Abstract
Our study investigated the possible impact of SP-IPT given to infants and children on the prevalence of SP-resistant haplotypes in the Plasmodium falciparum genes Pfdhfr and Pfdhps, comparing sites with and without IPTi/c. P. falciparum positive samples (N = 352) collected from children < 5 years were analyzed to determine the prevalence of SP resistance-related haplotypes by nested PCR followed by sequence-specific oligonucleotide probe-enzyme-linked immunosorbent assay. The prevalence of the Pfdhfr triple mutant haplotype (CIRN) increased in both groups, but only significantly in the IPTi/c group from 41% to 65% in 2011 (P = 0.005). Conversely, the Pfdhps 437G mutation decreased in both groups from 44.6% to 28.6% (P = 0.07) and from 66.7% to 47.5% (P = 0.02) between 2010 and 2011 in the control and the IPTi/c groups, respectively. A weak trend for decreasing prevalence of quadruple mutants (triple Pfdhfr + Pfdhps 437G) was noted in both groups (P = 0.15 and P = 0.34). During the two cross-sectional surveys some significant changes were observed in the SP resistance-related genes.
Introduction
During the last decades, Plasmodium falciparum has become broadly resistant to widely used antimalarial drugs such as chloroquine (CQ) and sulfadoxine-pyrimethamine (SP).1 Still, one of the essential strategies to control P. falciparum infections in malaria-endemic countries in sub-Saharan Africa is the use of SP as an intermittent preventive treatment (SP-IPT) for malaria in risk groups.2 For instance, many African countries have through their National Malaria Control Program (NMCP) implemented the use of SP-IPT during pregnancy (IPTp).3 In recent years, several studies in Africa, including Senegal, have explored the use of SP for malaria prevention in infancy (IPTi) through the Expanded Program on Immunization (EPI). In a pooled data analysis of six clinical SP-IPTi trials, the strategy was shown to provide a 30% overall protection against clinical malaria episodes (95% confidence interval [CI] = 19.8–39.4%) and overall reduction in the prevalence of anemia (< 8 g/dL) by 21.3% (95% CI = 8.3–32.5%).4,5 Consequently, the World Health Organization (WHO) recommended the implementation of the IPTi strategy.4 However, a SP-IPTi study in Tanzania, not included in the pooled analysis, contrarily found no protection at all, most likely a result of the high level of SP resistance in the P. falciparum populations prevailing in the study area.6 Thus, the protective efficacy of IPTi is highly dependent on regional levels of SP resistance in the P. falciparum populations.
In areas of markedly seasonal malaria transmission, such as the Sahel and sub-Sahel regions of Africa, the main burden of malaria is in older children rather than infants, and the risk of clinical malaria is restricted largely to a few months each year.7,8 In such areas, administration of IPT to children (IPTc) 3 months to 5 years of age monthly during the seasonal peak in malaria transmission seems like an attractive strategy in preventing malaria. A meta-analysis of 12 clinical IPTc studies in sub-Saharan Africa including Senegal using SP, but always combined with another drug (amodiaquine AQ), have demonstrated impressive protective efficacies against clinical malaria episodes ranging from 31% to 93% (overall protective efficacy of 82% (95% CI = 75–87%).9 Furthermore, it was shown that IPTc protected against all-cause mortality by 57% (95% CI = 24–76%).9 Thus, IPTc is highly effective and furthermore, well tolerated.10–12 Therefore, WHO recommended IPTc,13 now named Seasonal Malaria Chemoprevention (SMC) and the intervention will most likely be adopted large scale in areas where malaria transmission is seasonal. However, as long as SP is used, the protective efficiency of these strategies is highly dependent on the current level and spread of SP resistance in P. falciparum populations.
Plasmodium falciparum in vivo and in vitro resistance to pyrimethamine and sulfadoxine is associated with single nucleotide polymorphisms (SNPs) in the P. falciparum dihydrofolate reductase gene (Pfdhfr) and the dihydropteroate synthetase gene (Pfdhps), respectively. Resistance to pyrimethamine is related with SNPs in the Pfdhfr gene resulting in amino acid changes at mainly positions N51I, C59R, S108N/T, and I164L.14,15 In the Pfdhps gene, five SNPs, namely, S436A/F, A437G, K540E, A581G, and A613S/T is associated with P. falciparum resistance to sulfadoxine.16,17 The accumulation of SNPs in both Pfdhfr and Pfdhps genes leads to increased risk of clinical failures after SP treatment. The predictive value of the combination of these SNPs varies geographically, depending on, e.g., baseline prevalence, age, and levels of acquired immunity.18–22 In Africa, the Pfdhfr triple mutant, 51I-59R-108N, together with the Pfdhps double mutant, 437G-540E forms the quintuple mutant that predicts a high risk of treatment failure after SP treatment.23,21 Similarly, and specifically regarding IPTi, recent evidence suggests that high prevalence (> 50%) of the highly resistant Pfdhps 540E mutant—essentially the quintuple Pfdhfr/Pfdhps mutant, may undermine the required protective efficacy of SP-IPTi of 20%.24
However, cumulative effects of IPTi/c interventions and what happens when follow-up is performed at long term remain largely unknown. The aim of this study was to investigate the possible impact of IPTi/c on prevalence of SNPs in Pfdhfr/Pfdhps after long-term follow-up in Senegal. The study was conducted in one area where both IPTi and IPTc strategies have been implemented since 2007 and 2009, respectively, and compared with a study site where none of these strategies have been applied.
