Abstract
In endemic countries with soil-transmitted helminths mass drug administration with albendazole or mebendazole are being implemented as a control strategy. However, it is well known in veterinary helminths that the use of the same benzimidazole drugs can place selection on the β-tubulin gene, leading to resistance. Given the concern that resistance could arise in human soil-transmitted helminths, there is an urgent need to develop accurate diagnostic tools for monitoring resistance. In this study, we developed molecular assays to detect putative resistance genetic changes in Ascaris lumbricoides, Trichuris trichiura, and hookworms, and we optimized an egg hatch assay for the canine hookworm Ancylostoma caninum and applied it to Necator americanus. Both assays were tested on field samples. The molecular assays demonstrated their reproducibility and capacity to detect the presence of worms carrying putative resistance-associated genetic changes. However, further investigations are needed to validate our molecular and biological tests on additional field isolates.
Introduction
Soil-transmitted helminth (STH) infections, caused by the three most important STHs: Ascaris lumbricoides (roundworm), Trichuris trichiura (whipworm), and Necator americanus/Ancylostoma duodenale (hookworms) are a major health problem in developing countries. The number of cases of STHs is estimated to be 1,221 million people worldwide for A. lumbricoides, 795 million for T. trichiura, and 740 million for hookworms1; among them, 400 million school-age children are infected by STHs, resulting in children being physically and intellectually compromised by anemia, attention-deficit, and learning disabilities. In addition, 44 million pregnant women suffer from severe anemia caused by hookworms. These infections are endemic in tropical and sub-tropical regions of the developing world with the greatest burden in sub-Saharan Africa, East Asia, China, India, and South America.2–4 The STH infections are not only responsible for 135,000 deaths annually, but also impose a devastating socioeconomic burden on affected communities. It is estimated that 39 million disability-adjusted life years are lost worldwide each year because of STHs.5 The high prevalence, percentage of cases, and intensity of infections rank STHs as one of the major neglected tropical diseases.3,6 Control strategies through mass drug administration (MDA) programs have been implemented to decrease the prevalence, intensity, and morbidity associated with STHs and are based on a periodic distribution of a single dose of the benzimidazole (BZ) derivatives albendazole or mebendazole.7,8 Albendazole is the most commonly used BZ anthelmintic drug because it shows more activity against hookworms than other anthelmintics.9 Regular treatments in endemic countries have shown positive effects on health.10 However, in veterinary medicine, the widespread and frequent use of BZ drugs has led to extremely high rates of resistance in multiple nematode species in a wide range of hosts.11 The BZ resistance in parasitic nematodes is characterized by single nucleotide polymorphisms (SNPs), which cause amino acid substitutions from phenylalanine (Phe TTC) to tyrosine (Tyr TAC) in β-tubulin at codons 200 or 167.12,13 Furthermore, a glutamate to alanine change at codon 198 (Glu198Ala) has occasionally been associated with BZ resistance.14,15 In human STHs, there is a growing concern that repeated MDA with the same BZ drugs could exert similar selection pressure on parasites and favor the development of resistance. Some studies have suggested an emergence of drug resistance by reporting low cure rates and fecal egg count reductions.9,16,17 However, these major indicators, used for assessing drug efficacy, lack sufficient precision and accuracy to conclude the development of resistance.18–20 Thus, there is an urgent need to develop better diagnostic tools for detecting BZ resistance in human parasites. We have previously developed DNA-based assays to detect the Phe200Tyr SNP associated with BZ resistance in whipworms and roundworms.21 The objectives of this study were 1) to develop standardized molecular tests for A. lumbricoides, T. trichiura, and N. americanus for SNPs in the three critical positions associated with BZ resistance in other nematode parasites, and 2) to optimize the egg hatch assay (EHA) for assessing the response of hookworms to BZ anthelmintics.
Material and Methods
Acquisition of parasite material.
Molecular assay development and optimization were performed using DNA samples from A. lumbricoides, T. trichiura, and N. americanus collected in areas where MDA was not ongoing. The DNA from adult A. lumbricoides and T. trichiura had been extracted previously using the DNeasy blood and tissue extraction kit (QIAGEN Inc., Toronto, ON, Canada).21 The ability of the molecular assay to detect resistance-associated SNPs in human hookworms was tested by applying DNA from N. americanus collected from 28 persons in areas under MDA with albendazole in Haiti and from five children previously treated with mebendazole, on Pemba Island, Tanzania,22 and was provided by Dr. Marco Albonico to R. Kaplan at the University of Georgia (Table 1). Human stool samples were collected as part of a study of MDA for lymphatic filariasis and STH in Haiti, and were conducted by Dr. Patrick Lammie of the Centers for Disease Control and Prevention (CDC), Atlanta, GA. The samples were collected before MDA in Cayes-Jacmel located in the Southeast Department of Haiti. Haiti is known to be endemic for STHs, including N. americanus23; in samples from three adults, the response of hookworm eggs to BZ was assessed by the EHA. The optimization of the biological assay was done using both dog and human hookworm parasites. Fresh canine stool samples were collected at a dog pound in Athens, GA. Dogs at the pound carried natural infections of the canine hookworm. In addition, stool samples were provided by TRS Laboratories, Athens, Georgia from dogs used to maintain a culture of A. caninum.
Table 1.
Origin and treatment history of Necator americanus DNA samples used in the molecular assays for human hookworms*
| Pemba Island | Haiti | |
|---|---|---|
| Specimen | DNA from adult worms and larvae | DNA from eggs |
| Sample size | 2 DNA samples of individual adult worms | 27 DNA samples, each of 10 eggs |
| 7 DNA samples, each of 50 larvae | ||
| Treatment history | Post-treatment | Post-treatment |
| Mebendazole | Albendazole | |
| Round of treatment | 13 | Unknown |
Adult and larvae samples from Pemba Island were recovered from five infected children.21 In Haiti, eggs were recovered from 28 persons.
Ethical approval.
Ethical approval (study 2535) was obtained (Dr. Patrick Lammie) from the CDC, Atlanta, GA, and included the collection of stool samples, examination of stool samples for helminth eggs, and DNA analysis of helminth eggs. Oral informed consent was obtained from all human adult participants and from parents or legal guardians of minors.
Wild-type (WT) and mutant-type (MT) plasmid constructs.
From a previous study, genetic markers, validated with control plasmids, were constructed for position 200 in A. lumbricoides and T. trichiura.21 In this work, we also investigated codons 167 and 198 of the β-tubulin gene, involved in resistance in other parasitic nematodes.15 Diagnostic tests were optimized by using control plasmids to investigate codons 167 and 198 in β-tubulin of A. lumbricoides, T. trichiura, N. americanus, and codon 200 in N. americanus. For each parasite species, WT plasmids, and MT plasmids carrying a mutation either at codon 167, 198, or 200, generated by site-directed mutagenesis, were produced as described previously.21 The WT and MT plasmids contained the β-tubulin sequences of interest (with or without mutations). Primers for WT (Table 2) and MT plasmid (Table 3) were designed in the exonic regions of genomic DNA, for A. lumbricoides (GenBank accession no. FJ501301.1) and T. trichiura (GenBank accession no. AF034219.1) and in exonic and intronic regions for N. americanus (GenBank accession no. EF392851). All polymerase chain reactions (PCR) contained: 2 μL 10 × PCR buffer, 1 μL [50 mM] MgSO4, 1 U Platinum Taq DNA Polymerase High Fidelity, 1 μL of sense and antisense primers [10 μM], 1 μL dNTP mix [10 μM], 2 μL template DNA, and distilled H2O to 20 μL. Negative controls without DNA were also included. The PCR conditions remained the same for all parasites, except for annealing temperatures that varied according to the species and the position amplified (Tables 2 and 3). All PCR reactions included an initial denaturation at 94°C for 45 s followed by 35 cycles of 94°C for 45 s, a specific annealing temperature (see Table 2), for 45 s, an elongation at 68°C for 1 min, and a final extension at 68°C for 5 min. The WT and MT amplified fragments were ligated into a TOPO-TA cloning vector (Invitrogen, Life Technologies, Burlington, ON) and subsequently sequenced at the McGill University/Genome Quebec Innovation Center, Montreal, Quebec.
