Abstract
Tungiasis is an ectoparasitic skin disease caused by Tunga penetrans and Tunga trimamillata. There is a lack of histopathological studies that evaluate the recognition of this flea in tissues. We describe the ex vivo dermoscopic and the histopathological patterns of six cases and relate the findings to the developmental stage of the parasite as defined by the Fortaleza classification: two were classified as Fortaleza 3b, 3 as 4a, and 1 as 4b. Two dermoscopic patterns were observed: a brown pigmented ring and a radial crown with a central pore. The most common histopathological findings were an eosinophilic cuticle, eggs in different stages of development, tracheal rings (parasite), and basal hyperplasia (host). The eosinophilic cuticle, eggs in different stages of evolution, and tracheal rings can help to establish the diagnosis when other parts of the parasite are lacking. The Fortaleza staging may represent a tool for pathology reporting purposes.
Introduction
Tunga penetrans and Tunga trimamillata are the only two species that are known to infect humans among the 11 species of Tunga.1–3 The sand flea has been endemic to Peru for almost 14 centuries, as evidenced by written documentation and anthropomorphic ceramics that depict pathognomonic lesions of this infection.4 Although it is an ancient skin infection, there are no systematic epidemiological studies on the distribution or impact in economically deprived populations in Peru.5 The causes of underreporting possibly include the lack of interest in this ectoparasite among health care providers, the habit of the removal of the embedded insect by individuals themselves, and the lack of awareness of inexperienced physicians.
Studies comparing the external morphology and phenotypic characteristics of the two species of Tunga spp. have been performed.6–9 Others have emphasized a few histological characteristics of Tunga spp. that may aid the histological diagnosis when lesions have been previously manipulated and whose sections are, subsequently, technically difficult to study.10,11 There are only three isolated cases reported in the literature from Peru.5,12,13
Our objectives are to describe the ex vivo dermoscopic patterns through scanning, to describe the histopathological characteristics of human tungiasis in individuals who received care in hospitals in the capital suburbs of Lima, and to apply the Fortaleza classification (Eisele and others, 200314), a clinico-histopathological staging system, to better characterize and report the stages of infection.
Material and Methods
Population.
Between 2007 and 2010, 10 of 23 suburban hospitals were prospectively included in our study, most of them located in the outskirts of Lima.
Scanning, histopathology, and staging.
Personal data, including sex, age, localization of the lesion, and differential diagnoses were recorded. The biopsies were performed by a general physician in each hospital.
All of the samples were properly placed in clean containers with 10% formaldehyde, transported to our Laboratory of Anatomic Pathology on the same day of sampling, processed overnight with conventional methods, and stained with hematoxylin-eosin (HE). We reviewed all of the skin samples that were macroscopically suggestive of tungiasis: characteristic central dark spot, black nodule, or intra-epidermic foreign body-like structures.
To perform ex vivo dermoscopy, each tissue sample was carefully placed upside down on a scanner (Hewlett Packard ScanJet 5370C, Greeley, Colorado; magnification 10×), and the images were saved for subsequent reports. This concept of the magnification of the skin was applied analogous to dermoscopy to characterize the macroscopic patterns of tungiasis. Using this method of magnification, we screened for all of the macroscopic criteria of tungiasis documented in the literature: 1) a brown pigmented ring with a central pore that represents the posterior end of the exoskeleton, 2) a grey-blue blotch that may represent either the eggs or hematin in the parasite gut, and 3) a radial crown that represents columnar hemorrhagic parakeratosis in a radial arrangement.15–18
For staging purposes, we applied the Fortaleza classification described by Eisele and others in 2003, a dynamic classification system that permits the staging of the cycle of infection of Tunga spp. in the host.14
Fortaleza stages:
Stage 1: Penetration (3–7 h)
Stage 2: Beginning of hypertrophy (1–2 days after penetration), which lasts for 2–3 days
- Stage 3: White halo divided into two substages:
- First (3a): 2–3 days, lasts for 2–3 days
- Second (3b): 6–7 days, lasts for 2 weeks
- Stage 4: Involution of the lesion:
- First (4a): live flea, 3–4 weeks after penetration
- Second (4b): 4–6 weeks, dead flea
Stage 5: Residues
Finally, we used the results of works by Pampiglione and others8,9 in an attempt to identify any specific histological in situ hallmark of T. trimamillata that differentiates it from T. penetrans. We particularly screened for midgut diverticula, a presumed anatomical difference present in gravid T. trimamillata, but not in T. penetrans.9
Results
During a 3-year period (2007–2010), 1,665 skin biopsies were studied. A total of six cases of human tungiasis were diagnosed by HE.
