Abstract
Although the dengue iceberg phenomenon is well known, there is a paucity of data on inapparent dengue. Results from a seroepidemiological study conducted during a dengue epidemic in 2007 in Singapore showed a seroprevalence of 65.9% and an inapparent dengue rate of 78%. Older adults (> 45 years old) had significantly higher rates of inapparent dengue infections (P < 0.05).
Since the first report of a dengue epidemic in 1901, Singapore has had outbreaks of dengue fever (DF). Unlike many countries in the region, dengue cases in Singapore are largely reported among the adult population,1,2 and dengue mortality is mainly observed in adults.3 Dengue infection is often epitomized by the iceberg effect, in which most cases are clinically inapparent.4 Although inapparent dengue has been reported in many countries,5–7 there is little data on its epidemiology or the extent of its severity. During the dengue epidemic in Singapore in 2007, we conducted cross-sectional seroepidemiologic surveys in seven outbreak areas (Figure 1) to estimate the dengue attack rate and the rate of inapparent dengue, and to further our understanding of the disease epidemiology.
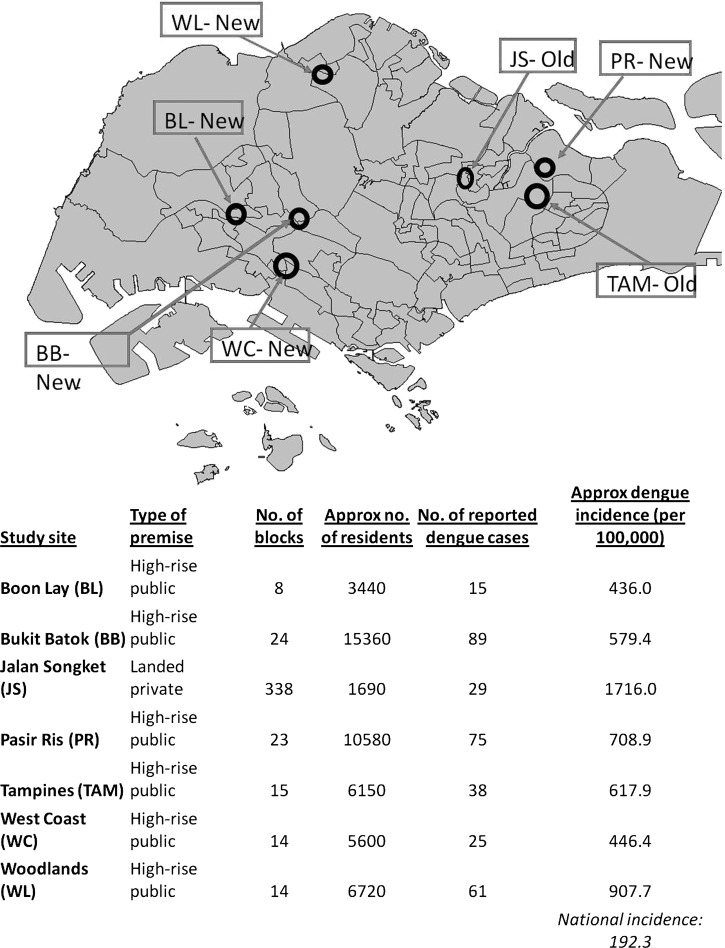
Figure 1.
Distribution of the seven study sites in Singapore, and estimated dengue incidences in each of the study sites. Because there were no census data available, calculations were based on number of units in each block of high-rise public housing, and the average household size based on data collected during the study.
All residents at each site were invited to participate in the study, which involved obtaining blood samples and a face-to-face, interviewer-administered questionnaire on demographics, clinical symptoms of dengue, and epidemiologic information. The study was reviewed and approved by the National Environment Agency Bioethics Committee. Signed, informed consent was provided by all study participants.
