Abstract
The genus Halicephalobus consists of eight species of free-living nematodes. Only one species (H. gingivalis) has been reported to infect vertebrates. Human infection is extremely rare, and only four cases have been reported in the literature. These nematodes seem to exhibit neurotropism, but their life cycle, mode of infection, and risk factors are poorly understood. Neurohelminthiases are not commonly recognized in the United States and when they do occur, pose great diagnostic challenges because of lack of appropriate non-invasive screening and/or confirmatory tests. We report a challenging case of meningoencephalomyelitis caused by a Halicephalobus sp., in which the patient had a rapidly deteriorating clinical course. The case did not raise any clinical suspicion of neurohelminthiases, although increased eosinophils were present in the cerebrospinal fluid. This case presents an opportunity to highlight the importance of considering parasitic infection in meningoencephalitis or meningoencephalomyelitis presenting atypically.
Introduction
Halicephalobus gingivalis,1 also referred to as Micronema deletrix or H. deletrix, is a free-living saprophytic nematode belonging to the order Tylenchida and class Chromadorea. It is capable of infecting and reproducing in vertebrates, including horses,2–5 zebras,6 and humans.7–9 The genus Halicephalobus contains many species, of which only H. gingivalis has been reported to infect vertebrates and is morphologically distinct from the other seven species.2 The other seven species are identified only as free-living forms in soil, rotting plant matter, and fresh and salt water. Unless a well-preserved specimen of adult H. gingivalis worms is available, it is not possible to delineate the species on morphology from histologic sections. The other alternative is to use molecular methods. We report the fifth case of human infection caused by H. gingivalis. The clinicopathologic features and differential diagnosis are discussed.
Case Report
A 65-year-old Caucasian woman from Pensacola, Florida, was given a diagnosis of a urinary tract infection caused by a Klebsiella sp. She was treated with ciprofloxacin and discharged. Soon after being discharged, nausea and vomiting developed in the patient. A week later, she had a fever and was admitted to a hospital. She also had mild congestion of sinusoidal air spaces, which was relieved by treatment with the antihistamine loratidine. She had no other symptoms and denied having any sick contacts. Her medical history was significant for chronic back pain, hypertension, osteoarthritis, manic depression, vertebral compression fractures, lumbar stenosis, tonsillectomy, appendectomy, and hysterectomy.
She was single and lived alone. She had a pet dog. She smoked but did not consume alcohol. Her admitting temperature was 102.5°F and she appeared ill, but was in no acute distress. Results of a physical examination were unremarkable. She was anemic, but her leukocyte count was within reference limits. There was no eosinophilia and her eosinophil percentage was 0.5%.
On day 3 of admission, she had blurring of vision in the left eye and tenderness in the left temporal area, and temporal arteritis was clinically suspected. On day 4, she became confused and her mental status deteriorated quickly thereafter. Although she never showed clinical signs of meningism, meningoencephalitis was suspected. Approximately 12.5 mL of clear and colorless cerebrospinal fluid was tapped. The cerebrospinal fluid leukocyte count was slightly increased (160 cells/μL), and she had a differential count of 14% neutrophils, 20% lymphocytes, 35% macrophages, 27% eosinophils, and 4% other cells.
Computed tomography of the head showed mild cerebral atrophy and no other abnormality. Magnetic resonance imaging of the brain showed mild chronic ischemic changes in the brain, but was otherwise unremarkable. Magnetic resonance imaging of the spine showed left lateral disc protrusion at L4–L5, with 50% narrowing of spinal canal, which explained her back pain. Results of an electroencephalogram were abnormal with moderate diffuse slowing of electrocerebral activity, suggestive of encephalopathy of toxic, metabolic or degenerative nature. Although her erythrocyte sedimentation rate was increased, the result of a left temporal artery biopsy was unremarkable.
A viral or fungal meningoencephalitis was suspected and she was tested for cryptococcal antigen, arbovirus panel by serologic analysis, and herpes simplex virus types 1 and 2 DNA by polymerase chain reaction; all results were negative. On day 5, she was intubated for respiratory failure because of pulmonary edema and became comatose. She died approximately 2 weeks after the onset of her symptoms.