Ex vivo dermoscopy and staging.
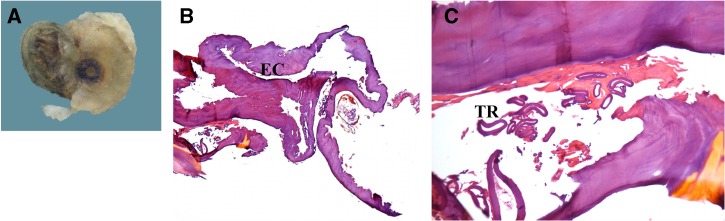
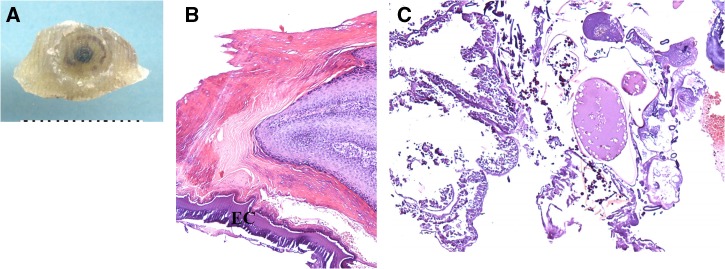
After scanning the samples, we observed two dermoscopic patterns of tungiasis: a brown pigmented ring with a central pore in patients 2, 3, 4, and 5 (panels A, Figures 1–3), and a radial crown in patient 3 (Figure 2A). No grey-blue blotch was observed. Scanning could not be appropriately performed on patient 1 because of inadequate sampling, and was missing for patient 6. Patient 5, a case of ectopic tungiasis, was previously reported and pictures are not presented in this work. Table 1 describes the district of origin, anatomical area of biopsy, and dermoscopic findings of patients 1–6. Two cases were classified as Fortaleza substage 3b (patients 3 and 4), 3 cases as substage 4a (patients 1, 5, and 6), and 1 case as substage 4b (patient 2).
Figure 1.
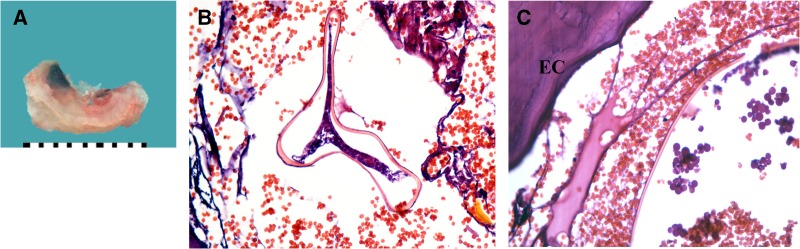
Patient 2. Ex vivo dermoscopy: wrinkled, darker black spot with a more dysmorphic and wider central pore compared with Patient 3 (A). Histopathology: Eosinophilic cuticle (EC) and the carcass of dead parasite (B, hematoxylin-eosin [HE], 100×) with tracheal rings (TR). Pinkish material surrounding the TR represents signs of cell degradation (C, HE, 200×).
Figure 3.
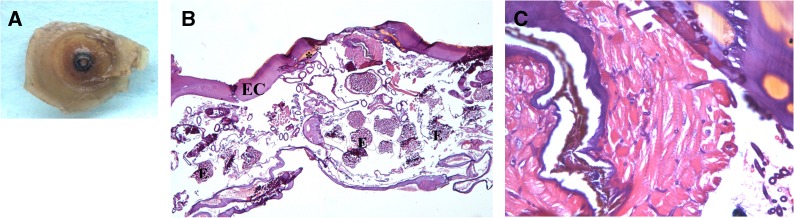
Patient 4. Ex vivo dermoscopy: pigmented central black spot with central pore (A). Histopathology: Eosinophilic cuticle (EC) with yellowish pigmented areas on top (*), eggs (E) in different stages of development, branching tracheal rings, and oviducts (B, hematoxylin-eosin [HE], 40×). Muscle fibers of the flea in transversal and longitudinal disposition (C, HE, 200×).
Figure 2.
