Abstract
An 85-year-old female farmer was admitted to our hospital for fever, general fatigue, and skin rash. Cephalosporin was not effective and minocycline was dramatically effective. An eschar was discovered on her inguinal region after the defervescence. Laboratory examination of serum taken 12 days after onset of the illness showed elevated titers of antibodies against the Shimokoshi strain of Orientia tsutsugamushi. The gene sequence analysis of specimen from the patient's eschar revealed high similarity to the Shimokoshi strain by nested polymerase chain reaction. Therefore, this patient was diagnosed as a case of Shimokoshi-type tsutsugamushi disease, which has not previously been reported in Western Japan. Recently, cases of this type have also been confirmed in northeastern Japan, suggesting the need for further epidemiological studies.
Introduction
Tsutsugamushi disease is a rickettsiosis caused by Orientia tsutsugamushi. The vector is the larval form of the trombiculid mite and the infection occurs through bites of the infected mite.1 Fever, skin rash, and eschar constitutes the known triad of clinical symptoms of this infection. To our knowledge, the present patient is the first case of Shimokoshi-type disease from Western Japan, where some well-known types of O. tsutsugamushi have generally been found.
Case report
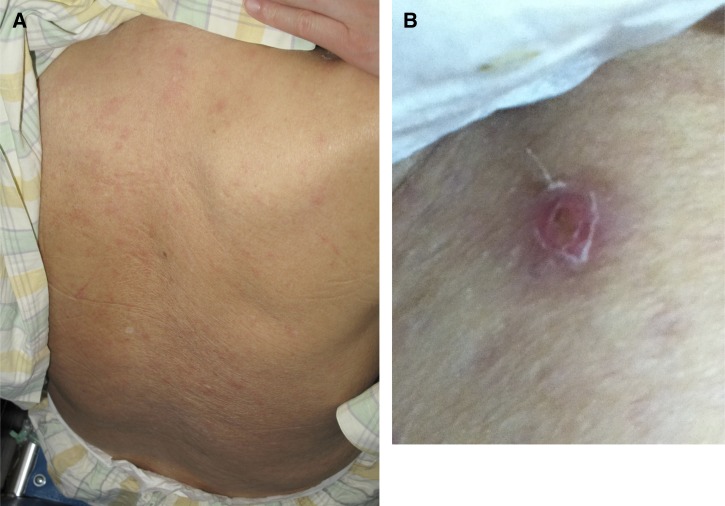
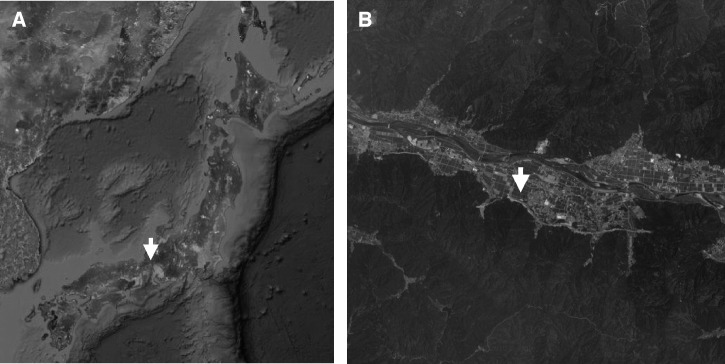
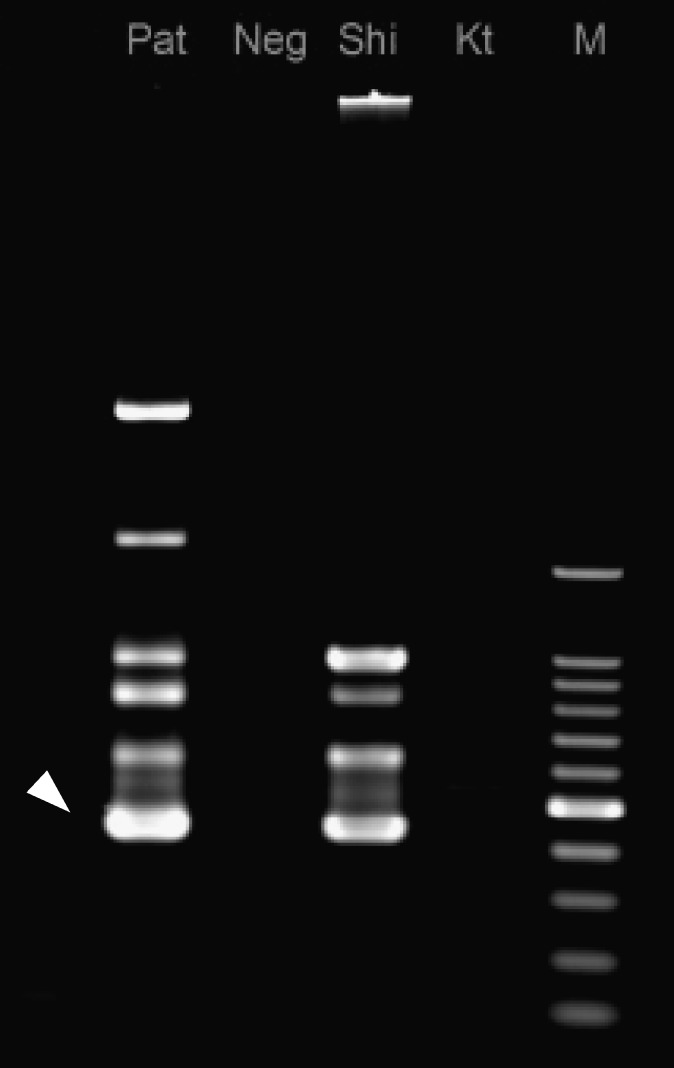
An 85-year-old female farmer was admitted to our hospital for fever and general fatigue on April 29, 2012. Physical examination on admission showed a fever of 38.1°C (axillary); blood pressure was 138/70 mm of Hg, heart rate was 80/min, and the patient's consciousness was clear. Various sizes of edematous skin erythema were seen mainly at the trunk of the body (Figure 1A). Hepatosplenomegaly and lymphadenopathy were not apparent. Clinical laboratory data did not suggest any severe abnormality (white cell count 7.6 × 109/L, hemoglobin 13.5 g/dL, platelet count 20.6 × 109/L, aspartate aminotransferase 37 IU/L, alanine aminotransferase 22 IU/L, lactate dehydrogenase 268 IU/L, C-reactive protein 2.97 mg/dL), and disseminated intravascular coagulation markers were within normal ranges. Chest x-ray and abdominal computed tomography did not show any significant findings of infectious focus. Ceftriaxone was administered on the suspicion of urinary tract