Table 2.
Species and SNP-specific primers for wild-type plasmids of the three STH β-tubulins*
| Codon | Species | Primer sequences (5′–3′) | T°ann (°C) |
|---|---|---|---|
| 167 | A. lumbricoides | Forward: ACTCGCTTGGTGGAG | 58.4 |
| Reverse: CAACCAACTGATGAACGGAC | |||
| T. trichiura | Forward: CCTCGGCGGCGGAACTG | 56.4 | |
| Reverse: GGTGGACTGACAGAGTTGC | |||
| N. americanus | Forward: TGACTGTCTCCAGGTAATTCG | 58 | |
| Reverse: CGATGATACGCGGGATG | |||
| 198 | A. lumbricoides | Forward: CCGTGAAGAATACCCCGACA | 58.7 |
| Reverse: TATGTGGGATTTGTAAGCTTCAG | |||
| T. trichiura | Forward: TTTCAGATACAGTTGTAGAACC | 57 | |
| Reverse: CAAATGATTTAAGTCTCCG | |||
| N. americanus | Forward: GTTTCCGACACTGTGGTTGAG | 59 | |
| Reverse: AGTTCGTTACTAGCCAGCTCACC | |||
| 200 | N. americanus | (same as position 198) |
SNP = single nucleotide polymorphism; STH = soil-transmitted helminth; T°ann = primer annealing temperature.
Table 3.
Species-specific primers for mutant type constructs in the three STH β-tubulins*
| Codon | Species | Primer sequences (5′–3′) | T°ann (°C) |
|---|---|---|---|
| 167 | A. lumbricoides | Forward: GCACTCGCTTGGTGGAG | 58.4 |
| Reverse: CAACCAACTGATGAACGGAC | |||
| SNP-Fwd: GCTCGTACTCAGTTGTTCCATC | |||
| SNP-Rev: GATGGAACAACTGAGTACGAGC | |||
| T. trichiura | Forward: CCTCGGCGGCGGAACTG | 58.5 | |
| Reverse: GGTGGACTGACAGAGTTGC | |||
| SNP-Fwd: CAACTTATAGTGTCGTTCCGTC | |||
| SNP-Rev: GACGGAACGACACTATAAGTTG | |||
| N. americanus | Forward: TGACTGTCTCCAGGTAATTCG | 59 | |
| Reverse: CGATGATACGCGGGATG | |||
| SNP-Fwd: CGTTATCCGTTGTACCCTC | |||
| SNP-Rev: CAACGGAATACGAGGACAT | |||
| 198 | A. lumbricoides | Forward: TGAAGAATACCCCGACA | 58.7 |
| Reverse: CTGAAGCTTACAAATCCCACATA | |||
| SNP-Fwd: ACACCGATGCAACCTTC | |||
| SNP-Rev: GAAGGTTGCATCGGTGT | |||
| T. trichiura | Forward: GTTTCAGATACAGTTGTAGAACC | 50 | |
| Reverse: CAAATGATTTAAGTCTCCG | |||
| SNP-Fwd: ACACGGACGCAACATTC | |||
| SNP-Rev: GAATGTTGCGTCCGTGT | |||
| N. americanus | Forward: GTTTCCGACACTGTGGTTGAG | 57 | |
| Reverse: GATTCAGATCTCCATATGTTGGG | |||
| SNP-Fwd: AGATGCGACCTTCTGTATTGATAATG | |||
| SNP-Rev: CATTATCAATACAGAAGGTCGCATCT | |||
| 200 | N. americanus | Forward: GTTTCCGACACTGTGGTTGAG | 58 |
| Reverse: GATTCAGATCTCCATATGTTGGG | |||
| SNP-Fwd: AGATGAGACCTACTGTATTGATAATG | |||
| SNP-Rev: CATTATCAATACAGTAGGTCTCATCT |
SNP-Fwd = forward primer mutated for a single nucleotide; SNP-Rev = reverse primer mutated for a single nucleotide; T° ann = primer annealing temperature.
Diagnosis of resistance-associated mutations in A. lumbricoides, T. trichiura, and N. americanus.
Mutated and susceptible alleles were detected for all parasites by pyrosequencing. To optimize our assays the sense, antisense, and sequencing primers required for pyrosequencing were designed by PyroMark Assay Design Software (QIAGEN Inc., version 2.0) (Table 4). The PCR reactions contained 5 μL 10 × PCR buffer, 2 μL [50 mM] MgSO4, 1 U Platinum Taq DNA Polymerase High Fidelity, 1 μL of sense and antisense primers [10 μM], 1 μL dNTP mix [10 μM], 3 μL template DNA, and distilled H2O to 50 μL. Antisense primers were biotinylated at their 5′ end to generate single-stranded template using streptavidin beads (Amersham Biosciences, Piscataway, NJ). The amplification conditions were 94°C for 45 s followed by 50 cycles of 94°C for 45 s, 59°C for 45 s, 68°C for 1 min, and a final extension at 68°C for 5 min. Sequencing primers (Table 4) were used for SNP analysis in the pyrosequencer.
Table 4.
Allele-specific primers and sequencing primers used for pyrosequencing reactions for the three STH β-tubulins
| Codon | Species | Primer sequences (5′–3′) | T°ann (°C) |
|---|---|---|---|
| 167 | A. lumbricoides | Forward: CCGTGAAGAATACCCCGAC | 59 |
| Reverse: GGTGGACTGACAGAGTTGC | |||
| Seq primer: ACCCCGACAGAATCATGAGCTCG | |||
| T. trichiura | Forward: CCTCGGCGGCGGAACTG | 59 | |
| Reverse: GGTGGACTGACAGAGTTGC | |||
| Seq primer: TGACCGAATTATGACAACT | |||
| N. americanus | Forward: CCGGATCAGGAATGGGAAC | 59 | |
| Reverse: GGCTCAACCACAGTGTCG | |||
| Seq primer: GATAGAATCATGTCCTCGT | |||
| 198 | A. lumbricoides | Forward: AGGTTTCTGATGTGGTGTTGGA | 59 |
| Reverse: CAAATGATTTAAGTCTCCG | |||
| Seq primer: GGTTGAGAACACCGAT | |||
| T. trichiura | Forward: AGGTTTCAGATACAGTTGTAG | 59 | |
| Reverse: CAAATGATTTAAGTCTCCG | |||
| Seq primer: GGTAGAGAACACGGACG | |||
| N. americanus | Forward: GTTTCCGACACTGTGGTTGAG | 58 | |
| Reverse: GATTCAGATCTCCATATGTTGGG | |||
| Seq primer: GTTGAGAATACAGATG | |||
| 200 | N. americanus | Forward: GTTTCCGACACTGTGGTTGAG | 58 |
| Reverse: GATTCAGATCTCCATATGTTGGG | |||
| Seq primer: GTTGAGAATACAGATG |
T° ann = primer annealing temperature; Seq primer = sequencing primer.