A total of 3,939 blood samples (participation rates = 3–15%) (Figure 2) were collected, stored on ice during transport, processed, and stored at –80°C until testing. Serum samples were tested by using a capture dengue IgM enzyme-linked immunosorbent assay (ELISA) and an indirect dengue IgG ELISA (PanBio, Windsor, Queensland, Australia) according to manufacturer's instructions. One study reported the sensitivity and specificity of the indirect dengue IgG ELISA to be 100% and 98%, respectively, and those of the capture dengue IgM ELISA to be 87% and 96%, respectively.8 The possibility that rheumatoid factors interfering with interpretation of IgM results was eliminated by removal of these factors before samples were assayed. A reverse transcription polymerase chain reaction (RT-PCR) was performed on 400 randomly selected samples by using an in-house assay.9 Seroprevalence was calculated by adding the number of samples that were positive for IgG and those positive for IgM and dividing this total by the total number of samples.
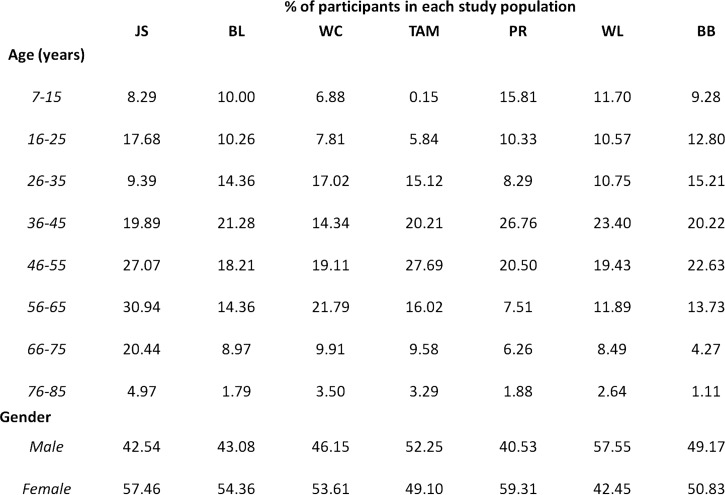
Figure 2.
Age and gender distribution of participants per study site, Singapore. Values are percentages. No significant difference in age and gender was observed between populations.
Historical dengue areas were areas that have consistently reported dengue cases annually over the past 20 years. New dengue areas were those that have reported dengue cases only since 2000. Overall seroprevalence was 65.9% (range = 57.7–81.4%). Historical dengue areas had an average seroprevalence of 76.5%, and new dengue areas had an average seroprevalence of 63.5% (P =0.17).
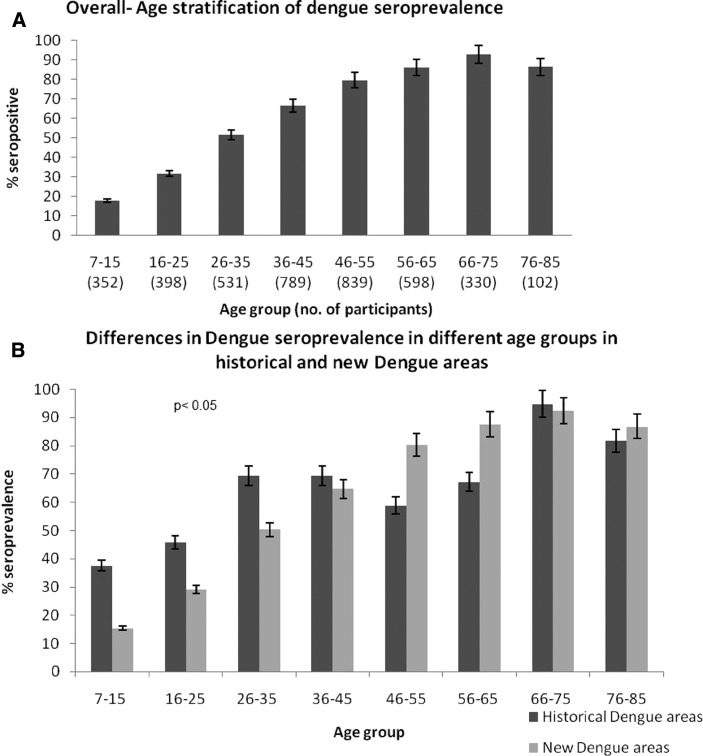
Seroprevalence was also observed to increase with age (Figure 3A). Generally, less than 20% of persons under 15 years of age had previously had dengue. This prevalence increased to 32% in persons 16–25 years of age, and was > 50% for adults 26–35 years of age. Seroprevalence steadily increased until > 90% in persons 66–75 years of age. When analyzed by location, historical dengue areas reported higher seroprevalences in persons ≤ 45 years of age compared with persons in new dengue areas (P < 0.05) (Figure 3B). This finding indicated that living in historical dengue areas predisposed one to having had dengue at a younger age. There were no gender differences in past exposure to dengue (P = 0.39); seroprevalence was 64.8% (range = 63.7–65.9%) in males, and 67.0% (range = 66.0–68.1%) in females.
