Abstract
The expanding economy of Qatar in the last two decades has attracted immigrants, often from countries with poor socio-economic levels. Many arrive with patent intestinal parasitic infections, and recent analyses have indicated consistently rising trends in the prevalence of some infections. Here, we examined 18,563 hospital records of subjects in Qatar seeking medical assistance for a variety of ailments, combining data from 2009 to 2011 with the earlier dataset from 2005 to 2008 to enable trends to be identified across a 7-year period. We found that 8.6% were infected with one or more species of parasites, however in contrast to the earlier period (2005–2008), in the latter 3 years there were falling trends of prevalence providing some optimism that parasitic infections among the resident immigrants have begun to decline. We identified also geographic regions from which resident workers still maintain a relatively high prevalence of helminth infections despite their long-term residence in Qatar.
Introduction
In recent decades the Arabian Gulf region in the Middle East has seen an enormous transformation in the standard of living of the inhabitants of cities, mostly facilitated by income from oil and gas reserves, and the growing economies have spawned building and modernization programs. This in turn has attracted immigrants seeking work, many of whom originate from less affluent states in Asia and Africa and from further afield. Qatar, in particular, has seen an enormous influx of immigrant workers drawn to the country by the extensive building programs that have dominated in Doha and also for work in the rapidly expanding food and domestic service industries in the city. The rate of change has been considerable and infrastructure, notably with respect to housing and sanitation, has not always kept pace with the expanding human population.
All new immigrants are obliged to report to the Medical Commission for thorough medical inspections but fecal examinations were abandoned about a decade ago as they were considered to be superfluous following the introduction of compulsory treatment of all immigrant workers seeking employment in the food industries and/or as housemaids with albendazole.1 On gaining a work permit, workers are no longer obliged to undergo further health inspections, except those engaged in the food industries who have to undergo an annual re-examination to maintain the validity of their work permit. Subjects with work permits can obtain residency status and are then eligible for treatment at Hamad Medical Corporation (HMC) hospitals as the need arises.
In our earlier work we exploited the computerized database held at HMC to examine the prevalence of intestinal helminth and protozoan infections among long-term residents in Qatar who had reported with a range of medical problems.2 Although not a randomly chosen selection of the population, and therefore a somewhat biased subsample, we argued nevertheless that analysis of these data would provide useful information on the persistence of infections among the resident population. Our retrospective analyses of HMC data for the period 2005–2008, indicated that 10.2% of the subjects were infected with at least one species of parasite. The prevalence of helminth infections was 2.6% and that of intestinal protozoa 8.0%, however in both cases there were worrying upward trends across these four years. We also identified Western Asian immigrants, especially 20.0- to 39.9-year-old male Nepalese workers as the major carriers of intestinal nematode infections, largely comprising hookworms. The question then arose as to why these national groups, in particular, continued to harbor helminth infections, whereas immigrants from other regions (e.g., N and NE Africa, East Asia), originally also showing a high prevalence of intestinal nematodes, did not show nearly as high a prevalence after obtaining residency status.3
In this work, we report on intestinal helminth and protozoan infections in Qatar over an extended period spanning 7 years. We combined data for the years 2009, 2010, and 2011, with that in our earlier database (2005–2008)2 and examined the trends in prevalence for individual species and for higher taxonomic groups across this entire period. A particular objective was to determine whether the upward trends in prevalence identified previously2 continued to climb further in the latter 3 years. Again, we included countries of origin of the subjects in the study, to enable identification of regions supplying workers most likely to perpetuate parasitic infections in Qatar even after gaining residency status. Our primary purpose was to provide the health and administrative authorities with information to enable them to focus control measures on these essential and much valued immigrant workers, who nevertheless represent high risk groups for perpetuation of parasitic infections in Qatar and whose own health and productivity would be improved by follow-up health inspections and remedial therapy.
Materials and Methods
Study subjects and sample collection.
The subjects for this study were 18,563 individuals from different departments of the HMC hospitals, including maternity, pediatrics, internal medicine, and gastroenterology, who had been referred for a routine stool test. This study was based on a retrospective survey of intestinal parasitic infections from the records held at HMC database (MediCom) maintained at the Department of Laboratory Medicine and Pathology at HMC and its outpatient clinics between 2005 and 2011. We combined a previously analyzed dataset for the period from 2005 to 2008 with that for the following 3 years to December 31, 2011. The latter dataset also contained records of 1,063 children from 2 days to 7 months of age, and in order not to bias long-term trends in our analysis, these were removed because the earlier analysis had been confined to those over 7 months of age. On examination of these records only one was diagnosed as infected (with Endolimax nana, a 3-month-old Qatari girl in 2010). A further 275 records comprising subjects from Europe (N = 122), North America (N = 130), Central America (N = 6), Australasia (N = 10), and South America (N = 7) were also removed, because these continents had not been considered in our earlier analysis. Among these subjects 15 were infected (5.5%, CL95 3.56–8.24), 11 with Blastocystis hominis. There were also 2 Entamoeba coli, 3 E. nana, and 1 Giardia duodenalis infections among this group, with three subjects infected concurrently with two species (B. hominis + E. nana, B. hominis + E. coli, and E. nana + Iodamoeba buetschlii).
In addition, the dataset used in the current analysis included 135 subjects from 28 countries not represented in the earlier analysis, but within the regions delimited in our earlier study. The wider range of resident workers is consistent with Qatar's policy for encouraging visiting and eventually resident workers from an increasingly broad range of countries. However, as noted later, some analyses in the current work were restricted to subjects from countries represented in both studies.
Ethical approval.
Ethical approval for access to these data was obtained from the Medical Research Center and Research Committee at HMC, Qatar (Research protocol no. 11110/11(NPRP 4- 1283-3-327).
Fecal samples were obtained from subjects referred for examination at HMC as part of a routine screening policy for the diagnosis of diseases associated with intestinal infections. Confidentiality was maintained throughout and the identity of subjects was not available to us, other than through each individual's reference number. Age, sex, and geographical region were recorded for each patient before taking the specimen. Fresh fecal specimens were collected in 25 mL clean wide-mouth, covered plastic containers. Stool samples were then immediately transported to the Microbiology Laboratory at HMC.
Stool examination.
Stool examination was carried out in a safety cabinet, where stool specimen was preserved in an ecofix preservative vial (Meridin Biosciences, Inc., Cincinnati, OH). The contents were stirred with fine clean disposable wooden sticks to remove large clumps and mixed vigorously by vortex to homogenize the sample. To ensure adequate fixation of the homogenized stool, the sample was kept for half an hour at room temperature. The preserved specimen was mixed by vortex and filtered through a macro-con filtration unit for the removal of bulky debris. After filtration, 10% formalin and ethyl acetate were added, the sample was centrifuged for 10 min at 3,000 rpm, and the fluid containing diethyl ether and formalin was discarded. The pellet was resuspended by agitation, poured onto a microscope slide containing one drop of iodine, and examined microscopically for the presence/absence of parasite eggs/cysts and to enable identification of parasites in positive samples. Amoeba species other than Entamoeba histolytica/dispar including E. coli, Entamoeba hartmanni, E. nana, Chilomastix mesnili, and I. buetschlii were pooled together and recorded as non-pathogenic amoebae in the first period (2005–2008) because the cysts are not easily distinguishable.1,2 However, in the second period, these stages were separated by the microscopists and relevant data are presented. We refer to G. duodenalis (= lamblia/intestinalis).
Definition of variables.
All birth dates and examination dates were recorded meticulously and the ages of subjects were classified into ranges by years. Thirteen age classes were then constructed to span ≤ 1 year, 1.1–1.9, 2.0–4.9, 5.0–9.9, 10.0–14.9, 15.0–19.9, 20.0–29.9, 30.0–39.9, 40.0–49.9, 50.0–59.9, 60.0–69.9, 70.0–79.9, and < 79.9 years.
