Abstract
We assessed risk factors for vectors of dengue and chikungunya viruses near a new hydroelectric project, Nam Theun 2, in Laos. Immature stages of Aedes aegypti were found only in sites within 40 km of the urban provincial capital, but Aedes albopictus was found throughout. Aedes aegypti pupae were most common in water storage jars (odds ratio [OR] = 4.72) and tires (OR = 2.99), and Ae. albopictus pupae were associated with tires in 2009 (OR = 10.87) and drums, tires, and jars in 2010 (drums OR = 3.05; tires OR = 3.45, jars OR = 6.59). Compared with water storage vessels, containers used for hygiene, cooking, and drinking were 80% less likely to harbor Ae. albopictus pupae in 2010 (OR = 0.20), and discarded waste was associated with a 3.64 increased odds of infestation. Vector control efforts should focus on source reduction of water storage containers, particularly concrete jars and tires.
Introduction
Dengue is common in towns and cities in Laos. In 2010, there were 22,772 cases and 42 deaths1 (total population = 6.2 million in 20102). Over the past decade, the number of dengue cases has increased and control of this disease is now a major public health issue. The status of chikungunya is less well understood, but given that disease outbreaks have been reported in neighboring Thailand3 it is also likely to occur in Laos.
The major vector of dengue virus (DENV) and chikungunya virus (CHIKV) is Aedes aegypti, which has been reported in Vientiane capital city, as well as other urban areas of Laos.4,5 The immature stages (eggs, larvae, and pupae) of this species are predominantly found in natural and artificial container habitats within human settlements.6 The adaptation of Ae. aegypti to the urban environment means that transmission of DENV and CHIKV predominantly occurs in these settings. Aedes albopictus, a secondary vector for DENV and primary vector for recent outbreaks of chikungunya in Malaysia and Thailand,7,8 also exhibits a preference for oviposition in container habitats, but is generally more associated with peri-urban and rural environments.9–11
Dengue and chikungunya are neither vaccine preventable, nor are there drugs to treat infections. Therefore, attempts to reduce the burden of disease are reliant on vector control. Aedes spp. mosquitoes typically feed during the early evening when humans are not protected by bed nets. Thus, control strategies focus on source reduction of larval/pupal habitats or other techniques targeting the immature stages of the mosquito such as larval habitat source reduction through frequent emptying and cleaning of water-holding containers, or killing larvae using insecticides or biological control agents.12 For source reduction to be effective, it is essential to understand which containers form the most productive habitats and identify the features that increase their suitability as breeding sites.
It was hypothesized that development of the large Nam Theun 2 hydroelectric project (NT2) (www.namtheun2.com) in Khammouane Province in south central Laos, and the resettlement of more than 6,500 persons in the area would result in more opportunities for Aedes spp. breeding because of increased socioeconomic development and urbanization. Proximity of the NT2 project to a highly urban center, Thakhek, to which dengue is endemic and in which Ae. aegypti is abundant,4 led to concerns that the vector and the virus could spread to the NT2 resettlement area where surveys had indicated the absence of Ae. aegypti and low level of exposure to DENV.13 An understanding of vector distribution and habitat preference was needed to determine the extent of DENV transmission risk across NT2 affected areas and to better understand options for vector control in this area.
The aim of the study was to investigate risk factors for the presence of Ae. aegypti and Ae. albopictus in domestic water-holding containers in settlements of Khammouane Province, which were distributed along a 90-km stretch of road between the provincial capital city, Thakhek, and the rural NT2 resettlement area.
Methods
Study area.
Surveys took place during the hot, rainy seasons of 2009 and 2010 (July–September). Settlements could be broadly categorized as resettlement or traditional. Villages of the resettlement area (Figure 1) were built to relocate persons who had been living in the area that was flooded during creation of the NT2 reservoir. These villages were established in 2007 and 2008 before reservoir inundation. They were located along the southern shoreline of the reservoir, connected to one another by a dirt road. Pumped water was available from wells. The primary occupations of persons in the resettlement community were fishing, growing rice and sugarcane, gathering food from the forest, and animal husbandry (cows, buffaloes, goats, pigs, chickens, and ducks). Resettlement houses were of a uniform style, constructed from locally sourced hardwood with iron roofs, and elevated on stilts 2.5–3 meters above ground level. Each house had its own latrine in a hut built at ground level approximately 5 meters from the main house.
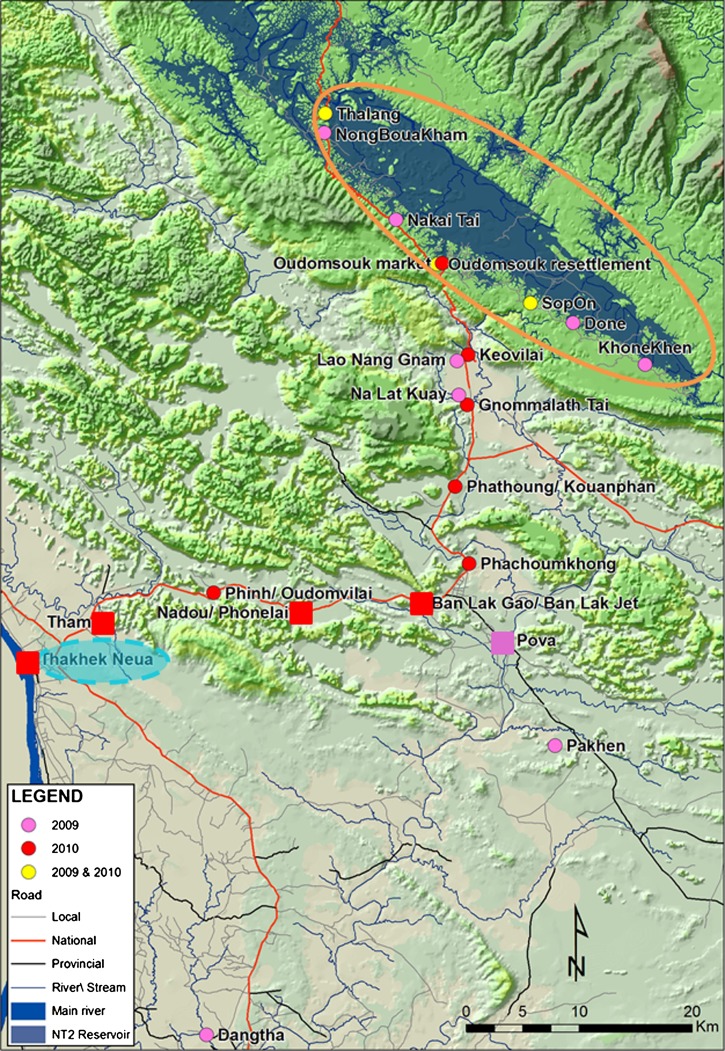
Figure 1.
Locations of settlements in Laos in which surveys for dengue virus and chikungunya virus vectors were conducted during 2009 and 2010. Settlements located in the NT2 resettlement area are enclosed in the orange circle and settlements outside this area are downstream villages. The urban center, Thakhek Neua, is enclosed by a blue circle. Locations of settlements in which Aedes aegypti was sampled are indicated by squares and locations in which only Ae. albopictus was present are indicated by circular icons.
