Abstract
Anti-malarial 8-aminoquinolines drugs cause acute hemolytic anemia in individuals with glucose-6-phosphate dehydrogenase deficiency (G6PDD). Efforts to develop non-hemolytic 8-aminoquinolines have been severely limited caused by the lack of a predictive in vivo animal model of hemolytic potential that would allow screening of candidate compounds. This report describes a G6PDD mouse model with a phenotype closely resembling the G6PDD phenotype found in the African A-type G6PDD human. These G6PDD mice, given different doses of primaquine, which used as a reference hemolytic drug, display a full array of hemolytic anemia parameters, consistently and reproducibly. The hemolytic and therapeutic indexes were generated for evaluation of hemotoxicity of drugs. This model demonstrated a complete hemolytic toxicity response to another known hemolytic antimalarial drug, pamaquine, but no response to non-hemolytic drugs, chloroquine and mefloquine. These results suggest that this model is suitable for evaluation of selected 8-AQ type candidate antimalarial drugs for their hemolytic potential.
Introduction
Glucose-6-phosphate dehydrogenase (G6PD) deficiency (G6PDD) is a common X-linked genetic disorder affecting over 400 million people worldwide. The G6PDD results in an impaired ability of red blood cells (RBCs) to deal with oxidative stress, which eventually leads to their lysis after exposure to certain foods, infectious microorganisms, or even medications.1 The G6PD is a ubiquitous enzyme that plays a central role in the support of cellular redox balances. Circulating RBCs lack the capacity of de novo protein synthesis, and thus erythrocytes rely on the level of G6PDH activity that is present at the end of their maturation in the bone marrow. Thus, G6PDH deficiency primarily affects RBC functions.2
The G6PDD was characterized in a classic series of experiments in the 1950s following the observation of hemolytic anemia (HA) in African-American men given primaquine.3 The 8-AQs are an unusually useful class of antimalarials with a broad spectrum of activity, including activity against the pre-erythrocytic liver stage (both true causal prophylactic activity and anti-hypnozoite activity) as well as blood schizonticidal and gametocytocidal activities, and many 8-AQs appear to have sporonticidal activity in mosquitoes.4–9 Thus, 8-AQs are considered to be essential to current strategies for malaria treatment and control because they have both causal and suppressive activity and also reduce transmission. As mentioned previously, however, the major liability associated with the use of 8-AQs in G6PDD individuals is their propensity to cause dose-dependent HA and as such cannot usually be used to treat G6PDD patients without dose and regimen modifications. Testing 8-AQs using in vitro hemolysis test systems does not accurately reflect the hemolytic potential of these drugs seen in vivo. One possible reason for the poor predictive ability of in vitro models has been that metabolism of the parent drug is usually required for hemolytic activity.10 This highlights that an in vivo model is needed to accurately predict the hemolytic potential of new 8-AQ drugs.
In the current study, we tested hemolytic potentials of two known hemolytic antimalarial 8-AQ drugs, primaquine and pamaquine, and two non-hemolytic antimalarial drugs, chloroquine and mefloquine in the G6PDD mouse model. The efficacy dose of reference drug, primaquine was established using an In vivo Imaging System (IVIS) with a Plasmodium berghei mouse liver stage malaria model. We used an array of clinical parameters of HA as toxicity indicators to include complete blood counts, reticulocyte counts, Heinz body counts, and quantitation of mouse haptoglobin.
In this study, we tested whether a G6PDD mouse model,11–14 with a moderated degree of G6PD deficiency (10–15% of normal enzyme activity) is similar to that seen in the most common African type A-G6PDD phenotype in humans, and could be used to predict primaquine-induced hemolysis.
Materials and Methods
Animals.
Male G6PD mutant mice (y/–, G6PDD mice), 10–12 weeks old, with a C3H background were obtained initially as a gift from Dr. Zoltan Spolarics of the University of Medicine and Dentistry of New Jersey, New Jersey Medical School (Newark, NJ, bred in the Taconic Farm) and wild-type male C3H mice, 10–12 weeks old were purchased from Taconic Farm (Germantown, NY).12 The studies were performed in accordance with the 8th edition of the Guide for the Care and Use of Laboratory Animals, 2011 and were approved by the Institutional Animal Care and Use Committee of the Walter Reed Army Institute of Research.
Genotyping.
The mutant G6PD variant was identified as described by Spolarics and others,12 and the mutation was induced chemically in the G6PD promoter region. The particular mutation in the G6PD gene results in the disappearance of a DdeI restriction site in the mutant gene. Briefly, total genomic DNA was isolated from tail clippings, and the target gene was amplified with sense (5′-GGAAACTGGCTGTGCGCTAC) and antisense (5′-TCAGCTCCGGCTCTCTTCTG) primers. Polymerase chain reaction samples were incubated in the presence or absence of DdeI. Products were visualized with ethidium bromide staining and polyacrylamide gel electrophoresis.
G6PD enzyme activity assay.
Animals were phenotyped with respect to G6PD enzyme activity by using tail vein blood tested in a G6PD enzyme activity assay kit from Trinity Biotech (Berkeley Heights, NJ) following the manufacturer's instructions.13,14
Drugs.
Primaquine, pamaquine, and mefloquine were obtained from ASH Stevens Inc. (Detroit, MI). Chloroquine was purchased from Sigma (St. Louis, MO). Primaquine was used as the referenced hemolytic drug for this study. Pamaquine, an 8-AQ drug that is closely related to primaquine, was used as the known hemolytic drug for model validation15,16; the two non-hemolytic 4 aminoquinoline drugs, chloroquine and mefloquine (Larium), used in the prevention and treatment of malaria, were used in the model validation. Primaquine and chloroquine were diluted in isotonic saline (0.9%, w/v), whereas pamaquine and mefloquine were dissolved in 0.5% hydroxyethyl cellulose/1% Tween 80 just before administrations. All dosages in this study were calculated using the molecular weight of the base compound excluding the molecular weight of the salt.