The subjects in this study came from 57 countries. For the purpose of analysis, the subjects were grouped into four geographical groups for comparison with Qatari nationals (N = 6,413). These were as follows: from five countries in the Arabian Peninsula (N = 974, Bahrain, Oman, Saudi Arabia, United Arab Emirates, and Yemen); from seven countries in the Eastern Mediterranean (N = 1,676, Iraq, Jordan, Kuwait, Lebanon, Palestine, Syria, and Turkey); from 25 countries in Africa (N = 2,998, Algeria, Burkina Faso, Cameroon, Chad, Djibouti, Egypt, Eritrea, Ethiopia, Gambia, Ghana, Guinea, Kenya, Libya, Malawi, Mauritania, Morocco, Mozambique, Nigeria, Senegal, Somalia, South Africa, Sudan, Tanzania, Tunisia, and Uganda); and from 19 countries in Asia (N = 6,502, Afghanistan, Azerbaijan, Bangladesh, Burma, China, India, Indonesia, Iran, Japan, Malaysia, Nepal, Pakistan, Philippines, Singapore, Sri Lanka, Tajikistan, Thailand, Uzbekistan, and Vietnam).
The analysis was based on data recorded at the Department of Laboratory Medicine and Pathology, HMC from January 1, 2005 until December 31, 2011, and is coded by year of study. However, since an analysis has already been published for the period 2005–2008,2 we call this period 1 and in some analyses compare prevalence rates in this period with period 2 covering the years 2009–2011.
Statistical analysis.
Prevalence data (percentage of subjects infected) are shown with 95% confidence limits (CL95), calculated as described in Reference 4 employing bespoke software. In some figures we have omitted confidence levels so as not to obscure trends in the illustrated data (but these are available from the authors on request). Prevalence was analyzed by maximum likelihood techniques based on log linear analysis of contingency tables using the software package SPSS (version 16.0.1, SPSS, Inc., Chicago, IL). Initially, full factorial models were fitted, incorporating as factors SEX (2 levels, males and females), AGE (13 levels as shown in Table 1), YEAR of study (7 levels, for each of the years from 2005 to 2011), and REGION of origin (5 levels, Africa, Arabian Peninsula, Asia, Eastern Mediterranean and Qatar). In some analyses PERIOD was fitted rather than YEAR because we wanted to know whether prevalence had changed between the first period (years 2005–2008) and the second period (years 2009–2011). Infection was considered as a binary factor (PRESENCE/ABSENCE of parasite). These explanatory factors were fitted initially to all models that were evaluated. For each level of analysis in turn, beginning with the most complex model, involving all possible main effects and interactions, those combinations that did not contribute significantly to explaining variation in the data were eliminated in a stepwise fashion beginning with the highest level interaction (backward selection procedure). A minimum sufficient model was then obtained, for which the likelihood ratio of χ2 was not significant, indicating that the model was sufficient in explaining the data (these values are given in the legends to the figures as relevant). The importance of each term (i.e., interactions involving infection) in the final model was assessed by the probability that its exclusion would alter the model significantly and these values relating to interactions that included presence/absence of infection are given in the text. The remaining terms in the final model that did not include presence/absence of infection are not given but can be made available from the authors on request.
Table 1.
Number of subjects in each category and the prevalence (%) of the four species of helminths by age, sex, region, and year
| No. subjects | Hookworms | T. trichiura | A. lumbricoides | H. nana | Combined | ||
|---|---|---|---|---|---|---|---|
| Age class age (years) | |||||||
| 1 | 0–1 | 783 | 0 | 0 | 0 | 0 | 0 |
| 2 | 1.1–1.9 | 1334 | 0 | 0 | 0.15 | 0 | 0.15 |
| 3 | 2.0–4.9 | 2253 | 0.04 | 0.09 | 0.13 | 0.22 | 0.49 |
| 4 | 5.0–9.9 | 1818 | 0 | 0.22 | 0.17 | 0.22 | 0.50 |
| 5 | 10.0–14.9 | 837 | 0.12 | 0 | 0 | 0.60 | 0.72 |
| 6 | 15.0–19.9 | 546 | 1.5 | 0.37 | 0.18 | 0.37 | 1.8 |
| 7 | 20.0–29.9 | 2600 | 6.3* | 1.3 | 0.69 | 0.35 | 7.5 |
| 8 | 30.0–39.9 | 2566 | 3.7 | 0.74 | 0.39 | 0.08 | 4.5 |
| 9 | 40.0–49.9 | 2288 | 1.3 | 0.52 | 0.44 | 0 | 2.1 |
| 10 | 50.0–59.9 | 1615 | 0.43 | 0.5 | 0.06 | 0.06 | 1.1 |
| 11 | 60.0–69.9 | 1024 | 0.29 | 0.29 | 0 | 0 | 0.49 |
| 12 | 70.0–79.9 | 639 | 0.16 | 0 | 0 | 0 | 0.16 |
| 13 | > 79.9 | 260 | 0 | 0 | 0 | 0 | 0 |
| χ2 | 279.1 | 39.0 | 21.9 | 37.5 | ** | ||
| P | < 0.001 | < 0.001 | 0.038 | < 0.001 | |||
| Sex | |||||||
| Males | 10791 | 2.8 | 0.65 | 0.37 | 0.18 | 3.6 | |
| Females | 7772 | 0.17 | 0.17 | 0.10 | 0.12 | 0.49 | |
| χ2 | ** | – | – | – | ** | ||
| P | NS | NS | NS | ||||
| Region | |||||||
| Arabian Pen. | 974 | 0 | 0 | 0 | 0.21 | 0.21 | |
| Eastern Med. | 1676 | 0 | 0 | 0 | 0 | 0 | |
| Africa | 2998 | 0.20 | 0.1 | 0.07 | 0.27 | 0.60 | |
| Asia | 6502 | 4.7 | 1.23 | 0.71 | 0.28 | 6.2 | |
| Qatar | 6413 | 0.05 | 0 | 0 | 0 | 0.05 | |
| χ2 | ** | 112.3 | 67.5 | 33.2 | ** | ||
| P | < 0.001 | < 0.001 | < 0.001 | ||||
| Year | |||||||
| 2005 | 2559 | 0.98 | 0.27 | 0.23 | 0.04 | 1.3 | |
| 2006 | 2120 | 1.3 | 0.24 | 0.33 | 0.05 | 1.7 | |
| 2007 | 2220 | 3.1 | 0.68 | 0.45 | 0 | 3.7 | |
| 2008 | 2309 | 3.0 | 0.78 | 0.35 | 0.30 | 4.0 | |
| 2009 | 3314 | 1.6 | 0.48 | 0.30 | 0.27 | 2.5 | |
| 2010 | 2990 | 1.7 | 0.50 | 0.10 | 0.17 | 2.2 | |
| 2011 | 3051 | 0.66 | 0.23 | 0.13 | 0.16 | 1.1 | |
| χ2 | 67.5 | 12.9 | – | 15.6 | 72.9 | ||
| P | < 0.001 | 0.045 | NS | 0.016 | <0.001 | ||
The highest prevalence in each category is in bold italics for emphasis.
In these cases see text for significant interactions between some of the variables in the model.
The statistical outputs were derived from minimum sufficient models, after first fitting for each species in turn, all variables into a single full factorial model, and then stepwise backward deletion of non-significant terms. The degrees of freedom can be calculated from the number of levels within each factor minus 1. The χ2 values are not shown for non-significant terms because these had been removed from the model during backward elimination in the derivation of the minimum sufficient model. The χ2 values for goodness of fit of the minimum sufficient models for hookworms, T. trichiura, A. lumbricoides, H. nana, and all helminths combined was as follows: 805.7 (dof = 1560, P = 1), 785.9 (dof = 1565, P = 1), 782.3 (dof = 1571, P = 1), 774.8 (dof = 1565, P = 1), and 902.7 (dof = 1548, P = 1), respectively. Additional terms in the final models, that did not incorporate the presence/absence of parasites are not shown, however can be made available on request from the authors.
