Abstract
Objectives
To determine the association of self-reported traumatic brain injury (TBI) with loss of consciousness (LOC) with late-life re-injury, dementia diagnosis and mortality.
Design
Ongoing longitudinal population-based prospective cohort study.
Setting
Seattle-area integrated health system.
Participants
4225 dementia-free individuals age 65 and older were randomly selected and enrolled between 1994 and 2010. Participants were seen every 2 years, with mean (range) follow-up of 7.4 (0–16) years. 606 (14%) participants reported a lifetime history of TBI with LOC at enrolment. 3466 participants provided information regarding lifetime history of TBI and completed at least one follow-up visit.
Main outcome measures
Self-reported TBI with LOC after study entry, incident all-cause dementia and Alzheimer’s disease (AD), and all-cause mortality.
Results
There were 25 567 person-years of follow-up. History of TBI with LOC reported at study enrolment was associated with increased risk for TBI with LOC during follow-up, with adjusted HRs ranging from 2.54 (95% CI 1.42 to 4.52) for those reporting first injury before age 25 to 3.79 (95% CI 1.89 to 7.61) for those with first injury after age 55. History of TBI with LOC was not associated with elevated risk for developing dementia or AD. There was no association between baseline history of TBI with LOC and mortality, though TBI with LOC since the previous study visit (‘recent TBI’) was associated with increased mortality (HR 2.12, 95% CI 1.62 to 2.78).
Conclusions
Individuals aged 65 or older who reported a history of TBI with LOC at any time in their lives were at elevated risk of subsequent re-injury. Recent TBI with LOC sustained in older adulthood was associated with increased risk for mortality. Findings support the need for close clinical monitoring of older adults who sustain a TBI with LOC.
Long-term health consequences of traumatic brain injury (TBI) remain poorly understood. Individuals who have sustained a TBI may be at increased risk for negative health outcomes as they age, including increased medical morbidity,1 dementia2 and early mortality.3,4 Older adults who sustain a TBI may experience particularly poor outcomes.5 There is some evidence that younger individuals who have sustained a TBI may be at elevated risk for re-injury,6 though risk for re-injury in older adults (who tend to have greater functional decline after TBI) has not been examined.
The association between TBI and risk of dementia remains controversial due to discrepant findings across studies. Two large prospective studies7,8 and one retrospective study9 did not find increased risk of dementia or Alzheimer’s disease (AD) associated with TBI. A cohort study,10 a case–control study11 and a prospective longitudinal cohort study12 each found associations between TBI and dementia. Two meta-analyses similarly report increased AD risk.13,14 The Institute of Medicine (IOM) recently concluded that TBI with loss of consciousness (LOC) is associated with increased AD risk.15 Studies that have examined the effect of apolipoprotein-E (APOE) genotype on dementia risk after TBI are similarly discordant.7,11,12,16 Additional studies have documented increased mortality rates among individuals with TBI, with average reductions in life expectancy of 4–7 years.3,4 Mortality risk may diminish over time,17 though this has not been investigated more than 1 year post-injury, and it is not known whether age at the time of injury impacts mortality risk.
The current study investigates whether lifetime history of TBI with LOC is associated with increased risk for subsequent TBI with LOC in older adulthood, AD and other dementias, and death in a large community-based sample.
METHODS
Participants
Participants were drawn from Adult Changes in Thought (ACT), an ongoing longitudinal population-based prospective cohort study of incident dementia and AD. Detailed study methods have been published.18,19 Seattle-area Group Health Cooperative (GHC) members aged 65 and older were randomly sampled, evaluated to confirm they were free of dementia (see below), and invited to enrol. A total of 4249 dementia-free older adults were enrolled in 1994–2011. Participants were excluded due to missing head injury data (n=24), leaving 4225 (99.4%) with baseline data. Of the 3466 participants with follow-up data, one was excluded due to missing education information; 620 with missing APOE data were excluded from models with APOE. The research protocol for this study was approved by GHC and University of Washington institutional review boards.
Study design
At all study visits, participants were interviewed by trained professionals with structured questionnaires to obtain demographic characteristics and lifetime (baseline) or interval (follow-up) medical history, including TBI with LOC (see below). Participants completed standardised cognitive assessments and a blood draw for APOE genotyping. Participants were followed prospectively and re-evaluated every 2 years.