Figure 3.
A, Overall dengue seroprevalence and age stratification in outbreak areas, Singapore. Past exposure to dengue increased with age. B, Differences in dengue seroprevalence in different age groups in historical and new dengue areas. Younger persons (< 45 years old) who lived in historical dengue areas had a significantly higher chance of being exposed compared with their counterparts in new dengue areas (P < 0.05). Error bars indicate mean ± SD.
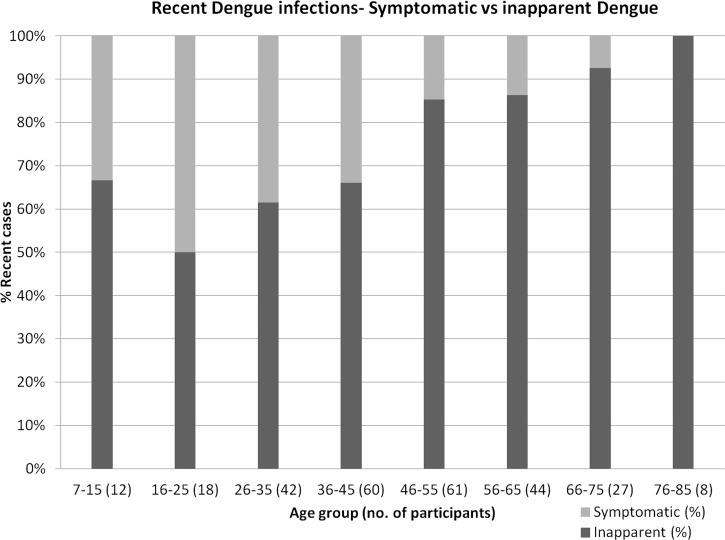
The presence of IgM against dengue indicates a recent infection. The dengue incidence rate was 6.8% (6,803.8 cases/100,000 population) in these outbreak areas during the three months prior to this study. Persons with no recollection of symptoms in the previous three months but who were positive for IgM against dengue were considered as having inapparent dengue. Among persons with recent infections, 78.0% had no recollection of any fever or dengue symptoms in the preceding three months. When stratified into age groups, the rate of inapparent dengue increased with age (Figure 4). Older (≥ 45 years of age) adults had a significantly higher (P < 0.005) rate of inapparent dengue (87.9%, range = 85.1–90.6%) than persons < 45 years of age (62.5%, range = 58.2–66.8%).
Figure 4.
Dengue symptomatic and inapparent rates in different age groups, Singapore. Inapparent dengue rates increased with age.
One person, who did not report any symptoms when a blood sample was obtained, was positive for dengue by RT-PCR (dengue virus serotype 2) and had a viral load of 100 plaque-forming units/mL. As all samples were anonymised before laboratory tests were performed, we could not establish if this person subsequently developed any dengue symptoms. Of persons with recent symptoms of dengue, only five (8.2%) consulted a physician, and only one person was given a diagnosis of dengue.
As our study was conducted in dengue outbreak areas, we report a seroprevalence rate of 65.9%, which was higher than those reported in recent local outbreaks (45–59%). Residents in historical dengue areas, having had more encounters with dengue compared with those in new areas, experienced a higher degree of past exposure. In addition, these persons are more likely to have had dengue at a younger age than persons in new dengue areas.