Methods
Study sites and sample collection.
The samples for this study were collected in the southern part of Senegal in Velingara, Saraya, and Tambacounda health districts located 500, 400, and 700 km from the capital city of Dakar, respectively (Figure 1). In Velingara and Saraya districts, a pilot study of IPTi was conducted from 2006 to 2009. In the same area, IPTc was also implemented from 2009 until now. Tambacounda district was the control for both strategies. Malaria transmission in these areas is seasonal and in 2008 the entomological inoculation rate was 264-infected bites/person/year25; before blood sample collection, written informed consent was obtained from the parent or guardian of each child. During the study, if children presented to health huts with symptoms consistent with uncomplicated malaria, including fever and a positive rapid diagnostic test (RDT), they were offered standard artemisinin combination therapy first-line treatment, and children with severe malaria were referred to the nearest health center and received quinine treatment.
Figure 1.
Study area Velingara and Saraya: IPTi/c districts Tambacounda: Control district (without IPTi/c).
Blood samples were collected using finger prick blood. Thick and thin smears were stained with Giemsa. Parasite density was determined by counting the number of asexual parasites per 200 white blood cells, and calculated per μL using the following formula: number of parasites × 8,000/200, assuming a white blood cell count of 8,000 cells/μL. The absence of malaria parasites in 200 high power ocular fields of the thick film was considered as negative. Additionally, the finger prick blood was also blotted onto Whatman filter paper 3MM. Samples were stored at room temperature and protected with silica gel desiccant. Filter papers corresponding to thick P. falciparum positives were selected for later P. falciparum DNA isolation.
DNA extraction and Pfdhfr/Pfdhps SNPs analysis.
DNA was extracted from positive P. falciparum blood spots by the Chelex-100 method described by Wooden and others26 with some modifications described by Pearce and others27 A nested polymerase chain reaction (PCR) (was used to amplify fragments of the Pfdhfr and Pfdhps genes as described by Alifrangis and others.28 The 20-μL Pfdhfr/Pfdhps outer PCR mixture consisted of 0.3 mM of each dNTP, 0.25 μM of either primer set M1/M7 (Pfdhfr) or N1/N2 (Pfdhps), one unit of DNA HotStart polymerase (Ampliqon III; VWR-Bie Berntsen, Denmark), buffer containing 1.5 mM MgCl2, as recommended by the manufacturer, and 1 μL of extracted DNA. Genomic DNA preparation of laboratory isolates 3D7, FRC3, K1, Dd2, and 7G8 were included as controls with known Pfdhfr/Pfdhps haplotypes. The nested Pfdhfr and Pfdhps PCR reaction mixture was the same as the outer PCR mixture using primer sets M3b/M9 and R2/R/ for the dhfr and dhps PCR, respectively. Amplifications were performed in 96-well PCR microplates. The M9 and R/primers for the Pfdhfr and Pfdhps, nested PCRs were biotinylated at the 5_-end by the supplier (MWG Biotech, Riskov, Denmark). The nested PCR products were confirmed by running the controls by electrophoresis on 1.5% agarose gel.
The SNPs at Pfdhfr (position 50/51, 59, and 108), Pfdhps (position 436/437, 540, 581, and 613) were determined by the sequence-specific oligonucleotide probe (SSOP)-enzyme-linked immunosorbent assay (ELISA)-based technique of PCR amplified fragments, as described in Reference 28. Briefly, biotin-conjugated nested PCR amplified DNA were fixed on streptavidin-coated ELISA plates and mixed with digoxigenin-labeled oligonucleotide probes with specificity for the SNPs of interest. The mixtures were washed with high stringency at set temperatures using Tetramethyl ammonium chloride (TMAC; Sigma Aldrich Chemie, Seelze, Germany) solution (3 M TMAC, 50 mM Tris, pH 8.0, 0.1% sodium dodecyl sulfate, 2 mM EDTA, pH 8.0), before incubated with peroxidase-conjugated anti-digoxigenin antibodies and visualized by o-phenylenediamine (OPD).28 For each SNP analyses, parasite samples were categorized into single or mixed infections as follows: infections were considered to be single genotype when only one SNP was present at optical density (OD) values above the threshold of positivity. Samples were considered to be mixed but containing a majority SNP genotype when the OD value of the weakly reacting SSOP was less than half the OD value of the strongly reacting SSOP. Conversely, if the OD value of the weakly reacting SSOP was higher than half the OD value of the strongly reacting SSOP, the infection was categorized as mixed with no dominant genotype. For the construction of Pfdhfr or Pfdhps haplotypes, samples that contained mixed SNP genotypes without one being dominant at more than one codon were omitted from the analysis.
Statistical analysis.
Statistical analyses of data were performed using Epi Info (version 06; CDC, Atlanta, GA). The χ2 test was used to compare differences in proportions in Pfdhfr/Pfdhps comparing parasite populations. The significance level of statistical tests was set at 0.05, with a two-sided test. Data from this study were also compared with previous Pfdhfr/Pfdhps studies conducted in the same study area using the χ2 test.
Ethical approval.
This study received the approval of the National Ethical Committee (000169 MSP/DS/CNERS) and the administrative approval of the Ministry of Health and Prevention of Senegal (004883 MSP/DS/CNERS). Informed consent was signed by all parents or legal representatives of children before any blood sampling.