Houses in the traditional settlements, located downstream of the reservoir in a predominantly rice farming area, were more heterogeneous in design. Traditional settlements are those located outside the resettlement area (Figure 1). Most residents of traditional settlements had access to pumped water, but some collected water from wells and nearby rivers. Villages were connected to dirt roads and were near the major tarmac road connecting Thakhek with the resettlement area. Houses were mostly made from wood or bamboo, with iron roofs, but with a higher proportion of brick houses closer to Thakhek (Figure 1). The primary occupations of local people were similar to those in the resettlement area.
During 2009, surveys took place in seven resettlement and five traditional settlements. In 2010, the geographic range of the study area was expanded to include the urban area around Thakhek, and surveys took place in four resettlement and nine traditional settlements. Global positioning system coordinates for all settlements are shown in Table 1.
Table 1.
Relative abundance of container types alongside pupae per person in each settlement surveyed during 2009 and 2010*
| Year and village name | GPS positional data | Container type as a proportion (%) of water-holding containers in each village | Pupae per person | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2009 | North | East | Elevation above sea level (meters) | Drum(n = 161) | Tire (n = 141) | Jar (n = 137) | Other (n = 268) | Remaining (n = 392) | Aedes aegypti | Aedes albopictus |
| Thalang | 17°50′10.6″ | 105°02′59.9″ | 541 | 19.8 | 14 | 1.7 | 24.8 | 39.7 | 0 | 0.046 |
| NongBouakham | 17°49′15.8″ | 105°02′57.3″ | 544 | 20.5 | 4.5 | 5.7 | 23.9 | 45.5 | 0 | 0.109 |
| Nakai Tai | 17°45′04.3″ | 105°06′32.8″ | 553 | 3.7 | 7.9 | 4.3 | 28 | 56.1 | 0 | 0.030 |
| Oudomsouk | 17°42′57.2″ | 105°08′35.7″ | 559 | 25.7 | 13.9 | 0.7 | 13.9 | 45.8 | 0 | 0.085 |
| SopOn | 17°41′04.5″ | 105°13′16.4″ | 543 | 4.6 | 8 | 1.1 | 37.9 | 48.3 | 0 | 0.118 |
| Done | 17°40′07.1″ | 105°15′24.4″ | 551 | 6.7 | 6.7 | 2.2 | 28.1 | 56.2 | 0 | 0.043 |
| KhoneKhen | 17°38′.5″ | 105°19′00.7″ | 565 | 3.4 | 10.3 | 0 | 17.2 | 69 | 0 | 0.009 |
| Lao Nang Gnam | 17°38′17.4″ | 105°09′34.6″ | 184 | 21.1 | 28.9 | 5.3 | 40.8 | 3.9 | 0 | 0.313 |
| Na Lat Kuay | 17°36′39.5″ | 105°09′40.0″ | 172 | 21.1 | 36.8 | 7 | 31.6 | 3.5 | 0 | 0.099 |
| Pova | 17°24′53.5″ | 105°12′00.5″ | 158 | 21.3 | 8.5 | 17 | 27.7 | 25.5 | 0.045 | 0.067 |
| Pakhen | 17°19′49.8″ | 105°14′28.8″ | 160 | 5.6 | 21.1 | 62 | 7 | 4.2 | 0 | 0.060 |
| Dangtha | 17°05′57.3″ | 104°57′02.8″ | 147 | 16.5 | 6.3 | 64.6 | 10.1 | 2.5 | 0 | 0.091 |
| 2010 | Drum (n = 325) | Tire (n = 296) | Jar (n = 451) | Other (n = 341) | Remaining (n = 1,015) | Aedes aegypti | Aedes albopictus | |||
| Thalang | 17°50′10.6″ | 105°02′59.9″ | 541 | 17.1 | 6.3 | 4 | 14.3 | 58.3 | 0 | 0.149 |
| Oudomsouk | 17°42′57.2″ | 105°08′35.7″ | 559 | 29.5 | 12.5 | 2.3 | 14.2 | 41.5 | 0 | 0.294 |
| Oudomsouk Market | 17°42′599.2″ | 105°08′51.6″ | 544 | 15.6 | 15.6 | 0 | 14.4 | 54.4 | 0 | 0.147 |
| SopOn | 17°41′04.5″ | 105°13′16.4″ | 543 | 7.4 | 5.4 | 4.4 | 21.7 | 61.1 | 0 | 0.248 |
| Keovilai | 17°38′36.2″ | 105°10′09.1″ | 188 | 10.5 | 14.9 | 22.7 | 9.9 | 42 | 0 | 0.818 |
| Gnommalath Tai | 17°36′11.7″ | 105°10′05.8″ | 159 | 19.3 | 13.6 | 10.3 | 16.3 | 40.5 | 0 | 0.741 |
| Phathoung-Kouanphan | 17°32′16.5″ | 105°09′29.3″ | 157 | 16 | 29 | 18.2 | 13.4 | 23.4 | 0 | 1.354 |
| Phachoumkhong | 17°28′34.4″ | 105°10′11.1″ | 160 | 15.5 | 6.2 | 35.4 | 3.7 | 39.1 | 0 | 1.023 |
| Lak Gao-Lak Jet | 17°26′41.4″ | 105°07′45.0″ | 157 | 4.7 | 8.5 | 38 | 4.7 | 44.2 | 0.006 | 0.222 |
| Nadou-Phonlai | 17°26′14.5″ | 105°01′42.6″ | 157 | 4.2 | 11.3 | 28 | 10.7 | 45.8 | 0.017 | 0.295 |
| Phinh-Oudomvilai | 17°27′10.3″ | 104°57′24.6″ | 160 | 9.6 | 14.4 | 30.5 | 11.4 | 34.1 | 0 | 0.669 |
| Tham | 17°25′42.2″ | 104°51′50.0″ | 150 | 7.6 | 5.6 | 45.1 | 13.2 | 28.5 | 0.503 | 0.211 |
| Thakhek | 17°24′00.2″ | 104°48′07.7″ | 148 | 9.4 | 9.4 | 17.4 | 24.3 | 39.6 | 0.427 | 0.080 |
Downstream villages are shown in bold. Other villages are NT2 resettlement villages. GPS = global positioning system.
Entomologic surveys.
In 2009, study villages in the resettlement area were selected to provide an even distribution along the shore of the reservoir and the area immediately downstream of the reservoir. In 2010, the selection of settlements was designed to give an even distribution along the main road leading from Thakhek to the resettlement area, and one settlement was selected approximately every 10 km along the main road.
Household container surveys.
To reduce the effects of seasonal or temporal variation on catch sizes, surveys took place in each settlement over the course of 1–3 days. During 2009, 20–25 households were surveyed per settlement and in 2010 this was increased to 30 households per settlement. A household was defined as a single residential building, including any storage buildings, kitchen, or latrine huts, as well as the outside area up to the fenced partition separating one house from its neighbor. Where no obvious partition was in place, all containers within an approximately 10-meter radius of the house were surveyed.