Sample collection.
Blood (0.1 mL from each mouse) was collected in an EDTA-treated BD microcontainer tube (BD, Franklin Lakes, NJ) by nicking the tail with an EDTA-treated syringe and a 22-gauge needle. On the last day of the experiment, intracardiac puncture was also performed and 1.0 mL of blood was obtained from each mouse.
Complete blood counts.
Complete blood counts, which include RBC count, hemoglobin (Hb), and hematocrit (HCT), were performed on a Hemavet 960 blood analyzer (Drew Scientific, Oxford, CT) with 20 μL of peripheral blood following the manufacturer's protocol.
Reticulocyte count.
Reticulocyte counts were performed using both light microscopy and flow cytometry methods. The microscopic method was based on staining of whole-blood cells with Wright-Giemsa and New Methylene Blue (Sigma-Aldrich, St. Louis, MO) to identify reticulocytes17; each count was performed in triplicate, with a total of 1,000 erythrocytes being examined for each animal. For flow cytometry counting, 2 μL of whole blood was added to 1 mL of a working thiazole orange solution (0.1 μg/mL). Blood (2 μL) was also added to 1 mL phosphate-buffered saline alone as an unstained control; both tubes were then incubated for 60 min at room temperature in the dark before analysis. A total of 1×104 erythrocytes were analyzed on a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA). Cell gating was set to include only RBCs. Using excitation at 488 nm, green fluorescence (emission range 500–560 nm) of gated RBCs (10,000 events) was measured and analyzed.18 The unstained control was analyzed first to determine the cutoff threshold (cursor). Any reticulocytes present had higher fluorescence intensity than the autofluorescence of mature RBCs and appeared to the right of the cursor, whereas mature RBCs remained on the left. The value of the control sample was deducted from the number of positive cells to give the corrected reticulocyte count. The number of mature RBCs was calculated by subtracting the reticulocyte count from total RBC count.
Heinz body count.
Heinz bodies were counted by mixing one volume of 10 g/L crystal violet (Sigma-Aldrich) in citrate saline with one volume of whole blood19; this mixture was incubated at 37°C for 60 min before being spread onto a clean glass slide. Each count was performed in triplicate, with a total of 1,000 RBCs being examined for each sample.
Quantification of mouse haptoglobin in serum.
Serum haptoglobin was quantified using a mouse-specific haptoglobin ELISA kit (Immunology Consultants, Newberg, OR) following the instructions of the manufacturer.20
Assay for reduced glutathione (GSH).
The reduced GSH assay was performed with whole blood samples using the GSH-Glo Glutathione Assay kit (Promega, Madison, WI).21 Blood was diluted 1:5 in the reaction buffer and incubated and lysed on ice for 15 min. Samples were then centrifuged at 10,000×g for 15 min at 4°C, and the supernatants were collected. The lysates were then diluted 1:30 in deionized water. A 10-μL aliquot of each diluted lysate was transferred to triplicate wells of a 96-well plate, and GSH-Glo Reagent (100 μL) was added to each well. After mixing on a plate shaker, the plate was incubated at room temperature for 30 min. The Luciferin Detection Reagent (100 μL) was added to each well, and the plate was mixed briefly and incubated for 15 min. Luminescence was measured with a Tecan GENios microplate reader (Tecan Group, Männedorf, Switzerland) and compared with assays containing known standards to quantify GSH in each sample.
Hemolytic index (HI) calculation.
Primaquine was used as a standard hemolytic drug to generate a normalized hemolytic dose-response curve for comparing the hemolytic potential of candidate 8-AQ drugs measured by the percentage of RBC loss observed after treatment. All drug doses were converted to moles of base compound administered per kg of body weight. The reduction in the RBC count after administration of primaquine given at 0.29 mmol/kg (15 mg/kg/day, the causal prophylactic ED100 dose), was set as 100% hemolysis on the standard curve. All other values of the reduction in the RBC were normalized against this value. The HI value was calculated by dividing the percentage of hemolysis with a test drug by the percentage of hemolysis with an equimolar dose of primaquine.
Therapeutic index (TI) Calculation.
A statistically valid hemolytic dose of primaquine that reliably gave a 50% drop in red blood cells (HD50) was determined. The PQ HD50 was defined as the dose of primaquine that produced a 50% decrease in RBCs. The TI was defined as the 50% hemolytic dose (HD50) divided by the 50% effective dose (ED50) (TI = HD50/ED50) of a test drug. To generate a normalized drug hemolytic dose-response curve versus efficacy, ED doses were calculated at multiples of the ED100. The reduction in RBC count as a result of primaquine administered at 15 mg/kg/day was used as the ED100 and set on the chart as 1 and as 100% hemolysis on curve. The percentages of RBC loss observed were normalized against this value.
Statistical analysis.
Data were expressed as mean ± SE 1-way analysis of variance with Bonferroni's multiple comparison post-test against pre-test (baseline) was performed at a 95% confidence interval for all statistical analysis.
Results
Physical characteristics of G6PDD mice after primaquine treatment.