Patient 3. Ex vivo dermoscopy: pigmented, brownish punctate rim (radial crown) around central black spot, desquamation of the surface of the borders of the lesion (A). Histopathology: Marked hyperkeratosis, hyperplasia, and parakeratosis with an eosinophilic cuticle (EC) (B, hematoxylin-eosin [HE], 200×). Dermal papillae with lymphocytes (C, HE, 100×).
Table 1.
District of origin, area of biopsy, ex vivo dermoscopy findings, and Fortaleza classification staging in individuals with tungiasis*
| Patient | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| District of origin | Lince | Lince | San Juan de Lurigancho | Surquillo | Villa el Salvador | Lince |
| Area of biopsy | Right 4th toe | Plantar area | Plantar area | Right ankle | Knee | Plantar area |
| Ex vivo dermoscopy | ||||||
| Brown pigmented ring with central pore | NA | + | + | + | + | NA |
| Grey-blue blotch | NA | – | – | – | – | NA |
| Radial crown with central pore | NA | – | + | – | – | NA |
| Fortaleza classification | 4a | 4b | 3b | 3b | 4a | 4a |
NA = not available. (+): present, (–): absent.
Histopathological changes.
The most common parasitic findings in our series were the presence of the eosinophilic cuticle, eggs in different stages of development and tracheal rings, which were observed in all of the cases (6 of 6) (Figures 1–5). A yellowish-brown intracuticular chitin representing the last abdominal segment of the embedded flea was identified almost consistently (5 of 6) (Figure 1B and C and Figure 3B and C). Other structures, such as oviducts, midgut, and intraparasitic red blood cells (2 of 6), and hypodermis, hypertrophied striated muscle (Figure 3C), and bacterial colonies (cocci in clusters and chains) (1 of 6) (not shown), were less frequently observed.
Figure 5.
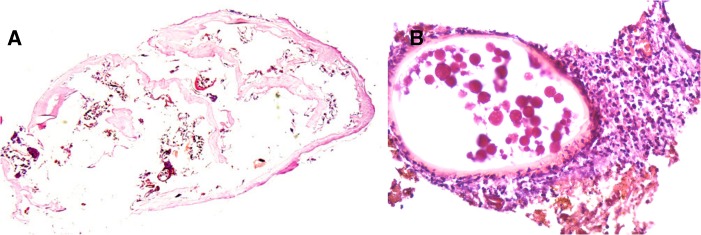
Patient 6. Ex vivo dermoscopy: Not available. Histopathology: Carcass of decaying parasite and egg shells (A, hematoxylin-eosin [HE], 20×). Neutrophilic infiltrates around the egg (B, HE, 400×).
Figure 4.
Patient 1. Ex vivo dermoscopy: Lateral black necrotic tissue (A). Histopathology: Hemorrhagic area in dermis with a cystic, collapsed structure (B, hematoxylin-eosin [HE], 200×) surrounded by an eosinophilic cuticle (C, HE, 200×).
Regarding the host histopathological changes, basal hyperplasia was invariably observed in all of the cases (6 of 6). Additionally, acanthosis (5 of 6), hyperkeratosis, parakeratosis, hypergranulosis, and papillomatosis (4 of 6), spongiosis (3 of 6), and microabscesses (2 of 6) were identified. The demographic and histopathological characteristics are summarized in Table 2.
Table 2.
Histopathological results of parasites and the parasite-host interaction in the epidermis and dermis
| Patients | 1 | 2 | 3 | 4 | 5 | 6 | Proportion |
|---|---|---|---|---|---|---|---|
| Demographic characteristics | |||||||
| Age | 30 | 38 | 7 | 17 | 8 | 10 | |
| Sex | F | F | F | M | F | F | |
| Histopathological findings | |||||||
| Parasite | |||||||
| Cuticle | + | + | + | + | + | + | 6/6 |
| Eggs | + | + | + | + | + | + | 6/6 |
| Oviducts | – | – | – | + | + | – | 2/6 |
| Tracheal rings | + | + | + | + | + | + | 6/6 |
| Striated muscle | – | – | – | + | – | – | 1/6 |
| Hypodermis | – | – | – | – | + | – | 1/6 |
| Midgut | – | – | + | + | – | – | 2/6 |
| Intraparasitic red blood cell | + | – | – | + | – | – | 2/6 |
| Yellowish brown chitin | + | + | + | + | – | + | 5/6 |
| Bacterial colonies | – | + | – | – | – | – | 1/6 |
| Host | |||||||
| Epidermis | |||||||
| Hyperkeratosis | + | – | + | + | + | – | 4/6 |
| Parakeratosis | + | – | + | + | + | – | 4/6 |
| Spongiosis | – | – | + | + | – | + | 3/6 |
| Acanthosis | + | + | + | + | + | – | 5/6 |
| Basal hyperplasia | + | + | + | + | + | + | 6/6 |
| Hypergranulosis | + | – | + | + | + | – | 4/6 |
| Papillomatosis | – | + | + | + | + | – | 4/6 |
| Microabscesses | – | – | + | + | – | – | 2/6 |
| Epidermis/Dermis | |||||||
| Lymphocytes | –/– | –/N | +/+ | +/+ | +/+ | –/N | 3/6–3/4 |
| Neutrophils | –/– | –/N | +/+ | +/+ | –/– | –/N | 2/6–2/4 |
| Eosinophils | –/– | –/N | +/+ | +/+ | –/– | –/N | 2/6–2/4 |
| Histocytes | –/– | –/N | –/+ | +/+ | –/– | –/N | 1/6–2/4 |
M = male; F = female; PMNs = polymorphonuclear cells found in the epidermis and dermis; N = no dermis available.