Household selection was performed in a systematic random manner. From a randomly selected start point at the periphery of each settlement, every ith house was selected for inclusion in the survey, where i was the total number of households in the settlement, divided by 25 in 2009 and by 30 in 2010. If it was not possible to include a house in the study because nobody was at home to give permission for sampling, the neighboring house (i + 1) was chosen. This sampling strategy meant that study houses were uniformly distributed across each settlement and that any house, regardless of size, construction, number of occupants, or socioeconomic status, was included in the sampling frame.
Every accessible water-holding container in and around a house was sampled for the presence of immature mosquitoes. Small containers (≤ 20-liter capacity volume) were completely drained through a sieve into a white larval sampling tray (25 × 20 × 4 cm) to collect larvae and pupae. Larger containers were sampled using a 250-mL larval dipper. Five dips were taken from the surface water of each container (four dips evenly spaced around the edges of the container and one at the center). All larvae and pupae were returned in labeled bottles to the field laboratory with a small amount of detritus for nutrition.
Every water-holding container was categorized according to type of container, container function (2010 only), shape, maximum capacity, volume of water in the container (as a percentage of the maximum capacity), material, presence of a cover, location within the house (2010 only), and degree of shade.
Measuring the degree of urbanization.
Rather than categorizing villages according to a classic urban/rural dichotomy, settlements were placed on a scale of urbanization according to a numerical score. The urbanization score assigned to each settlement was the sum of scores allocated to a number of specific characteristics, and high scores were related to greater degrees of urbanization. For example, the more businesses present in a community, the higher the score assigned to a village on the basis of business. Attributes contributing to the urbanization score were land use, proportion of community working in agriculture or fisheries, proximity to a bus station, car and motorbike ownership, road condition, spatial density of housing, house construction materials, water supply, distance to services such as markets, post offices, gas stations, and healthcare centers, telephone network coverage, types of school or higher education establishment in the community, electricity supply, number of restaurants, number of businesses, and population of the settlement. Full details of scoring for each criterion are provided in Supplemental Table 1.
Laboratory methods.
In the laboratory, third-stage and fourth-stage larvae and all pupae were counted and transferred to holding cups covered with permeable gauze. Pupae were allowed to develop and emergent adults were identified morphologically. At least five larvae were identified from each container within 48 hours of sampling. If a mixture of species were present, the minimum number of larval identifications was increased to 10. Any remaining larvae were kept for 15 days to develop into adults. Larvae were fed Sakura Gold fish food for small aquarium fish (See-All Aquariums Co., Ltd., Bangkok, Thailand). Adult and larval identifications were carried out morphologically by using keys to the mosquitoes of Thailand and Vietnam.14–16
Statistical analysis.
Initial analyses characterized containers with the greatest pupal densities during each year of the study. To determine whether the presence of one species of Aedes was independent of the presence of the other species within the same container, data was analyzed for the four settlements in which both species were found. Assuming that the probability of species presence in a container is constant, the probability of finding each species in any container was calculated. The number of containers in which both species would be expected to occur simultaneously, if they are independent of one another, was estimated. Under the null hypothesis, the observed number of containers with both species would not differ significantly from the expected number of containers with both species, and P values for this test were calculated by using the binomial cumulative distribution function.
For each settlement in which surveys took place, the number of pupae per person was calculated because pupal density has been reported to be a good indicator of adult Ae. aegypti population size,17,18 and there is a close correlation between pupal densities and adult emergence rates.19 This index forms an appropriate way to assess risk of disease transmission in different geographic areas.20
During 2009, only 9 containers had any Ae. aegypti and risk models were not created for this species. In 2010, risk factors for Ae. aegypti pupae were identified by using data from only the four villages in which this species was found. Risk factors for Ae. albopictus pupae were estimated for all villages surveyed during both years of the study.
For each species, univariate logistic regression analyses were conducted to identify possible risk factors for the presence/absence of pupae in a container. All individually significant variables with P < 0.1 were included in a multivariable model, and predictors were eliminated from the multivariable model by using a backwards stepwise approach, retaining terms for which the result of a likelihood ratio test gave P < 0.1 because they were deemed to contribute significantly to the overall fit of the model.
The following variables were excluded from the multivariable model on the basis of confounding with other predictors: material that was associated with container type and elevation and village that were associated with urbanization score in 2010 (all assigned at the village level). Container function and room were only recorded during the 2010 surveys. Therefore, these factors were not included in 2009 risk models.
Statistical analyses were performed by using Microsoft Excel 2010 (Microsoft, Redmond, CA), Stata Statistical Software Release 10 (StataCorp LP, College Station, TX), and Matlab (The Mathworks Inc., Natick, MA).
Ethical approval.
This study was approved by the Lao Ministry of Health, the Khammouane Provincial Health Office, and the district health offices of Nakai, Gnommalath, Mahaxay, Xe Bang Fai, and Thakhek, the Health Program Management Unit of NT2, and the ethics committee of the London School of Hygiene and Tropical Medicine. Meetings were held with the heads of each village before commencement of surveys, and informed oral consent was given by the head of each household before entering any home.
Results
General findings.
Surveys took place in 704 households (3,546 containers) during July 2009–August 2010. Aedes aegypti comprised 4.1% of all larvae (n = 3,973) and 1.3% of pupae (n = 458) in 2009 and 10.1% of larvae (n = 9,067) and 10.4% of pupae (n = 1,526) in 2010. In contrast, Ae. albopictus was much more abundant and formed 37.9% of all larvae and 39% of pupae sampled in 2009 and 61.5% of larvae and 65% of pupae sampled in 2010. Although Ae. albopictus was present in all study settlements in both years, the distribution of Ae. aegypti was limited to four settlements located within 40 km of Thakhek (Thakhek, Tham, Nadou-Phonlai, and Lak Gao-Jet). This species was never found in the NT2 resettlement area (Table 1).
Distribution of Aedes immature mosquitoes among containers and households.