infection; however, the patient failed to improve. In light of the patient's worsening skin rash and general fatigue, the antibiotic agent was changed to intravenous minocycline (MINO). The case improved dramatically after administration of MINO, with fever decreasing within 12 hours. After examining the whole body again under the suspicion of tsutsugamushi disease, an eschar was discovered at the left inguinal region (Figure 1B). The patient was shown to have been bitten by mites while farming in the fields close to her home, which is located among the rivers and mountains of the Fukui prefecture (Figure 2A and B). The patient was discharged from the hospital on Day 10 post-admission, the administration of MINO was changed from intravenous to oral form. The immunoperoxidase and immunofluorescence tests for antibodies against Gilliam, Karp, Kato, Kawasaki, Kuroki, and Shimokoshi-type antigens were performed. Of these antigens, the Shimokoshi type is rarely used in routine tests for diagnosis of this disease, but was attempted here in response to a recent discussion of the diversity of O. tsutsugamushi types. Specifically, the tests were performed at the University of Fukui (Table 1A), at the Ohara Research Laboratory (Table 1B), and at the Miyazaki Prefectural Public Health Laboratory (Table 1C). The serum drawn at 12 or 28 days after the onset of the illness exhibited elevated titers of antibodies against the Shimokoshi strain of O. tsutsugamushi. Polymerase chain reaction (PCR) with nested primer pairs was done by Medico-field study support (Fukui, Japan) according to the previously reported method.2 The primers for Shimokoshi type, p34 (5′-TCAAGCTTATTGCTAGTGCAATGTCTGC-3′) and pShi1 (5′-CTGCCCTTGCCCCTGAACTA-3′) were used for the first PCR, and p10 (5′-GATCAAGCTTCCTCAGCCTACTATAATGCC-3′) and pShi4 (5′-CTCAGAAATTTGAACGGA-3′) were used for the second PCR. Gene sequence analysis of the 436-bp product amplified from a sample of the patient's eschar revealed 100% and 99% similarities to the corresponding sequences from the Shimokoshi strain (accession nos.: AB536749 and M63381, respectively) (Figure 3). Therefore, we diagnosed the patient with Shimokoshi-type tsutsugamushi disease. To our knowledge, this is the first report of this disease in Western Japan.
Figure 1.
(A) Skin rash and (B) eschar at left inguinal region.
Figure 2.
(A and B) The geographic location where the patient was infected.
Table 1.