Wild-type and G6PDD mice were treated with primaquine, a known hemolytic 8-AQ drug, which we used as our positive control for qualifying this model for its intended use as a drug screening tool. The doses of primaquine used, frequency of dosing, routes of administration, G6PD enzyme activity, and physical characteristics observed in this initial study are shown in Table 1. Our previous studies of the efficacy of primaquine using the IVIS liver stage in vivo efficacy model showed that the causal prophylactic ED100 dose is 15 mg/kg/day dosed over a 3-day period (data not shown). Accordingly, we tested a range of doses above and below the ED100 to assess the physical observations of treatment and associated measures of hemotoxicity observed. One example of the characteristics observed in this initial study is shown in G6PDD mice dosed with primaquine at 25 mg/kg/day for 5 days. These G6PDD animals treated with PQ showed pale skin, fatigue, weakness, and apathy about 24 hr after treatment at the 25 mg/kg dose of primaquine, which is the maximal daily tolerable dose. When this dose was given for 5 consecutive days, the mice exhibited dark tea-colored urine starting at Day 3 of dosing and lasted 3–4 days. No hemolytic signs and symptoms were observed in G6PDD mice or wild-type mice treated with primaquine dosed at 17.5, 8.75, or 4.375 mg/kg/day for 5 days (see Table 1). There was a trend of slightly decreased body weight in G6PDD mice treated with primaquine at 25 mg/kg/day for 5 days as compared with wild-type mice treated with the same treatment regimen (P < 0.01).
Table 1.
Outcomes of drug dosing*
| Daily dose (mg/kg/day) | Days of dosing | Total dose (mg/kg) | Drug route | No. of animal | G6PD activity (% of WT) | Death | CNS toxicity | Hemolysis |
|---|---|---|---|---|---|---|---|---|
| Primaquine | ||||||||
| 57† | 1 | 57 | i.p. | 3 | 14.9 ± 1.3 | 3 | 0 | 0 |
| 42.5‡ | 1 | 42.5 | i.p. | 8 | 14.8 ± 1.1 | 4 | 4 | 4 |
| 42.5† | 1 | 42.5 | i.p. | 8 | 15.0 ± 1.2 | 0 | 0 | 8 |
| 42.5† | 3 | 127.5 | i.p. | 8 | 14.5 ± 1.4 | 2 | 2 | 6 |
| 28.5† | 7 | 200 | p.o. | 8 | 14.9 ± 1.2 | 0 | 0 | 8 |
| 28.5† | 7 | 200 | p.o. | 7 | 15.0 ± 1.7 | 0 | 0 | 7 |
| 25‡ | 5 | 125 | p.o. | 5 | 14.8 ± 1.6 | 0 | 0 | 5 |
| 17.5‡ | 5 | 87.5 | p.o. | 5 | 15.0 ± 1.7 | 0 | 0 | 5 |
| 15‡ | 5 | 75 | p.o. | 5 | 14.7 ± 1.4 | 0 | 0 | 5 |
| 8.75‡ | 5 | 43.75 | p.o. | 5 | 14.3 ± 1.2 | 0 | 0 | 5 |
| 4.375‡ | 5 | 21.9 | 5 | 14.8 ± 1.8 | 0 | 0 | 2 | |
| Pamaquine | ||||||||
| 14‡ | 5 | 70 | p.o. | 5 | 14.9 ± 1.5 | 0 | 0 | 5 |
| Chloroquine | ||||||||
| 25‡ | 7 | 175 | p.o. | 8 | 14.5 ± 1.2 | 0 | 0 | 0 |
| 60‡ | 5 | 300 | p.o. | 5 | 14.7 ± 1.7 | 0 | 0 | 0 |
| Mefloquine | ||||||||
| 117‡ | 2 | 234 | p.o. | 5 | 14.5 ± 1.4 | 0 | 0 | 0 |
WT = wild-type; CNS = central nervous system. Values are mean ± SE.
Daily dose was spited to two with 6 hours apart.
Single dosing.
CNS toxicities were displayed by a characteristic seizure pattern and decreased spontaneous motor activity.
Known hemolytic 8-AQs induce hemolytic toxicity in G6PDD mice.
The known hemolytic 8-AQ drug primaquine was given orally or by mouth in different doses either for a single day or for 5 consecutive days. Hemolytic parameters, such as RBC count, HCT, Hb levels, haptoglobin, reticulocyte counts, and Heinz body counts were measured on Day 0 (before drug treatment) and Day 7 (post drug treatment). After primaquine treatments, RBC counts, Hb, and HCT decreased in a drug dose-dependent manner in G6PDD mice (P < 0.01) (Table 2). Wild-type mice showed no changes in these parameters throughout the experiment.
Table 2.
Hemolytic effects of primaquine in G6PDD mice*
| Daily dose (mg/kg/d) | 25† | 17.5 | 15‡ | 8.75 | 4.375 | |||||
| Days of dosing | 5 | 5 | 5 | 5 | 5 | |||||
| Total dose (mg/kg) | 125 | 87.5 | 75 | 43.75 | 21.9 | |||||
| Route | p.o. | p.o. | p.o. | p.o. | p.o. | |||||
| No. of animal (n) | 7 | 7 | 5 | 5 | 5 | |||||
| G6PD activity (% of wild-type) | 14.8 ± 1.6 | 15.0 ± 1.7 | 14.7 ± 1.4 | 14.3 ± 1.2 | 14.8 ± 1.8 | |||||
| Day | Day | Day | Day | Day | ||||||
| 0 | 7 | 0 | 7 | 0 | 7 | 0 | 7 | 0 | 7 | |
| RBC (106/μL) | 10.2 ± 0.6 | 6.9 ± 1.1* | 9.9 ± 0.2 | 7.8 ± 0.8* | 9.9 ± 0.3 | 8.0 ± 0.2* | 9.7 ± 0.4 | 8.3 ± 1.1* | 9.6 ± 0.3 | 8.4 ± 0.1* |
| HCT (%) | 58.1 ± 1.2 | 46.0 ± 9.2* | 52.7 ± 1.4 | 45.0 ± 4.3* | 54.3 ± 1.2 | 47.7 ± 1.4* | 54.0 ± 2.1 | 44.2 ± 2.1* | 52.4 ± 2.3 | 42.5 ± 0.5* |
| Hb (g/dL) | 11.8 ± 0.8 | 9.8 ± 0.7* | 11.3 ± 0.4 | 9.0 ± 0.9* | 13.1 ± 0.0 | 12.8 ± 0.4* | 11.9 ± 0.2 | 9.3 ± 0.9* | 11.9 ± 0.6 | 9.6 ± 0.5* |
| Haptoglobin (μg/mL) | 82.1 ± 21.6 | 11.5 ± 3.4* | 56.0 ± 27 | 36.0 ± 8.4* | 131 ± 20 | 118 ± 44* | 9.4 ± 3.2 | 9.7 ± 1.2 | ||
| Reticulocyte (% of RBC) | 2.5 ± 0.4 | 9.7 ± 1.4* | 2.5 ± 0.5 | 6.9 ± 0.9* | 2.9 ± 0.2 | 6.1 ± 0.2* | 2.7 ± 0.3 | 6.4 ± 1.9* | 2.8 ± 0.2 | 4.6 ± 0.4* |
| Heinz body (% of RBC) | 0.0 ± 0.0 | 6.1 ± 0.5* | 0.0 ± 0.0 | 4.4 ± 0.7* | 0.0 ± 0.0 | 4.4 ± 0.7* | ||||
Values are mean ± SE. RBC = red blood cell; HCT = hematocrit; Hb = hemoglobin.