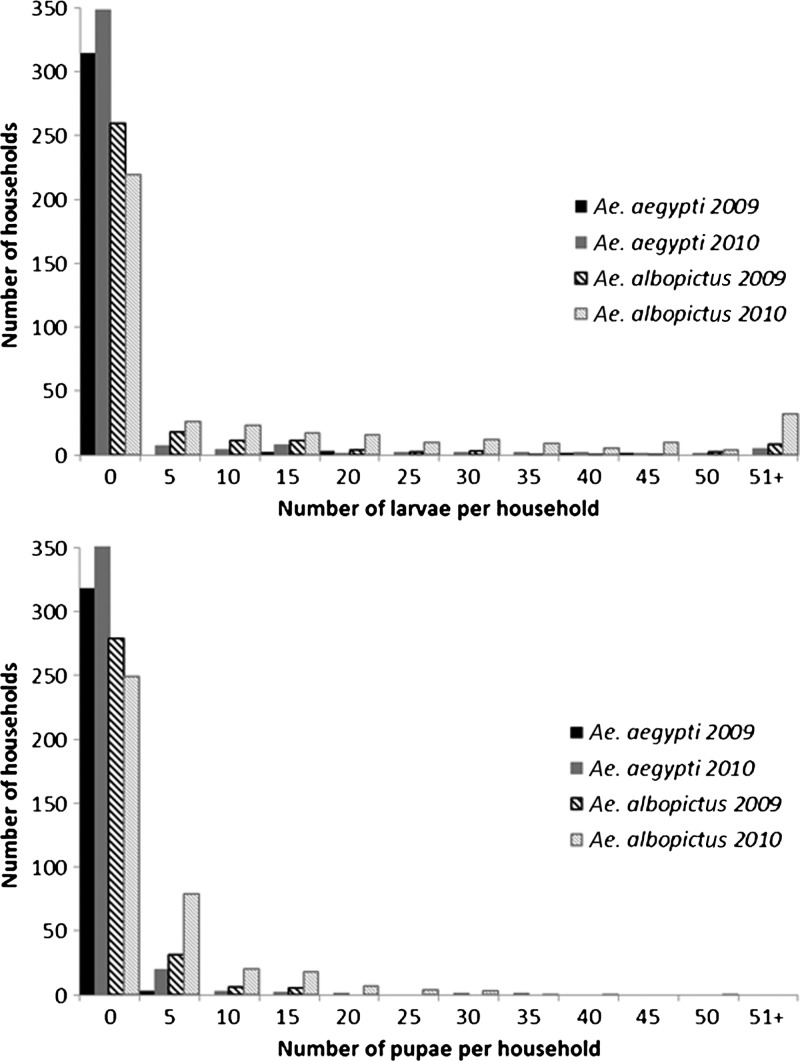
During both years of the study, mosquito populations were over-dispersed, and there was much greater mosquito densities in some households than others (Figure 2). Among water-holding containers, the distribution of larvae and pupae was also over-dispersed, and most containers had few or no aquatic stages and some containers had high larval and pupal counts.
Figure 2.
Over-dispersed abundance of Aedes aegypti and Ae. albopictus larvae (upper) and pupae (lower) among houses in Laos during 2009 and 2010. Distribution between houses remained fairly consistent between years.
Key container habitats.
During 2009, 5 of 6 Ae. aegypti pupae were sampled from jars and drums without covers. During 2010, 68.1% of Ae. aegypti pupae were found in jars and containers categorized as other (n = 160 pupae).
For Ae. albopictus, most pupae collected in 2009 were in tires and containers classified as other, which did not have a cover (39.5% of all pupae, n = 178 pupae). In 2010, tires, jars, and containers classified as other held 75.2% of all pupae (n = 992 pupae).
Simultaneous occurrence of Ae. aegypti and Ae. albopictus in the same container.
In 2010, Ae. aegypti and Ae. albopictus occurred simultaneously in 26 containers (3.8% of 676 containers in the four villages where both species were present). The observed number of containers with both species was higher than would be expected under a binomial distribution in which the species occur independently of one another (Table 2). Thus, the null hypothesis that the presence of one species in a container was independent of the presence of the other species was rejected.
Table 2.
Observed and expected number of containers with Aedes aegypti and Ae. albopictus in four settlements where both species were found during 2010*
| Settlement (n = total number of containers) | No. containers with Aedes aegypti and Ae. albopictus | ||
|---|---|---|---|
| Observed | Expected | P | |
| All settlements (n = 676) | 26 | 7.36 | < 0.001 |
| Lak Gao-Lak Jet (n = 129) | 1 | 0.1 | 0.0047 |
| Nadou-Phonlai (n = 168) | 5 | 0.833 | < 0.001 |
| Tham (n = 144) | 10 | 3.97 | 0.0023 |
| Thakhek (n = 235) | 10 | 2.36 | 0.0023 |
P values were calculated after comparing observed and expected values by using the binomial cumulative distribution function.
Container types.
The relative abundance of jars, drums, tires and other containers differed between years (Table 1) (2009: χ2 = 688.3, degrees of freedom = 55, P < 0.001; 2010: χ2 = 531.7, degrees of freedom = 60, P < 0.001). Jars were more common in traditional villages (5.3–64.6% in 2009; 10.3–45.1% in 2010) than resettlement villages (0–5.7% in 2009; 0–4% in 2010). Although the relative abundance of tires, drums, and other containers differed between villages, there did not appear to be a clear-cut difference between resettlement and traditional villages.
Risk factors for pupae of Aedes spp.
For 2010, risk factor analysis for pupae of Ae. aegypti was conducted for the 676 water-holding containers sampled in the four villages where Ae. aegypti was found. Pupae of Ae. aegypti were present in 7.0% of these containers (47 of 676), and the final multivariable model is shown in Table 3.
Table 3.
Summary results of multivariable logistic regression analysis for pupae of Aedes aegypti and Ae. albopictus during 2009 and 2010*
| Risk factor | Aedes aegypti (2010) | Ae. albopictus (2009) | Ae. albopictus (2010) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Adjusted Odds ratio (95% confidence interval) | P | No. | Adjusted odds ratio (95% confidence interval) | P | No. | Adjusted odds ratio (95% confidence interval) | P | |
| Degree of urbanization (2010) | 676 | 1.04 (1.02–1.06) | < 0.001 | 2,428 | 0.96 (0.94–0.98) | < 0.001 | |||
| Type of water container | 676 | 1,099 | 2,428 | ||||||
| Bucket | 174 | 1.00 | 296 | 1.00 | 653 | 1.00 | |||
| Drum | 46 | 2.26 (0.54–9.45) | 0.263 | 161 | 0.40 (0.04–3.60) | 0.414 | 325 | 3.05 (1.35–6.89) | 0.007 |
| Tire | 60 | 2.99 (1.01–8.86) | 0.049 | 141 | 10.87 (3.60–32.88) | < 0.001 | 296 | 3.45 (1.63–7.31) | 0.001 |
| Pot or bowl | 94 | 0.32 (0.03–3.14) | 0.328 | 96 | 1.67 (0.31–9.12) | 0.552 | 362 | 1.61 (0.67–3.85) | 0.288 |
| Jar | 202 | 4.72 (2.05–10.89) | < 0.001 | 137 | 3.04 (0.81–11.46) | 0.100 | 451 | 6.59 (3.25–13.35) | < 0.001 |
| Other | 100 | 3.30 (0.96–11.40) | 0.059 | 268 | 4.03 (1.40–11.56) | 0.01 | 341 | 2.34 (1.10–5.01) | 0.028 |
| Container function (2010) | 2,428 | ||||||||
| Water storage | 296 | 1.00 | |||||||
| Hygiene/cooking/drinking | 1,438 | 0.20 (0.10–0.39) | < 0.001 | ||||||
| Discarded waste | 496 | 3.64 (1.84–7.17) | < 0.001 | ||||||
| Other | 198 | 1.38 (0.67–2.87) | 0.384 | ||||||
| Cover | 676 | 2,428 | |||||||
| No | 545 | 1.00 | 2,074 | 1.00 | |||||
| Yes | 131 | 0.19 (0.06–0.63) | 0.006 | 354 | 0.25 (0.08–0.74) | 0.013 | |||
| How full (%) | 1,099 | 1.01 (1.00–1.02) | 0.020 | 2,428 | 1.00 (1.00–1.00) | 0.198 | |||
No. is the number of containers sampled at each exposure level. Significant associations are show in bold.