The antibody titers against six strains of Orientia tsutsugsmushi in three laboratory institutions, days means the period between onset of the illness and drawing serum immunoperoxidase (A and B), immunofluorescence tests (C)
| A | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days | Kawasaki | Kuroki | Gilliam | Karp | Kato | Shimokoshi | ||||||
| IgM | IgG | IgM | IgG | IgM | IgG | IgM | IgG | IgM | IgG | IgM | IgG | |
| 4 | – | – | – | – | – | – | – | – | – | – | – | – |
| 6 | – | – | – | – | – | – | – | – | – | – | 80 | – |
| 12 | – | 160 | 160 | 160 | – | 80 | 320 | 640 | 80 | 80 | 1280 | 1280 |
| 28 | – | 160 | 160 | 1280 | – | 160 | 320 | 1280 | – | 80 | 1280 | 2560 |
| B | ||||||||||||
| Days | Kawasaki | Kuroki | Gilliam | Karp | Kato | Shimokoshi | ||||||
| IgM | IgG | IgM | IgG | IgM | IgG | IgM | IgG | IgM | IgG | IgM | IgG | |
| 4 | – | – | – | – | – | – | – | – | – | – | 160 | – |
| 6 | – | – | – | – | – | – | – | – | – | – | 640 | – |
| 12 | 40 | 320 | 320 | 320 | 80 | 160 | 640 | 1280 | 160 | 160 | 10240 | 10240 |
| 28 | – | 320 | 320 | 2560 | 80 | 160 | 640 | 2560 | 160 | 160 | 10240 | 10240 |
| C | ||||||||||||
| Days | Kawasaki | Kuroki | Gilliam | Karp | Kato | Shimokoshi | ||||||
| IgM | IgG | IgM | IgG | IgM | IgG | IgM | IgG | IgM | IgG | IgM | IgG | |
| 4 | – | – | – | – | – | – | – | – | – | – | – | – |
| 12 | – | 40 | 160 | 40 | – | 40 | 80 | 40 | – | 80 | 1280 | 320 |
Figure 3.
Orientia tsutsugamushi genome detection using nested polymerase chain reaction. Pat = patient's skin eschar; Neg = negative control; Shi = Shimokoshi strain (positive control); Kt = Kato strain; M = marker (100 bp ladder).
Discussion
Definitive diagnosis of tsutsugamushi disease is often difficult, given that the disease presents with common, non-specific symptoms such as fever and skin rash3; in the case presented here, diagnosis of tsutsugamushi disease was complicated (by chance) by the presence of simple cystitis caused by Escherichia coli. The beta lactams have no efficacy against rickettsial infections, making prompt administration of tetracycline important to treatment of tsutsugamushi disease.4 A previous report suggests that tetracycline is effective in most cases, with a majority of patients showing dramatic defervescence within 24 h.5 The patients are diagnosed by elevation of serum antibody titer.6 Orientia tsutsugamushi genome detection using nested PCR also is reported to be useful.2
Orientia tsutsugamushi consists of several antigenic variants. There are three classical types: Gilliam, Karp, and Kato strains (from Southeast Asia); more recently, serotypes such as Kawasaki, Kuroki, and Shimokoshi have been isolated in Japan.7 A correspondence between rickettsial serotype and the species of vector chiggers has been reported, such as the transmission of the Kato serotype by Leptotrombidium akamushi, of Japanese Karp and Gilliam by Leptotrombidium pallidum, and of Kawasaki by Leptotrombidium scutellare.8,9 However, the vector for Shimokoshi type has not yet been defined.
The Shimokoshi type has remained epidemiologically rare following the first observations in the Niigata Prefecture.10 To our knowledge, there has been only one report in the Yamagata Prefecture,11 but some retrospective or speculative cases have been reported in northeastern Japan (Akita and Fukushima prefectures.). The Shimokoshi-type tsutsugamushi disease reported here appears to be the first case reported in Western Japan. Most Japanese laboratories usually measure the antibody titers against five antigens (Kawasaki, Kuroki, Karp, Gilliam, and Kato), with the inclusion of Shimokoshi as necessary.12 Because elevation of IgM antibodies against five strains were not recognized up to the sixth day after onset of the illness, Shimokoshi titer was determined for the case described here. Other researchers have reported that the Shimokoshi strain exhibits lower virulence in mouse,10 but clinical manifestations of the Shimokoshi serotype in human appear to be similar to those of other types. The fact that this type was found in Western Japan strongly suggests that the distribution of Shimokoshi type is much wider than previously supposed. The Shimokoshi type may be endemic in the northeast of Japan, and may potentially spread beyond Hokuriku to the Kansai area. Further epidemiological studies are required.
ACKNOWLEDGMENTS
We thank Hiromi Fujita of Ohara Research Laboratory for his examination of serum titers of antibodies.
Footnotes
Financial support: This work was supported by Grant-in-Aid for Scientific Research 24591478 and a grant from The Ministry of Health, Labour and Welfare, Japan.