Molecular assay applied to field hookworm samples.
Hookworm DNA from pools of eggs or larvae and individual worms were amplified using the same primers, and PCR conditions described previously. All PCR products were checked on agarose gel before performing genotyping of the Phe200Tyr SNP with the pyrosequencer.
Allele frequency and sequence analysis.
Plasmids and hookworm genotypes were obtained by pyrosequencing and the allele frequencies of each SNP were determined by the AQ module in the PSQ 96 Single Nucleotide Position Software (Biotage AB, Charlottesville, VA). Conventional Sanger sequencing was also performed for each plasmid and chromatograms resulting from sequencing were analyzed with Sequencher (version 5.0, Ann Arbor, MI). The WT and MT plasmids sequences were aligned using CLUSTALW on Geneious (version 5.5.6: www.geneious.com).
EHA for hookworms.
Before being applied to human hookworms, the EHA was optimized using dog hookworms (A. caninum). One gram of fecal material was used to perform an egg count and the rest were stored under anaerobic conditions to avoid larval development until egg isolation was performed. To isolate the eggs, one gram of feces was suspended in distilled water and the mixture was poured into a tube through surgical gauze to remove large debris. After centrifugation for 10 min at 300 × g the supernatant was discarded and a saturated sucrose solution (Sp. Gr. 1.27) was added. The sediment was thoroughly mixed with the sugar solution until fully resuspended, and then additional saturated sucrose solution was added to form a slight positive meniscus. Before centrifugation, a cover slip was placed on the top of the tube and then after centrifugation the tube was left for 10 minutes before removing the cover slip. The cover slip was rinsed with distilled water to remove the eggs, and the number of eggs recovered was counted using low power microscopy (40× magnification). Residual sucrose was removed by successive washing with distilled water, and centrifugation. Additional one-gram samples were used to isolate more eggs as needed. After all eggs for a given sample were recovered, the isolated eggs were vortexed and three separate 20 μL aliquots were taken to count the number of eggs present in the sample. The sample volume was then adjusted to yield a concentration of ∼1 egg/μL.
An agar-based assay system was used as previously described24; a stock solution of thiabendazole (Sigma-Aldrich, St. Louis, MO) was serially diluted in dimethyl sulfoxide (DMSO, Sigma Aldrich, St. Louis, MO) by 2-fold dilutions. Ten drug solutions ranging from 0.8 to 0.0015125 μg/mL were produced. In the assay plates, the first two wells of each row served as non-drug controls (2 μL DMSO), the third well contained the lowest concentration chosen, and the last well the highest. The 200 μL of 2% agar (Bacto Agar, VWR Becton Dickinson Sparks, MD) was then added to each well containing the drug or DMSO. Assay plates were allowed to cool so that agar would harden, and then sealed with parafilm and placed in sealed zip-lock plastic bags in the refrigerator at 4°C until used. At the time of the assay, plates were removed from the refrigerator and allowed to warm to room temperature. Forty microliters, containing ∼40 hookworm eggs were then added to each well and the plates placed into an incubator at 29°C. Plates were incubated for 16, 18, or 45 hours, and assays were defined as completed when 95–100% of the eggs had hatched in the control wells. At the end of the assay each well was scored by counting the number of larvae (hatched eggs) and unhatched eggs. All assays were performed in triplicate for each drug concentration tested. Various experimental parameters were tested (summarized in Table 5) to assess their impact on the dose response and to determine the optimal conditions for assaying human hookworms under laboratory field conditions. The qualitative parameters tested included the duration of the assay, the composition of the plate (agar based versus liquid based), the drug concentration, the storage condition of the stool containing eggs, the A. caninum isolates (either laboratory isolates of A. caninum [TRS strain] or field isolates, the addition of antifungal amphotericin B [10 μg/10 mL]) (Fisher, Pittsburgh, PA), and finally the addition of Lugol's iodine solution after the incubation to stain eggs and larvae, and sodium thiosulfate to destain the background.
Table 5.
Conditions applied to each egg hatch assay
| End point (hrs) | Solutions | Storage type and temperature | |
|---|---|---|---|
| Experiment A* | 18 | N/A | N/A |
| 45 | N/A | N/A | |
| Experiment B-1† | 18 | N/A | Anaerobic in zip-loc bag at RT§ |
| Experiment B-2† | 18 | N/A | Anaerobic in air tight container at RT§ |
| Experiment B-3† | 18 | N/A | Anaerobic in air tight container at 4°C |
| Experiment C† | 18 | 50% Lugol's iodine¶ + 20% sodium thiosulfate¶ | Anaerobic in air tight container at RT |
| Experiment D† | 18 | Amphotericin B∥+ 50% Lugol's iodine + 20% sodium thiosulfate | Anaerobic in air tight container at RT |
| Experiment E‡ | 16 | Amphotericin B + 50% Lugol's iodine + 20% sodium thiosulfate | N/A |
Experiment A was split into two assays (A-1 and A-2), in experiment A-1 eggs were recovered from feces of dogs infected with laboratory isolates of Ancylostoma caninum (TRS strain), whereas in experiment A-2, eggs were recovered from feces of dogs infected with field isolates of A. caninum.
Eggs recovered from stool were stored under anaerobic conditions before the assays.
Eggs were recovered from fresh stool samples before the assays.
The temperature given refers to the storage temperature of the feces and not the incubation temperature of the assay.
Staining and distaining solutions applied into wells at the end of the assay to facilitate the scoring.
Antifungal solution added to the egg solution before the assay.
In assays from all experiments drug concentrations tested were from 0.8 to 0.003125 μg/mL.
RT = room temperature; N/A = not applicable.
Experiment A: A. caninum isolates, duration of the assay, and plate composition.
In this experiment two assays were set up and in each of them five drug concentrations (from 0.8 to 0.003125 μg/mL) were tested. For both assays, the plates used were made using either an agar or a liquid matrix. Experiment A was split into two assays, in the first (A-1), 40 μL of egg suspension from laboratory isolates was added to each well filled either with agar or liquid. Eggs and larvae were scored in each well after 18 hours of incubation, and a second time after 45 hours of incubation. In the second assay (A-2), the conditions remained the same except that field isolates of A. caninum were used.
Experiment B: Storage conditions of stool-containing eggs.
Fresh stool samples from dogs infected with the laboratory isolate of A. caninum were stored under different anaerobic conditions: The first consisting of putting a fresh fecal sample in a specialized zip-lock plastic bag where air was removed through a port using a vacuum pump (Reynolds Handi-Vac, Walmart, Athens, GA) and left at room temperature until needed (Experiment B-1). In the second, fresh stool samples were placed in two containers of 50 mL with 15 glass beads and water, and shaken to disrupt the stool. Each container was then filled completely with water and shaken again to produce anaerobic conditions. One container was placed at room temperature (Experiment B-2) and the other at 4°C (Experiment B-3). Before the anaerobic storage of the stool sample, an egg count was performed to determine the number of eggs per gram. Three days after the anaerobic storage, three assays were setup (one for each storage condition). Six drug concentrations were tested (from 0.8 to 0.003125 μg/mL). All plates used were agar-based and the plates were incubated for 18 hours.
Experiment C: Staining and destaining of eggs and larvae.