For every one unit increase in degree of urbanization, which ranged from 16 in the least urban settlement (Nadou-Phonlai) to 50 in the most urban settlement (Thakhek), the odds of finding Ae. aegypti pupae in a container increased by 0.04 (odds ratio [OR] = 1.04, 95% confidence interval [CI] = 1.02–1.06, P < 0.001). Container type was also an important predictor of Ae. aegypti pupal presence during 2010 with tires 2.99 times more likely to contain pupae than buckets (OR = 2.99, 95% CI = 1.01–8.86, P = 0.049) and jars 4.72 times more likely to contain pupae than buckets (OR = 4.72, 95% CI = 2.05–10.89, P < 0.001). The presence of a cover significantly reduced the odds of a container having pupae, and covered containers were 81% less likely be infested with Ae. aegypti than one without a cover (OR = 0.19, 95% CI = 0.06–0.63, P = 0.006). Univariate analysis indicated there was evidence that the function of a container influenced the probability of infestation with Ae. aegypti pupae because containers used for hygiene, cooking, and drinking were associated with 73% lower risk of having pupae than a container used for water storage (OR = 0.27, 95% CI = 0.10–0.73, P = 0.010). However, the addition of container function to the multivariable model did not increase the fit of the model and this predictor was subsequently dropped.
During 2009, risk factor analysis for Ae. albopictus pupae included the data from 1,099 domestic water-holding containers. Pupae of Ae. albopictus were present in 4.2% of these containers (46 of 1,099). The likelihood of finding Ae. albopictus pupae in a container was strongly affected by container type, and tires had odds of infestation 10.87 times greater than buckets during 2009 (OR = 10.87, 95% CI = 3.60–32.88, P < 0.001) (Table 3). Discarded waste items, watering cans, bottles, wheelbarrows, trees, and ditches pooled as other were 4.03 times more likely to contain Ae. albopictus pupae than buckets (OR = 4.03, 95% CI = 1.40–11.56, P = 0.010). In addition to the physical attributes of a container, how full it was at the time of sampling was also an important predictor of Ae. albopictus pupal presence with every 1% increase in fullness associated with a 0.01 increase in the odds of pupae (OR = 1.01, 95% CI = 1.00–1.02, P = 0.020).
In 2010, risk factor analysis for Ae. albopictus pupae included data from 2,431 domestic water-holding containers. Pupae of Ae. albopictus were present in 8.8% of these containers (213 of 2,431).Urbanization was also a significant predictor for Ae. albopictus pupae, but in contrast with the results for Ae. aegypti, for every one unit increase in urbanization, the odds of finding Ae. albopictus in a container was reduced by 0.04 (OR = 0.96, 95% CI = 0.94–0.98, P < 0.001). Container type remained an important risk factor in 2010, with the odds of infestation 3.05 times greater in drums (OR = 3.05, 95% CI = 1.35–6.89, P = 0.007), 3.45 times greater in tires (OR = 3.45, 95% CI = 1.63–7.31 P = 0.001), 6.59 times greater in jars (OR = 6.59, 95% CI = 3.25–13.35, P < 0.001) and 2.34 times greater in other types of container (OR = 2.34, 95% CI = 1.10–5.01, P = 0.028), all compared with buckets. For Ae. albopictus, discarded waste items had 3.64 times greater odds (OR = 3.64, 95% CI = 1.84–7.17, P < 0.001) of infestation than a container used to store water. Containers used for hygiene, cooking, and drinking purposes were 80% less likely to have pupae than those used for water storage (OR = 0.20, 95% CI = 0.10–0.39, P < 0.001). The risk of Ae. albopictus pupae in 2010 was also less in covered containers compared with uncovered containers (OR = 0.25, 95% CI = 0.08–0.74, P = 0.013).
Discussion
The investigations described in this report address previously unknown features of the biology and spatial distribution of DENV and CHIKV vectors in areas affected by the NT2 hydroelectric project in south central Laos. Aedes aegypti was strongly associated with urban environments and was found only in settlements near the provincial capital, Thakhek, and within the city itself. In the remainder of this rural area, Ae. albopictus dominated the collections. This urban-rural split for the two vector species is a common finding in other parts of Southeast Asia,9–11 although the reasons for this finding are unclear.
The different distributions may be partly caused by local climate factors. The climate of the resettlement area, which is situated on a mountain plateau, is cooler than in the traditional settlements and urban center downstream of the reservoir. Local recordings from meteorologic stations show that the resettlement area was 3.3°C cooler than downstream areas in 2009 and 2.6°C cooler in 2010. Aedes albopictus may be better adapted to the cooler weather than Ae. aegypti. Similar findings have been reported in Vietnam where Ae. albopictus dominates in cool mountainous areas in the north of the country, and Ae. aegypti is found more commonly in the hotter south,21 and it appears that climatic differences between the regions explain most of these differences in species composition, even after testing the effects of urbanization. In Madagascar, Ae. albopictus dominates on the cool central plateau, whereas Ae. aegypti was primarily found at lower altitudes on the hotter parts of the island.22 In Taiwan, the northern limit of Ae. aegypti appeared to be restricted by low temperatures that Ae. albopictus was better able to tolerate.23 During the hot, rainy season, study period in Laos, temperatures exceeded the average winter minimums of 15.8–17.8°C from areas of Taiwan where Ae. aegypti was absent. However, during the cool, dry season of 2010, temperatures in Nakai decreased to an average minimum 17°C in January and 16.8°C in December, which are below the likely threshold limit for Ae. aegypti survival, and temperatures in Thakhek remained within the favorable limits for Ae. aegypti survival (minimums of 21°C in January and 20°C in December 2010).
Although these differences in winter temperature provide a plausible explanation for the absence of Ae. aegypti from the NT2 resettlement area, temperature alone cannot explain the absence of this species from settlements in the traditional downstream area that are more than 40 km from Thakhek, but have a climate similar to that in Thakhek. Differences in the availability of water storage jars as breeding sites may also contribute to the limited distribution of Ae. aegypti. In downstream settlements where Ae. aegypti was present, jars were much more abundant than in the resettlement area, and risk factor analysis indicated that jars were a preferred habitat for this species in Laos.
During the 1970s, studies in Singapore suggested that Ae. aegypti will out compete Ae. albopictus,24 but a greater body of more recent literature demonstrates that Ae. albopictus is competitively superior at the larval stage.25–28 The results of these studies indicate that cohabitation of the same container habitat occurs relatively rarely for these species in Laos because only 26 containers were found with both species, However, this number of dual infestations was still significantly higher than that expected by chance alone. Dual infestation can probably be attributed to similar preferences of the two species for oviposition in jars and tires. The issue of interspecific competition and shared preferences for oviposition sites should be investigated further to determine to what extent control of specific container types could reduce populations of both species simultaneously.