Results
Overall, 1,903 samples were collected in the health district of Tambacounda without IPTi/c intervention; e.g., the control zone (804 in 2009 and 1,099 in 2010) and a total of 2,457 samples (1,215 in 2009 and 1,242 in 2010) in the health district of Velingara and Saraya where IPTi/c has been implemented (IPTi/c zone).
The prevalence of P. falciparum infections in the control zone (based on RDT determination) increased significantly from 10.1% (81 of 804) in 2009 to 13.9% (153 of 1,099) in 2010 (χ2 = 5.00, P = 0.02). Similarly, in the IPTi/c zone the prevalence increased significantly from 3.7% (46 of 1,215) in 2009 to 10.1% (125 of 1,242) in 2010 (χ2 = 32.57, P ≤ 0.001). Overall, 234 samples were P. falciparum RDT positive in the control group and 176 in the IPTi/c zone. Out of these, 164 samples (88 samples in 2009 and 88 in 2010) were randomly selected in each zone for further PCR analysis.
Prevalence of SNPs in the Pfdhfr and Pfdhps genes in samples from IPTi/c and control zones in 2009 and 2010.
In the control zone, the prevalence of mutant SNPs at N51I, C59R, and S108N in Pfdhfr including mixed infections, were 65.3% (47 of 72), 65.3% (47 of 72), and 72.6% (53 of 73), respectively, in 2009, whereas 75.0% (48 of 64), 76.6% (49 of 64), and 91.0% (69 of 76), respectively, in 2010 (Table 1). Though a trend for an increase in the prevalence of all mutant SNPs was observed, only S108N was significant (χ2 = 8.30, P = 0.003).
Table 1.
Prevalence of Pfdfr/Pfdhps mutation before and after IPTi/c in Senegal
| Mutations | Control zone | P values | IPTi/c zone | P values | |||
|---|---|---|---|---|---|---|---|
| 2009 | 2010 | 2009 | 2010 | ||||
| Pfdhfr | 51I | 65.3% (47/72) | 75.0% (48/64) | 0.21 | 60.8% (48/79) | 60.7% (51/84) | 0.99 |
| 59R | 65.3% (47/72) | 76.6% (49/64) | 0.14 | 67.5% (54/80) | 71.2% (52/73) | 0.61 | |
| 108N | 72.6% (53/73) | 90.7% (69/76) | 10−3 | 73.0 (54/74) | 79.7% (59/74) | 0.33 | |
| Pfdhps | 437G | 44.6% (25/56) | 66.7% (54/81) | 0.01 | 28.6% (16/56) | 47.5% (29/61) | 0.03 |
| 581G | 0.0% (0/64) | 2.7% (2/73) | – | 2.4% (2/83) | 0.0% (0/78) | – | |
| 613S | 4.8% (3/62) | 7.1% (5/70) | 0.85 | 4.9% (4/81) | 0.0% (0/72) | – | |
In the IPTi/c zone, the prevalence of N51I, C59R, and S108N including mixed infections, were 60.8% (48 of 79), 67.5% (54 of 80), and 73.0% (54 of 74), respectively, in 2009, whereas in 2010 it was 60.7% (51 of 84), 71.2% (52 of 73), and 79.7% (59 of 74), respectively, and no significant difference was observed between the years (Table 1). For both zones, only wild-type at codon 164 was detected. Between the control and the IPTi/c zone in 2009, there was no difference between the codons of Pfdhfr (data not shown).
For the Pfdhps gene, in the control zone, a trend for a decrease in the prevalence of Pfdhps A437G including mixed infections was observed from 44.6% (25 of 56) in 2009 to 28.6% (16 of 56) in 2010 (χ2 = 3.12; 0.07), whereas in the IPTi/c zone a significant decrease was noted from 66.7% (54 of 81) in 2009 to 47.5% (29 of 61) in 2010 (χ2 = 5.92, P = 0.01) (Table 1). The Pfdhps A581G was identified in the IPTi/c zone at 2.4% (2 of 83) in 2009, however absent in the 2010 samples, whereas it was 2.7% (2 of 73) in 2010 in the control zone. In 2009, the prevalence of Pfdhps 613S was 4.8% (3 of 62) and 4.9% (4 of 81) in the control and IPTi/c zone, respectively, although in 2010 it rose to 7.1% (5 of 70) in the control zone and was absent in the IPTi/c zone (Table 1). For both zones, only wild-types (540 K) were detected.
Prevalence of constructed Pfdhfr and Pfdhps haplotypes in samples from IPTi/c and control zones in 2009 and 2010.