In this experiment, eggs recovered from the non-refrigerated stool sample (as in Experiment B-2) were used. Again, six drug concentrations were tested (from 0.8 to 0.003125 μg/mL) and the incubation time was 18 hours. To evaluate if staining the eggs and larvae made them easier to count, 10 μL of 50% Lugol's iodine solution followed by 20% sodium thiosulfate were added to each well at the end of the assay.
Experiment D: Addition of an antifungal solution, Amphotericin B.
In this experiment, before adding the egg suspension to the assay wells, 90 μL of the amphotericin B [10 μg/10 mL] antifungal solution was added to each 1 mL egg suspension. All conditions of the assay remained the same as in Experiment C.
Experiment E: Optimized assays.
In this experiment agar plates were prepared under the same conditions that would be used in the field to assay human hookworms. In all plates seven drug concentrations were tested (from 0.8 to 0.003125 μg/mL). Eggs were recovered from fresh fecal material obtained from dogs infected with the A. caninum laboratory isolate (TRS strain). Two assays were performed using the same conditions as are described in Experiment D. In both assays egg hatching was scored at 16 and 18 hours.
Application of the EHA to human hookworms.
The assays were performed using the protocol optimized with A. caninum. Assay plates containing agar and drug were prepared in the laboratory at McGill University and transported to the field site in Haiti. Plates were prepared with seven drug concentrations (from 0.8 to 0.003125 μg/mL) (as in Experiment E). Hookworm eggs were recovered from fresh fecal samples as described previously. Aliquots of egg suspension containing amphotericin B were disposed into each well, with all assays performed in duplicate. The average number of eggs allocated to each well of assays 3010019, 3010022, and 3010028 was 39, 11, and 10, respectively. Plates were incubated for 16 hours at room temperature at ∼29°C and staining and destaining solutions were added to each well at the termination of the assays.
Statistical analyses.
Data from EHAs were analyzed by Logit models that describe the statistical model25 and its application by fitting the data of each individual EHA and showing the relationship between the expected proportions of unaffected eggs (not hatching) at each concentration and the log concentration.26 The dose response data were obtained by a non-linear regression curve. The drug sensitivity was calculated as the LC50, which is respectively the drug concentration required to decrease the number of eggs hatching to 50% of that observed in the control wells (no drug). The LC50 values and 95% confidence intervals (CIs) were calculated in GraphPad Prism (v5.0 for Windows, GraphPad software, San Diego, CA).
Results
Development of molecular markers for each species.
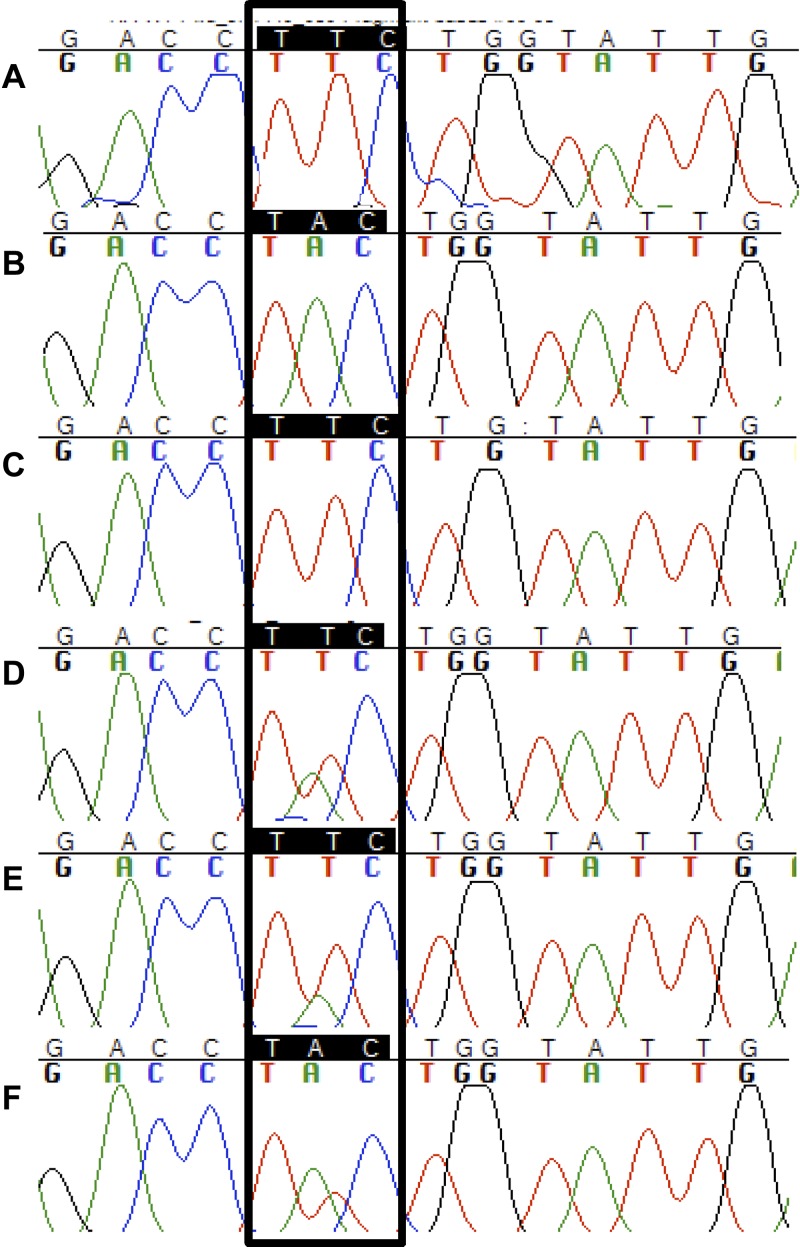
Sequencing of the WT and MT plasmids clearly showed that we had generated the desired mutations at codons 167 and 198 for the three species and at codon 200 for N. americanus. From the pyrosequencing we obtained distinct genotype profiles illustrated by pyrograms for WT and MT (Figure 1). For codons 167 and 198, WT plasmids identified the homozygote susceptible alleles T and A, respectively. The MT plasmids identified, at codon 167 and 198, the homozygote-resistant alleles A and C, respectively (data not shown). Those results confirmed that all diagnostic tests were efficient as they could identify easily and clearly the susceptible and resistant-type alleles for A. lumbricoides, T. trichiura, and N. americanus. From a previous study21 the Phe200Tyr SNP diagnostic assays using pyrosequencing were applied to A. lumbricoides and T. trichiura field samples. The results obtained validated the reproducibility and efficacy of those assays on field samples. The WT and MT plasmids served as positive controls when running pyrosequencing assays for positions 167, 200, or 198. In this study, the pyrosequencing assay was used to screen N. americanus samples from Pemba Island and Haiti (Table 1). All samples were tested for the Phe200Tyr SNP. In samples from Pemba Island, the mean allele frequency of the susceptible allele T was 100% in individual adult worms (N = 2). In pool samples (N = 7 pools; each pool of 50 larvae) the mean percentage of the susceptible allele T was 90% and the mean allele frequency of the resistance allele A was 10%. The same samples had previously been screened for the Phe200Tyr SNP by allele-specific PCR and real-time PCR. In hookworm samples from a treated area in Haiti (N = 27 pools of 10 eggs), two samples failed the PCR amplification. Among the remaining 25 samples, the mean allele frequency of the susceptible allele was 64% and the mean of the resistance-type allele was 36% (Table 6). Genotypes for some of these samples were confirmed by conventional Sanger sequencing. In samples carrying the resistance-type allele (TAC), (chromatograms D, E, F), a double peak at the second nucleotide (T and A) of the codon 200 was always detected and for each of these samples, and the pyrosequencer never showed an allele frequency of the resistance-type allele (TAC) higher than 65%. Chromatograms of the control plasmids were included to compare with those of the field samples (Figure 1).