Aedes aegypti has a limited flight range and most adults disperse no more than 100 meters, although females sometimes fly further in search of oviposition sites.29–31 The limited dispersal of adults means that the spread of this species to new areas is most likely to occur through the transportation of eggs, larvae, pupae, or adults alongside humans. It is probable that this species has been introduced to the NT2 resettlement area on numerous occasions because of the high degree of human movement between Thakhek and Nakai but has not become established. Climate change and increasing levels of urbanization in these villages may make the area more suitable for this species in the future. Therefore, continued vigilance and monitoring of mosquito species composition is encouraged. This suggestion is particularly important because low levels of past infection with DENV are reported from the NT2 resettlement population.13 Therefore, this population is highly susceptible to the introduction of any DENV. Development of an Aedes vector control program in the NT2 resettlement area is strongly recommended to prevent the establishment of Ae. aegypti and to control Ae. albopictus, which is a secondary vector of DENV,32 as well as a vector of CHIKV.
Multivariable risk factor models for Ae. aegypti and Ae. albopictus suggest that targeting specific types of water-holding container would enable a more focused approach to vector control than attempting to eliminate all water-holding containers. A multi-country study compared targeted with non-targeted approaches to dengue vector control and found that both approaches led to significant reductions in entomologic indices (Breteau Index and Pupae Per Person Index) but targeted approaches were in most instances more cost-effective than non-targeted ones.33 For Ae. aegypti, draining jars of water once a week would be an effective way to kill larvae and pupae because the generation time from larva to adult takes two or more weeks. Elimination of breeding in jars could reduce Ae. aegypti pupal populations by approximately one-third, leading to reduced adult population size and risk of disease transmission. Covering water-holding containers should also reduce the risk of breeding by preventing female mosquitoes access to water in which to oviposit. Vector control efforts for this species should focus primarily in urban and warm lowland areas of Laos.
For Ae. albopictus, elimination of waste tires should be a priority for vector control. Old tires could be cut up so that they cannot hold water or they could be stored in dry environments. As for Ae. aegypti, jars formed important breeding sites for Ae. albopictus in this area and they should be frequently drained of water to kill larvae and pupae. Metal drums should also be targeted for control activities.
It has been reported that Ae. aegypti are more likely to oviposit in containers already inhabited by larvae and pupae of conspecifics,34 and the gains achieved through targeted control of productive containers would be short-lived if females were diverted to oviposit in alternative sites. The creation of an egg sink, as proposed by Wong and others,34 could resolve this problem through the addition of an insect growth regulator, such as pyriproxyfen, to productive habitats. An insect growth regulator kills mosquitoes late in their development. Thus, maintaining the attractive properties of a site with conspecifics and preventing the diversion of females to other sites that are not being targeted for control. The results described here also indicate that containers could be covered to prevent access by females seeking an oviposition site.
Jars and drums held 7.4–29.6% of Ae. albopictus pupae in the villages where these surveys took place. Therefore, targeted control of this habitat type could contribute to large reductions in Ae. albopictus populations in villages of this part of Laos. The sampling of immature mosquitoes by using our dipping method is likely to be less efficient in large water containers than in smaller ones. Thus water storage jars and 200-liter drums are likely to be even more important as sources of Ae. aegypti and Ae. albopictus than we estimate here.
Multivariable models show that containers that are in frequent use for hygiene, cooking, and drinking purposes are less likely to become breeding sites than long-term water-storage containers. In a similar vein, containers that have been discarded and are not in active use are much more likely to be colonized by Ae. albopictus than containers specifically used to store water. Therefore, in addition to considering which types of container should be targeted in control activities, whether a container is in active use or not should be a factor in deciding whether vector control is needed. Storing water need not exacerbate the problems of mosquito breeding as long as water is used frequently. As a result of the work described in this report, local health authorities in areas affected by the NT2 project are in a stronger position to make predictions regarding areas at risk of DENV and CHIKV transmission. This investigation identified Ae. albopictus in all study villages. Thus, the potential for CHIKV transmission exists throughout this region of Laos. The risk of DENV transmission is greater in the urban center, Thakhek, and in surrounding villages where Ae. aegypti was present.
Drums, jars, and tires have been recognized as primary sources of Aedes mosquitoes elsewhere in Southeast Asia,4,24,35,36 and community education programs run in collaboration with district health staff and village health volunteers should promote the idea of a clean living environment, as well as regular emptying of water storage containers and keeping dry those containers that are not in use. Regular community clean-up campaigns have been suggested as a way forward, although there is still a lack of robust scientific evidence in support of successful, sustainable community-based control.37 In Vietnam, use of up to six locally occurring species of Mesocyclops to control Aedes container breeding has been highly successful in field trials,38–42 and this approach could be used in Laos to control breeding in large containers such as drums and rainwater collection tanks, which are permanently wet. Other studies have demonstrated some success using insecticide-treated curtains, bed nets, and water container covers for dengue control.43–45 Pre-intervention feasibility and community acceptance studies would be absolutely essential before proceeding with any approach that requires a high degree of community cooperation.
Concerns raised at the commencement of the NT2 project regarding the potential for ingress of Ae. aegypti to the rapidly developing resettlement area have as yet proven unfounded. However, continued vigilance is essential. Monitoring of Aedes species composition and container risk factors will continue in the NT2 resettlement area as the communities continue to develop and reach a new equilibrium after resettlement.
Supplementary Material
ACKNOWLEDGMENTS
We thank the district health offices of Nakai, Gnommalath, Mahaxay, Thakhek, and Xe Bang Fai Districts and the Khammouane provincial health office for support during this study; Mr. Joy and Mr. Amphone for assistance during field sampling; entomologists at the National Institute for Hygiene and Epidemiology, Hanoi for providing training to Alexandra Hiscox regarding mosquito identifications; Liankham Payasane (Nam Theun 2 Power Company) for assistance during creation of Figure 1; the anonymous reviewers for their recommendations that helped to improve the paper; and local people in Khammouane Province who allowed us into their homes and lives during the course of these surveys.
Disclaimer: Alexandra Hiscox, Angela Kaye, Ian Banks, Nigel Hill, and Paul T. Brey conceived and designed the study. Alexandra Hiscox and Angela Kaye conducted field investigations and sample identifications. Phasouk Khammanithong, Pany Sananikhom, and Surinder Kaul, liased with district and provincial health offices, as well as villages heads regarding the final methods. Alexandra Hiscox, Angela Kaye, Khamsing Vongphayloth, Michele Piffer, and Steven W. Lindsay performed the statistical analyses. Alexandra Hiscox drafted the manuscript with Steven W. Lindsay and Paul T. Brey. All authors read and approved the final manuscript.
Footnotes
Financial support: This study was supported by the Fondation EDF Diversiterre. Steven W. Lindsay was supported by the Research and Policy for Infectious Disease Dynamics Program of the Science and Technology Directory, Department of Homeland Security, and Fogarty International Center, National Institutes of Health.