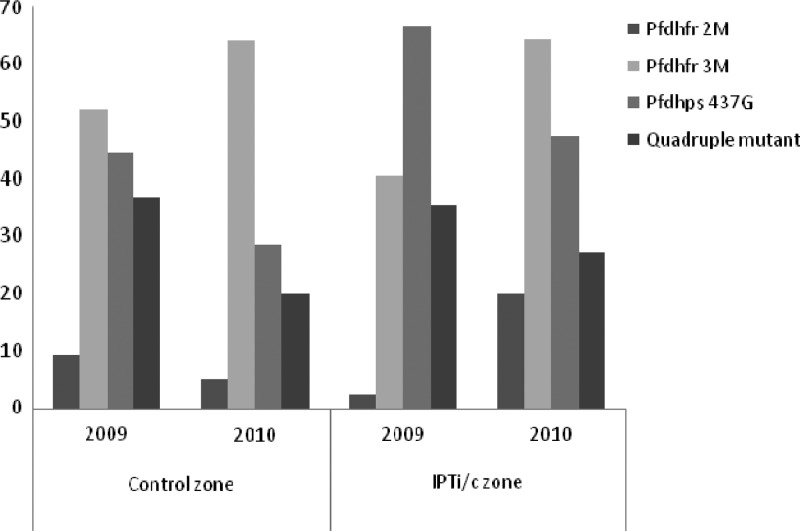
In the control zone, the prevalence of parasites harboring the double mutant haplotypes (CICN, CNRN) including mixed haplotype infections (excluding haplotypes with more than one mixed SNP) was 9.3% (6 of 64) in 2009 and 5.1% (2 of 39) in 2010 with no significant difference between the years (P = 0.68) (Figure 2). In the IPTi/c zone, the prevalence of double mutant haplotype was 2.3% (1 of 43) in 2009, although increasing significantly to 20.0% (10 of 50) in 2010 (χ2 = 6.92, P = 0.008). Regarding the triple mutant Pfdhfr haplotype (CIRN), there was a small trend for an increase in prevalence in the control group from 52.1% (37 of 71) in 2009 to 64.3% (36 of 56) in 2010 (χ2 = 1.90, P = 0.17), whereas in the IPT group, it was significant, from 40.6% (28 of 69) in 2009 to 64.6% (42 of 65) in 2010 was observed (χ2 = 7.75, P = 0.005). A decrease of Pfdhfr wild-types and/or single mutants was noted in the IPTi/c zone.
Figure 2.
Prevalence of Pfdhfr/Pfdhps mutant haplotype in the study area.
There was a trend for a decrease in the prevalence of parasites harboring the Pfdhps 437G mutation (as SGKAA, AGKAA, and FGKAA) from 44.6% (25 of 56) in 2009 to 28.6% (16 of 56) in 2010 in the control group (χ2 = 3.12, P = 0.08), whereas this was significant in the IPT-group from 66.7% (54 of 81) in 2009 to 47.5% (29 of 61) in 2010 (χ2 = 5.24; P = 0.02).
When both Pfdhfr and Pfdhps haplotypes were jointly examined by constructing Pfdhfr-Pfdhps haplotypes, the prevalence of quadruple mutant parasites (CIRN/SGKAA or CIRN/AGKAA), a limited decrease in both groups from 36.8% (14 of 38) to 20.0% (5 of 25) (χ2 = 2.03, P = 0.15) and from 35.6% (21 of 59) to 27.1% (13 of 48) was observed in 2009 and 2010 for the control and the IPTi/c group, respectively (χ2 = 0.88, P = 0.35).
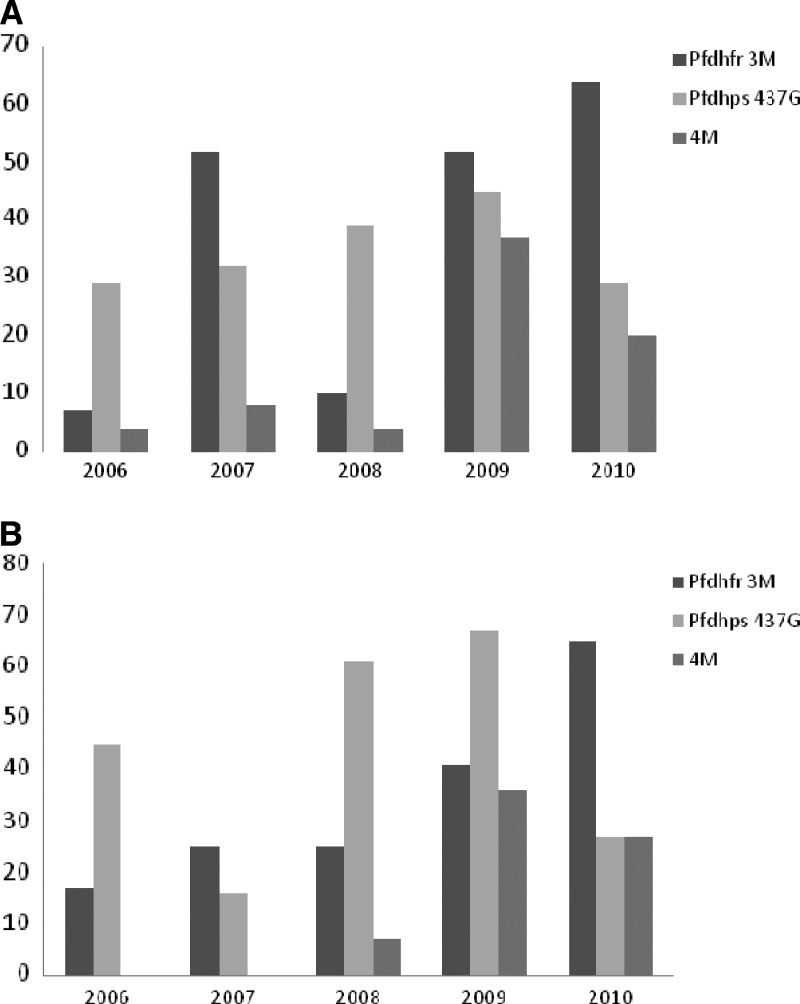
Temporal distribution of Pfdhfr/Pfdhps SNP/haplotypes prevalence in samples from IPTi/c and control zones between 2006 and 2010.