Authors' addresses: Satoshi Ikegaya, University of Fukui, Division of Hematology and Oncology, Fukui, Japan, E-mail: sikegaya@u-fukui.ac.jp. Hiromichi Iwasaki, University of Fukui, Division of Infection Control, Fukui, Japan, E-mail: hiwasaki@u-fukui.ac.jp. Nobuhiro Takada, University of Fukui, Senior Fellow Laboratory, Fukui, Japan, E-mail: acari@u-fukui.ac.jp. Seigo Yamamoto, Miyazaki Perfectural Institute of Public Health, Division of Bacteria, Miyazaki, Japan, E-mail: yamamoto-seigo@pref.miyazaki.lg.jp. Takanori Ueda, University of Fukui, Division of Hematology and Oncology, Fukui, Japan, E-mail: tueda@u-fukui.ac.jp.
References
- 1.Koh GC, Maude RJ, Paris DH, Newton PN, Blacksell SD. Diagnosis of scrub typhus. Am J Trop Med Hyg. 2010;82:368–370. doi: 10.4269/ajtmh.2010.09-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furuya Y, Yoshida Y, Katayama T, Yamamoto S, Kawamura A., Jr Serotype-specific amplification of Rickettsia tsutsugamushi DNA by nested polymerase chain reaction. J Clin Microbiol. 1993;31:1637–1640. doi: 10.1128/jcm.31.6.1637-1640.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajapakse S, Rodrigo C, Fernando D. Scrub typhus: pathophysiology, clinical manifestations and prognosis. Asian Pac J Trop Med. 2012;5:261–264. doi: 10.1016/S1995-7645(12)60036-4. [DOI] [PubMed] [Google Scholar]
- 4.Botelho-Nevers E, Raoult D. Fever of unknown origin due to rickettsioses. Infect Dis Clin North Am. 2007;21:997–1011. doi: 10.1016/j.idc.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Blacksell SD, Bryant NJ, Paris DH, Doust JA, Sakoda Y, Day NP. Scrub typhus serologic testing with the indirect immunofluorescence method as a diagnostic gold standard: a lack of consensus leads to a lot of confusion. Clin Infect Dis. 2007;44:391–401. doi: 10.1086/510585. [DOI] [PubMed] [Google Scholar]
- 6.Iwasaki H, Mizoguchi J, Takada N, Tai K, Ikegaya S, Ueda T. Correlation between the concentrations of tumor necrosis factor-alpha and the severity of disease in patients infected with Orientia tsutsugamushi. Int J Infect Dis. 2010;14:e328–e333. doi: 10.1016/j.ijid.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Ohashi N, Koyama Y, Urakami H, Fukuhara M, Tamura A, Kawamori F, Yamamoto S, Kasuya S, Yoshimura K. Demonstration of antigenic and genotypic variation in Orientia tsutsugamushi which were isolated in Japan, and their classification into type and subtype. Microbiol Immunol. 1996;40:627–638. doi: 10.1111/j.1348-0421.1996.tb01120.x. [DOI] [PubMed] [Google Scholar]
- 8.Kawamori F, Akiyama M, Sugieda M, Kanda T, Akahane S, Uchikawa K, Yamada Y, Kumada N, Furuya Y, Yoshida Y. Epidemiology of Tsutsugamushi disease in relation to the serotypes of Rickettsia tsutsugamushi isolated from patients, field mice, and unfed chiggers on the eastern slope of Mount Fuji, Shizuoka Prefecture, Japan. J Clin Microbiol. 1992;30:2842–2846. doi: 10.1128/jcm.30.11.2842-2846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogawa M, Ono T. Epidemiological characteristics of tsutsugamushi disease in Oita Prefecture, Japan: yearly and monthly occurrences of its infections and serotypes of its causative agent, Orientia tsutsugamushi, during 1984–2005. Microbiol Immunol. 2008;52:135–143. doi: 10.1111/j.1348-0421.2008.00024.x. [DOI] [PubMed] [Google Scholar]
- 10.Tamura A, Takahashi K, Tsuruhara T, Urakami H, Miyamura S, Sekikawa H, Kenmotsu M, Shibata M, Abe S, Nezu H. Isolation of Rickettsia tsutsugamushi antigenically different from Kato, Karp, and Gilliam strains from patients. Microbiol Immunol. 1984;28:873–882. doi: 10.1111/j.1348-0421.1984.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 11.Otani K, Kaneko A, Aoki T, Fujita H. A case report of Shimokoshi type Orientia tsutsugamushi from Yamagata Prefecture, Japan. Med Entomol Zool. 2009;60:317–321. [Google Scholar]
- 12.Furuya Y. Re-emerging infectious disease – Tsutsugamushi disease. Bull Kanagawa Ins of PH. 2012;42:1–10. [Google Scholar]