Discussion
Before the implementation of these suburban hospitals in the city of Lima, we rarely diagnosed tungiasis with histopathology. The strategic location of these hospitals close to impoverished shanty towns might explain this trend. Among physicians, the presence of piques or niguas (as Tunga spp. are known in Peru) is an overlooked and most likely underreported infection. The general physician who extracts the arthropod at primary health care centers commonly discards the samples. This underreporting is reflected by the absence of epidemiological studies in Peru, with only three documented isolated cases in scientific journals, the first reported by Urteaga and others in Archivos Peruanos de Patologia y Clinica in 1955.12 However, the indirect evidence of tungiasis in Peru dates back to pre-Incan cultures that flourished between c. 1200 and 1420 AD and that depicted the infection in anthropomorphic pottery.4
By magnifying the details of the upper layer of the skin through scanning the biopsies similar to entodermoscopy some ex vivo patterns of tungiasis were revealed.19,20 In addition to the classic brown pigmented ring with a central pore observed in patients 2, 3, 4, and 5, we observed a radial crown in patient 3 consistent with marked parakeratosis, as described by Marraza and others.18
To date, there are no studies on the epidemiology of T. trimamillata in Peru, and its histological and differences of infection, if at all, remain unknown. Clearly, the principal anatomic differences between these species are easily distinguished by the stereoscopic examination of non-gravid adults and gravid parasitic females. The presence of three anterior abdominal humps observed by naked eye, a greater length of the first maxillary palps, and the presence of spines in the tibia of the third legs are the main anatomical features that differentiate T. trimamillata from the rest of the Tungidiae.7,8
The most common parasitic findings in our series were the eosinophilic cuticle, eggs, and tracheal rings in section studies. These findings are in agreement with Smith and Procop,10 who unequivocally observed all of these structures as a histopathological hallmark of tungiasis. In contrast to their findings, portions of the digestive tract (midgut) and hypodermis were observed in only 2 and 1 out of 6 cases, respectively, in our series and not in all of the cases studied. This result may be explained by the patients' manipulation of the lesions, which could have distorted their histological appearance, or because of a more advanced stage of involution because the midgut in our patients was observed in Fortaleza stage 3b (patients 3 and 4) and not in the more advanced stages 4a and b (patients 1, 2, 5, and 6). It is worth noting that the only patient in Fortaleza stage 4b (patient 2) had colonies of cocci in clusters and chains, which most likely represents bacterial superinfection, a common complication of tungiasis.21
Concerning the host histopathologic features in the skin, basal hyperplasia was invariably seen in all individuals. Other reactive inflammatory changes such as acanthosis, hyperkeratosis, parakeratosis, papillomatosis, hypergranulosis, and spongiosis were also present. Microabscesses were only observed in patients 3 and 4 and helped classify these cases into Fortaleza stage 3b (Table 2). These microabscesses, constantly present in substage 3b, together with an infiltrate of lymphocytes, neutrophils, eosinophils, and histiocytes in the epidermis and dermis, suggest earlier infection. Furthermore, these are absent in stage 4, a more advanced stage of gravid flea involution. These findings are in accordance with a larger series of cases from Brazil where microabscesses were invariably present in stage 3b.14 The bacterial colonies and the carcass of the dead parasite, both observed only in patient 2, suggested a more advanced stage of involution, and also helped classify this case into stage 4b.