Results
This study comprised tests on 18,563 subjects across 7 years, and Table 1 shows the number of subjects by each of the primary factors analyzed (main effects). Of these 1,593 (8.6%, CL95 8.18–8.98) were infected with one or more of the parasites in the study. The overall prevalence of each of the species in the study is given in Table 2, and prevalence is given also for the first period of study from January 2005 until December 2008, and for the second period from January 2009 to December 2011.
Table 2.
Prevalence (%) of protozoan and helminth parasites in the study population in the first period (2005–2008), the second (2009–2011), and overall
| Prevalence (95% confidence limits) | ||||||
|---|---|---|---|---|---|---|
| 2005–2008 | 2009–2011 | Combined | ||||
| Protozoa | ||||||
| Blastocystis hominis | 4.3 | (3.91–4.74) | 2.9 | (2.60–3.28) | 3.6 | (3.36–3.89) |
| Giardia duodenalis | 1.9 | (1.66–2.23) | 1.4 | (1.19–1.67) | 1.7 | (1.50–1.87) |
| Chilomastix mesnili | Nd | 0.04 | (0.012–0.109) | Na | ||
| Entamoeba coli | Nd | 0.51 | (0.38–0.68) | Na | ||
| Entamoeba hartmanni | Nd | 0.03 | (0.007–0.094) | Na | ||
| Endolimax nana | Nd | 0.86 | (0.678–1.064) | Na | ||
| Iodamoeba buetschlii | Nd | 0.16 | (0.090–0.264) | Na | ||
| All Non pathogenic amoebae* | 2.5 | (2.20–2.84) | 1.4 | (1.13–1.60) | 1.9 | (1.74–2.14) |
| Pathogenic amoeba* | 0.29 | (0.193–0.427) | 0.25 | (0.156–0.369) | 0.27 | (0.200–0.355) |
| All protozoa combined | 8.0 | (7.43–8.54) | 5.3 | (4.82–5.72) | 6.6 | (6.26–6.97) |
| Helminths | ||||||
| Hookworms | 2.1 | (1.76–2.34) | 1.3 | (1.08–1.55) | 1.7 | (1.50–1.87) |
| Trichuris trichiura | 0.5 | (0.36–0.65) | 0.4 | (0.29–0.56) | 0.4 | (0.36–0.55) |
| Ascaris lumbricoides | 0.3 | (0.23–0.48) | 0.2 | (0.11–0.29) | 0.3 | (0.19–0.34) |
| Hymenolepis nana† | 0.1 | (0.04–0.19) | 0.2 | (0.12–0.32) | 0.2 | (0.10–0.22) |
| All helminths combined | 2.6 | (2.30–2.95) | 1.9 | (1.67–2.23) | 2.3 | (2.07–2.50) |
| All the above species combined | 10.2 | (9.62–10.8) | 6.9 | (6.43–7.46) | 8.6 | (8.18–8.98) |
Non-pathogenic amoebae are E. coli, E. hartmanni, E. nana, and I. buetschlii.
Pathogenic amoebae E. histolytica/dispar, which cannot be distinguished on cyst morphology. See text for further explanation
This species is also known as Rodentolepis nana.
Nd = not done, these species were not assessed independently in the first period; Na = not applicable.
All parasites combined.
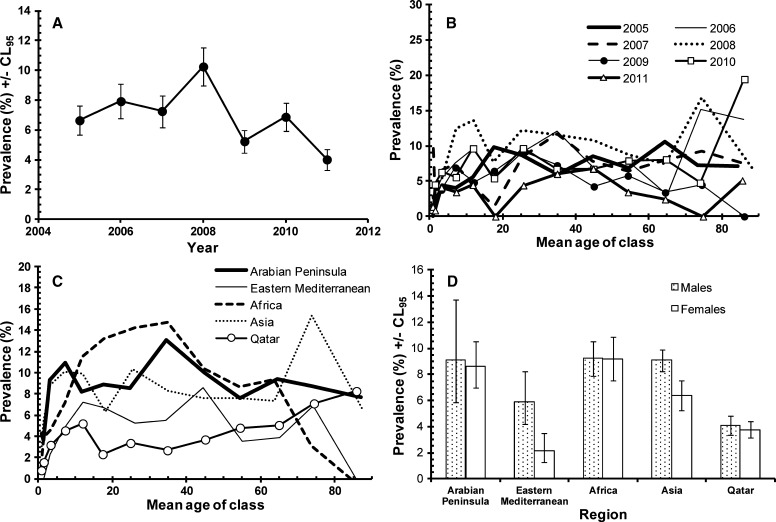
When all the parasite species were combined, over the first 4 years there was a significant steady increase with prevalence almost doubling over this period to peak in 2009 at 13.4%, as reported previously.2 However, over the following 3-year period prevalence fell by over 60% to the low observed in 2011 (4.9%) as shown in Figure 1A. This change in prevalence over the 7-year period was confounded by an interaction with age (YEAR*AGE CLASS*PRESENCE/ABSENCE of infection, χ272 = 114.2, P = 0.001), and this is illustrated in Figure 1B. Although there are common features to the age-prevalence profiles there are also distinct differences responsible for generating the significant effect. Among the similarities are the dip in prevalence in the 10–19.9 age group (age classes 5 and 6, in 5 of the 7 years), the increased prevalence with age in the following age classes, peaking in the 20–29.9 age range (age class 7) in each of the 7 years, and the fall in prevalence among the older age classes.
Figure 1.
Factors affecting the prevalence of all parasites combined. (A) Changes in prevalence during the 7 years of the study. (B) Differences between the age-prevalence profile between the years of the study. (C) Differences in the age-prevalence profile by region of origin of subjects. (D) Difference between the sexes in age-prevalence profile. The overall likelihood ratio for the goodness of fit of the model together with the REGION*SEX* PRESENCE/ABSENCE of infection term cited in the text and two additional terms not incorporating presence/absence of infection was χ21428 = 1335, P = 0.96). For the assessment of the significance of individual terms see the text.
Variation among regions of origin of settled immigrants and the local Qatari residents was dependent on sex (SEX*REGION*PRESENCE/ABSENCE of infection, χ24 = 31.5, P < 0.001). In the present combined dataset the pattern was much the same as that observed and reported earlier for 2005–2008.2 As earlier, the greatest discrepancy in terms of prevalence between sexes was among Asian workers, where prevalence was more than twice as high in males compared with females (16.0%, CL95 14.98–17.06 and 7.5%, CL95 6.26–8.71, respectively). A greater relative discrepancy in favor of males was found among workers from the Eastern Mediterranean region (males 5.9%, CL95 4.17–8.26 and females 2.1%, CL95 1.29–3.54) but among the other three regions (Arabian Peninsula, Africa, and Qatar), there was no difference in prevalence between the sexes. Prevalence (sexes combined) was lowest among the Qataris (4.0%, CL95 3.50–4.45), and similar among subjects from the Arabian Peninsula (8.9%, CL95 7.15–11.02) and Africa (9.6%, CL95 8.58–10.70).
The age-prevalence profiles also varied significantly between subjects from the five regions (age class*region*presence/absence of infection, χ248 = 81.8, P = 0.002) as shown in Figure 1C. The increased prevalence in the 15.0 to 49.9 age group (age classes 6–9) was most marked among Asians, subjects from the other four regions showing less change in prevalence with increasing age after the initial rise from age class 1. The age-prevalence profile for the Qataris was the lowest, although there was a very consistent upward drift with increasing age among these subjects, to eventually peak in the oldest age class (those over 79.9 years of age, N = 206).