An autopsy was performed at the request of her physician. A complete autopsy showed the following findings. Results of the external examination were unremarkable. The liver appeared congested and weighed 1,900 grams. The heart weighed 490 grams and showed mild atherosclerotic coronary artery disease. The brain weighed 1,140 grams and appeared autolytic and did not retain its shape. There was bilateral pulmonary congestion.
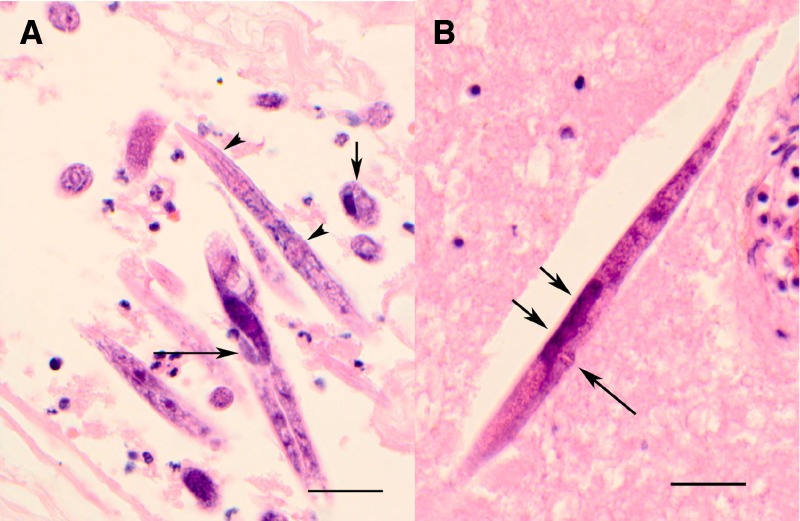
Microscopic examination of brain, spinal cord, and meninges showed extensive inflammation and nematodes morphologically consistent with Halicephalobus sp. The morphology observed in the sections included the presence of only adult female worms with a maximum diameter of approximately 25 μm; a smooth cuticle; a rhabtidiform esophagus with corpus, isthmus, and posterior bulb; a female genital tract with single dorsiflexed ovary; a single uterus with eggs; protuberant vulval lips, and a tail that was long and tapered to a point (Figure 1). Also noted were numerous larval forms that were somewhat smaller but with the same features as the adult females, but lacking a developed reproductive system.
Figure 1.
Photomicrographs of brain tissue containing Halicephalobus sp. A, Numerous sections of females and larvae, showing rhabditoid esophagus (arrowheads), flexed ovary (long arrow), and a cross section (short arrow) showing intestine (on the right) and single reproductive tube (on left), (H&E stain, ×600). B, Longitudinal section of a female worm showing protuberant vulva (long arrow) and two eggs (short arrows) (Hematoxylin and eosin stained, original magnification ×600, scale bars = 50 μm).
The worms seem to be more abundant in the perivascular region of the brain parenchyma. The inflammatory cells were predominantly neutrophils, but also included lymphocytes, macrophages and rare eosinophils. Rare worms were seen in myocardium and lung parenchyma.
Discussion
Halicephalobus gingivalis is an uncommon cause of meningoencephalitis in humans. All previous cases were reported from North America and meningoencephalitis with myelitis was reported in half the cases. The mode of infection appears to be by penetration of existing lesions in the skin, although in half of the cases, including the present case, no apparent entry lesion was noted. Based on the diffuse perivascular distribution of these organisms in the meninges and the brain parenchyma, it is likely that these nematodes reach the central nervous system by hematogenous spread and penetrate the blood–brain barrier. Clinical changes common to the reported cases include fever, mental changes, and lethargy with cerebrospinal studies showing elevated leukocyte counts. Grossly, there were diffuse changes such as edema or hyperemia of brain parenchyma and dullness of meninges. Microscopically, all cases had a mixed inflammatory infiltrate containing neutrophils, eosinophils, lymphocytes, and multinucleate histiocytes. It should be noted that the age range in the previous case-patients was 5–54 years, and that the present case-patient is the oldest to date.