Although it has not been applied prospectively, the Fortaleza classification may represent a valuable tool for pathology reporting purposes. This classification can estimate the chronicity of infection by recognizing different histopathological changes and dermoscopic features characteristic of each stage of infection. Most of the patients (4 of 6) were in stage 4 compared with stage 3 (2 of 6), which reflects some patients seeking medical treatment 3 to 6 weeks after the penetration of the flea.
Our study has important limitations, including the small number of individuals and the restriction of the data to a certain number and geographic location of hospitals in Lima's suburbs. This first series of cases of human tungiasis in Peru represents an attempt to report an old disease whose geographic distribution remains largely unstudied.
In summary, we have shown that some dermoscopic patterns of tungiasis are revealed by scanning the lesions ex vivo before their inclusion in paraffin blocks, similar to entodermoscopy. Common histopathological features of tungiasis are the eosinophilic cuticle, the eggs in different stages of evolution, and the tracheal rings observed in different stages of tungiasis, which can help to establish the diagnosis when distorted material or advanced lesions are encountered. The application of Fortaleza staging in histological reports of tungiasis can help not only to understand the dynamic biology of the sand flea but also estimate the time frame after penetration. Finally, there is a need for epidemiological studies in humans and animals to describe the distribution of this ectoparasite in Peru, with special emphasis on the histological features of the new species T.trimamillata that will allow us to differentiate it from the old T. penetrans.
ACKNOWLEDGMENTS
The American Committee on Clinical Tropical Medicine and Travelers' Health (ACCTMTH) assisted with publication expenses.
Footnotes
Authors' addresses: Vicente Maco, Beth Israel Medical Center, Albert Einstein College of Medicine, New York, New York, and Instituto de Medicina Tropical Alexander von Humboldt, Universidad Peruana Cayetano Heredia (UPCH), Lima, Peru, E-mail: vicente_maco@hotmail.com. Vicente P. Maco, Servicios de Salud Guadalupe, Lima, Peru, E-mail: vmaco@speedy.com.pe. Manuel E. Tantalean, Facultad de Medicina Veterinaria y Zootecnia, UPCH, Lima, Peru, E-mail: mtantaleanv@hotmail.com. Eduardo Gotuzzo, Instituto de Medicina Tropical Alexander von Humboldt, UPCH, Lima, Peru, E-mail: eduardo.gotuzzo@upch.pe.
References
- 1.Pampiglione S, Fioravanti ML, Gustinelli A, Onore G, Mantovani B, Luchetti A, Trentini M. Sand flea (Tunga spp.) infections in humans and domestic animals: state of the art. Med Vet Entomol. 2009;23:172–186. doi: 10.1111/j.1365-2915.2009.00807.x. [DOI] [PubMed] [Google Scholar]
- 2.De Avelar DM, Linhares AX, Linardi PM. A new species of Tunga (Siphonaptera: Tungidae) from Brazil with a key to the adult species and neosomes. J Med Entomol. 2012;49:23–28. doi: 10.1603/me11111. [DOI] [PubMed] [Google Scholar]
- 3.Heukelbach J. Tungiasis. Rev Inst Med Trop Sao Paulo. 2005;47:307–313. doi: 10.1590/s0036-46652005000600001. [DOI] [PubMed] [Google Scholar]
- 4.Maco V, Tantalean M, Gotuzzo E. Evidence of tungiasis in pre-Hispanic America. Emerg Infect Dis. 2011;17:855–862. doi: 10.3201/eid1705.100542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maco V, Maco VP, Gotuzzo E. An ectopic case of Tunga spp. infection in Peru. Am J Trop Med Hyg. 2010;82:1076–1078. doi: 10.4269/ajtmh.2010.10-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pampiglione S, Trentini M, Fioravanti ML, Onore G, Rivasi F. Additional description of a new species of Tunga (Siphonaptera) from Ecuador. Parasite. 2003;10:9–15. doi: 10.1051/parasite/2003101p9. [DOI] [PubMed] [Google Scholar]