Finally, with age and region taken into account, there was a highly significant effect of sex on the age-prevalence profile (SEX*AGE CLASS*PRESENCE/ABSENCE of infection, χ212 = 30.3, P = 0.003) as illustrated in Figure 1D. Clearly, there was no difference in prevalence between the sexes among the youngest five classes (up to and including 19.9 years of age). However, in the next age class there was a marked difference, and although the discrepancy between the sexes was not as extensive in older age classes it was nevertheless maintained right until age class 12 (70- to 79.9-year-old subjects).
All helminths combined.
Helminth infections were rarer than protozoan infections, only 424 subjects (2.3%) were recorded as carrying these parasites (Table 2). Prevalence increased between 2006 and 2008 (Table 1), peaking in 2008 and then falling to a low of just 1.1% in 2011, and this change in prevalence between years was significant (Table 1). Among subjects from Asia, prevalence of helminths was much higher than among individuals from other regions (for example ×10 higher than among Africans), and in fact 94.6% of all helminths were detected among this regional group. However, the regional effect on prevalence was confounded by host sex (SEX*REGION χ24 = 14.9, P = 0.005) because among Asians prevalence was considerably higher among male (7.9%, CL95 7.15–8.68) compared with female (1.5%, CL95 0.96–2.16) subjects. Despite the low prevalence in other regions discrepancies between the sexes were either non-existent (e.g., Arabian Peninsula prevalence = 0.2% in both sexes and among those from the Eastern Mediterranean no infections were detected in either sex) or much lower, and in some cases in the opposite direction (e.g., among Africans prevalence in males = 0.4%, CL95 0.19–0.87), whereas among females = 0.8%, CL95 0.40–1.55).
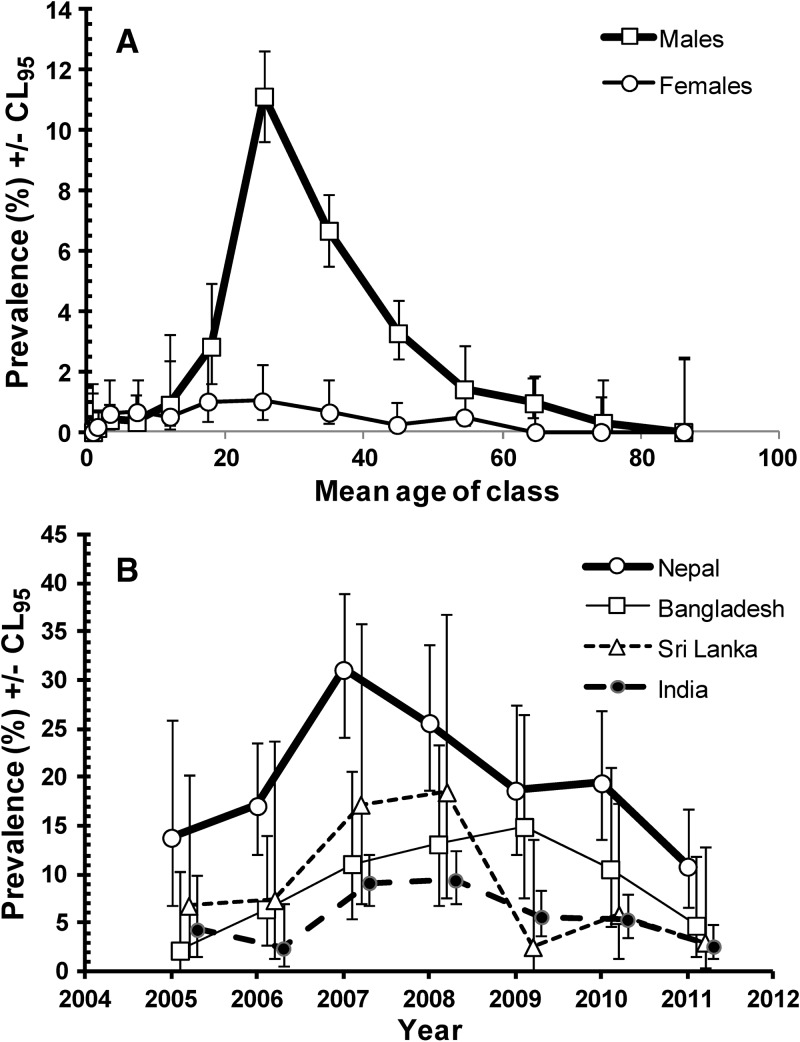
There was also a significant, albeit weaker difference between the age-prevalence profiles of the two sexes (SEX*AGE*PRESENCE/ABSENCE of helminths χ212 = 23.2, P = 0.026). As Figure 2A shows this arose because the prevalence of helminths was relatively high among males of working age (15.0–69.9) but especially so among those in the 20.0–29.9 age class, compared with females. Overall, 86.6% of helminth infections were among male subjects aged 15.0–69.9 years and 44.8% among those aged 20.0–29.9 years. There were only rare cases among younger individuals, with a total of just two in those aged 1.1–1.9 years and 11 among those aged 2.0–4.9 years (see Table 1 for sample sizes). Among older individuals, there were no helminth infections in women more than 60 years of age (N = 938), only six among males 60.0–79.9 years of age (N = 857), and none among older males (N = 128).
Figure 2.
Prevalence of helminth infections. (A) Age-prevalence profiles of helminth infections in male and female subjects (all regions, years, and age classes combined). (B) Changes in prevalence of helminth infections over the period of 7 years in male subjects (all age classes) from the four countries showing overall the highest prevalence rates in the study population.
Examination of the countries of origin of Asian subjects, and confining this to those countries with more than 10 individuals in the current sample, revealed that prevalence was highest among the Nepalese (19.7%, CL95 16.45–23.44, N = 989), and lower among those from Bangladesh (8.3%, CL95 6.57–10.40, N = 626), Sri Lanka (6.8%, CL95 4.57–10.05, N = 307), and India (4.4%, CL95 3.54–5.33, N = 2,221). Prevalence was below 1.7% in the remaining countries in this region, so clearly resident workers from Nepal were most likely to carry helminth infections, and among them prevalence was twice as high as that among individuals from Bangladesh with the next highest prevalence. Among the Nepalese sample only 16 were female and only one of these was infected, so the majority of helminth infections were among male Nepalese (N = 973), and these were all confined to subjects 15.0–59.9 years of age, prevalence varying from 17.2–22.5% among the four age classes in this age range, but numerically most infections were among the 20.0–29.9 year age group, which comprised 56.9% of the 973 male Nepalese subjects in the study.
It was of interest next to examine how helminth infections had changed over the 7 years of the study among male Nepalese workers, in comparison to those from other countries showing a relatively high prevalence with helminths (Figure 2B). Among male Nepalese prevalence peaked in 2007, and has since been declining. Among male Bangladeshi subjects prevalence rose steadily until 2009 and then fell over the next 2 years. Males from Sri Lanka had the highest prevalence of 18.5% in 2008, after which there was a marked drop by 86.2% to just 2.6% in 2009 and prevalence has since remained low. Indian males showed less overall change with year of study and lowest overall prevalence of helminths but peak years were 2007 and 2008 and prevalence was only 2.6% in 2011, the lowest among the males of these four countries.
Individual species of helminths.
Analysis of each of the four species of helminths separately yielded much the same pattern as previously mentioned for the analysis of the combined helminth data. Hookworm infections were most common (prevalence = 1.7%), and the other helminths all had lower overall prevalence (Table 2). Table 1 shows prevalence rates for each species in the different subsets of the data. The prevalence of the nematode species increased between 2006 and 2008 and as with all helminths combined, prevalence of hookworms and Trichuris trichiura was lower in the second period compared with the earlier one, the fall in prevalence being most marked in the case of hookworms (by 35.9%). Despite a similar pattern in the case of Ascaris lumbricoides there was no significant difference between years when other factors had been taken into account. In contrast, despite the low overall prevalence of Hymenolepis nana in the study population, prevalence of this species rose from 0.04% in 2005 to 0.30% in 2008, the peak year for this cestode, and there were signs of a reduction in prevalence in the following years (fall from 0.27% in 2009 to 0.16% in 2011), although this is not reflected in the means for periods 1 and 2 (Table 2).