Figure 1.
Chromatograms resulting from conventional Sanger sequencing of Necator americanus control plasmids and field samples of the β-tubulin gene around codon 200. (A) The chromatogram of the wild-type (WT) plasmid showed the presence of a single peak (T) at the second nucleotide of the codon (TTC), this characterizes the homozygous susceptible type (TTC/TTC). (B) The chromatogram of the mutant type (MT) plasmid revealed the presence of the codon associated with resistance to benzimidazoles (TAC). Chromatograms C–F show polymerase chain reaction (PCR) products of pooled egg samples from Haiti, C (pool ID no. 10 in Table 6) shows a single peak “T” at the second position of the nucleotide, whereas the sequences shown in chromatograms D (pool ID no. 11 in Table 6), E (ID no. 12 in Table 6), F (ID no. 1 in Table 6) have two peaks “T” and “A” at the same position.
Table 6.
Frequency of the susceptible allele “T” and the resistance allele “A” detected by the pyrosequencer in 25 pools, each of 10 Necator americanus eggs, collected in Haiti
| Pool IDs | Allele frequency (%) | |
|---|---|---|
| T | A | |
| 1 | 40.0 | 60.0 |
| 2 | 61.9 | 38.1 |
| 3 | 61.1 | 38.9 |
| 4 | 95.6 | 4.4* |
| 5 | 77.6 | 22.4 |
| 6 | 63.2 | 36.8 |
| 7 | 61.9 | 38.1 |
| 8 | 42.9 | 57.1 |
| 9 | 54.6 | 45.4 |
| 10 | 93.1 | 6.9* |
| 11 | 53.5 | 46.5 |
| 12 | 62.9 | 37.1 |
| 13 | 90.8 | 9.2 |
| 14 | 70.7 | 23.3 |
| 15 | 43.7 | 56.3 |
| 16 | 73.6 | 26.4 |
| 17 | 89 | 11 |
| 18 | 34.8 | 65.2 |
| 19 | 68.1 | 31.9 |
| 20 | 60.6 | 39.4 |
| 21 | 77.5 | 22.5 |
| 22 | 72.3 | 27.7 |
| 23 | 58.4 | 41.6 |
| 24 | 46.8 | 53.2 |
| 25 | 22.7 | 77.3 |
Percentages below 7% may be misleading because of pyrosequencing errors on pooled samples.
EHA.
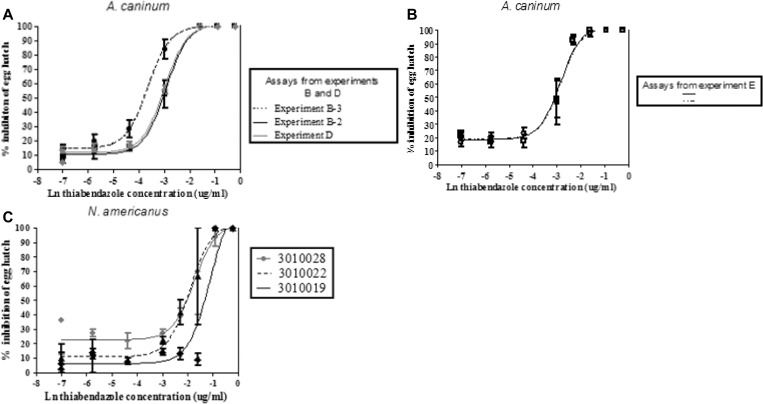
In addition to molecular assays, a biological assay was developed for A. caninum and then adapted to human hookworms to detect resistance to BZ drugs. In all assays, eggs and larvae were counted in each well and the relationship between drug concentration and egg hatching were established using a logistic regression model for dose-response, which permitted the calculation of LC50 (Figure 2 ). Data are summarized in Table 7.
Figure 2.
Dose-response curves for Ancylostoma caninum and Necator americanus to thiabendazole. Dose responses of A. caninum in egg hatch assays with thiabendazole, after 18 hours (A) obtained from three experiments B-2, B-3, and D where different conditions were used. In experiment B-3 eggs in anaerobic condition were refrigerated before the assay, whereas in experiment B-2 they were kept at room temperature. In experiment D, antifungal solution was added to the egg suspension before the assay. Panel B shows the dose responses of A. caninum to thiabendazole after 16 hours, obtained from experiment E. Panel C shows the dose responses of field N. americanus, after 16 hours exposure, obtained from three subjects naturally infected in Haiti (3010028, 3010022, and 3010019). All curves were obtained with the logit model; the dots represent the observed data obtained in all assays.
Table 7.
LC50 for thiabendazole in the egg hatch assay with Ancylostoma caninum*
| Assay from experiments: no. | LC50 (μg/mL) (95% confidence interval) |
|---|---|
| B-3 | 0.0258 (0.0205–0.0324) |
| B-2 | 0.0522 (0.0415–0.0655) |
| D | 0.0495 (0.0394–0.0622) |
| E | 0.0547 (0.0435–0.0687) |
| E | 0.0357 (0.0284–0.0448) |
The 50% lethal concentration was determined after 16 or 18 hours of exposure of A. caninum eggs to thiabendazole (see text). In experiment E, two assays were conducted under the same conditions.
Experiment A.
In this experiment, three parameters were evaluated: the hookworm isolates, the duration of the assay, and the use of an agar-based assay versus a liquid-based assay. No differences in dose response were seen between the two A. caninum isolates; at the same drug concentrations the proportions of eggs hatching were similar. However, agar-based assays were easier to score than liquid-based assays, and the data were more consistent. For both assays, 45 hours of incubation made the counting of eggs and larvae difficult because of fungal growth and the dryness of the agar plate. The long incubation did not increase the number of larvae that hatched; the scoring of eggs and larvae at 18 hours remained the same as at 45 hours (data not shown).
Experiments B–D.
In these three experiments, we tested the impact of the following parameters on the dose response: the storage conditions of the stool containing eggs (anaerobic conditions and temperature), the addition of Lugol's iodine, destainer, and amphotericin B. Experiment B-1, showed that the storage of the fecal material in specialized zip-lock style bags, left at room temperature, was not an appropriate anaerobic environment as it did not prevent the hatching of eggs. Thus, it was not possible to perform the EHA using these samples. We observed a difference in egg hatch response to thiabendazole between assays performed with refrigerated and non-refrigerated eggs stored in a tight air container. Indeed, the LC50 of the assay obtained from Experiment B-3 with refrigerated eggs (0.0258) was significantly different from the LC50 of the assays from Experiment B-2 and D where non-refrigerated eggs were used (0.0522 and 0.0496, respectively) as the 95% CIs of the LC50 did not overlap. In contrast, the assays performed with non-refrigerated eggs (Experiments B2 and D), the 95% CIs for LC50 overlapped, showing no significant difference in dose response. In Experiments C and D, addition of the antifungal solution, Lugol's iodine and sodium thiosulfate showed qualitative improvement as they facilitated the scoring of unhatched eggs and larvae (Figure 2A and Table 7).
Experiment E.
All conditions shown to quantitatively and qualitatively improve the EHA were applied in this experiment. By 16 hours larvae had hatched in the control wells and were motile. The same observation was found in the previous experiments after 18 hours incubation. Sixteen hours of incubation was therefore considered as the end point of the assay. Using these assay conditions there was no significant difference between the LC50 (overlapping 95% CIs) in the two assays. Overall the replicates of the assays showed consistent results (Figure 2B).