Every visit included a structured interview that addressed participants’ history of physical injuries, including specifically whether they had sustained an injury to the head that resulted in loss of consciousness. Participants were asked at baseline and follow-up, ‘Have you ever had an injury so severe that you lost consciousness?’ If an injury was reported, participants were further queried to determine whether the injury was a head injury or other type of injury (eg, near drowning, electric shock), and the age at which this injury was sustained. Data were not collected on injury severity. Structured interviews were also conducted at baseline and follow-up to assess activities of daily living (ADLs). Subjects were asked whether they had any difficulty walking around a house, bathing or taking shower, dressing themselves, getting out of a bed or chair, feeding themselves, and using a toilet. Participants who reported any such difficulty were asked how much difficulty they had, and responses were coded on a 4-point scale: 0 (no difficulty), 1 (some difficulty), 2 (a lot of difficulty), and 3 (unable to do the activity). Responses to six items were summed to create an ADL total score.
Main outcome measures
Outcomes of interest included incident late life TBI or re-injury (recurrent TBI), incident all-cause dementia and AD, and all-cause mortality. Late life injury or re-injury was defined as incident TBI with LOC after baseline.
Methods for identifying incident dementia cases have been described elsewhere.18,19 Briefly, individuals were screened with the Cognitive Abilities Screening Instrument (CASI),20 a 100-point brief assessment of attention, concentration, orientation, memory, language, visual construction, verbal fluency and judgement that has favourable sensitivity for measuring cognitive decline compared to commonly used cognitive screening tools such as the Mini-Mental State Examination.20 Individuals with CASI20 scores <86 or symptoms suggesting onset of dementia underwent a standardised diagnostic evaluation, including physical and neurological examinations by a neurologist, geriatrician or internist, a 1-h battery of neuropsychological tests, and a battery of laboratory tests and brain imaging.18,19 All-cause dementia diagnoses were determined at consensus conferences using the Diagnostic and statistical manual of mental disorders, 4th edition (DSM-IV) criteria.21 Probable and possible AD diagnoses were determined using National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association (NINCDS–ADRDA) criteria.22 Participants with low baseline CASI scores but no dementia were enrolled in the study. The ACT study does not formally assess for mild cognitive impairment. These procedures were repeated every 2 years to identify incident dementia and AD cases. Dementia and AD rates are consistent with published data.18
Vital status was sought for participants who did not appear for study visits by checking group health records, local obituaries and informant contacts. Death dates were available for decedents.
Covariates of interest included age, self-reported years of education, sex, and presence of one or more copies of the APOE ε4 allele.18
Statistical analyses
We used Stata V.12.0 for all analyses.23 The differences between participants with and without follow-up data were examined in a logistic regression model that included baseline age, education, gender, race, APOE, and CASI and ADL scores. We initially modelled time to incident outcomes with Cox proportional hazards models. We censored analyses at last follow-up for the TBI with LOC, dementia and AD analyses, and at last known date alive for the mortality analysis. We adjusted all models for age (continuous), sex, and education (continuous), and report adjusted results. We used graphical and statistical tests based on Schoenfeld residuals to evaluate the proportional hazards assumption. We evaluated the functional form of continuous variables using Martingale residuals. To assess overall model fit and outlier effects, we used Cox–Snell and deviance residuals and measures of influence that assess how each coefficient is changed by deleting each observation. For re-injury models, we chose age categories for previous TBI with LOC of <25 years, 25–54 years, and 55-baseline; cut-offs of 25 and 55 were based on our understanding of brain development and cognitive decline, and also on previous literature classifying TBI by age group.6,24 For dementia models, we included direct effects and interactions for TBI and presence of ≥APOE ε4 allele.
The proportional hazards assumption was violated for dementia models. We compared a variety of parametric models on the basis of their Akaike Information Criterion values. The best of these were loglogistic, reflecting increased risk over time. For these models we report time ratios. Ratios >1 indicate factors associated with longer survival. Some analyses examined combined effects of lifetime TBI with LOC reported at baseline and ‘recent TBI with LOC’ defined as TBI with LOC reported at the most recent follow-up visit (2 years prior). We combined risk groups due to sparse data. Final groupings included: (a) recent TBI but no TBI prior to baseline; (b) TBI before age 25 and recent TBI; (c) TBI before age 25 but no recent TBI; (d) TBI between age 25 and baseline, with or without recent TBI; and (e) no reported TBI (reference category). All final survival models had acceptable fit and negligible outlier effects.
We used 2000 bootstrap samples to generate population attributable risks (PAR) with 95% CIs. We used 2-sided p values of 0.05 for significance.
RESULTS
The mean (SD) enrolment age was 74.9 (6.4) years, and 59% were female (see table 1). As in many longitudinal studies of aging, those without follow-up were more likely to be male and older, and had higher CASI scores (p<0.05). Individuals with and without follow-up data did not differ significantly by race or APOE status. Those missing APOE data were younger, more highly educated, and more likely to be female. Of 4225 participants with exposure data, 606 (14%) reported a history of TBI with LOC at baseline. Rates of TBI reported here are consistent with those reported using similar methods,10,7 but higher than Centers for Disease Control estimates of 2–3% which omit individuals who are not treated in hospitals or emergency rooms.25 Of the 759 people with only baseline data (no follow-up), 125 (16%) reported a history of TBI.