Our finding of a high rate (78%) of inapparent dengue is consistent with rates previously reported in Singapore,10,11 and also reaffirmed findings in other countries, in which there are silent transmissions of dengue, resulting in large proportion of dengue infections being subclinical or inapparent.5–7 The national incidence rate of dengue in Singapore in 2007 was 192.3 cases/100,000 population (Ministry of Health, Singapore). We report the incidence rate in dengue outbreak areas as 6,803.8 cases/100,000 population, which is 35 times higher than the national average. The higher incidence rate maybe attributed to unreported cases, including those that are inapparent, and the higher incidences in dengue outbreak areas where the study was conducted.
Some studies have reported adults with dengue having more symptoms than children with dengue.1,12 In contrast, our study demonstrated adults, especially older adults, are less symptomatic. Lye and others also reported no increased morbidity in older adults than in other age groups.13 The pathogenicity of this phenomenon is not understood. As dengue is known to be an immunogenic disease, high rates of inapparent dengue could be caused by decreased immunologic responses in elderly persons. With advancing age, cellular and humoral immune responses are modified, and such changes likely affect the general decreased immunoresponsiveness in the elderly.14 This finding could also be caused by partial cross-protection conferred by previous multiple exposures to other dengue virus serotypes. It is interesting that although older adults are less symptomatic or have inapparent infections, this group is also more vulnerable to dengue mortality.15 Co-mobidity has been shown to play a significant role in these fatal cases, and more studies are needed for this age group, particularly in Singapore.
Another interesting finding was the one person who was dengue positive by RT-PCR and had a viral load of 100 plaque-forming units/mL. We do not know if dengue transmission is sustainable with such a low viremia. Likewise, we do not know if persons with inapparent infections were capable of maintaining dengue transmission. To explain the epidemiology of inapparent dengue infections, future studies are planned to ascertain the minimal viral load necessary for uptake of dengue virus by mosquitoes.
The health-seeking behavior and low dengue diagnostic rate demonstrated in this study, despite the presence of a convenient and accessible primary health care system in Singapore, serve to further corroborate the iceberg phenomena that most dengue infections are either asymptomatic or mild (undifferentiated fever).
There are three noteworthy limitations to this study. First, because this study was a cross-sectional study, selection bias cannot be ruled out. The participants in this study might have had a different serologic profile for dengue than persons who did not participate. The low response rate is also a concern and can bias the findings. Nevertheless, the generalizabilty of these research findings is limited. Second, there is a potential of recall bias influencing the research findings. As data were collected by using a questionnaire, some degree of subjectivity is inevitable. However, it is also worth noting that this is not a study to estimate the association between a risk factor and a health problem, in which case any selection and information bias may lead to erroneous conclusions. Third, because the duration of IgM against dengue can range from three to nine months, our prevalence of inapparent infections may be an overestimate because our questionnaire asked about symptoms restricted only to the preceding three months. Nevertheless, because we conducted our study in areas that were having dengue outbreaks and, by inference, because there were no dengue cases in these areas before the outbreaks, IgM against dengue infections more than three months ago should not be present.
Supplementary Material
ACKNOWLEDGMENTS
We thank Lim Hwee Hua, Amy Khor, Ngeow Pack Hua, Arthur Fong, Ong Kian Min, and Teo Chee Hean for assistance during the study; the staff and students of the Environmental Health Institute for providing field and laboratory assistance; Professor Chia Kee Seng for providing technical advice; and the volunteers for participating in the study.
Footnotes
Financial support: This study was supported by the National Environment Agency, Singapore.
Authors' addresses: Grace Yap, Chenny Li, Adeliza Mutalib, Yee-Ling Lai, and Lee-Ching Ng, Environmental Health Institute, National Environment Agency, Singapore, E-mails: grace_yap@nea.gov.sg, chenny.li@gmail.com, adeliza_mutalib@nea.gov.sg, lai_yee_ling@nea.gov.sg, and ng_lee_ching@nea.gov.sg.