EHA on human hookworms.
Application of the EHA on field N. americanus eggs recovered from stool samples of three study participants produced egg hatch responses that were less sensitive to thiabendazole. The LC50 of the assays with N. americanus ranged from 0.1237 to 0.2566 μg/mL, compared with results obtained with A. caninum (LC50 ranged from 0.0258 to 0.0547 μg/mL). There was no inhibition of hatching at lower concentrations, and this shift in sensitivity meant that the concentration range tested was not high enough to achieve a maximal effect (Figure 2C). However, it was still possible to calculate the LC50, and these were not significantly different (the 95% CIs for the LC50 overlapped) among the three N. americanus samples. The major issue encountered when attempting to perform the assays were the low egg counts in the N. americanus positive participants, which limited the number of eggs we could recover and use. Less than optimal egg numbers caused us to use fewer eggs per well and to perform the assays in duplicate, rather than triplicate. This led to high variability in the data that reduced the precision of the assays.
Discussion
The development of accurate tools to detect, at an early stage, the possible development of resistance in human parasites is now a priority with large-scale MDA programs being launched for STHs. During the past 10 years, there has been some suggestion of BZ selection occurring in filarial nematodes,27 human hookworms,17,22 and in human whipworms.21 However, in these studies evidence of genetic selection was not directly linked with resistance. Nevertheless, diagnostic tools based on SNP markers for codon 200 have been developed and validated through pyrosequencing and the real-time PCR.30 In this study two molecular markers for codon 167 and 198 were developed in A. lumbricoides, T. trichiura, and N. americanus and, in addition, for codon 200 in N. americanus using the pyrosequencing method. The WT and MT control plasmids were genotyped and these showed two distinct genotypic profiles for each parasite species and for each SNP position. Thus, for the three most important human STHs, we have developed molecular makers for the three positions in the β-tubulin gene, which based on experience in veterinary nematodes are likely to be critical for BZ resistance.
The pyrosequencing method, based on “sequencing by synthesis,” has several advantages, including accuracy in detecting the frequency of single or multiple SNPs, high throughput, reliability, and the ability to determine proportions of alternative nucleotides at a SNP site in pools of eggs, larvae, or worms.27–29 The goal in developing such assays was to have reproducible procedures for SNP detection that could be used to monitor for possible resistance selection in MDA programs for human STHs. In our protocol, primer sequences for each reaction were determined and tested against plasmid constructs, each step in the procedure has been tested on field samples of human STHs and the procedures are detailed. We are aware that one of the major limitations to the use of this molecular technique, in a field context, could be the cost of the pyrosequencer. However, once the samples are collected, the genotyping could be done in laboratories where the equipment is available. Our molecular techniques are therefore suitable for resistance monitoring on field samples, where multiple samples are to be collected. The accurate assessment of SNP frequencies can then be performed in a short period of time.
We also applied the assay of the Phe200Tyr SNP on N. americanus from areas treated with albendazole and mebendazole for many years. In samples from Pemba Island, DNA from two individual adult worms showed the susceptible SNP and in a pool of hookworm larvae the percentage of the susceptible SNP was close to 100% even though those samples had been recovered from children in which mebendazole sensitivity had been lower than those found in previous years.16 However, our genotyping result was consistent with the previous molecular analyses performed by conventional Sanger sequencing and real-time PCR methods.22,30 Because of a lack of sufficient DNA, analyses of codons 167 and 198 were not performed. However, they have been investigated by others30 who failed to detect the presence of the resistance-associated SNP at either position. As suggested by these authors, further analyses on the β-tubulin gene are necessary to determine if it is possible to link the low BZ efficacy with other molecular changes in β-tubulin. In pools of 10 eggs/person of N. americanus recovered from 28 persons in areas in Haiti under MDA, the resistance-type SNP (codon 200) was identified (frequency = 36%). According to the genotype frequency obtained in each pool, we could estimate (assuming Hardy-Weinberg equilibrium) that the egg population was mainly composed of homozygous susceptible and heterozygous resistant eggs, though some homozygous resistant eggs may be present. Because BZ resistance has been shown to be a recessive trait in several nematode parasites of animals,31 it is likely that overall the population will appear susceptible, however any increase in the frequency of the resistance allele with further treatments could lead to the appearance of a resistance phenotype in the population. Genotypes were confirmed by conventional Sanger sequencing.
The pyrosequencing method has an error rate in determining SNP frequency of ∼5–7%29; this means at low allele frequency (≤ 7%) the estimate may be unreliable. Taking this error rate at low SNP frequencies into account, the resistance allele was identified in 92% of the samples and confirmed by conventional Sanger sequencing. The results indicate that the proportion of the resistance-type allele in the total population was moderately high. The finding of resistance-associated polymorphisms in N. americanus from a treated area raises the question whether further repeated treatment may cause an increase in the frequency of the resistance allele. To answer this question it will be important to perform longitudinal studies where egg samples will be collected before treatment and after several rounds of treatment, and to verify if, in the same host population, the allele frequency increases after treatment. To understand better the correlation between both parameters (treatment and the resistant allele frequency), it would be interesting to mathematically model any change in resistance allele frequency after a given number of rounds of treatment, assuming an initial resistance allele frequency as determined in this or other studies. This kind of model has been applied to filarial nematodes and been used to predict the spread of the resistance by considering different parameters.32
In this study, we also described the development of an in vitro EHA for dog hookworms, which can be used to assess sensitivity to a BZ drug. We investigated the dose response of A. caninum to thiabendazole, the best analogue of the BZ class for measuring resistance in vitro. Before applying the same assay to human hookworms, we optimized it with canine hookworms, which are close to human hookworms in terms of phylogeny and dose response to anthelmintics.33,34 In veterinary parasites, the EHA has been standardized, even if from one study to another some variations remain.35 For instance, it has been clearly defined for some veterinary nematodes that when the LC50 is in excess of 0.1 μg/mL of thiabendazole that the parasite isolate is considered to be BZ resistant.36 Since 1999, the development of the EHA in human hookworms has been supported by the World Health Organization (WHO) and the assays have been applied in several studies that proved its ability to give quantitative and qualitative measurements of the effect of thiabendazole on hatching of the eggs.24,37,38 However, the assay has not been standardized because of the lack of resistant isolates of human hookworms.38 The attempt to optimize our assay was based on quantitative and qualitative criteria and on the suitability of the assay for human hookworm eggs collected in the field. The EHA described in this study was adapted from the protocol described by Kotze and colleagues.24
First of all, it was observed that increasing the duration of incubation from 16 to 18 hours did not have any effect on the LC50, but longer incubation (45 hours) had an adverse effect on the dose response as the scoring of egg hatching was made difficult. In addition, the use of an agar-based assay was better than a liquid-based assay for two reasons: it is more practical to prepare and transport plates with the solid-phase of agar,38 as our final objective was to use the EHA in the field, and agar-based plates could be more easily standardized.38 In addition, the agar-based assay showed less variation in the drug concentrations affecting egg hatching compared with the liquid-based assay.24 However, in a recent study on dose-response assays, it was shown that both agar and liquid-based assays have the same sensitivity to discriminate known resistant and sensitive Haemonchus contortus isolates.38 In this study, we also compared the impact of fecal storage conditions and temperature on the egg hatch response. Based on our data, we recommend anaerobic storage of eggs at room temperature because it is unknown how storage at 4°C may affect the fitness of the eggs and their ability to develop. Furthermore, refrigeration may not be available in the field. Additional work is needed to assess the value of refrigerating the eggs to delay development before commencement of an EHA. We also observed that more stringent washing of the egg preparation reduced bacterial growth, and the use of Lugol's iodine improved qualitatively scoring egg hatching but had no significant impact on the dose response. Thus, in our study we determined important qualitative and quantitative parameters that have an impact on the egg hatch response assessment in A. caninum and showed the reproducibility of the test once optimized.