Table 1.
Demographic characteristics of entire baseline cohort, stratified by self-reported TBI with LOC before and after baseline, compared with people who never self-reported TBI with LOC*
| Characteristics | No TBI with LOC either before or after baseline (n=3553)† | TBI with LOC before baseline but not after (n=576)‡ | TBI with LOC after baseline but not before (n=66) | TBI with LOC both before and after baseline (n=30) | Total (n=4225)§ |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | |
| Age (years) | |||||
| 65–69 | 874 (25) | 135 (23) | 10 (15) | 6 (20) | 1025 (24) |
| 70–79 | 1798 (51) | 321 (56) | 40 (61) | 16 (53) | 2175 (52) |
| 80–89 | 800 (23) | 111 (19) | 12 (18) | 8 (27) | 931 (22) |
| 90+ | 81 (2) | 9 (2) | 4 (6) | 0 (0) | 94 (2) |
| Female sex | 2196 (62) | 242 (42) | 46 (70) | 11 (37) | 2495 (59) |
| Education¶ | |||||
| <12 years | 399 (11) | 57 (10) | 8 (12) | 4 (13) | 468 (11) |
| 12 years | 841 (24) | 117 (20) | 19 (29) | 5 (17) | 982 (23) |
| ≥13 years | 2312 (65) | 402 (70) | 39 (59) | 21 (70) | 2774 (66) |
| Self-reported race | |||||
| White | 3209 (90) | 541 (94) | 62 (94) | 30 (100) | 3842 (91) |
| Black | 154 (4) | 16 (3) | 0 (0) | 0 (0) | 982 (23) |
| Asian-American | 131 (4) | 11 (2) | 2 (3) | 0 (0) | 144 (3) |
| Other or mixed | 54 (2) | 8 (1) | 2 (3) | 0 (0) | 64 (2) |
| Presence of ≥1 copies of APOE ε4 allele (n=3148) | 684 (26) | 91 (22) | 15 (28) | 4 (15) | 794 (25) |
| Baseline ADL | |||||
| 0 | 2777 (78) | 436 (76) | 49 (74) | 22 (73) | 3284 (78) |
| 1–2 | 450 (13) | 80 (14) | 7 (11) | 4 (13) | 541 (13) |
| 3–6 | 232 (7) | 40 (7) | 7 (11) | 2 (7) | 281 (7) |
| 7–24 | 83 (2) | 16 (3) | 3 (5) | 2 (7) | 104 (2) |
| Baseline CASI | |||||
| <86 | 255 (7) | 31 (5) | 3 (5) | 1 (3) | 290 (7) |
| 86–92.9 | 1040 (30) | 157 (27) | 20 (32) | 5 (17) | 1222 (29) |
| 93–100 | 2199 (63) | 387 (67) | 40 (63) | 23 (79) | 2649 (64) |
Post-baseline TBI with LOC status is not known for 759 individuals who were lost to follow-up; 259 of those lost to follow-up are known to have subsequently died.
Includes 634 people with no follow-up data.
Includes 125 people with no follow-up data.
Educational history is missing for one individual in the ‘no TBI-LOC’ group.
Data from the 321 participants who were missing injury data were excluded from the table.
ADL, activities of daily living; APOE ε4, apolipoprotein-E ε4 allele; CASI, Cognitive Abilities Screening Instrument; LOC, loss of consciousness; TBI, traumatic brain injury.
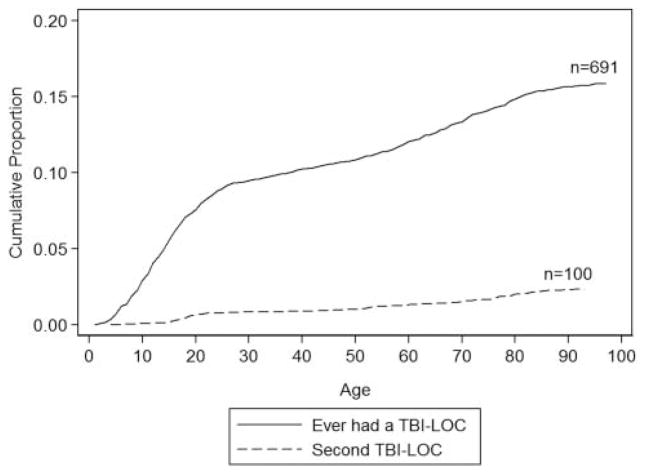
Figure 1 shows a plot of the cumulative incidence of first and second lifetime self-reported TBI with LOC. The steepest period of incidence was in the childhood and teenage years.