Results of the Pfdhfr/Pfdhps SNPs/haplotypes in samples from 2009 and 2010 was compared with previously published results obtained in the years 2006, 2007, and 2008 from the same study area, Faye and others29 (Figure 3). Except for a sudden high prevalence of the Pfdhfr triple mutant haplotype (52%) observed in 2007 in the control zone (Figure 3A), a general trend of increase of Pfdhfr triple mutant (3M) parasites was noted from 2006 to 2010 in both groups: from a prevalence of 7% and 17% in 2006 to 64.3% and 64.6% in 2010 for the control and IPTi/c zones, respectively (χ2 = 23.7; P ≤ 0.001) (Figure 3A and B). A significant increase of the quadruple mutation (Pfdhfr triple mutant plus Pfdhps 437G) was noted: from 3.7% and 4.4% in 2006 to 38.6% and 35.6% in 2009 in the control and IPT zone, respectively (χ2 = 9.7; P ≤ 0.001; χ2 = 33.2; P ≤ 0.001) (Figure 3B). However, in 2010, as described, there was a trend for a decrease in prevalence in both groups (Figure 3A and B).
Figure 3.
(A) Temporal distribution of Pfdhfr/Pfdhps single nucleotide polymorphisms (SNPs) mutant haplotype from 2006 to 2010 in control zone (without IPTi/c). (B) Temporal distribution of Pfdhfr/Pfdhps SNPs mutant haplotype from 2006 to 2010 in IPTi/c zone Pfdhfr 3M: haplotype dhfr triple mutant (Pfdhfr 51I, 59R, 108N) 4M: haplotype quadruple mutant (Pfdhfr 51I, 59R, 108N + Pfdhps 437G) 2006: Baseline data IPTi: Pilot study from 2007 to 2008: after 2 years of IPTi a significant increase of Pfdhps 437G was noted IPTc: started from 2009 to 2010.
Discussion
In malaria-endemic settings, IPT using mainly SP have shown high protective efficacy against malaria, anemia, and death in IPTp,30–32 IPTi,4 and IPTc12; despite the beneficial impact of these strategies, mass implementation of IPT raises overall concern on whether the strategy may drive the spread of SP resistance further. Resistance to SP is well established in East Africa, whereas not as pronounced in West Africa, mainly as a result of continuing low prevalence of the Pfdhps 540E mutation and thus, also low prevalence of the quintuple Pfdhfr/Pfdhps haplotype. In large areas of East Africa SP for IPTi and SMC should not be applied because above 50% prevalence of the Pfdhps 540E mutation and thus other options or combinations are currently tested such as artesunate plus amodiaquine (AS+AQ), artesunate plus sulfadoxine pyrimethamine (AS+SP), dihydroartemisinin plus piperaquine (DHA+PPQ), sulfadoxine pyrimethamine plus piperaquine (SP+PPQ). Regarding IPTc (now SMC), further research is also needed to evaluate the potential benefits of these drugs. For IPTc, to ensure a maximum protective effect in areas of highly seasonal malaria, it is recommended to combine two long half-life drugs and the combination of SP/AQ is currently the most optimal regimen.10 With concerns of continuing accumulation of SP resistance possibly establishing quintuple Pfdhfr/Pfdhps haplotypes (or similar) in West Africa as well, periodical monitoring of molecular markers of SP resistance is necessary to evaluate these strategies in the region. This study performed in Senegal evaluated the possible impact of IPTi/c on SP resistance markers after long-term follow-up in areas where both strategies were implemented.
The study observed a general trend of an increase in the prevalence of triple Pfdhfr haplotypes in both intervention and control areas over just 1 year. Furthermore, a high prevalence of the Pfdhps 437G mutants was observed in both groups in 2009, however the prevalence seemed to decrease in 2010. Finally, a combined, high prevalence of quadruple mutant (triple mutant Pfdhfr + 437G mutations) haplotype was noted in both areas in 2009 but as well seemed to decrease in 2010. Compared with the baseline study conducted by Faye and others29 in the same study area, a general increase in all mutant haplotypes was noted. However, comparing the two groups in 2009 and 2010, it appears that, although changes are observed as a trend for an increase of triple Pfdhfr haplotypes and a trend for a decrease in Pfdhps 437G this is not only seen in the IPTi/c group and thus, IPTi/c is not driving the selection of SP resistance in the study area. Other factors may impose drug pressure in both the control and the IPTi/c zone; possibility of availability of SP in the private market and as well, the use of other sulfa-drugs such as cotrimoxazole. Similarly, in Mali, a study showed that the prevalence of SP resistance markers did not increase over a 1-year period of SP-IPTi intervention but remained unchanged at 34.6% and 8.1% regarding triple and quadruple mutations.33 Conversely, in Mozambique, Mayor and others (2008) observed that the prevalence of Pfdhfr/Pfdhps quintuple mutants nearly doubled in the IPTi-SP group compared with the placebo group.34 For IPTc in a study in Senegal, Cissé and others (2007) have shown that post-intervention prevalence of Pfdhfr triple mutants plus Pfdhps A437G mutants was significantly higher in the SP-artesunate treatment arm than the placebo arm.10 The same tendency was also observed in Mali; the post-intervention prevalence of Pfdhfr/Pfdhps quadruple mutants was significantly higher in the SP-amodiaquine group than the placebo group.12 In areas with a long and high malaria transmission SMC will be given an all year-round treatment, there may then be a stronger selection of resistance. This could explain why this strategy is not recommended in areas of high malaria transmission (as Uganda).