The suitability of the conditions of the EHA in a field context was another factor considered. Indeed, the temperature of incubation (29°C) of the assays was set according to the ambient temperature that could be reached in a field laboratory in a tropical country where electricity shut downs may be frequent or incubators might not be available. Another important consideration was the low cost of the procedure in terms of labor and time. Agar-based plates could be made beforehand and stored at 4°C or at room temperature for 78 days without showing any change in dose response.38 Finally, the EHA was applied in the field on N. americanus using the same conditions that had been established for A. caninum. Unfortunately, a small sample size and low intensity of N. americanus infection prevented conclusions on the in vitro susceptibility of N. americanus in the area investigated. Beyond this limitation, two additional ones prevented the standardization of the EHA on human hookworms. Indeed, as previously highlighted38 the lack of information on the dose response of known resistant and susceptible field isolates make decisions on the susceptibility of hookworm samples difficult. In this study, even though the three assays with N. americanus showed a lower sensitivity to thiabendazole compared with A. caninum and N. americanus used in other studies,24,37,38 this does not mean that the field isolates used in this study were resistant, as there is no data available, so far, on the response of a fully characterized field-resistant strain as a reference. In addition, the lack of data on the LC50 for human hookworms was also a challenge for attempts to standardize the assay. The range of drug concentrations used was not sufficient to achieve a maximal effect. As suggested by Kotze and colleagues, a broader range of drug concentration should have been tried to achieve maximal drug response.38 Finally, to validate this assay as a diagnostic tool to detect resistance, it will be interesting to correlate the LC50, the frequency of the resistance alleles, and response to treatment in terms of egg count reduction.
ACKNOWLEDGMENTS
We are grateful to Patrick Lammie from the Centers for Diseases Control and Prevention for his contribution to the study. We thank Thomas Streit from Notre Dame University for the logistic support in Haiti. We also thank the staff members of the Hôpital Sainte-Croix in Léôgane for their commitment and their support: Luccene Desir, Wesly Pierre, Dardith Desire, Wilman Metelus, Rose-Guerda Louis-Charles, Wilfrid Jean, Jean Makatu Innocent, Shiler Emile, Eudson Romulus, Max Rosemond, and Michelet Leriche. We also thank Marco Albonico for providing hookworm DNA samples, and John McCall and TRS Labs Inc. for providing canine stool samples containing A. caninum eggs.
Footnotes
Financial support: This work was supported by a grant from the Atlanta Research and Education Foundation, Inc. in collaboration with the Centers for Diseases Control and Prevention (CDC), and the research at the Institute of Parasitology was supported by the Québec Centre for Host Parasite Interactions/FQRNT.
Authors' addresses: Aïssatou Diawara and Roger K. Prichard, Institute of Parasitology, McGill University, Ste-Anne-de-Bellevue, Quebec, Canada, E-mails: aissatou.diawara@mail.mcgill.ca and roger.prichard@mcgill.ca. Jan M. Schwenkenbecher and Ray M. Kaplan, Department of Infectious Diseases, College of Veterinary Medicine, University of Georgia, Athens, GA, E-mails: janschwenkenbecher@yahoo.de and rkaplan@uga.edu.
References
- 1.WHO . Informal Consultation on Monitoring Drug Efficacy in the Control of Schistosomiasis and Intestinal Nematodes. Geneva: World Health Organization; 1999. pp. 1–59. [Google Scholar]
- 2.Brooker S, Clements AC, Bundy DA. Global epidemiology, ecology and control of soil-transmitted helminth infections. Adv Parasitol. 2006;62:221–261. doi: 10.1016/S0065-308X(05)62007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotez PJ, Kamath A. Neglected tropical diseases in sub-Saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis. 2009;3:e412. doi: 10.1371/journal.pntd.0000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotez PJ. A constitutional amendment for deworming. PLoS Negl Trop Dis. 2009;3:e454. doi: 10.1371/journal.pntd.0000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan MS. The global burden of intestinal nematode infections–fifty years on. Parasitol Today. 1997;13:438–443. doi: 10.1016/s0169-4758(97)01144-7. [DOI] [PubMed] [Google Scholar]
- 6.Molyneux DH, Hotez PJ, Fenwick A, Newman RD, Greenwood B, Sachs J. Neglected tropical diseases and the Global Fund. Lancet. 2009;373:296–297. doi: 10.1016/S0140-6736(09)60089-1. [DOI] [PubMed] [Google Scholar]
- 7.Albonico M, Stoltzfus RJ, Savioli L, Chwaya HM, d'Harcourt E, Tielsch JM. A controlled evaluation of two school-based anthelminthic chemotherapy regimens on intensity of intestinal helminth infections. Int J Epidemiol. 1999;28:591–596. doi: 10.1093/ije/28.3.591. [DOI] [PubMed] [Google Scholar]
- 8.Albonico M, Montresor A, Crompton DW, Savioli L. Intervention for the control of soil-transmitted helminthiasis in the community. Adv Parasitol. 2006;61:311–348. doi: 10.1016/S0065-308X(05)61008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett A, Guyatt H. Reducing intestinal nematode infection: efficacy of albendazole and mebendazole. Parasitol Today. 2000;16:71–74. doi: 10.1016/s0169-4758(99)01544-6. [DOI] [PubMed] [Google Scholar]
- 10.Taylor-Robinson D, Jones A, Garner P. Does deworming improve growth and school performance in children? PLoS Negl Trop Dis. 2009;3:e358. doi: 10.1371/journal.pntd.0000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan RM, Vidyashankar AN. An inconvenient truth: global worming and anthelmintic resistance. Vet Parasitol. 2012;186:70–78. doi: 10.1016/j.vetpar.2011.11.048. [DOI] [PubMed] [Google Scholar]
- 12.Kwa MS, Veenstra JG, Roos MH. Benzimidazole resistance in Haemonchus contortus is correlated with a conserved mutation at amino acid 200 in beta-tubulin isotype 1. Mol Biochem Parasitol. 1994;63:299–303. doi: 10.1016/0166-6851(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 13.Silvestre A, Cabaret J. Mutation in position 167 of isotype 1 beta-tubulin gene of Trichostrongylid nematodes: role in benzimidazole resistance? Mol Biochem Parasitol. 2002;120:297–300. doi: 10.1016/s0166-6851(01)00455-8. [DOI] [PubMed] [Google Scholar]
- 14.Ghisi M, Kaminsky R, Maser P. Phenotyping and genotyping of Haemonchus contortus isolates reveals a new putative candidate mutation for benzimidazole resistance in nematodes. Vet Parasitol. 2007;144:313–332. doi: 10.1016/j.vetpar.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Mottier M, Prichard RK. Genetic analysis of a relationship between macrocyclic lactone and benzimidazole anthelmintic selection on Haemonchus contortus. Pharmacogenet Genomics. 2008;18:129–140. doi: 10.1097/FPC.0b013e3282f4711d. [DOI] [PubMed] [Google Scholar]