Figure 1.
Cumulative incidence of first and second head injury with loss of consciousness (LOC) reported at baseline or follow-up. TBI, traumatic brain injury.
Late life injury or re-injury
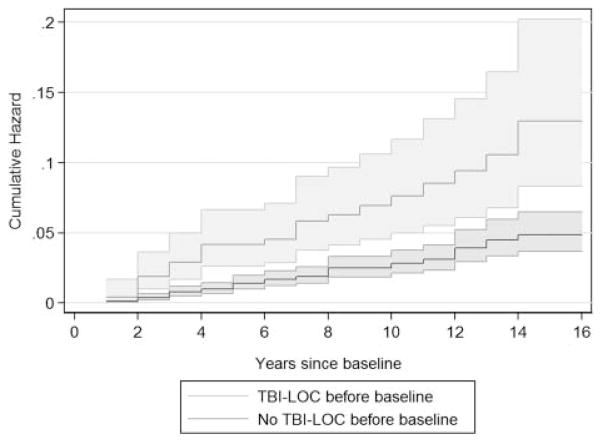
There were 3465 people with complete data (other than APOE) and at least one follow-up visit (82% of the total sample presented in table 1). There were 25 567 person-years of follow-up data, with a mean (SD, range) per person of 7.4 (4.2, 0.9–16.1) years. Ninety-six people reported at least one TBI with LOC after baseline, yielding an incidence rate of 3.8 per 1000 person-years. Of these, 30 were re-injuries. The incidence rates for people with and without a history of TBI with LOC were 8.3 and 3.0 per thousand person-years, respectively (see figure 2 and table 2). We found strong associations with lifetime re-injury regardless of reported age at initial injury. Nearly 20% of the population risk of TBI with LOC after baseline was attributable to baseline history of TBI with LOC.
Figure 2.
Nelson–Aalen cumulative hazard estimates for traumatic brain injury (TBI) with loss of consciousness (LOC) reported at follow-up visits, with 95% confidence bands.
Table 2.
Baseline report of age at first TBI with LOC as a predictor of TBI with LOC after study enrolment, controlling for age, sex and years of education, with the PAR
| Age at first TBI with LOC | Late life TBI with LOC cases/person years | HR (95% CI) | PAR (95% CI) |
|---|---|---|---|
| None prior to baseline | 66/21945 | 1 (reference) | – |
| <25 | 15/2147 | 2.54 (1.42 to 4.52) | 9.4% (2.8 to 15.4) |
| 25–54 | 6/678 | 3.24 (1.40 to 7.52) | 3.9% (0.5 to 8.0) |
| 55-baseline | 9/798 | 3.79 (1.89 to 7.62) | 6.7% (1.3 to 12.3) |
LOC, loss of consciousness; PAR, population attributable risk; TBI, traumatic brain injury.
The risk of late-life re-injury was highest among people who sustained a TBI with LOC later in adulthood and thus closer to study baseline (log-rank test for trend p<0.0001). Nevertheless, the population attributable risk was largest for those with TBI with LOC reported during childhood or young adulthood. The association between baseline TBI history and late-life re-injury was not confounded by cognition as measured by baseline CASI score or by functional disability as measured by ADLs, and we did not find any evidence of effect modification by either CASI or ADL scores.
Associations with dementia and AD
Among our sample of 3465 people, there were 592 incident cases of DSM-IV all-cause dementia, and 510 incident cases of NINCDS-ADRDA probable or possible AD. Baseline history of TBI with LOC was not associated with risk for incident dementia or incident probable or possible AD, regardless of age of reported TBI with LOC (table 3). There was no indication of an interaction between APOE ε4 alleles and history of TBI with LOC in increasing dementia (likelihood ratio test p=0.60) or probable or possible AD (likelihood ratio test p=0.82) risk.
Table 3.
Time ratios baseline report of TBI with LOC for any dementia and Alzheimer’s disease, adjusting for age, sex, education and APOE ε4
| Age at first TBI with LOC | TR (95% CI) |
|---|---|
| Any dementia | |
| <25 | 1.02 (0.87 to 1.20) |
| 25–54 | 1.04 (0.78 to 1.38) |
| 55-baseline | 1.06 (0.81 to 1.39) |
| Probable or possible AD | |
| <25 | 0.99 (0.84 to 1.15) |
| 25–54 | 1.01 (0.76 to 1.34) |
| 55-baseline | 1.15 (0.86 to 1.53) |
Time ratios indicate the ratio of the time to dementia or AD in the given group, compared to those with no TBI with LOC; time ratios greater than one correspond to longer time to dementia or AD.