P < 0.01 (Day 7 vs. Day 0).
The maximum tolerated dose (MTD)
A 100% effective causal prophylactic dose (CP ED100) and minimal curing dose (MCD) in C3H mouse P. berghei sporozoite challenge model.
Another known hemolytic 8-AQ drug, pamaquine, showed a similar hemolytic response in G6PDD mice. Pamaquine (14 mg/kg/day for 5 days) treated G6PDD mice showed a reduction of 67% in RBC, a 69% decline in HCT, a 48% drop in Hb and a 38% decrease in haptoglobin, whereas the reticulocyte count increased 2.4-fold (Table 3).
Table 3.
Hemolytic effects of pamaquine in G6PDD mice
| Daily dose (mg/kg/d) | 14 | |
| Regimens | Split doses | |
| Days of dosing | 5 | |
| Total dose (mg/kg) | 70 | |
| Route | p.o. | |
| G6PD activity (% of wild-type) | 14.9 ± 1.5 | |
| Day | ||
| 0 | 7 | |
| RBC (106/μL) | 9.9 ± 0.3 | 3.3 ± 0.7* |
| HCT (%) | 53.6 ± 1.6 | 16.7 ± 4.7* |
| Hb (g/dL) | 11.8 ± 0.7 | 6.1 ± 0.8* |
| Haptoglobin (μg/mL) | 41 ± 20 | 25.5 ± 17* |
| Reticulocyte (% of RBC) | 2.7 ± 0.2 | 6.5 ± 0.3* |
| Heinz body (% of RBC) | 0.0 ± 0.0 | 13.5 ± 1.2* |
Values are mean ± SE. RBC = red blood cell; HCT = hematocrit; Hb = hemoglobin.
P < 0.01 (Day 7 vs. Day 0).
Treatment of G6PD deficient mice with chloroquine (either 25 mg/kg daily for 7 days, or 60 mg/kg daily for 5 days), and mefloquine (117 mg/kg daily for 2 days), which are non-hemolytic anti-malarial drugs resulted in no hemolytic effects (Table 4).
Table 4.
Effects of non-hemolytic drugs in G6PDD mice*
| Drugs | Chloroquine | Chloroquine | Mefloquine | |||
|---|---|---|---|---|---|---|
| Daily dose (mg/kg/d) | 25 | 60 | 117 | |||
| Days of dosing | 7 | 5 | 2 | |||
| Total dose (mg/kg) | 175 | 300 | 234 | |||
| Route | p.o. | p.o. | p.o. | |||
| No. of animal (n) | 8 | 5 | 5 | |||
| G6PD activity (% of wild-type) | 14.5 ± 1.2 | 14.7 ± 1.7 | 14.5 ± 1.4 | |||
| Day | Day | Day | ||||
| 0 | 7 | 0 | 7 | 0 | 7 | |
| RBC (106/μL) | 9.0 ± 0.4 | 9.0 ± 0.4 | 9.8 ± 0.45 | 9.7 ± 0.4 | 9.4 ± 0.8 | 9.38 ± 0.8 |
| HCT (%) | 55.3 ± 2.1 | 55.6 ± 2.4 | 52.6 ± 1.0 | 52.9 ± 1.1 | 51.6 ± 5.0 | 58.7 ± 3.8 |
| Hb (g/dL) | 11.8 ± 0.6 | 12.1 ± 0.8 | 11.6 ± 0.4 | 11.8 ± 0.4 | 12.0 ± 1.3 | 12.7 ± 1.5 |
| Haptoglobin (μg/mL) | 16.9 ± 6.1 | 25.5 ± 7.2 | 21.2 ± 4.6 | 20.2 ± 6.9 | 31.5 ± 18 | 30.1 ± 11 |
| Reticulocyte (% of RBC) | 2.0 ± 0.3 | 1.9 ± 0.3 | 2.5 ± 0.1 | 3.4 ± 0.3 | 3.7 ± 1.1 | 3.9 ± 0.7 |
| Heinz body (% of RBC) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
Values are mean ± S.E. RBC = red blood cell; HCT = hematocrit; Hb = hemoglobin.
To see the dynamic hemolytic changes as a result of primaquine treatment of G6PDD mice, primaquine was administered in a dosing regimen of 6.16 mg daily for 30 days; this regimen was determined as the estimated human equivalent dose by allometric scaling.22 Hemolysis and recovery were monitored until Day 35.The results showed hemolysis beginning on Day 6, and the initiation of recovery was observed on Days 10–14 with near full recovery observed on day 21 (Figure 1).
Figure 1.
