyg. 2009;103:255–261. doi: 10.1016/j.trstmh.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Yong TS, Seo JH, Yeo IS. Serodiagnosis of human paragonimiasis by ELISA-inhibition test using monoclonal antibodies. Korean J Parasitol. 1993;31:141–147. doi: 10.3347/kjp.1993.31.2.141. [DOI] [PubMed] [Google Scholar]
- 19.Lee JS, Lee J, Kim SH, Yong TS. Molecular cloning and characterization of a major egg antigen in Paragonimus westermani and its use in ELISA for the immunodiagnosis of paragonimiasis. Parasitol Res. 2007;100:677–681. doi: 10.1007/s00436-006-0324-7. [DOI] [PubMed] [Google Scholar]
- 20.Yang SH, Park JO, Lee JH, Jeon BH, Kim WS, Kim SI, Yun KJ, Jeong ET, Lee KW, Kim YM, Lee MH, Park H. Cloning and characterization of a new cysteine proteinase secreted by Paragonimus westermani adult worms. Am J Trop Med Hyg. 2004;71:87–92. [PubMed] [Google Scholar]