All three nematodes were mainly encountered among subjects from Asia. In contrast the prevalence of the cestode H. nana was very similar among subjects from the Arabian Peninsula, Africa, and Asia, but was not encountered at all among those from the Eastern Mediterranean countries and among the Qataris.
All helminths showed a higher prevalence among male subjects, but the sex effect was not significant in any of the species when other factors were taken into consideration. In the case of hookworm infections, there was a significant interaction with region of origin (SEX*REGION*PRESENCE/ABSENCE of hookworms χ24 = 13.5, P = 0.009), reflecting the marked sexual dichotomy of hookworm prevalence among Asian subjects (6.2%, CL95 5.54–6.91 among males, but only 0.5%, CL95 0.20–0.89 among females) but not among those from other regions. Hookworms were essentially restricted to Asian subjects (97.1% of hookworm cases), there being only three cases among the Qataris and six among African subjects, therefore differences between the sexes from other regions were not detectable.
There was a significant age effect in all four helminths (Table 1), peak prevalence of all three species of nematodes being in the 20.0–29.9 age class, and the 10.0–14.9 age class in the case of H. nana.
Combinations of helminths.
Four subjects carried three species of helminths, 39 had double infections and the remaining 381 all had one of the four species that were detected. The multiple helminth species infections were mostly confined to Asian subjects (97.4% of the double helminth infections and all four triple species infections), males (90.7%), and subjects aged 15.0–49.9 years (93.0% of cases). All four triple species infections were among the 20.0–29.9 age class. Of the 42 multiple species among Asian subjects, 47.6% were among the Nepalese, 23.8% among the Bangladeshi, and 21.4% among Indian subjects.
All protozoan infections combined.
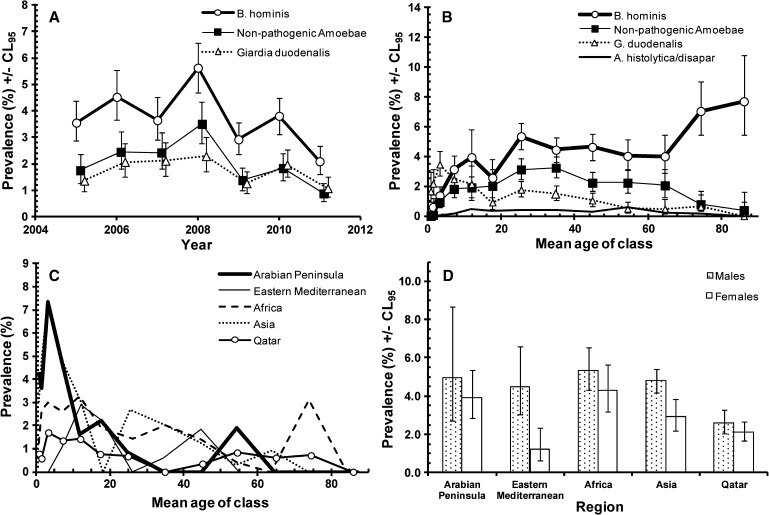
Protozoan infections were more common than helminth infections and the prevalence of individual species is given in Table 2. Altogether 1,228 subjects (6.6%, see Table 2) in the study were infected by at least one of the species tested. Figure 3A shows that there were signs of falling prevalence in 2009 and 2011, after the peak year 2008. The difference between years was significant but confounded by an interaction with age (Figure 3B; for AGE*YEAR*PRESENCE/ABSENCE of infection χ272 = 106.6, P = 0.005). Although the age-prevalence profiles had many similarities (dip in prevalence in age classes 5 or 6 in 6 of the 7 years of the study, falling prevalence in older classes), the height of the peaks and rates of change with increasing age varied significantly between years.
Figure 3.
Factors affecting the prevalence of all protozoan parasites combined. (A) Changes in prevalence during the 7 years of the study. (B) Differences between the age-prevalence profile between the years of the study. (C) Differences in the age-prevalence profile by region of origin of subjects. (D) Difference between the sexes among subjects from the five regions. The overall likelihood ratio for the goodness of fit of the model based on the three terms summarized in B, C, and D and two additional terms not incorporating presence/absence of infection was χ21440 = 1365.7, P = 0.92). For the assessment of the significance of individual terms see the text.
There was also a highly significant difference in the age-prevalence profiles between subjects from the five different regions (Figure 3C; REGION*AGE*PRESENCE/ABSENCE of infection, χ248 = 81.1, P = 0.002). In subjects from Africa, the Arabian Peninsula, and Eastern Mediterranean there was a protracted high plateau of infection among those in-between the ages of 10.0 and 49.9. In subjects from Asia there was a sharp additional peak among individuals 70–79.9 years of age, but in Qataris who showed the lowest prevalence overall (3.9%, CL95 3.45–4.41), there was a clear upward trend with age, achieving the peak level only in those over 80 years of age (8.3%, CL95 6.09–11.09).
As with the data for all parasitic infections a combined prevalence of protozoan infections was numerically higher in male (7.5%, CL95 7.02–8.01) compared with female (5.4%, CL95 4.86–5.87) subjects, but this difference between the sexes was confounded by region of origin (SEX*REGION*PRESENCE/ABSENCE of protozoan infection, χ24 = 17.0, P = 0.002). Figure 3D shows that the sex effect was only evident in individuals from Asia and the Eastern Mediterranean, whereas in those from the Arabian Peninsula, Africa, and Qatar the prevalence of protozoan infections was very similar in both sexes.
Factors affecting individual species of protozoa.
Overall prevalence.
Blastocystis hominis was the commonest species of intestinal protozoan, with an overall prevalence of 3.6%, almost twice that of the next most common, the non-pathogenic amoebae (Table 2) with an overall prevalence of 1.9%, and then G. duodenalis with a prevalence of 1.7%. In the second period (2009–2011) the prevalence of non-pathogenic amoebae was 1.4% and the dominant species were E. nana (0.86%) and E. coli (0.51%) with the remaining two species being detected only sporadically. The pathogenic E. histolytica/dispar combination was also rare in the study population.
Changes in prevalence over time.
The three most common protozoan species all showed significant changes in prevalence over the 7 years of the current study (for YEAR*PRESENCE/ABSENCE of B. hominis, non-pathogenic amoebae, and G. duodenalis χ26 = 48.3, 51.7 and 23.3, respectively, and P = 0.001 or less in each case). As can be seen from Figure 4A, there was an upward drift in each of the first 4 years of the study with prevalence rising steadily as reported previously,2 peaking in 2008 and then dropping in the following years. In each case prevalence was lower in the second period compared with the first (Table 2). Despite the generally lower prevalence values for E. histolytica/dispar in the second period relative to the first, there was no significant difference in prevalence of this species between years.
Figure 4.
Factors affecting individual species of protozoa. (A) Changes in prevalence of the dominant three species by year of study. (B) The age prevalence profiles of four species of protozoa in the study. (C) The age-prevalence profiles of Giardia duodenalis in subjects from each of the five regions of origin. (D) Differences between the sexes in prevalence of Blastocystis hominis among the five regions of origin of the subjects in the study.
Change in prevalence with increasing age.