Authors' addresses: Alexandra Hiscox, Laboratory of Entomology, Wageningen University and Research Centre, Wageningen, The Netherlands, E-mail: alexandra.hiscox@wur.nl. Angela Kaye and Ian Banks, Department of Disease Control, London School of Hygiene and Tropical Medicine, London, UK, E-mails: angela.kaye@lshtm.ac.uk and ian.banks@lshtm.ac.uk. Khamsing Vongphayloth, Institut Pasteur du Laos, Sisattnanak District, Vientiane, Laos, E-mail: khamsing-v@live.com. Michele Piffer, Department of Economics, London School of Economics, London, UK, E-mail: m.piffer@lse.ac.uk. Phasouk Khammanithong, Khammouane Provincial Health Office, Thakhek City, Khammouane Province, Laos, E-mail: pphaso@yahoo.com. Pany Sananikhom and Surinder Kaul, Nam Theun 2 Power Company, Nongbone Village, Vientiane, Laos, E-mails pany@namtheun2.com and surinder@namtheun2.com. Steven W. Lindsay, School of Biological and Biomedical Sciences, Durham University, Durham, UK, E-mail: s.w.lindsay@durham.ac.uk. Paul T. Brey, Institut Pasteur du Laos, Sisattnanak District, Vientiane, Laos, E-mail: paul.brey@pasteur.fr.
References
- 1.World Health Organization . Dengue in the Western Pacific Region. Geneva: World Health Organization; 2010. [Google Scholar]
- 2.World Bank . World Development Indicators: Lao PDR. Washington, DC: World Bank; 2010. [Google Scholar]
- 3.Rianthavorn P, Prianantathavorn K, Wuttirattanakowit N, Theamboonlers A, Poovorawan Y. An outbreak of chikungunya in southern Thailand from 2008 to 2009 caused by African strains with A226V mutation. Int J Infect Dis. 2010;14:e161–e165. doi: 10.1016/j.ijid.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Tsuda Y, Kobayashi J, Nambanya S, Miyagi I, Toma T, Phompida S, Manivang K. An ecological survey of dengue vector mosquitos in central Lao PDR. Southeast Asian J Trop Med Public Health. 2002;33:63–67. [PubMed] [Google Scholar]
- 5.Jennings CD, Phommasack B, Sourignadeth B, Kay BH. Aedes aegypti control in the Lao People's Democratic Republic, with reference to copepods. Am J Trop Med Hyg. 1995;53:324–330. doi: 10.4269/ajtmh.1995.53.324. [DOI] [PubMed] [Google Scholar]
- 6.Christophers SR. Aedes aegypti (L.): The Yellow Fever Mosquito. London: Cambridge University Press; 1960. [Google Scholar]
- 7.Sam IC, Chan YF, Chan SY, Loong SK, Chin HK, Hooi PS, Ganeswrie R, Abubakar S. Chikungunya virus of Asian and Central/East African genotypes in Malaysia. J Clin Virol. 2009;46:180–183. doi: 10.1016/j.jcv.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Thavara U, Tawatsin A, Pengsakul T, Bhakdeenuan P, Chanama S, Anantapreecha S, Molito C, Chompoosri J, Thammapalo S, Sawanpanyalert P, Siriyasatien P. Outbreak of chikungunya fever in Thailand and virus detection in field population of vector mosquitoes, Aedes aegypti (L.) and Aedes albopictus Skuse (Diptera: Culicidae) Southeast Asian J Trop Med Public Health. 2009;40:951–962. [PubMed] [Google Scholar]
- 9.Braks MA, Honorio NA, Lourencqo-De-Oliveira R, Juliano SA, Lounibos LP. Convergent habitat segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in southeastern Brazil and Florida. J Med Entomol. 2003;40:785–794. doi: 10.1603/0022-2585-40.6.785. [DOI] [PubMed] [Google Scholar]
- 10.Chan YC, Chan KL, Ho BC. Aedes aegypti (L.) and Aedes albopictus (Skuse) in Singapore City. 1. Distribution and density. Bull World Health Organ. 1971;44:617–627. [PMC free article] [PubMed] [Google Scholar]
- 11.Tsuda Y, Suwonkerd W, Chawprom S, Prajakwong S, Takagi M. Different spatial distribution of Aedes aegypti and Aedes albopictus along an urban-rural gradient and the relating environmental factors examined in three villages in northern Thailand. J Am Mosq Control Assoc. 2006;22:222–228. doi: 10.2987/8756-971X(2006)22[222:DSDOAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization . Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva: World Health Organization; 2009. New Edition WHO/HTM/NTD/DEN/2009.1. [PubMed] [Google Scholar]
- 13.Hiscox A, Winter CH, Vongphrachanh P, Sisouk T, Somoulay V, Phompida S, Kaul S, Sananikhom P, Nguyen TY, Paul RE, Brey P, Bryant JE. Serological investigations of flavivirus prevalence in Khammouane Province, Lao People's Democratic Republic, 2007–2008. Am J Trop Med Hyg. 2010;83:1166–1169. doi: 10.4269/ajtmh.2010.09-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rattanarithikul R, Harbach RE, Harrison BA, Panthusiri P, Coleman RE, Richardson JH. Illustrated keys to the mosquitoes of Thailand. VI. Tribe Aedini. Southeast Asian J Trop Med Public Health. 2010;41((Suppl 1)):1–225. [PubMed] [Google Scholar]
- 15.Stojanovich CJ, Scott HG. Illustrated Key to Mosquitoes of Vietnam. Atlanta, GA: U.S. Department of Health, Education and Welfare, Public Health Service, Communicable Disease Center; 1966. [Google Scholar]
- 16.Huang YM. Medical entomology studies - XI. The subgenus Stegomyia of Aedes in the Oriental region with keys to the species (Diptera: Culicidae) Contrib Am Entomol Inst. 1979;15:1–82. [Google Scholar]
- 17.Southwood TR, Murdle G, Yasuno M, Tonn RJ, Reader PM. Studies on the life budget of Aedes aegypti in Wat Samphaya, Bangkok, Thailand. Bull World Health Organ. 1972;46:211–226. [PMC free article] [PubMed] [Google Scholar]
- 18.Focks DA, Sackett SR, Bailey DL, Dame DA. Observations on container-breeding mosquitoes in New Orleans, Louisiana, with an estimate of the population density of Aedes aegypti (L.) Am J Trop Med Hyg. 1981;30:1329–1335. doi: 10.4269/ajtmh.1981.30.1329. [DOI] [PubMed] [Google Scholar]
- 19.Knox TB, Nguyen YT, Vu NS, Kay BH, Ryan PA. Quantitative relationships between immature and emergent adult Aedes aegypti (Diptera: Culicidae) populations in water storage container habitats. J Med Entomol. 2010;47:748–758. doi: 10.1603/me09297. [DOI] [PubMed] [Google Scholar]
- 20.Focks DA, Chadee DD. Pupal survey: an epidemiologically significant surveillance method for Aedes aegypti: an example using data from Trinidad. Am J Trop Med Hyg. 1997;56:159–167. doi: 10.4269/ajtmh.1997.56.159. [DOI] [PubMed] [Google Scholar]
- 21.Higa Y, Yen NT, Kawada H, Son TH, Hoa NT, Takagi M. Geographic distribution of Aedes aegypti and Aedes albopictus collected from used tires in Vietnam. J Am Mosq Control Assoc. 2010;26:1–9. doi: 10.2987/09-5945.1. [DOI] [PubMed] [Google Scholar]