APOE ε4, apolipoprotein-E ε4 allele; AD, Alzheimer’s disease; LOC, loss of consciousness; TBI, traumatic brain injury; TR, time ratio.
Individuals who reported a recent TBI (mean (SD) age of injury 82.0 (6.8) years) were not at significantly increased risk for dementia. The adjusted time ratio (aTR) was 0.87 (95% CI 0.60 to 1.27). An aTR of 0.87 would indicate a 13% shorter mean time to dementia for those with recent TBI; however this difference was not statistically significant. Recent TBI did not influence AD risk, with an aTR of 0.95 (95% CI 0.65 to 1.38), and there was no indication of an interaction between recent TBI and APOE genotype for AD risk (table 4). There was a possible interaction between APOE genotype and recent TBI in increasing all-cause dementia risk (p=0.08) (table 4).
Table 4.
Time ratios and population attributable risks for any dementia and Alzheimer’s disease for recent TBI with LOC, stratified by APOE ε4 genotype†
| No APOE ε4
|
≥1 APOE ε4
|
|||
|---|---|---|---|---|
| TR (CI) | PAR (CI) | TR (CI) | PAR (CI) | |
| Any dementia | ||||
| No recent TBI-LOC | 1 (Ref) | – | 0.69 (0.63 to 0.76) | 17.3 (12.6 to 21.8) |
| Recent TBI-LOC | 1.15 (0.69 to 1.91) | −0.2 (−1.6 to 0.5) | 0.50 (0.23 to 1.10)* | 0.8 (0.2 to 1.6) |
| Probable or possible AD | ||||
| No recent TBI-LOC | 1 (Ref) | – | 0.71 (0.64 to 0.78) | 18.0 (13.0 to 22.7) |
| Recent TBI-LOC | 1.07 (0.66 to 1.73) | −0.1 (−1.4 to 0.6) | 0.70 (0.32 to 1.54) | 0.5 (0.1 to 1.1) |
Time ratios indicate the ratio of the time to dementia or AD in the given group, compared to those with no recent TBI with LOC and no APOE ε4; time ratios greater than one correspond to longer time to dementia or AD.
p=0.08.
Hazard models are adjusted for age, age-squared, gender, and education.
APOE ε4, apolipoprotein-E ε4 allele; AD, Alzheimer’s disease; LOC, loss of consciousness; PAR, population attributable risk; TBI, traumatic brain injury; TR, time ratio.
Fifteen people reported sustaining a TBI with LOC before age 25 and re-injury at the most recent visit. Of these, six became demented, with an estimated time to dementia of 57% of those who never had a TBI (aTR 0.57, 95% CI 0.31 to 1.06; p=0.075). The other combinations of early and late-life TBI with LOC (listed in the Statistical analyses section) had aTR values close to one.
Associations between lifetime TBI with LOC and mortality
Median (SD, range) follow-up time for mortality analyses was 8.2 (4.9, 0.0–16.6) years, during which there were 1620 deaths. A history of TBI with LOC at baseline was not associated with increased mortality risk, regardless of age at injury, with an adjusted HR of 1.03 (95% CI 0.90 to 1.19). However, individuals with recent TBI were at increased risk for mortality, with an adjusted HR of 1.97 (95% CI 1.51 to 2.58).
DISCUSSION
We found that lifetime history of TBI with LOC reported at study entry was associated with increased risk for subsequent TBI with LOC, particularly among individuals whose first TBI with LOC was sustained after age 55. History of TBI with LOC was not associated with elevated risk for developing subsequent dementia or probable or possible AD. We did not find an association between history of TBI with LOC reported at baseline and mortality. By contrast, report of a TBI with LOC at the previous study visit (‘recent TBI’) was associated with increased mortality.
Limitations to the current study should be considered carefully. Study participants were alive and non-demented at enrolment (age 65 and older), so individuals who died due to severe injuries or who had already developed dementia were not enrolled. Data on gait instability and Parkinsonian features were not routinely collected. It should also be noted that only TBI with LOC was recorded; TBIs not resulting in LOC (ie, very mild TBIs) were not assessed and thus could not be analysed. The TBIs recorded in the current study likely include many mild TBIs that fall on the more severe end of the ‘mild’ spectrum, in addition to moderate and severe TBIs. Unfortunately the data available in the current study do not allow for more refined categorisations of injury severity. We were unable to study the dose effects of trauma severity or multiple recurrent traumas. Those with severe or frequent traumas may be at higher risk. The results of this study are best generalised to the population we studied: individuals who are over the age of 65, have health insurance coverage, are alive, and dementia-free. Unmeasured confounders and residual confounding could be present as they could be in any observational study.