Figure 4B illustrates differences between the age-prevalence profiles of B. hominis, the non-pathogenic amoebae, G. duodenalis and E. histolytica/dispar. Prevalence of B. hominis increased steadily with increasing host age to peak in the oldest individuals of the study (AGE*PRESENCE/ABSENCE of B. hominis χ212 = 204.3, P < 0.001). In contrast the age-prevalence profile for the non-pathogenic amoebae showed initially a steady rise with age, with a plateau in the 20–49.9 age class, and then a steady fall in prevalence in the older age groups (AGE*PRESENCE/ABSENCE of B. hominis χ212 = 104.7, P < 0.001). The age-prevalence profile for G. duodenalis showed a much earlier peak in the 2.0–4.9 year age class, and then a steady drop, but this was confounded by a significant interaction with region of origin (AGE*REGION*PRESENCE/ABSENCE of G. duodenalis χ248 = 70.6, P = 0.019). In fact, G. duodenalis infections were relatively rare among those from the Eastern Mediterranean and among Qataris but more common among those from the Arabian Peninsula, Africa, and Asia (Table 3), and these differences in regional prevalence generated slightly different age-prevalence profiles as shown in Figure 4C. A marked peak among the young children can be seen clearly among those from the Arabian Peninsula and Asia, and a lesser peak among Africans and Qataris. Prevalence then generally declined, but among some regions there was a resurgence of infection in particular age classes (Figure 4C). In the case of E. histolytica/dispar there was also a significant difference between age classes (AGE*PRESENCE/ABSENCE E. histolytica/dispar χ212 = 24.4, P = 0.018), prevalence ranging between 0.26% and 0.56% among subjects 10.0–59.9 years of age with the peak among the 50.0–59.9 age class (0.56%, CL95 0.25–1.06). No infections with this species were detected in those < 2 years of age or more than 80 years of age (Figure 4B).
Table 3.
Prevalence (%) of protozoan parasites by region of origin in the first period (2005–2009), the second (2009–2011), and overall
| Prevalence (95% confidence limits) | ||||||
|---|---|---|---|---|---|---|
| 2005–2008 | 2009–2011 | Combined | ||||
| Blastocystis hominis | ||||||
| Arabian Peninsula | 6.1 | (4.69–7.72) | 2.6 | (1.11–5.70) | 4.4 | (2.93–6.52) |
| Eastern Mediterranean | 4.1 | (2.76–6.05) | 1.9 | (1.07–3.26) | 3.0 | (2.27–4.00) |
| Africa | 5.4 | (4.20–6.81) | 4.6 | (3.63–5.73) | 4.9 | (4.16–5.71) |
| Asia | 5.2 | (4.46–6.00) | 3.4 | (2.79–4.03) | 4.3 | (3.81–4.80) |
| Qatar | 2.8 | (2.27–3.44) | 1.9 | (1.42–2.42) | 2.4 | (1.98–2.73) |
| Non-pathogenic amoebae | ||||||
| Arabian Peninsula | 2.9 | (2.03–4.22) | 1.9 | (0.71–4.87) | 2.5 | (1.41–4.19) |
| Eastern Mediterranean | 1.3 | (0.61–2.55) | 0.7 | (0.34–1.76) | 1.0 | (0.59–1.62) |
| Africa | 5.1 | (3.93–6.46) | 2.5 | (1.78–3.34) | 3.6 | (2.94–4.27) |
| Asia | 3.3 | (2.68–3.92) | 1.6 | (1.21–2.11) | 2.4 | (2.07–2.82) |
| Qatar | 1.0 | (0.71–1.44) | 0.6 | (0.34–0.92) | 0.8 | (0.61–1.06) |
| Giardia duodenalis | ||||||
| Arabian Peninsula | 3.1 | (2.19–4.44) | 2.2 | (0.84–5.16) | 2.7 | (1.55–4.44) |
| Eastern Mediterranean | 0.7 | (0.30–1.73) | 0.4 | (0.17–1.18) | 0.5 | (0.25–1.02) |
| Africa | 2.5 | (1.75–3.57) | 1.4 | (0.91–2.10) | 1.9 | (1.44–2.46) |
| Asia | 2.8 | (2.22–3.41) | 2.3 | (1.85–2.93) | 2.6 | (2.17–2.94) |
| Qatar | 1.1 | (0.74–1.47) | 0.6 | (0.39–1.00) | 0.9 | (0.65–1.12) |
| Amoeba histolytica/dispar | ||||||
| Arabian Peninsula | 0 | (0–0.38) | 0.22 | (0.04–2.11) | 0.10 | (0.04–0.89) |
| Eastern Mediterranean | 0.11 | (0.05–0.83) | 0.12 | (0.06–0.79) | 0.12 | (0.01–0.43) |
| Africa | 0.54 | (0.22–1.11) | 0.35 | (0.13–0.77) | 0.43 | (0.23–0.74) |
| Asia | 0.50 | (0.28–0.81) | 0.40 | (0.21–0.68) | 0.45 | (0.30–0.64) |
| Qatar | 0.09 | (0.02–0.26) | 0.06 | (0.01–0.23) | 0.08 | (0.03–0.18) |
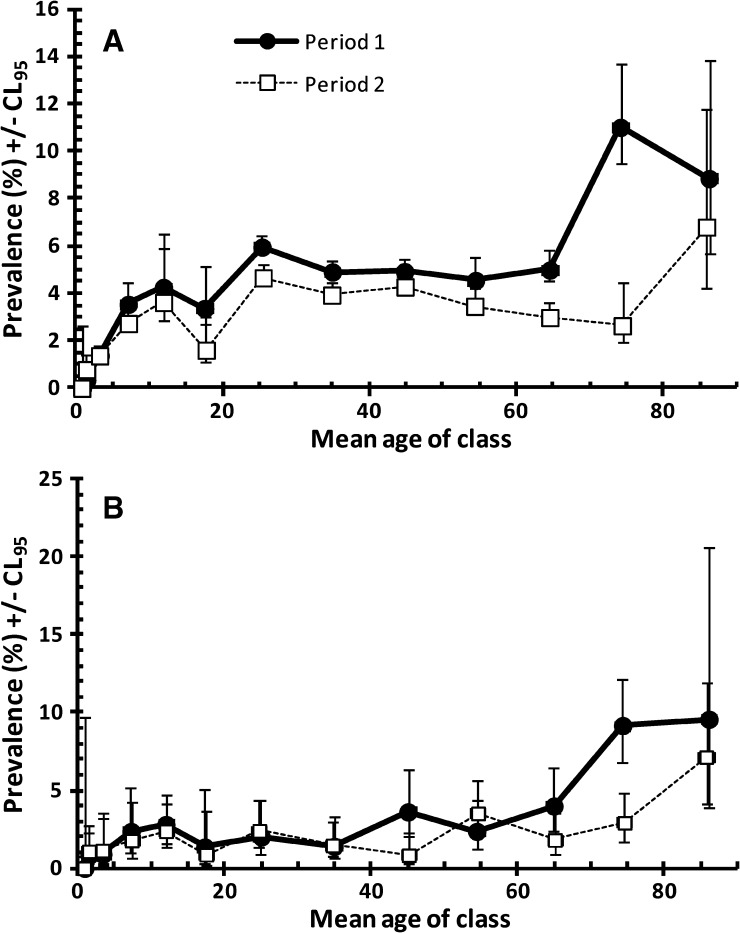
Because the age-prevalence profile for B. hominis showed a rise among the elderly, we dissected this further, comparing the profiles for the first and second periods to see whether the reduction in overall prevalence was evident in all age classes and particularly whether the upward rise among the elderly had been affected by the falling overall prevalence rate. The data in Figure 5A show that indeed prevalence was lower in the second period in all age classes and this was significant (PERIOD*PRESENCE/ABSENCE of B. hominis χ21 = 18.5, P < 0.001), but the upward trend among the elderly was still evident albeit this time confined to the very oldest age class (> 79.9 years). Moreover, because among the Qatari nationals an upward trend in the prevalence of all protozoa (mainly B. hominis) combined had been shown (Figure 3C), we repeated this analysis confining it to the Qataris. The data in Figure 5B show that here also between period 1 and 2, a lower prevalence was evident in most age classes (PERIOD*PRESENCE/ABSENCE of B. hominis χ21 = 5.1, P = 0.024), but the upward drift in prevalence among the elderly was still evident in period 2.