Hemolytic course of primaquine in G6PDD mice. G6PDD mice were administered primaquine orally at 6.6 mg/kg/day for 30 consecutive days. Hemolytic parameters were determined on the day before starting primaquine treatment (Day zero) and at about weekly intervals until 5 weeks. Data points are mean ± SE from 5 animals in each group. **Statistically significant difference as compared with Day zero in the same treatment group (P < 0.01).
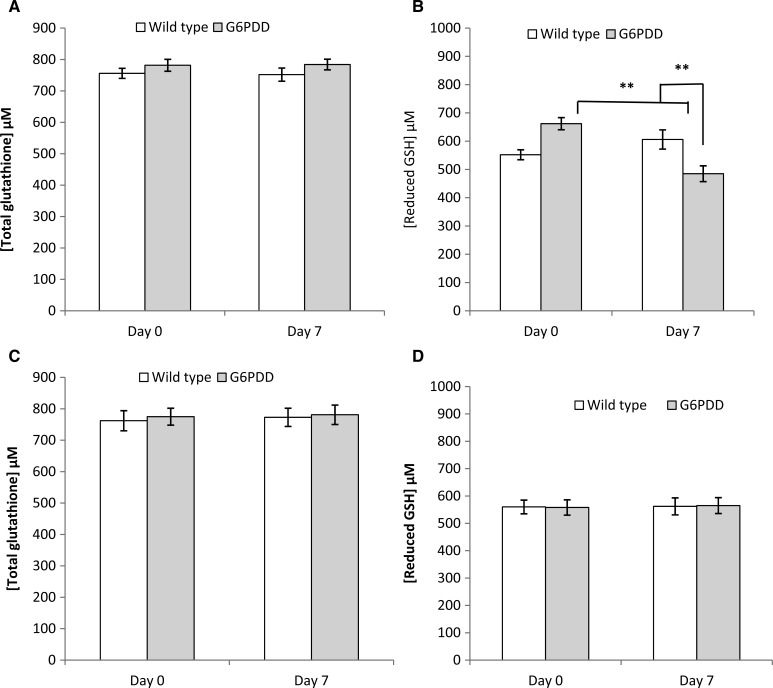
To determine whether primaquine hemotoxicity is related to the RBC GSH status in G6PDD mice, total and reduced GSH in RBC from wild-type and G6PDD mice before and after primaquine treatment were measured. Treatment with primaquine (25 mg/kg/day for 5 days) did not alter total GSH in wild-type and G6PDD mice (Figure 2A). Administration of primaquine decreased the reduced GSH level by 26.7% in G6PDD mice (P < 0.01), but did not affect the levels of reduced GSH of wild-type mice (Figure 2B). In contrast, the known non-hemolytic antimalarial drug chloroquine did not have any effect on either total or reduced GSH levels in both wild-type or G6PDD mice (Figure 2C and D).
Figure 2.
Primaquine and chloroquine effects on total and reduced glutathione (GSH) levels in wild-type and G6PDD mice. Groups (N = 7) of wild-type and G6PDD mice were administered primaquine orally at 25 mg/kg/d for 5 consecutive days, or administered chloroquine orally at 25 mg/kg/d for 7 consecutive days. Total and reduced GSH levels were determined on the day before drug administration (Day zero) and on Day 7. (A) Changes in total GSH level caused by primaquine, (B) Changes in reduced GSH level caused by primaquine, (C) Changes in total GSH level caused by chloroquine, (D) Changes in reduced GSH level caused by chloroquine. Data points are mean ± SE. Statistically significant difference as compared with Day zero in the same treatment group, and between wild-type and G6PDD mice in the same day of dosing (**P < 0.01).
HI of test compounds.
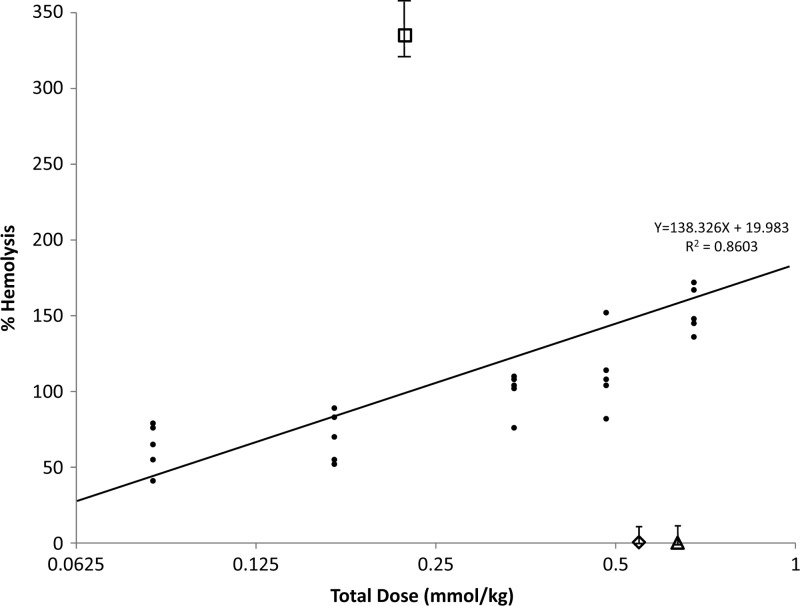
The hemolytic potential of 8-AQ candidate drugs can be compared with the hemolytic potential of primaquine in this model at equimolar doses to calculate an HI. We set the percentage of RBC loss induced by primaquine given at the ED100 dose at 100% hemolysis. The reduction in the RBC counts observed after administration of other test compounds were normalized against this value. The standard curve of primaquine induced hemolysis was generated by plotting the various doses of primaquine against the RBC (Table 5, Figure 3). The percentage of RBC hemolysis observed after administration of test compounds was compared with the standard curve of primaquine at equimolar doses. An HI value of > 1 or < 1 indicates a relatively higher or lower hemolytic toxicity, respectively, compared with primaquine. The hemolytic indices of pamaquine, chloroquine, and mefloquine were derived from this standard curve and found to be 6.66, 0.003, and 0.001, respectively, indicating that pamaquine has more hemolytic potential than primaquine, whereas chloroquine and mefloquine have far less hemolytic potential at equimolar doses (Figure 3).