Several methodological strengths of the study should also be considered. This prospective population-based study contains 25 569 person-years of follow-up data, which far exceeds the follow-up data available in the largest study we are aware of that reported no increased risk of dementia associated with TBI history (13 377 person-years)7 and also exceeds that of the European Community Concerted Action on the Epidemiology and Prevention of dementia (EURODEM) pooled analyses.26 Exposure to TBI with LOC was collected prior to onset of dementia in the current study, minimising (but not eliminating) the effects of recall bias. History of TBI with LOC was elucidated in a structured interview format referencing only injuries with LOC, maximising exposure recall. The current study utilised strict diagnostic criteria and consensus-based diagnoses for dementia and AD,22 unlike some previous studies.9 These design features, which include excellent exposure data and research-quality diagnoses of incident dementia cases, protect the current study from the prevalence/incidence bias that can inflate exposure–outcome relationships in cross-sectional or case–control studies.27,28
To our knowledge, this is the first known prospective population-based study to quantify the risk of self reported later life re-injury among survivors of TBI with LOC. One early study found a threefold greater risk for subsequent TBI among individuals who were hospitalised for TBI and survived the first injury.6 Repeated head injury is typically discussed in the context of sports concussion among athletes, who are at high risk of re-injury due to environmental factors on return to play. Documentation of recurrent head injury in non-athletes is limited, and the factors associated with vulnerability to re-injury are poorly understood.29 One study reported that risk for subsequent TBI is greatest (ie, five times greater risk of re-injury) among individuals who sustain their first injury after age 25, and that after sustaining two TBIs the risk rises to eight times that seen in the uninjured population.6 The current study specifically examined the risk of re-injury in older adulthood (after the age of 65) when TBI is known to have particularly detrimental and lasting effects.5,30,5 We found a 3.8-fold greater risk of re-injury after study enrolment among individuals who sustained a TBI with LOC after age 55. While vulnerability to re-injury could be attributable to external/environmental factors (ie, hazardous home environment), lifestyle choices (ie, alcohol use, choice of leisure time activities or profession), biologically-based vulnerability to otherwise insignificant injury,27 age-related changes or vulnerabilities, or some combination of these factors, the greater risk for re-injury with increasing age in the current study may suggest that age-related factors play a role. Previous research indicates that older adults tend to recover more slowly and have more lasting disability relative to younger people,5 and it is possible that injury-related physical impairments (imbalance, dizziness) and cognitive changes (impulsivity, inattention) make these individuals more vulnerable to subsequent injury. We suspect that the aging human brain is less able to recover and may sustain injury related brain changes which are more consequential later in life.
We did not find an association between TBI with LOC and increased risk of developing dementia, and did not find an interaction between carrying the APOE ε4 allele and risk for dementia or AD associated with self-reported lifetime history of TBI. These findings are consistent with results of the large EURODEM pooled analysis of four European population-based studies of incident AD and TBI with LOC,26 though duration of follow-up in the current study far exceeds that of the EURODEM studies (mean (SD) follow-up of 2.24 (0.73) years). Our results are not entirely consistent with the conclusions of the recent report from the IOM, which state that there is evidence of an association between moderate or severe TBI and dementia, and limited/suggestive evidence of an association between mild TBI with LOC and dementia.15 The IOM report did not include three published studies which did not report an association of TBI and dementia.7,8,9 Also in contrast to previous research,3,4 the current study did not find an association between baseline history of TBI with LOC and mortality in late life.
Our results do suggest that advanced age at injury is associated with negative outcomes. In particular, individuals who reported recent TBI had twice the mortality rate of people who did not (see last sentence of Results section), and those with the APOE ε4 allele who report a recent TBI may be diagnosed with dementia twice as soon (see table 4). These results are consistent with one prior study31 and previous findings that older adults who sustain a TBI tend to have poorer outcomes and are more likely to experience significant decline post-injury as compared to younger adults.5 These findings suggest a model of increased mortality risk for TBI in the years following the event. However, if one survives to late life, there was no subsequent relationship between lifetime TBI and mortality risk. There was some indication in the current study that individuals who sustain both early and late-life TBIs may be at elevated risk of becoming demented. While this is the largest prospective cohort study of TBI with LOC ever reported in the literature, statistical power for this analysis was somewhat limited due to the small number of people with both early and late-life TBI in this study. Much of the research on long-term outcomes of multiple head injuries has been limited to athletes.32 Whether re-injury elevates dementia risk among non-athletes who generally sustain far fewer repeat injuries than athletes is less certain. Research in the sports concussion literature suggests that post-injury neurochemical changes can create biological vulnerability to the effects of subsequent injury,27 and both the duration of this potential vulnerability and the extent to which this applies to non-athletes warrant further study.