All reported cases of human Halicephalobus infections were fatal and because all of them were diagnosed post mortem, none of them were treated with anthelmintic drugs. Treatment of H. gingivalis infections in other animals is mostly unsuccessful and may be caused by the inability of anthelmintic drugs to cross the blood–brain barrier and penetrate the granulomatous lesions in the brain or a lack of sensitivity of H. gingivalis to anthelmintic drugs such as ivermectin and benzimidazole.4 There have been only two reports of successful treatment of extra-central nervous system–localized halicephalobus infections in horses with ivermectin alone or with diethylcarbamazine.10,11 An approach similar to treatment of disseminated strongyloidiasis may be prudent, specifically use of ivermectin alone or in combination with albendazole,12 but this approach would require antemortem clinical suspicion of halicephalobiasis.
Nematode infections of the brain are not common. The differential diagnosis of tissue nematodes in the brain includes Toxocara canis, Angiostrongylus cantonensis, Strongyloides stercoralis, Gnathostoma spinigerum, Baylisascaris procyonis, and Lagochilascaris minor.13 Size and morphologic features enable ready distinction from most of these other nematodes. Ascarid larvae (Toxocara, Baylisascaris, Lagochilascaris) are characterized by lateral alae, prominent excretory ducts, and thick, columnar intestinal cells. There is no development of reproductive structures in these larvae. Toxocara larvae are typically smaller (20 μm wide), whereas Baylisascaris larvae are larger (on average 50 μm in diameter) than Halicephalobus larvae. Immature Angiostrongylus spp. have two reproductive tubes, dome-shaped lateral chords, and the intestine has few, yet multinucleate, cells. When seen in the brain, Angiostrongylus are also larger (150–250 μm in diameter). Gnathostoma larvae are generally greater than 200 μm in diameter, making them much larger than either the adults or larvae of Halicephalobus. In addition, the presence of body spines, robust musculature, distinct intestine with prominent nuclei in each cell, and absence of developed reproductive structures allows easy differentiation from Halicephalobus. Strongyloides stercoralis is the helminth most-similar morphologically to Halicephalobus. In extraintestinal infection, filariform larvae may be seen but are of smaller diameter than Halicephalobus larvae and have small double lateral alae. Gravid female Strongyloides are usually not seen in the brain in cases of disseminated strongyloidiasis, and adult females have two reproductive tubes whereas in Halicephalobus the females have a single reproductive tube.
Neurohelminthiases in general are extremely rare in United States. As a result, physicians in the United States do not routinely consider parasitic infections in the differential diagnosis of meningoencephalitis and it is unlikely that halicephalobiasis would be in the differential diagnosis even if other helminthic infections were considered. This feature is complicated by the fact that there are no easy screening or confirmatory tests for parasitic meningoencephalitis and a definitive diagnosis of halicephalobiasis is difficult to confirm in the absence of accessible lesions for biopsy. Although extremely uncommon, clinicians may wish to entertain a diagnosis of Halicephalobis infection, especially in rapidly progressing neurologic cases in which other possible infections have been ruled out. Unfortunately, at present, arriving at a definitive diagnosis has only been possible postmortem.
ACKNOWLEDGMENTS
We thank the laboratory of University of South Alabama Medical Center for support. The American Committee on Clinical Tropical Medicine and Travelers' Health (ACCTMTH) assisted with publication expenses.
Footnotes
Authors' addresses: Bhavesh Papadi, National Cancer Institute, National Institutes of Health, Bethesda, MD, E-mail: bhavesh2papadi@yahoo.com. Carole Boudreaux and J. Allan Tucker, Pathology Department, University of South Alabama Medical Center, Mobile, AL, E-mails: cboudrea@usouthal.edu and atucker@usouthal.edu. Blaine Mathison, Henry Bishop, and Mark E. Eberhard, Center for Global Health, Division of Parasitic Diseases and Malaria, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: gqa4@cdc.gov, hsb2@cdc.gov, and mle1@cdc.gov.