Overall, during a short period of the two cross-sectional surveys some significant changes were observed in Pfdhfr and Pfdhps. However, because these changes were observed in both the intervention and in the control zones, the IPTi/c strategy does only seem to have limited impact on resistance development and other factors that impact as well. However, continuous monitoring will be needed as a result of the up scaling of the IPTc strategy in Senegal according to WHO recommendations.
ACKNOWLEDGMENTS
We express our gratitude to all the study participants, particularly the study population and administrative authorities, the entire staff of the Parasitology and Mycology Department (Senegal) and the Center for Medical Parasitology (CMP, Copenhagen).
Disclaimer: None of the authors declared a conflict of interest.
Footnotes
Financial support: This work was supported by the Malaria Capacity Development Consortium, which is funded by the Wellcome Trust (Grant number WT084289MA) and the Bill & Melinda Gates Foundation (Grant no. 51941) website: http://www.mcdconsortium.org.
Authors' addresses: Magatte Ndiaye, Roger Tine, Babacar Faye, Jean Louis Ndiaye, Ami Colle Lo, Khadime Sylla, Annie Abiola, Daouda Ndiaye, and Oumar Gaye, Cheikh Anta Diop University - Parasitology and Mycology, Dakar, Senegal, E-mails: magou22000@yahoo.fr, rogertine@hotmail.com, bfaye67@yahoo.fr, jlndiaye@yahoo.fr, amlosn@yahoo.fr, khadimesylla@yahoo.fr, annie_abiola@yahoo.fr, dndiaye@hsph.harvard.edu, and ogaye@refer.sn. Yémou Dieng, Dakar Faculty of Medicine - Parasitology-Mycology, Dakar, Senegal, E-mail: yemoud1@yahoo.fr. Rachel Hallett, UCL - Gynaecological Oncology, London, UK, E-mail: rachel.hallet@lshtm.ac.uk. Michael Alifrangis, Centre for Medical Parasitology - Department of International Health, Immunology and Microbiology, University of Copenhagen, and the Department of Infectious Diseases, Copenhagen University Hospital (Rigshospitalet), Copenhagen, Denmark, E-mail: micali@sund.ku.dk.
References
- 1.Le Bras J, Musset L, Clain J. Antimalarial drug resistance. Med Mal Infect. 2006;36:401–405. doi: 10.1016/j.medmal.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Greenwood B. The use of anti-malarial drugs to prevent malaria in the population of malaria-endemic areas. Am J Trop Med Hyg. 2004;70:1–7. [PubMed] [Google Scholar]
- 3.National Malaria Control Program . National Consensus Workshop on Malaria Treatment Policy in Senegal. Dakar, Senegal: Ministry of Health, Hygiene and Prevention; 2003. p. 71. [Google Scholar]
- 4.Aponte JJ, Schellenberg D, Egan A, Breckenridge A, Carneiro I, Critchley J, Danquah I, Dodoo A, Kobbe R, Lell B, May J, Premji Z, Sanz S, Sevene E, Soulaymani-Becheikh R, Winstanley P, Adjei S, Anemana S, Chandramohan D, Issifou S, Mockenhaupt F, Owusu-Agyei S, Greenwood B, Grobusch MP, Kremsner PG, Macete E, Mshinda H, Newman RD, Slutsker L, Tanner M, Alonso P, Menendez C. Efficacy and safety of intermittent preventive treatment with sulfadoxine-pyrimethamine for malaria in African infants: a pooled analysis of six randomised, placebo-controlled trials. Lancet. 2009;374:1533–1542. doi: 10.1016/S0140-6736(09)61258-7. [DOI] [PubMed] [Google Scholar]
- 5.Grobusch MP, Egan A, Gosling RD, Newman RD. Intermittent preventive therapy for malaria: progress and future directions. Infect Dis. 2007;20:613–620. doi: 10.1097/QCO.0b013e3282f1ae3b. [DOI] [PubMed] [Google Scholar]
- 6.Gosling RD, Gesase S, Mosha JF, Carneiro I, Hashim R, Lemnge M, Mosha FW, Greenwood B, Chandramohan D. Protective efficacy and safety of three antimalarial regimens for intermittent preventive treatment for malaria in infants: a randomised, double- blind, placebo-controlled trial. Lancet. 2009;374:1521–1532. doi: 10.1016/S0140-6736(09)60997-1. [DOI] [PubMed] [Google Scholar]
- 7.Etard JF, Le Hesran JY, Diallo A, Diallo JP, Ndiaye JL, Delaunay V. Childhood mortality and probable causes of death using verbal autopsy in Niakhar, Senegal, 1998–2000. Int J Epidemiol. 2004;33:1286–1292. doi: 10.1093/ije/dyh259. [DOI] [PubMed] [Google Scholar]
- 8.Jaffar S, Leach A, Greenwood AM, Jepson A, Muller O, Ota MO, Bojang K, Obaro S, Greenwood BM. Changes in the pattern of infant and childhood mortality in upper river division, The Gambia, from 1989 to 1993. Trop Med Int Health. 1997;2:28–37. doi: 10.1046/j.1365-3156.1997.d01-131.x. [DOI] [PubMed] [Google Scholar]