- 16.Albonico M, Bickle Q, Ramsan M, Montresor A, Savioli L, Taylor M. Efficacy of mebendazole and levamisole alone or in combination against intestinal nematode infections after repeated targeted mebendazole treatment in Zanzibar. Bull World Health Organ. 2003;81:343–352. [PMC free article] [PubMed] [Google Scholar]
- 17.Flohr C, Tuyen LN, Lewis S, Minh TT, Campbell J, Britton J, Williams H, Hien TT, Farrar J, Quinnell RJ. Low efficacy of mebendazole against hookworm in Vietnam: two randomized controlled trials. Am J Trop Med Hyg. 2007;76:732–736. [PubMed] [Google Scholar]
- 18.Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008;299:1937–1948. doi: 10.1001/jama.299.16.1937. [DOI] [PubMed] [Google Scholar]
- 19.Montresor A. Cure rate is not a valid indicator for assessing drug efficacy and impact of preventive chemotherapy interventions against schistosomiasis and soil-transmitted helminthiasis. Trans R Soc Trop Med Hyg. 2011;105:361–363. doi: 10.1016/j.trstmh.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geerts S, Gryseels B. Drug resistance in human helminths: current situation and lessons from livestock. Clin Microbiol Rev. 2000;13:207–222. doi: 10.1128/cmr.13.2.207-222.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diawara A, Drake LJ, Suswillo RR, Kihara J, Bundy DA, Scott ME, Halpenny C, Stothard JR, Prichard RK. Assays to detect beta-tubulin codon 200 polymorphism in Trichuris trichiura and Ascaris lumbricoides. PLoS Negl Trop Dis. 2009;3:e397. doi: 10.1371/journal.pntd.0000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albonico M, Wright V, Bickle Q. Molecular analysis of the beta-tubulin gene of human hookworms as a basis for possible benzimidazole resistance on Pemba Island. Mol Biochem Parasitol. 2004;134:281–284. doi: 10.1016/j.molbiopara.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Champetier de Ribes GFM, Desormeaux AM, Eyma E, Montagut P, Champagne C, Pierre J, Pape JW, Raccurt CP. Intestinal helminths in school children in Haiti in 2002. Bull Soc Pathol Exot. 2005;98:127–132. [PubMed] [Google Scholar]
- 24.Kotze AC, Coleman GT, Mai A, McCarthy JS. Field evaluation of anthelmintic drug sensitivity using in vitro egg hatch and larval motility assays with Necator americanus recovered from human clinical isolates. Int J Parasitol. 2005;35:445–453. doi: 10.1016/j.ijpara.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Dobson RJ, Griffiths DA, Donald AD, Waller PJ. A genetic model describing the evolution of levamisole resistance in Trichostrongylus colubriformis, a nematode parasite of sheep. IMA J Math Appl Med Biol. 1987;4:279–293. doi: 10.1093/imammb/4.4.279. [DOI] [PubMed] [Google Scholar]
- 26.Waller PJ, Dobson RJ, Donald AD, Griffiths DA, Smith EF. Selection studies on anthelmintic resistant and susceptible populations of Trichostrongylus colubriformis of sheep. Int J Parasitol. 1985;15:669–676. doi: 10.1016/0020-7519(85)90014-1. [DOI] [PubMed] [Google Scholar]
- 27.Schwab AE, Boakye DA, Kyelem D, Prichard RK. Detection of benzimidazole resistance-associated mutations in the filarial nematode Wuchereria bancrofti and evidence for selection by albendazole and ivermectin combination treatment. Am J Trop Med Hyg. 2005;73:234–238. [PubMed] [Google Scholar]
- 28.Barrère V, Alvarez L, Suarez G, Ceballos L, Moreno L, Lanusse C, Prichard RK. Relationship between increased albendazole systemic exposure and changes in single nucleotide polymorphisms on the β-tubulin isotype 1 encoding gene in Haemonchus contortus. Vet Parasitol. 2012;186:344–349. doi: 10.1016/j.vetpar.2011.11.068. [DOI] [PubMed] [Google Scholar]
- 29.Eng JK, Blackhall WJ, Osei-Atweneboana MY, Bourguinat C, Galazzo D, Beech RN, Unnasch TR, Awadzi K, Lubega GW, Prichard RK. Ivermectin selection on beta-tubulin: evidence in Onchocerca volvulus and Haemonchus contortus. Mol Biochem Parasitol. 2006;150:229–235. doi: 10.1016/j.molbiopara.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Schwenkenbecher JM, Albonico M, Bickle Q, Kaplan RM. Characterization of beta-tubulin genes in hookworms and investigation of resistance-associated mutations using real-time PCR. Mol Biochem Parasitol. 2007;156:167–174. doi: 10.1016/j.molbiopara.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 31.Elard L, Humbert JF. Importance of the mutation of amino acid 200 of the isotype 1 beta-tubulin gene in the benzimidazole resistance of the small-ruminant parasite Teladorsagia circumcincta. Parasitol Res. 1999;85:452–456. doi: 10.1007/s004360050577. [DOI] [PubMed] [Google Scholar]
- 32.Schwab AE, Churcher TS, Schwab AJ, Basanez MG, Prichard RK. Population genetics of concurrent selection with albendazole and ivermectin or diethylcarbamazine on the possible spread of albendazole resistance in Wuchereria bancrofti. Parasitol. 2006;133:589–601. doi: 10.1017/S003118200600076X. [DOI] [PubMed] [Google Scholar]
- 33.Blaxter ML, De Ley P, Garey JR, Liu LX, Scheldeman P, Vierstraete A, Vanfleteren JR, Mackey LY, Dorris M, Frisse LM, Vida JT, Thomas WK. A molecular evolutionary framework for the phylum nematoda. Nature. 1998;392:71–75. doi: 10.1038/32160. [DOI] [PubMed] [Google Scholar]
- 34.Kotze AC, Clifford S, O'Grady J, Behnke JM, McCarthy JS. An in vitro larval motility assay to determine anthelmintic sensitivity for human hookworm and Strongyloides species. Am J Trop Med Hyg. 2004;71:608–616. [PubMed] [Google Scholar]
- 35.von Samson-Himmelstjerna G, Coles GC, Jackson F, Bauer C, Borgsteede F, Cirak VY, Demeler J, Donnan A, Dorny P, Epe C, Harder A, Hoglund J, Kaminsky R, Kerboeuf D, Kuttler U, Papadopoulos E, Posedi J, Small J, Varady M, Vercruysse J, Wirtherle N. Standardization of the egg hatch test for the detection of benzimidazole resistance in parasitic nematodes. Parasitol Res. 2009;105:825–834. doi: 10.1007/s00436-009-1466-1. [DOI] [PubMed] [Google Scholar]
- 36.Coles GC, Bauer C, Borgsteede FH, Geerts S, Klei TR, Taylor MA, Waller PJ. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol. 1992;44:35–44. doi: 10.1016/0304-4017(92)90141-u. [DOI] [PubMed] [Google Scholar]
- 37.Albonico M, Wright V, Ramsan M, Haji HJ, Taylor M, Savioli L, Bickle Q. Development of the egg hatch assay for detection of anthelminthic resistance in human hookworms. Int J Parasitol. 2005;35:803–811. doi: 10.1016/j.ijpara.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 38.Kotze AC, Lowe A, O'Grady J, Kopp SR, Behnke JM. Dose-response assay templates for in vitro assessment of resistance to benzimidazole and nicotinic acetylcholine receptor agonist drugs in human hookworms. Am J Trop Med Hyg. 2009;81:163–170. [PubMed] [Google Scholar]