- 7.Fioravanti ML, Pampiglione S, Trentini M. A second species of Tunga (Insecta, Siphonaptera) infecting man: Tunga trimamillata. Parasite. 2003;10:282–283. [PubMed] [Google Scholar]
- 8.Pampiglione S, Fioravanti ML, Gustinelli A, Onore G, Rivasi F, Trentini M. Anatomy of Tunga trimamillata Pampiglione et al., 2002 (Insecta, Siphonaptera, Tungidae) and developmental phases of the gravid female. Parasite. 2005;12:241–250. doi: 10.1051/parasite/2005123241. [DOI] [PubMed] [Google Scholar]
- 9.Pampiglione S, Trentini M, Fioravanti ML, Gustinelli A. Differential diagnosis between Tunga penetrans (L., 1758) and T. trimamillata Pampiglione et al., 2002 (Insecta, Siphonaptera), the two species of the genus Tunga parasitic in man. Parasite. 2004;11:51–57. doi: 10.1051/parasite/200411151. [DOI] [PubMed] [Google Scholar]
- 10.Smith MD, Procop GW. Typical histologic features of Tunga penetrans in skin biopsies. Arch Pathol Lab Med. 2002;126:714–716. doi: 10.5858/2002-126-0714-THFOTP. [DOI] [PubMed] [Google Scholar]
- 11.Feldmeier H, Eisele M, Van Marck E, Mehlhorn H, Ribeiro R, Heukelbach J. Investigations on the biology, epidemiology, pathology and control of Tunga penetrans in Brazil: IV. Clinical and histopathology. Parasitol Res. 2004;94:275–282. doi: 10.1007/s00436-004-1197-2. [DOI] [PubMed] [Google Scholar]
- 12.Urteaga O, Salas A, Calderon J, Moncloa F. Foot tungiasis treated with Acarol [in Spanish] Arch Peru Patol Clin. 1955;9:37–51. [Google Scholar]
- 13.Beltran M. Tungiasis and Tunga penetrans [in Spanish] Rev Peru Med Exp Salud Publica. 2005;22:323–324. [Google Scholar]
- 14.Eisele M, Heukelbach J, Van Marck E, Mehlhorn H, Meckes O, Franck S, Feldmeier H. Investigations on the biology, epidemiology, pathology and control of Tunga penetrans in Brazil: I. Natural history of tungiasis in man. Parasitol Res. 2003;90:87–99. doi: 10.1007/s00436-002-0817-y. [DOI] [PubMed] [Google Scholar]
- 15.Bauer J, Forschner A, Garbe C, Rocken M. Dermoscopy of tungiasis. Arch Dermatol. 2004;140:761–763. doi: 10.1001/archderm.140.6.761. [DOI] [PubMed] [Google Scholar]
- 16.Bauer J, Forschner A, Garbe C, Rocken M. Variability of dermoscopic features of tungiasis. Arch Dermatol. 2005;141:643–644. doi: 10.1001/archderm.141.5.643. [DOI] [PubMed] [Google Scholar]
- 17.Di Stefani A, Rudolph CM, Hofmann-Wellenhof R, Mullegger RR. An additional dermoscopic feature of tungiasis. Arch Dermatol. 2005;141:1045–1046. doi: 10.1001/archderm.141.8.1045. [DOI] [PubMed] [Google Scholar]
- 18.Marazza G, Campanelli A, Kaya G, Braun RP, Saurat JH, Piguet V. Tunga penetrans: description of a new dermoscopic sign–the radial crown. Arch Dermatol. 2009;145:348–349. doi: 10.1001/archdermatol.2008.611. [DOI] [PubMed] [Google Scholar]
- 19.Zalaudek I, Giacomel J, Cabo H, Di Stefani A, Ferrara G, Hofmann-Wellenhof R, Malvehy J, Puig S, Stolz W, Argenziano G. Entodermoscopy: a new tool for diagnosing skin infections and infestations. Dermatology. 2008;216:14–23. doi: 10.1159/000109353. [DOI] [PubMed] [Google Scholar]
- 20.Tschandl P, Argenziano G, Bakos R, Gourhant JY, Hofmann-Wellenhof R, Kittler H, Rosendahl C, Minas S, Zalaudek I. Dermoscopy and entomology (entomodermoscopy) J Dtsch Dermatol Ges. 2009;7:589–596. doi: 10.1111/j.1610-0387.2009.07027.x. [DOI] [PubMed] [Google Scholar]
- 21.Feldmeier H, Heukelbach J, Eisele M, Sousa AQ, Barbosa LM, Carvalho CB. Bacterial superinfection in human tungiasis. Trop Med Int Health. 2002;7:559–564. doi: 10.1046/j.1365-3156.2002.00904.x. [DOI] [PubMed] [Google Scholar]