Figure 5.
Prevalence of Blastocystis hominis by age in all the subjects in the study (A) and among Qataris (B). The overall likelihood ratio for the goodness of fit of the minimum sufficient model, based on fitting PERIOD rather than YEAR was χ2337 = 346.1 (P = 0.354) for A, and for B where region of origin was omitted because the model was focused solely on Qataris, χ251 = 61.0, (P = 0.159). For the assessment of the significance of individual terms see the text.
Regional differences in prevalence of the protozoan infections.
Regional differences for G. duodenalis have been covered previously because of their association with different age-prevalence profiles of this parasite. For the non-pathogenic amoebae, there was a highly significant regional effect that was independent of other factors (REGION*PRESENCE/ABSENCE of non-pathogenic amoebae χ24 = 88.9, P < 0.001). Prevalence was highest among subjects of African origin, intermediate among those of Asian or Arabian Peninsula origin, and least among those from the Eastern Mediterranean and Qatar. In all regions prevalence was lower in the second period compared with the first (Table 3). Prevalence of B. hominis was higher in all regions compared with the other infections, again declining in all regions between period 1 and 2 (Table 3), but the regional effect was confounded by a significant interaction with sex (REGION*SEX*PRESENCE/ABSENCE of B. hominis χ24 = 10.6, P = 0.031) as illustrated in Figure 4D. Prevalence was higher among males in all regions, but there was a marked discrepancy between the sexes among subjects from the Eastern Mediterranean and to a lesser degree among subjects from Asia. In the remaining three regions there was little difference between the sexes. Entamoeba histolytica/dispar, despite its very low overall prevalence, also showed a significant regional effect (REGION*PRESENCE/ABSENCE of E. histolytica/dispar χ24 = 18.6, P = 0.001), being primarily concentrated among the African and Asian subjects, and rare among the other three regional groups. Among subjects from these two regions there was evidence of a slight reduction between period 1 and 2 (Table 3).
Discussion
Reassuringly, the present retrospective analysis of computerized records at HMC has indicated that in contrast to the period 2005–2008, prevalence of parasitic infections has begun to fall in the last 3 years. Without exception, all prevalence values for the period 2009–2011 were numerically lower than those recorded for 2005–2008. Combined intestinal protozoan infections fell from 8.0% to 5.3%, and combined helminth infections fell from 2.6% to 1.9% (see Table 2). Even within the 3 years 2008–2011, combined parasitic infections were lower in 2011 than 2008, and this was also the case for helminth infections among the high risk groups, i.e., the Nepalese, Bangladeshi, and Indians (see Figure 2B). By 2011 combined intestinal protozoan infections also fell from their peak in 2008 (see Figure 3A), and a downward trend was also clearly apparent for the dominant intestinal protozoan B. hominis as well as for G. duodenalis and the non-pathogenic amoebae. The explanation for the downward trends, from the peaks in 2007–2008, in all these taxa may lie in three notable changes that have occurred in recent years. First, because the population of Qatar has increased so markedly in the last decade, the HMC has changed its admittance policy and persons with minor ailments are no longer admitted, but rather redirected to their respective health centers for treatment. Admittance to HMC is now only possible if the problem is beyond the treatment possibilities of the doctors at the health center. This policy may have changed the demographic and social cross section of the subjects treated at the hospital, and in turn affected the prevalence statistics, a possibility that is difficult to investigate further because the social class and employments of subjects registered at the HMC were not available to us for the period 2005–2011. Second, the Ministry of Interior determines annually the quota of foreign workers that can be recruited for work in Qatar and the quotas for specific countries are not fixed but subject to change depending on current government policy, thereby affecting the ratios of workers from different countries entering Qatar each year. Because the prevalence of parasites varies between countries, the overall prevalence may change accordingly. Third, and perhaps most importantly, it is now mandatory for all applicants for jobs in Qatar to have pre-employment certificates (PEC), which are provided at local Qatar embassy-approved clinical centers in the countries of origin before arrival in Qatar, and require medical examinations that include fecal examination. Before obtaining a certificate, subjects who are found to be positive for parasites, are required to have treatment (usually albendazole for helminths and metronidazole for protozoa) and then re-examination. The PEC was introduced 3 years ago in 2009, and is particularly enforced by Qatar Embassy officials when providing visas for prospective workers. Therefore, the declining prevalence of protozoan infections in the last 3 years may be attributable in part to this initiative by the Qatar Public Health Authorities, and our data provide encouraging support for the success of this policy.
Equally interesting was our continued finding that as in the period 2005–2008, resident workers of Asian origin were the principal carriers of helminth infections, which were largely represented by hookworms and, as in the earlier study, the majority of these infections were among the 20.0- to 39.9-year-old Nepalese males. Prevalence levels among the Nepalese and subjects of Asian origins were the highest even when helminth infections were falling between 2009 and 2011 (see Figure 2B). Hookworm infections are known to be endemic in Nepal and a serious problem in many other Asian countries,5–8 therefore it is not surprising that the prevalence of hookworm infections in Nepalese workers initially arriving in Qatar is relatively high. However, unlike other nationalities, the persistence of infection among long-term residents of Nepalese origin is intriguing. Because construction workers with residency permits do not have to be treated with albendazole either on first arrival or on returning from short-term home visits, they are likely to maintain their worms in the long term, unless they treat themselves, which is highly unlikely (Abu-Madi and Behnke, unpublished observations). Hookworms are known to be able to persist in the human host for 17–18 years without reinfection,9,10 but the same should apply also to other nationals carrying hookworms. It is also possible that some transmission of hookworms takes place in the work camps of construction workers living in Doha, where housing may be inadequate with overcrowded conditions, but no evidence of local transmission is available as yet. In broader terms it is unlikely that the transmission of human hookworms is a major problem in Qatar itself, because of the arid and hot climatic conditions, although feline hookworms are a problem among the feral cat population in Doha,11 and Doha does experience 2 months of extremely humid weather in August–September each year. With the recent introduction of the PEC, and treatment of helminth carrying subjects with albendazole before arrival in Qatar, another possible explanation is that hookworms locally in Nepal have developed resistance to these benzimidazoles through the frequent de-worming programs that have been implemented in the country, especially among children.8The possibility of benzimidazole resistance having arisen in human hookworms has been recently reviewed,12 but there is still no indisputable evidence, although the efficacy of mebendazole in particular has been shown to have declined in some parts of the tropics, notably in Zanzibar, Vietnam, and Mali and instances of failure with albendazole have also been reported in Ghana.13–16
Another trend in the current analysis worth commenting on was the age-related increase in the prevalence of B. hominis (Figure 4B), which was much like that we reported earlier2 and hence largely unaffected after the addition of data from the three most recent years. Because these data generate some concern over B. hominis infections among the elderly, we also compared data for the first period with the second and we found that there was an overall reduction in all age groups and the upward drift in prevalence was now only evident in the very oldest age class. Although a very common parasite of humans worldwide, B. hominis is known to cause some pathology, especially in subjects with weakened immune systems17–20; consequently, this rise in prevalence among the elderly is likely to be a reflection of this pathology, and the elderly should therefore be the focus of attention to reduce prevalence rates even further.
Another age-related rising trend was for the combined protozoan infections among the Qatari residents (Figure 3C) where prevalence rose steadily from 2.3% among the 15.0- to 19.9-year-old class to 8.3% among the oldest class of subjects. Given that the prevalence of all protozoan parasites other than B. hominis was very low in the oldest age class, and that Qataris only rarely harbored helminth infections, this suggested that the steady increase must have been largely attributable to B. hominis infections. When the data were examined more closely and period 1 compared with period 2, despite the fall in overall prevalence rates, the upward drift with host age, particularly among the elderly was still evident (Figure 5B).