In summary, in our study of individuals from a community based population of persons over age 65 who did not have dementia at baseline, individuals who report having sustained a TBI with LOC during their lifetime were at increased risk for sustaining a subsequent TBI, and this risk was particularly high among individuals who reported a TBI with LOC later in life. Risk for the development of dementia or mortality was not significantly elevated compared to individuals who had not sustained a TBI with LOC, though mortality risk was greater for individuals who sustained a TBI with LOC during follow-up. This suggests that the risk for negative long-term outcomes (eg, dementia and premature mortality) may decrease with time since injury, such that individuals who survive to older adulthood and do not incur subsequent TBI may be at no greater risk for dementia or mortality than individuals who never sustained a TBI. Individuals who experience multiple TBI events may have a different dementia risk. Overall, the findings reported here underscore the need for effective strategies to prevent injury and re-injury in older adulthood.
Acknowledgments
Funding This project was supported by Grant Number #U01 AG006781 from the National Institute on Aging (NIA) of the National Institutes of Health (NIH) and Grant Number #R24 HD065702 from the National Institutes of Health (NIH). The NIH did not play any role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The authors submit this manuscript independent from the funding source.
Footnotes
Contributors KDO designed the study, wrote the statistical analysis plan, interpreted the data, drafted and revised the paper. LEG cleaned and analysed the data, and drafted and revised the paper. She is guarantor. JDB participated in data collection and revised the draft paper. SMM participated in data collection and revised the draft paper. EBL designed the study, designed data collection protocols, monitored data collection for the whole trial, and revised the paper. PKC wrote the statistical analysis plan, interpreted the data, drafted and revised the paper.
Ethics approval Institutional Review Board, University of Washington.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Statistical code available from the corresponding author at kristen.dams-o’connor@mountsinai.org. Consent for data sharing was not obtained from study participants but the presented data are anonymised and risk of identification is low.
Competing interests EBL and PKC received support from NIH/NIA for the submitted work. EBL is employed by Group Health Research Institute where the Adult Changes in Thought Study is run, and receives royalties from book chapters.
References
- 1.Masel BE, DeWitt DS. Traumatic brain injury: a disease process, not an event. J Neurotrauma. 2010;27:1529–40. doi: 10.1089/neu.2010.1358. [DOI] [PubMed] [Google Scholar]
- 2.Lye TC, Shores EA. Traumatic brain injury as a risk factor for Alzheimer’s disease: a review. Neuropsychol Rev. 2000;10:115–29. doi: 10.1023/a:1009068804787. [DOI] [PubMed] [Google Scholar]
- 3.Harrison-Felix C, Whiteneck G, DeVivo M, et al. Mortality following rehabilitation in the traumatic brain injury model systems of care. Neuro Rehabilitation. 2004;19:45–54. [PubMed] [Google Scholar]
- 4.Harrison-Felix C, Whiteneck G, Devivo MJ, et al. Causes of death following 1 year postinjury among individuals with traumatic brain injury. J Head Trauma Rehabil. 2006;21:22–33. doi: 10.1097/00001199-200601000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Marquez de la Plata CD, Hart T, Hammond FM, et al. Impact of age on long-term recovery from traumatic brain injury. Arch Phys Med Rehabil. 2008;89:896–903. doi: 10.1016/j.apmr.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Annegers JF, Grabow JD, Kurland LT, et al. The incidence, causes, and secular trends of head trauma in Olmsted County, Minnesota, 1935–1974. Neurology. 1980;30:912–9. doi: 10.1212/wnl.30.9.912. [DOI] [PubMed] [Google Scholar]
- 7.Mehta KM, Ott A, Kalmijn S, et al. Head trauma and risk of dementia and Alzheimer’s disease: the rotterdam study. Neurology. 1999;53:1959–62. doi: 10.1212/wnl.53.9.1959. [DOI] [PubMed] [Google Scholar]
- 8.Katzman R, Aronson M, Fuld P, et al. Development of dementing illnesses in an 80-year-old volunteer cohort. Ann Neurol. 1989;25:317–24. doi: 10.1002/ana.410250402. [DOI] [PubMed] [Google Scholar]
- 9.Williams DB, Annegers JF, Kokmen E, et al. Brain injury and neurologic sequelae: a cohort study of dementia, parkinsonism, and amyotrophic lateral sclerosis. Neurology. 1991;41:1554–7. doi: 10.1212/wnl.41.10.1554. [DOI] [PubMed] [Google Scholar]