- 9.Wilson AL. IPTc Taskforce A systematic review and meta-analysis of the efficacy and safety of intermittent preventive treatment of malaria in children (IPTc) PLoS ONE. 2011;6:e16976. doi: 10.1371/journal.pone.0016976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cisse B, Faye S, Dial Y, Faye O, Gaye O, et al. Pilot study of the implementation of seasonal intermittent treatment in children (IPTc) with community participation in Senegal. Trop Med Int Health. 2007;((Suppl 1)):46.025–40. [Google Scholar]
- 11.Chandramohan D, Owusu-Agyei S, Carneiro I, Awine T, Amponsa-Achiano K, Mensah N, Jaffar S, Baiden R, Hodgson A, Binka F, Greenwood B. Cluster randomized trial of intermittent preventive treatment for malaria in infants in area of high, seasonal transmission in Ghana. BMJ. 2005;331:727–733. doi: 10.1136/bmj.331.7519.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cissé B, Sokhna C, Boulanger D, Milet J, Bâ EH, Richardson K, Hallett R, Sutherland C, Simondon K, Simondon F, Neal A, Gaye O, Targett G, Lines J, Greenwood B, Trape JF. Seasonal intermittent preventive treatment with artesunate and sulfadoxine-pyrimethamine for prevention of malaria in Senegalese children: a randomised, placebo-controlled, double-blind trial. Lancet. 2006;367:659–667. doi: 10.1016/S0140-6736(06)68264-0. [DOI] [PubMed] [Google Scholar]
- 13.WHO Seasonal Malaria Chemoprevention (SMC) for Plasmodium falciparum Malaria Control in Highly Seasonal Transmission Areas of the Sahel Sub-Region in Africa. 2012. http://www.who.int/malaria/mpac/feb2012/mpac_article_03_2012.pdf Available at. Accessed October 15, 2012.
- 14.Cowman AF, Morry MJ, Biggs BA, Cross GA, Foote SJ. Amino acid changes linked to pyrimethamine resistance in the dihydrofolate reductase-thymidylate synthase gene of Plasmodium falciparum. Proc Natl Acad Sci USA. 1988;85:9109–9113. doi: 10.1073/pnas.85.23.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson DS, Walliker D, Wellems TE. Evidence that a point mutation in dihydrofolate reductase-thymidylate synthase confers resistance to pyrimethamine in falciparum malaria. Proc Natl Acad Sci USA. 1988;85:9114–9118. doi: 10.1073/pnas.85.23.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooks DR, Wang P, Read M, Watkins WM, Sims PF, Hyde JE. Sequence variation of the hydroxymethyldihydropterin pyrophosphokinase: dihydropteroate synthase gene in lines of the human malaria parasite, Plasmodium falciparum, with differing resistance to sulfadoxine. Eur J Biochem. 1994;224:397–405. doi: 10.1111/j.1432-1033.1994.00397.x. [DOI] [PubMed] [Google Scholar]
- 17.Triglia T, Cowman AF. Primary structure and expression of the dihydropteroate synthetase gene of Plasmodium falciparum. Proc Natl Acad Sci USA. 1994;91:7149–7153. doi: 10.1073/pnas.91.15.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alifrangis M, Lusingu JP, Mmbando B, Dalgaard MB, Vestergaard LS, Ishengoma D, Khalil IF, Theander TG, Lemnge MM, Bygbjerg IC. Five-year surveillance of molecular markers of Plasmodium falciparum antimalarial drug resistance in Korogwe District, Tanzania: accumulation of the 581G mutation in the P. falciparum dihydropteroate synthase gene. Am J Trop Med Hyg. 2009;80:523–527. [PubMed] [Google Scholar]
- 19.Staedke SG, Sendagire H, Lamola S, Kamya MR, Dorsey G, Rosenthal PJ. Relationship between age, molecular markers, and response to sulphadoxine-pyrimethamine treatment in Kampala, Uganda. Trop Med Int Health. 2004;9:624–629. doi: 10.1111/j.1365-3156.2004.01239.x. [DOI] [PubMed] [Google Scholar]
- 20.Happi CT, Gbotosho GO, Folarin OA, Akinboye DO, Yusuf BO, Ebong OO, Sowunmi A, Kyle DE, Milhous W, Wirth DF, Oduola AM. Polymorphisms in Plasmodium falciparum dhfr and dhps genes and age related in vivo sulfadoxine-pyrimethamine resistance in malaria-infected patients from Nigeria. Acta Trop. 2005;95:183–193. doi: 10.1016/j.actatropica.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 21.Kublin JG, Dzinjalamala FK, Kamwendo DD, Malkin EM, Cortese JF, Martino LM, Mukadam RA, Rogerson SJ, Lescano AG, Molyneux ME, Winstanley PA, Chimpeni P, Taylor TE, Plowe CV. Molecular markers for failure of sulfadoxine-pyrimethamine and chlorproguanil-dapsone treatment of Plasmodium falciparum malaria. J Infect Dis. 2002;185:380–388. doi: 10.1086/338566. [DOI] [PubMed] [Google Scholar]