References
- 1.Stefanski W. Rhabditis gingivalis sp. n. parasite trouve dans un granulome de la gencive chez un cheval. Acta Parasitol. 1954;1:329–334. [Google Scholar]
- 2.Anderson RC, Linder KE, Peregrine AS. Halicephalobus gingivalis (Stefanski, 1954) from a fatal infection in a horse in Ontario, Canada with comments on the validity of H. deletrix and a review of the genus. Parasite. 1998;5:255–261. doi: 10.1051/parasite/1998053255. [DOI] [PubMed] [Google Scholar]
- 3.Bryant UK, Lyons ET, Bain FT, Hong CB. Halicephalobus gingivalis-associated meningoencephalitis in a thoroughbred foal. J Vet Diagn Invest. 2006;18:612–615. doi: 10.1177/104063870601800618. [DOI] [PubMed] [Google Scholar]
- 4.Hermosilla C, Coumbe KM, Habershon-Butcher J, Schöniger S. Fatal equine meningoencephalitis in the United Kingdom caused by the panagrolaimid nematode Halicephalobus gingivalis: case report and review of the literature. Equine Vet J. 2011;43:759–763. doi: 10.1111/j.2042-3306.2010.00332.x. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson R, van Dreumel T, Keystone JS, Manning A, Malatestinic A, Caswell JL, Peregrine AS. Unsuccessful treatment of a horse with mandibular granulomatous osteomyelitis due to Halicephalobus gingivalis. Can Vet J. 2008;49:1099–1103. [PMC free article] [PubMed] [Google Scholar]
- 6.Isaza R, Schiller CA, Stover J, Smith PJ, Greiner EC. Halicephalobus gingivalis (Nematoda) infection in a Grevy's zebra (Equus grevyi) J Zoo Wildl Med. 2000;31:77–81. doi: 10.1638/1042-7260(2000)031[0077:HGNIIA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Hoogstraten J, Young WG. Meningo-encephalomyelitis due to the saprophagous nematode, Micronema deletrix. Can J Neurol Sci. 1975;2:121–126. doi: 10.1017/s0317167100020102. [DOI] [PubMed] [Google Scholar]
- 8.Gardiner CH, Koh DS, Cardella TA. Micronema in man: third fatal infection. Am J Trop Med Hyg. 1981;30:586–589. doi: 10.4269/ajtmh.1981.30.586. [DOI] [PubMed] [Google Scholar]
- 9.Ondrejka SL, Procop GW, Lai KK, Prayson RA. Fatal parasitic meningoencephalomyelitis caused by Halicephalobus deletrix: a case report and review of the literature. Arch Pathol Lab Med. 2010;134:625–629. doi: 10.5858/134.4.625. [DOI] [PubMed] [Google Scholar]
- 10.Dunn DG, Gardiner CH, Dralle KR, Thilsted JP. Nodular granulomatous posthitis caused by Halicephalobus (syn. Micronema) sp. in a horse. Vet Pathol. 1993;30:207–208. doi: 10.1177/030098589303000215. [DOI] [PubMed] [Google Scholar]
- 11.Pearce SG, Boure LP, Taylor JA, Peregrine AS. Treatment of a granuloma caused by Halicephalobus gingivalis in a horse. J Am Vet Med Assoc. 2001;219:1735–1738. doi: 10.2460/javma.2001.219.1735. [DOI] [PubMed] [Google Scholar]
- 12.Segarra-Newnham M. Manifestations, diagnosis, and treatment of Strongyloides stercoralis infection. Ann Pharmacother. 2007;41:1992–2001. doi: 10.1345/aph.1K302. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura K, Hung T. Current views on geographic distribution and modes of infection of neurohelminthic diseases. J Neurol Sci. 1997;145:5–14. doi: 10.1016/s0022-510x(96)00293-6. [DOI] [PubMed] [Google Scholar]