Table 5.
Hemolytic Index of tested drugs administered p.o.
| Drug | Daily dose (mmol/kg/d) | Total dose (mmol/kg) | % RBC loss* | %Hemolysis | Hemolytic index† |
|---|---|---|---|---|---|
| Primaquine | 0.1 | 0.5 | 32.25 ± 11 | 161 | |
| 0.067 | 0.335 | 21.21 ± 8 | 106 | ||
| 0.058‡ | 0.29 | 20.02 ± 6 | 100 | 1 | |
| 0.0335 | 0.168 | 14.43 ± 11 | 72 | ||
| 0.0168 | 0.084 | 12.5 ± 1 | 63 | ||
| Pamaquine | 0.044 | 0.22 | 66.67 ± 7.0 | 333 | 6.6 |
| Chloroquine | 0.188 | 0.94 | 1.0 ± 0.1 | 0.05 | 0.0003 |
| Mefloquine | 0.31 | 0.62 | 0.2 ± 0.01 | 0.01 | 0.0001 |
| Tafenoquine | 0.0286 | 0.086 | 4.3 ± 0.2 | 21.5 | 0.67 |
Values are mean ± SE.
The reduction in the red blood cell (RBC) count after administration of primaquine given at 0.29 mmol/kg (15 mg/kg/day, the causal prophylactic ED100 dose), was set as 100% hemolysis on the standard curve. All other values of the reduction in the RBC were normalized against this value. The Hemolytic Index (HI) value was calculated by dividing the percentage of hemolysis with a test drug by the percentage of hemolysis with an equimolar dose of primaquine.
A 100% effective causal prophylactic dose (CP ED100) in Imprinting Control Region (ICR) mouse Plasmodium berghei sporozoite challenge mode.
Figure 3.
Hemolytic index calculation. A scatter plot was created with the moles of drug/kg (Log2) of test drugs on the x axis, and percentage of red block cell (RBC) hemolysis on the y axis. The percentage of RBC loss induced by primaquine given at ED100 was defined as 100% hemolysis. The percent RBC hemolysis observed in animals administered test compounds was direct compared with a standard curve of primaquine at equimolar doses. •, Primaquine; □, pamaquine; ⋄, chloroquine; △, mefloquine.
TI of primaquine.
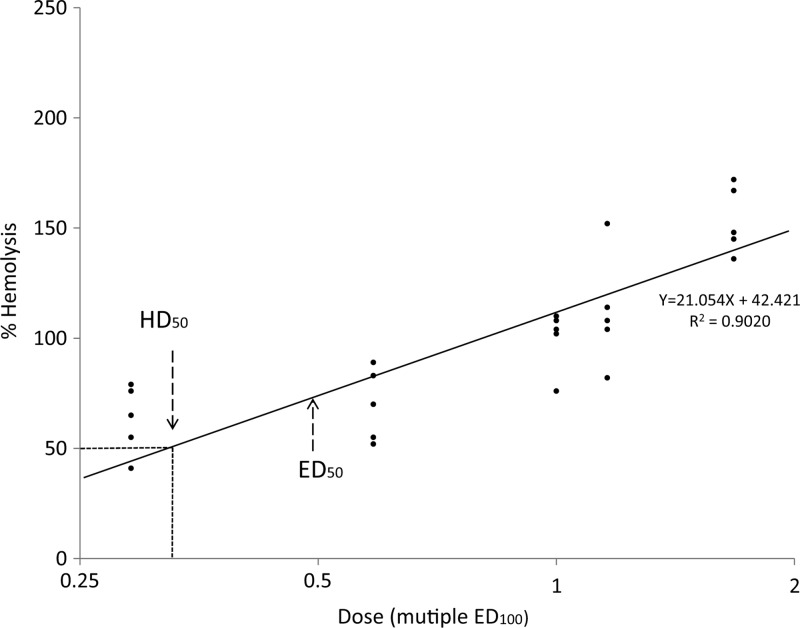
A scatter plot was created with the ED100 of test drugs on the x axis, and the percentage of RBC hemolysis on the y axis (Figure 4). The percentage of RBC loss induced by primaquine at the ED100 was set as 100% hemolysis. The TI)calculation for this plot was based on dividing the HD50 by the ED50. A TI value of < 1 would make a drug less desirable for use in a G6PDD individual. A TI value > 1 by this plot would suggest a candidate drug was more desirable for further development. The HD50 of primaquine was calculated as 0.36, and the TI of primaquine was calculated as 0.71, which is consistent with the known hemolytic potential of primaquine.
Figure 4.
Therapeutic index calculation. A scatter plot was created with effective dose (ED) doses (Log2) of test drugs on the x axis, and percent of red blood cell (RBC) hemolysis on the y axis. The percentage of RBC loss induced by primaquine given at ED100 was defined as 100% hemolysis. The Therapeutic Index was calculated as HD50 over ED50.
Discussion
An ideal in vivo G6PDD model should possess the following attributes: 1) have a naturally occurring G6PDD condition, 2) have a genotype and phenotype identical to or at least comparable with those of human G6PDD patients, 3) HA following at therapeutic doses of drug known to be hemolytic, 4) not exhibit HA at therapeutic doses with drugs known not to be hemolytic, and 5) produce statistically valid and reproducible assay results. We demonstrated in our G6PDD mouse model that primaquine treatment resulted in significant decreases in RBC count, HCT, Hb, and haptoglobin levels, and increases in reticulocyte counts and Heinz body formation. With the exception of Heinz body formation, these parameters constitute the routine clinical indicators of hemolytic anemia. After primaquine treatment, RBCs from G6PDD animals exhibited increased susceptibility to cellular thiol depletion and redox imbalance relative to the RBCs from wild-type animals. These results suggested that the decreases in reduced GSH observed after primaquine treatment are a consequence of G6PD deficiency. These data also suggest that G6PD deficiency impedes cellular regeneration of GSH from its oxidized form during primaquine challenge. Our mouse model provides valuable insights into how primaquine-induced hemolysis develops and progresses. Our results suggest that hemolytic progression after primaquine treatment is partly a result of G6PD deficiency that impedes cellular regeneration of GSH from its oxidized form.