- 22.Fontenille D, Rodhain F. Biology and distribution of Aedes albopictus and Aedes aegypti in Madagascar. J Am Mosq Control Assoc. 1989;5:219–225. [PubMed] [Google Scholar]
- 23.Chang LH, Hsu EL, Teng HJ, Ho CM. Differential survival of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) larvae exposed to low temperatures in Taiwan. J Med Entomol. 2007;44:205–210. doi: 10.1603/0022-2585(2007)44[205:dsoaaa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 24.Chan KL, Ho BC, Chan YC. Aedes aegypti (L.) and Aedes albopictus (Skuse) in Singapore City. 2. Larval habitats. Bull World Health Organ. 1971;44:629–633. [PMC free article] [PubMed] [Google Scholar]
- 25.O'Meara GF, Evans LF, Jr, Gettman AD, Cuda JP. Spread of Aedes albopictus and decline of Ae. aegypti (Diptera: Culicidae) in Florida. J Med Entomol. 1995;32:554–562. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- 26.Juliano SA. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition? Ecology. 1998;79:255–268. [Google Scholar]
- 27.Lounibos LP. Invasions by insect vectors of human disease. Annu Rev Entomol. 2002;47:233–266. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- 28.Barrera R. Competition and resistance to starvation in larvae of container-inhabiting Aedes mosquitoes. Ecol Entomol. 1996;21:117–127. [Google Scholar]
- 29.Service MW . Mosquito Ecology Field Sampling Methods. London: Elsevier Applied Science; 1993. [Google Scholar]
- 30.Reiter P, Amador MA, Anderson RA, Clark GG. Short report: dispersal of Aedes aegypti in an urban area after blood feeding as demonstrated by rubidium-marked eggs. Am J Trop Med Hyg. 1995;52:177–179. doi: 10.4269/ajtmh.1995.52.177. [DOI] [PubMed] [Google Scholar]
- 31.Harrington LC, Scott TW, Lerdthusnee K, Coleman RC, Costero A, Clark GG, Jones JJ, Kitthawee S, Kittayapong P, Sithiprasasna R, Edman JD. Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am J Trop Med Hyg. 2005;72:209–220. [PubMed] [Google Scholar]
- 32.Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol. 2004;18:215–227. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 33.Tun-Lin W, Lenhart A, Nam VS, Rebollar-Tellez E, Morrison AC, Barbazan P, Cote M, Midega J, Sanchez F, Manrique-Saide P, Kroeger A, Nathan MB, Meheus F, Petzold M. Reducing costs and operational constraints of dengue vector control by targeting productive breeding places: a multi-country non-inferiority cluster randomized trial. Trop Med Int Health. 2009;14:1143–1153. doi: 10.1111/j.1365-3156.2009.02341.x. [DOI] [PubMed] [Google Scholar]
- 34.Wong J, Stoddard ST, Astete H, Morrison AC, Scott TW. Oviposition site selection by the dengue vector Aedes aegypti and its implications for dengue control. PLoS Negl Trop Dis. 2011;5:e1015. doi: 10.1371/journal.pntd.0001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aldstadt J, Koenraadt CJ, Fansiri T, Kijchalao U, Richardson J, Jones JW, Scott TW. Ecological modeling of Aedes aegypti (L.) pupal production in rural Kamphaeng Phet, Thailand. PLoS Negl Trop Dis. 2011;5:e940. doi: 10.1371/journal.pntd.0000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seng CM, Setha T, Nealon J, Socheat D. Pupal sampling for Aedes aegypti (L.) surveillance and potential stratification of dengue high-risk areas in Cambodia. Trop Med Int Health. 2009;14:1233–1240. doi: 10.1111/j.1365-3156.2009.02368.x. [DOI] [PubMed] [Google Scholar]
- 37.Heintze C, Velasco Garrido M, Kroeger A. What do community-based dengue control programmes achieve? A systematic review of published evaluations. Trans R Soc Trop Med Hyg. 2007;101:317–325. doi: 10.1016/j.trstmh.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Vu SN, Nguyen TY, Kay BH, Marten GG, Reid JW. Eradication of Aedes aegypti from a village in Vietnam, using copepods and community participation. Am J Trop Med Hyg. 1998;59:657–660. doi: 10.4269/ajtmh.1998.59.657. [DOI] [PubMed] [Google Scholar]
- 39.Vu SN, Nguyen TY, Tran VP, Truong UN, Le QM, Le VL, Le TN, Bektas A, Briscombe A, Aaskov JG, Ryan PA, Kay BH. Elimination of dengue by community programs using Mesocyclops (Copepoda) against Aedes aegypti in central Vietnam. Am J Trop Med Hyg. 2005;72:67–73. [PubMed] [Google Scholar]
- 40.Kay B, Vu SN. New strategy against Aedes aegypti in Vietnam. Lancet. 2005;365:613–617. doi: 10.1016/S0140-6736(05)17913-6. [DOI] [PubMed] [Google Scholar]
- 41.Kay BH, Nam VS, Tien TV, Yen NT, Phong TV, Diep VT, Ninh TU, Bektas A, Aaskov JG. Control of Aedes vectors of dengue in three provinces of Vietnam by use of Mesocyclops (Copepoda) and community-based methods validated by entomologic, clinical, and serological surveillance. Am J Trop Med Hyg. 2002;66:40–48. doi: 10.4269/ajtmh.2002.66.40. [DOI] [PubMed] [Google Scholar]
- 42.Sinh Nam V, Thi Yen N, Minh Duc H, Cong Tu T, Trong Thang V, Hoang Le N, Hoang San L, Le Loan L, Que Huong VT, Kim Khanh LH, Thuy Trang HT, Lam LZ, Kutcher SC, Aaskov JG, Jeffery JA, Ryan PA, Kay BH. Community-based control of Aedes aegypti by using Mesocyclops in southern Vietnam. Am J Trop Med Hyg. 2012;86:850–859. doi: 10.4269/ajtmh.2012.11-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kroeger A, Lenhart A, Ochoa M, Villegas E, Levy M, Alexander N, McCall PJ. Effective control of dengue vectors with curtains and water container covers treated with insecticide in Mexico and Venezuela: cluster randomised trials. BMJ. 2006;332:1247–1252. doi: 10.1136/bmj.332.7552.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vanlerberghe V, Villegas E, Oviedo M, Baly A, Lenhart A, McCall PJ, Van der Stuyft P. Evaluation of the effectiveness of insecticide treated materials for household level dengue vector control. PLoS Negl Trop Dis. 2011;5:e994. doi: 10.1371/journal.pntd.0000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lenhart A, Orelus N, Maskill R, Alexander N, Streit T, McCall PJ. Insecticide-treated bednets to control dengue vectors: preliminary evidence from a controlled trial in Haiti. Trop Med Int Health. 2008;13:56–67. doi: 10.1111/j.1365-3156.2007.01966.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.