- 10.Schofield PW, Tang M, Marder K, et al. Alzheimer’s disease after remote head injury: an incidence study. J Neurol Neurosurg Psychiatry. 1997;62:119–24. doi: 10.1136/jnnp.62.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Meara ES, Kukull WA, Sheppard L, et al. Head injury and risk of Alzheimer’s disease by apolipoprotein E genotype. Am J Epidemiol. 1997;146:373–84. doi: 10.1093/oxfordjournals.aje.a009290. [DOI] [PubMed] [Google Scholar]
- 12.Plassman BL, Havlik RJ, Steffens DC, et al. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology. 2000;55:1158–66. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- 13.Fleminger S, Oliver DL, Lovestone S, et al. Head injury as a risk factor for Alzheimer’s disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry. 2003;74:857–62. doi: 10.1136/jnnp.74.7.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mortimer JA, van Duijn CM, Chandra V, et al. Head trauma as a risk factor for Alzheimer’s disease: a collaborative re-analysis of case-control studies. EURODEM risk factors research group. Int J Epidemiol. 1991;20(Suppl 2):S28–35. doi: 10.1093/ije/20.supplement_2.s28. [DOI] [PubMed] [Google Scholar]
- 15.Institute of Medicine Committee on Gulf War Health. Long-term consequences of traumatic brain injury. Gulf War and Health. 2009;7:197–264. [Google Scholar]
- 16.Mayeux R, Ottman R, Maestre G, et al. Synergistic effects of traumatic head injury and apolipoprotein-epsilon 4 in patients with Alzheimer’s disease. Neurology. 1995;45:555–7. doi: 10.1212/wnl.45.3.555. [DOI] [PubMed] [Google Scholar]
- 17.Ventura T, Harrison-Felix C, Carlson N. Mortality after discharge from acute care hospitalization with traumatic brain injury: a population-based study. Arch Phys Med Rehabil. 2010;91:21. doi: 10.1016/j.apmr.2009.08.151. [DOI] [PubMed] [Google Scholar]
- 18.Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002;59:1737–46. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- 19.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 20.Teng EL, Hasegawa K, Homma A, et al. The cognitive abilities screening instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994;6:45–58. doi: 10.1017/s1041610294001602. discussion 62. [DOI] [PubMed] [Google Scholar]
- 21.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press, Inc; 2000. Text Revision. [Google Scholar]
- 22.McKhann GM, Drachman D, Folstein MF. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS/ADRDA work group under the auspices of the department of health and human services task force on Alzheimer’s disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 23.StataCorp LP. Stata statistical software: Release 12. StataCorp LP; College Station, TX: 2011. [Google Scholar]
- 24.Thompson HJ, Weir S, Rivara FP, et al. Utilization and costs of health care after geriatric traumatic brain injury. J Neurotrauma. 2012;29:1864–71. doi: 10.1089/neu.2011.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faul M, Xu L, Wald MW, et al. Traumatic brain injury in the United States: Emergency department visits, hospitalizations, and deaths 2002–2006. Atlanta, GA, USA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- 26.Launer LJ, Andersen K, Dewey ME, et al. Rates and risk factors for dementia and Alzheimer’s disease: results from EURODEM pooled analyses. EURODEM incidence research group and work groups. European studies of dementia. Neurology. 1999;52:78–84. doi: 10.1212/wnl.52.1.78. [DOI] [PubMed] [Google Scholar]
- 27.GCC, Hovda DA. The neurometabolic cascade of concussion. J Athl Train. 2001;36:228–35. [PMC free article] [PubMed] [Google Scholar]
- 28.Hill G, Connelly J, Hebert R, et al. Neyman’s bias re-visited. J Clin Epidemiol. 2003;56:293–6. doi: 10.1016/s0895-4356(02)00571-1. [DOI] [PubMed] [Google Scholar]
- 29.Salcido R, Costich JF. Recurrent traumatic brain injury. Brain Inj. 1992;6:293–8. doi: 10.3109/02699059209029671. [DOI] [PubMed] [Google Scholar]
- 30.Ponsford J, McLaren A, Schonberger M, et al. The association between apolipoprotein E and traumatic brain injury severity and functional outcome in a rehabilitation sample. J Neurotrauma. 2011;28:1683–92. doi: 10.1089/neu.2010.1623. [DOI] [PubMed] [Google Scholar]
- 31.Luukinen H, Viramo P, Herala M, et al. Fall-related brain injuries and the risk of dementia in elderly people: a population-based study. Eur J Neurol. 2005;12:86–92. doi: 10.1111/j.1468-1331.2004.00953.x. [DOI] [PubMed] [Google Scholar]
- 32.Collins MW, Grindel SH, Lovell MR, et al. Relationship between concussion and neuropsychological performance in college football players. JAMA. 1999;282:964–70. doi: 10.1001/jama.282.10.964. [DOI] [PubMed] [Google Scholar]