In our study of primaquine-induced hemolysis using G6PD deficient mice, we observed that recovery from changes in hemolytic parameters was seen 14 days after primaquine dosing. This implies that the hemolysis observed after treatment with primaquine in the G6PDD mouse model is self-limited. This is a similar finding to what has been observed in human patients.23,24 We observed similar patterns of hemolysis and recovery where PQ was dosed in various regimens for short periods of 5 or 7 days, indicating the hemolysis might be triggered within the first several days of 8-AQ dosing. Normal hemodynamic status was recovered 14 days after treatment. This pattern of self-limited hemolysis might be caused by mice being relatively resistant to further primaquine challenge, or it could be the result of hemolysis and hematopoiesis processes reaching equilibrium.
To assist in rank ordering candidate 8-AQ drugs for their hemolytic potential, we used a calculated HI for all test drugs. The RBC hemolysis observed in G6PDD mice after administration of primaquine at various doses was used to create a standard curve to compare the hemolytic activity of novel 8-AQ candidates. The hemolytic potential of other test compounds can be compared with the primaquine standard curve with equimolar doses. As shown in Figure 3, pamaquine, a highly hemolytic drug, showed a much higher HI than equimolar amounts of primaquine, whereas chloroquine and mefloquine showed a complete lack of hemolysis at equimolar amounts. A similar plot of a TI was also created to rank order candidate 8-AQ compounds by reflecting both hemolysis and efficacy in the same figure. As shown in Figure 4, a TI plot comparing the efficacy and hemolytic toxicity of pamaquine and mefloquine was compared with a standard curve of primaquine. This type of assessment is very useful in comparing both hemolysis and efficacy in one visual display to rank order potential candidate compounds.
In conclusion, we evaluated a suitable G6PDD mouse model for prediction of the hemolytic potential of antimalarial drugs. These G6PDD mice show a full array of hemolytic toxicity responses to two known hemolytic 8-AQ drugs, primaquine and pamaquine, however, treatment of these mice with two known non-hemolytic drugs, chloroquine and mefloquine, had no effect. Our studies revealed the followings specific advantages of the model: 1) A genetically inherited G6PD deficiency naturally demonstrating characteristics of human G6PDD patients (class III); animals were otherwise healthy unless treated with drugs. 2) This model shows all clinical parameters of HA in human G6PDD. 3) This model facilitates studies on hematopoietic recovery after drug treatment. 4) This model produces reproducible and statistically valid results. This G6PDD mouse model is an excellent tool to be used to identify and to characterize potentially efficacious non-hemolytic 8-AQs by eliminating those that induce clinically intolerable levels of hemolysis so that safe drugs can be brought into clinical use. Furthermore, this in vivo model may be used to test the hemotoxic potential of other candidate drugs that are not 8-AQs during preclinical evaluation that may also be hemolytic when administered to G6PD individuals.
ACKNOWLEDGMENTS
We thank Dr. Zoltan Spolarics of UMDNJ for his generous gift of the initial set of G6PDD mice that helped us to initiate the breeding colony. We also thank William Ellis (WRAIR) for providing us with the experimental compounds. We are grateful to Dr. Larry Walker (U Miss) and the Non-Hemolytic 8-AQ Consortium for encouragement and financial support.
Disclaimer: The authors declare no competing financial interests. The views expressed in this article are those of the author(s) and do not reflect the official policy of the Department of Army, Department of Defense, or the U.S. Government. The experimental protocol was approved by the Animal Care and Use Committee at Walter Reed Army Institute of Research and all procedures were conducted in accordance with the principles stated in the Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act of 1966 (P.L. 89-544), as amended.
Footnotes
Financial support: This study was supported by the Military Infectious Diseases Research Program (MIDRP) Award #RQ0031_09_WR and the U.S. Army Medical Research & Materiel Command (USAMRMC) and the Telemedicine & Advanced Technology Research Center (TATRC), at Fort Detrick, MD, under award number: W81XWH-07-2-0095. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authors' addresses: Peng Zhang, Xiugong Gao, Hiroshi Ishida, Jack Amnuaysirikul, Peter J. Weina, Max Grogl, Michael T. O'Neil, Qigui Li, Diana Caridha, Colin Ohrt, Mark Hickman, Alan J. Magill, and Prabhati Ray, Walter Reed Army Institute of Research - Experimental Therapeutics, Silver Spring, MD, E-mails: peng.zhang@amedd.army.mil, Xiugong.Gao@fda.hhs.gov, hiroshi.ishida@amedd.army.mil, Jack.Amnuaysirikul@us.army.mil, peter.weina@us.army.mil, Max.Grogl@amedd.army.mil, michael.t.oneil@us.army.mil, Qigui.Li@us.army.mil, Diana.Caridha2@us.army.mil, Colin.Ohrt@amedd.army.mil, Mark.Hickman@amedd.army.mil, Alan.Magill@us.army.mil, alan.magill@gatesfoundation.org, and prabhati.ray@us.army.mil.