- 22.Nzila AM, Nduati E, Mberu EK, Sibley CH, Monks SA, Winstanley PA, Watkins WM. Molecular evidence of greater selective pressure for drug resistance exerted by the long-acting antifolate pyrimethamine/sulfadoxine compared with the shorter-acting chlorproguanil/dapsone on Kenyan Plasmodium falciparum. JID. 2000;181:2023–2028. doi: 10.1086/315520. [DOI] [PubMed] [Google Scholar]
- 23.Omar SA, Adagu IS, Warhurst DC. Can pretreatment screening for dhps and dhfr point mutations in Plasmodium falciparum infections be used to predict sulfadoxine-pyrimethamine treatment failure? Trans R Soc Trop Med Hyg. 2001;95:315–319. doi: 10.1016/s0035-9203(01)90250-0. [DOI] [PubMed] [Google Scholar]
- 24.WHO . Technical Consultation on Intermittent Preventive Treatment in Infants (IPTi), Technical Expert Group on Preventive Chemotherapy. Geneva: World Health Organization; 2009. pp. 1–11. WHO TEG IPTi Report April 2009. [Google Scholar]
- 25.Dia I, Diop T, Rakotoarivony I, Kengne P, Fontenille D. Bionomics of Anopheles gambiae Giles, An. arabiensis Patton, An. funestus Giles and An. nili (Theobald) (Diptera: Culicidae) and transmission of Plasmodium falciparum in a Sudano-Guinean zone (Ngari, Senegal) J Med Entomol. 2003;40:279–283. doi: 10.1603/0022-2585-40.3.279. [DOI] [PubMed] [Google Scholar]
- 26.Wooden J, Kyes S, Sibley CH. PCR and strain identification in Plasmodium falciparum. Parasitol Today. 1993;9:303–305. doi: 10.1016/0169-4758(93)90131-x. [DOI] [PubMed] [Google Scholar]
- 27.Pearce RJ, Drakeley C, Chandramohan D, Mosha F, Roper C. Molecular determination of point mutation haplotypes in the dihydrofolate reductase and dihydropteroate synthase of Plasmodium falciparum in three districts of northern Tanzania. Antimicrob Agents Chemother. 2003;47:1347–1354. doi: 10.1128/AAC.47.4.1347-1354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alifrangis M, Enosse S, Pearce R, Drakeley C, Roper C, Khalil IF, Nkya WM, Ronn AM, Theander TG, Bygjerg IB. A simple, high-throughput method to detect Plasmodium falciparum single nucleotide polymorphisms in the dihydrofolate reductase, dihydropteroate synthase, and P. falciparum chloroquine resistance transporter genes using polymerase chain reaction- and enzyme-linked immunosorbent assay-based technology. AJTMH. 2005;72:155–162. [PubMed] [Google Scholar]
- 29.Faye B, Ndiaye M, Ndiaye JL, Annie A, Tine RC, Lo AC, Ndiaye M, Sow D, De Sousa A, Gaye O. Prevalence of molecular markers of Plasmodium falciparum resistance to sulfadoxine–pyrimethamine during the intermittent preventive treatment in infants coupled with the expanded program immunization in Senegal. Parasitol Res. 2011;109:133–138. doi: 10.1007/s00436-010-2236-9. [DOI] [PubMed] [Google Scholar]
- 30.Menéndez C, D'Alessandro U, ter Kuile FO. Reducing the burden of malaria in pregnancy by preventive strategies. Lancet Infect Dis. 2007;7:126–135. doi: 10.1016/S1473-3099(07)70024-5. [DOI] [PubMed] [Google Scholar]
- 31.Crawley J, Hill J, Yartey J, Robalo M, Serufilira A, Ba-Nguz A, Roman E, Palmer A, Asamoa K, Steketee R. From evidence to action? Challenges to policy change and programme delivery for malaria in pregnancy. Lancet Infect Dis. 2007;7:145–155. doi: 10.1016/S1473-3099(07)70026-9. [DOI] [PubMed] [Google Scholar]
- 32.Mbaye A, Richardson K, Balajo B, Dunyo S, Shulman C, Milligan P, Greenwood B, Walraven G. A randomized, placebo-controlled trial of intermittent preventive treatment with sulphadoxine-pyrimethamine in Gambian multigravidae. Trop Med Int Health. 2006;11:992–1002. doi: 10.1111/j.1365-3156.2006.01649.x. [DOI] [PubMed] [Google Scholar]
- 33.Dicko A, Sagara I, Djimdé AA, Touré SO, Traore M, Dama S, Diallo AI, Barry A, Dicko M, Coulibaly OM, Rogier C, de Sousa A, Doumbo OK. Molecular markers of resistance to sulphadoxine-pyrimethamine one year after implementation of intermittent preventive treatment of malaria in infants in Mali. Malar J. 2010;9:9. doi: 10.1186/1475-2875-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayor A, Serra-Casas E, Sanz S, Aponte JJ, Macete E, Mandomando I, Puyol L, Berzosa P, Dobaño C, Aide P, Sacarlal J, Benito A, Alonso P, Menéndez C. Molecular markers of resistance to sulfadoxine-pyrimethamine during intermittent preventive treatment for malaria in Mozambican infants. J Infect Dis. 2008;197:1737–1742. doi: 10.1086/588144. [DOI] [PubMed] [Google Scholar]