References
- 1.Ooi EE, Goh KT, Chee Wang DN. Effect of increasing age on the trend of dengue and dengue hemorrhagic fever in Singapore. Int J Infect Dis. 2003;7:231–232. doi: 10.1016/s1201-9712(03)90057-9. [DOI] [PubMed] [Google Scholar]
- 2.Goh KT. Dengue: a re-emerging infectious disease in Singapore. Ann Acad Med Singapore. 1997;26:664–670. [PubMed] [Google Scholar]
- 3.Ong A, Sandar M, Chen MI, Sin LY. Fatal dengue hemorrhagic fever in adults during a dengue epidemic in Singapore. Int J Infect Dis. 2007;11:263–267. doi: 10.1016/j.ijid.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Guzman MG, Kouri G. Dengue: an update. Lancet Infect Dis. 2002;2:33–42. doi: 10.1016/s1473-3099(01)00171-2. [DOI] [PubMed] [Google Scholar]
- 5.Endy TP, Chunsuttiwat S, Nisalak A, Libraty DH, Green S, Rothman AL, Vaughn DW, Ennis FA. Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol. 2002;156:40–51. doi: 10.1093/aje/kwf005. [DOI] [PubMed] [Google Scholar]
- 6.Chen WJ, Chen SL, Chien LJ, Chen CC, King CC, Harn MR, Hwang KP, Fang JH. Silent transmission of the dengue virus in southern Taiwan. Am J Trop Med Hyg. 1996;55:12–16. doi: 10.4269/ajtmh.1996.55.12. [DOI] [PubMed] [Google Scholar]
- 7.Teixeira Mda G, Barreto ML, Costa Mda C, Ferreira LD, Vasconcelos PF, Cairncross S. Dynamics of dengue virus circulation: a silent epidemic in a complex urban area. Trop Med Int Health. 2002;7:757–762. doi: 10.1046/j.1365-3156.2002.00930.x. [DOI] [PubMed] [Google Scholar]
- 8.Groen J, Koraka P, Velzing J, Copra C, Osterhaus AD. Evaluation of six immunoassays for detection of dengue virus-specific immunoglobulin M and G antibodies. Clin Diagn Lab Immunol. 2000;7:867–871. doi: 10.1128/cdli.7.6.867-871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai YL, Chung YK, Tan HC, Yap HF, Yap G, Ooi EE, Ng LC. Cost-effective real-time reverse transcriptase PCR (RT-PCR) to screen for dengue virus followed by rapid single-tube multiplex RT-PCR for serotyping of the virus. J Clin Microbiol. 2007;45:935–941. doi: 10.1128/JCM.01258-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goh KT. Seroepidemiology of Dengue Virus Infection in Singapore. Dengue in Singapore. Singapore: WHO Collaborating Centre for Environmental Epidemiology, Ministry of the Environment; 1998. pp. 50–72. [Google Scholar]
- 11.Ye T, Ang LW, Chow A, Chew SK. Seroprevalence study on past and recent dengue virus infection in Singapore. Epidemiol News Bull (Singapore) 2007;33:36–41. [Google Scholar]
- 12.Seet RC, Quek AM, Lim EC. Post-infectious fatigue syndrome in dengue infection. J Clin Virol. 2007;38:1–6. doi: 10.1016/j.jcv.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Lye DC, Lee VJ, Sun Y, Leo YS. The benign nature of acute dengue infection in hospitalized older adults in Singapore. Int J Infect Dis. 2010;14:e410–e413. doi: 10.1016/j.ijid.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 14.Ginaldi L, De Martinis M, D'Ostilio A, Marini L, Loreto MF, Martorelli V, Quaglino D. The immune system in the elderly: II. Specific cellular immunity. Immunol Res. 1999;20:109–115. doi: 10.1007/BF02786467. [DOI] [PubMed] [Google Scholar]
- 15.Chan KP, Lau GK, Doraisingham S, Chan YC. Adult dengue deaths in Singapore. Clin Diagn Virol. 1995;4:213–222. doi: 10.1016/0928-0197(95)00004-r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.