References
- 1.Beutler E. G6PD deficiency. Blood. 1994;84:3613–3636. [PubMed] [Google Scholar]
- 2.Beutler E. G6PD: population genetics and clinical manifestations. Blood Rev. 1996;10:45–52. doi: 10.1016/s0268-960x(96)90019-3. [DOI] [PubMed] [Google Scholar]
- 3.Beutler E. The hemolytic effect of primaquine and related compounds: a review. Blood. 1959;14:103–139. [PubMed] [Google Scholar]
- 4.Baird JK, Fryauff DJ, Basri H, Bangs MJ, Subianto B, Wiady I, Purnomo, Leksana B, Masbar S, Richie TL, Jones TR, Tjitra E, Wignall ES, Hoffman SL. Primaquine for prophylaxis against malaria among nonimmune transmigrants in Irian Jaya, Indonesia. Am J Trop Med Hyg. 1995;52:479–484. doi: 10.4269/ajtmh.1995.52.479. [DOI] [PubMed] [Google Scholar]
- 5.Baird JK, Fryauff DJ, Hoffman SL. Primaquine for prevention of malaria in travelers. Clin Infect Dis. 2003;37:1659–1667. doi: 10.1086/379714. [DOI] [PubMed] [Google Scholar]
- 6.Baird JK, Hoffman SL. Primaquine therapy for malaria. Clin Infect Dis. 2004;39:1336–1345. doi: 10.1086/424663. [DOI] [PubMed] [Google Scholar]
- 7.Baird JK. Primaquine as anti-relapse therapy for Plasmodium vivax. Trans R Soc Trop Med Hyg. 1998;92:687. doi: 10.1016/s0035-9203(98)90814-8. [DOI] [PubMed] [Google Scholar]
- 8.Baird JK, Surjadjaja C. Consideration of ethics in primaquine therapy against malaria transmission. Trends Parasitol. 2011;27:11–16. doi: 10.1016/j.pt.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . Guidelines for the Treatment of Malaria. Second edition. Geneva: World Health Organization; 2006. pp. 52–58. [Google Scholar]
- 10.Beutler E. Drug-induced hemolytic anemia. Pharmacol Rev. 1969;21:73–103. [PubMed] [Google Scholar]
- 11.Pretsch W, Charles DJ, Merkle S. X-linked glucose-6-phosphate dehydrogenase deficiency in Mus musculus. Biochem Genet. 1988;26:89–103. doi: 10.1007/BF00555491. [DOI] [PubMed] [Google Scholar]
- 12.Spolarics Z, Condon MR, Siddiqi M, Machiedo GW, Deitch EA. Red blood cell dysfunction in septic glucose-6-phosphate dehydrogenase-deficient mice. Am J Physiol Heart Circ Physiol. 2004;286:H2118–H2126. doi: 10.1152/ajpheart.01085.2003. [DOI] [PubMed] [Google Scholar]
- 13.Wilmanski J, Siddiqi M, Deitch EA, Spolarics Z. Augmented IL-10 production and redox-dependent signaling pathways in glucose-6-phosphate dehydrogenase-deficient mouse peritoneal macrophages. J Leukoc Biol. 2005;78:85–94. doi: 10.1189/jlb.0105010. [DOI] [PubMed] [Google Scholar]
- 14.Spolarics Z, Siddiqi M, Siegel JH, Garcia ZC, Stein DS, Ong H, Livingston DH, Denny T, Deitch EA. Increased incidence of sepsis and altered monocyte functions in severely injured type A- glucose-6-phosphate dehydrogenase-deficient African American trauma patients. Crit Care Med. 2001;29:728–736. doi: 10.1097/00003246-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Beutler E, Duparc S. G6PD Deficiency Working Group Glucose-6-phosphate dehydrogenase deficiency and antimalarial drug development. Am J Trop Med Hyg. 2007;77:779–789. [PubMed] [Google Scholar]
- 16.Vale N, Moreira R, Gomes P. Primaquine revisited six decades after its discovery. Eur J Med Chem. 2009;44:937–953. doi: 10.1016/j.ejmech.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Johns JL, Shooshtari MP, Christopher MM. Development of a technique for quantification of reticulocytes and assessment of erythrocyte regenerative capacity in birds. Am J Vet Res. 2008;69:1067–1072. doi: 10.2460/ajvr.69.8.1067. [DOI] [PubMed] [Google Scholar]
- 18.Nobes PR, Carter AB. Reticulocyte counting using flow cytometry. J Clin Pathol. 1990;43:675–678. doi: 10.1136/jcp.43.8.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Summerfield M, Tudhope GR. Spectrophotometric method applicable to in vitro studies of Heinz body formation in erythrocytes. Acta Haematol. 1979;61:222–225. doi: 10.1159/000207660. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe J, Grijalva V, Hama S, Barbour K, Berger F, Navab M, Fogelman A, Reddy S. Hemoglobin and its scavenger protein haptoglobin associate with ApoA-1-containing particles and influence the inflammatory properties and function of high density lipoprotein. J Biol Chem. 2009;284:18292–18301. doi: 10.1074/jbc.M109.017202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neifer S, Jung A, Bienzle U. Characterization of erythrocytic glucose-6-phosphate dehydrogenase in a mouse strain with reduced G6PD activity. Biomed Biochim Acta. 1991;50:233–238. [PubMed] [Google Scholar]
- 22.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2007;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 23.Alving AS, Johnson CF, Tarlov AR, Brewer GJ, Kellrmeyer RW, Carson PE. Mitigation of the hemolytic effect of primaquine and enhancement of its action against exoerythrocytic forms of the Chesson strain of Plasmodium vivax by intermittent regimens of drug administration: a preliminary report. Bull World Health Organ. 1960;22:621–631. [PMC free article] [PubMed] [Google Scholar]
- 24.Charoenlarp R, Areekul S, Pholpothi T, Harinasuta T. The course of primaquine-induced hemolysis in G-6_PD deficient Thais. J Med Assoc Thail. 1973;56:392–397. [PubMed] [Google Scholar]