The analyses presented in this work, have shown that despite concerns expressed earlier2 about the rising prevalence rates of intestinal parasites among long-term residents in Qatar, the situation has much improved in the last 3 years and the falling trends provide optimism that in the years ahead intestinal parasitic infections will fall even lower. This is likely to be partly a result of the introduction of the PEC and the associated health inspections in the countries of origin of foreign workers. However, stricter control of the food industry, including distribution of food and regular auditing of restaurants and shops by the special unit from the Supreme Council of Health, responsible for policing food quality and safety, have probably also contributed to reducing the prevalence of parasitic infections in Qatar in recent years. The two outstanding problems that now require further attention are the still relatively high prevalence rates for hookworms among the Nepalese and other Asian workers and the rising trend in the prevalence of B. hominis with host age among both Qataris and foreign residents. Our attention is currently focused on unraveling the reasons for the relatively high prevalence of hookworm infections among resident Asians, and especially the Nepalese, in the hope that eventually the decline in these infections can be accelerated even more markedly than that reflected in this analysis.
ACKNOWLEDGMENTS
We thank the Qatar National Research Fund (QRNF) at Qatar Foundation for supporting this study through the National Priorities Research Program (NPRP) (Project No. NPRP 4-1283-3-327). The study was approved by the Medical Research Center & Research Committee at HMC, Qatar. Our grateful thanks go to all technical staff of the Microbiology Division, Department of Laboratory Medicine & Pathology at HMC, especial thanks to Miss Roda El-Ibrahim.
Disclaimer: The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of Qatar University and QRNF.
Footnotes
Financial support: This publication was made possible by a grant from Qatar National Research Fund (QRNF) at Qatar Foundation through National Priorities Research Program (NPRP) (Project No. NPRP 4-1283-3-327).
Authors' addresses: Marawan A. Abu-Madi, Department of Health Sciences, College of Arts and Sciences, Qatar University, Qatar, E-mail: abumadi@qu.edu.qa. Jerzy M. Behnke, School of Biology, University of Nottingham, University Park, Nottingham, UK, E-mail: jerzy.behnke@nottingham.ac.uk. Sanjay H. Doiphode, Department of Laboratory Medicine and Pathology, Hamad Medical Corporation, Qatar, E-mail: sdoiphode@hmc.org.qa.
References
- 1.Abu-Madi MA, Behnke JM, Ismail A. Patterns of infection with intestinal parasites in Qatar among food handlers and housemaids from different geographical regions or origin. Acta Trop. 2008;106:213–220. doi: 10.1016/j.actatropica.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Abu-Madi MA, Behnke JM, Doiphode SH. Changing trends in intestinal parasitic infections among long-term-residents and settled immigrants in Qatar. Parasite Vector. 2010;3:98. doi: 10.1186/1756-3305-3-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abu-Madi MA, Behnke JM, Ismail A, Al-Olaqi N, Al-Zaher K, El-Ibrahim R. Comparison of intestinal parasitic infection in newly arrived and resident workers in Qatar. Parasite Vector. 2011;4:211. doi: 10.1186/1756-3305-4-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rohlf FJ, Sokal RR. Statistical Tables. Third edition. San Francisco, CA: W.H. Freeman and Company; 1995. [Google Scholar]
- 5.Sharma1 BK, Rai SK, Rai DR, Choudhury DR. Prevalence of intestinal parasitic infestation in schoolchildren in the northeastern part of Kathmandu Valley, Nepal. Southeast Asian J Trop Med Public Health. 2004;35:501–504. [PubMed] [Google Scholar]
- 6.Khanal LK, Choudhury DR, Rai SK, Sapkota J, Barakoti A, Amatya R, Hada S. Prevalence of intestinal worm infestations among school children in Kathmandu, Nepal. Nepal Med Coll J. 2011;13:272–274. [PubMed] [Google Scholar]
- 7.Shah BK, Baig LA. Association of anemia with parasitic infestation in pregnant Nepalese women. Results from a hospital based study done in eastern Nepal. J Ayub Med Coll. 2005;17:5–9. [PubMed] [Google Scholar]
- 8.Shakya B, Bhargava D, Shrestha S, Rijal BP. Intestinal parasitosis. J Inst Med. 2009;31:3. [Google Scholar]
- 9.Palmer ED. Course of egg output over a 15 year period in a case of experimentally induced Necatoriasis americanus, in the absence of hyperinfection. Am J Trop Med Hyg. 1955;4:756–757. doi: 10.4269/ajtmh.1955.4.756. [DOI] [PubMed] [Google Scholar]
- 10.Beaver PC. Light, long-lasting Necator infection in a volunteer. Am J Trop Med Hyg. 1988;39:369–372. doi: 10.4269/ajtmh.1988.39.369. [DOI] [PubMed] [Google Scholar]
- 11.Abu-Madi MA, Behnke JM, Prabhaker KS, Al-Ibrahim R, Lewis JW. Intestinal helminths of feral cat populations from urban and sub-urban districts of Qatar. Vet Parasitol. 2010;168:284–292. doi: 10.1016/j.vetpar.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 12.Vercruysse J, Albonico M, Behnke JM, Kotze AC, Prichard RK, McCarthy JS, Montresor A, Levecke B. Is anthelmintic resistance a concern for the control of human soil-transmitted helminths? Int J Parasitol: Drugs and Drug Resistance. 2011;1:14–27. doi: 10.1016/j.ijpddr.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Clercq D, Sacko M, Behnke J, Gilbert F, Dorny P, Vercruysse J. Failure of mebendazole in treatment of human hookworm infections in the Southern Region of Mali. Am J Trop Med Hyg. 1997;57:25–30. doi: 10.4269/ajtmh.1997.57.25. [DOI] [PubMed] [Google Scholar]
- 14.Albonico M, Bickle Q, Ramsan M, Montresor A, Savioli L, Taylor M. Efficacy of mebendazole and levamisole alone or in combination against intestinal nematode infections after repeated targeted mebendazole treatment in Zanzibar. Bull World Health Organ. 2003;81:343–352. [PMC free article] [PubMed] [Google Scholar]
- 15.Flohr C, Tuyen LN, Lewis S, Minh TT, Campbell J, Britton J, Williams H, Hien TT, Farrar J, Quinnell RJ. Low efficacy of mebendazole against hookworm in Vietnam: two randomized controlled trials. Am J Trop Med Hyg. 2007;76:732–736. [PubMed] [Google Scholar]
- 16.Humphries D, Mosites E, Otchere J, Twum WA, Woo L, Jones-Sanpei H, Harrison LM, Bungiro RD, Benham-Pyle B, Bimi L, Edoh D, Bosompem K, Wilson LM, Cappello M. Epidemiology of hookworm infection in Kintampo North Municipality, Ghana: patterns of malaria coinfection, anemia, and albendazole treatment failure. Am J Trop Med Hyg. 2011;84:792–800. doi: 10.4269/ajtmh.2011.11-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boorom KF, Smith H, Nimri L, Viscogliosi E, Spanakos G, Parkar U, Li L-H, Zhou X-N, Ok UZ, Leelayoova S, Jones MS. Oh my aching gut: irritable bowel syndrome, Blastocystis and asymptomatic infection. Parasite Vector. 2008;1:40. doi: 10.1186/1756-3305-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stensvold CR, Nielsen HV, Mølbak K, Smith HV. Pursuing the clinical significance of Blastocystis – diagnostic limitations. Trends Parasitol. 2009;25:23–29. doi: 10.1016/j.pt.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Stensvold CR. Thinking Blastocystis out of the box. Trends Parasitol. 2012;28:306. doi: 10.1016/j.pt.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Scanlan PD. Blastocystis: pitfalls and future perspectives. Trends Parasitol. 2012;28:327–334. doi: 10.1016/j.pt.2012.05.001. [DOI] [PubMed] [Google Scholar]