Abstract
Congenital adrenal hyperplasia (CAH) refers to group of inherited diseases resulting from impaired adrenal steroidogenesis, and its most common cause is 21-hydroxylase deficiency. Testicular adrenal rest tumors (TARTs) are an important complication of CAH, which probably develop from ectopic remnants of intra-testicular adrenal tissue stimulated by Adrenocorticotropic hormone (ACTH) hypersecretion. These lesions are typically located within the rete testis and are bilateral, synchronous, nodular and multiple. TART usually, but not always, responses to suppressive medical therapy. TART leads to testicular structural damage, spermatogenesis disorders, infertility and most importantly, mass-forming lesions that could be mistaken for Leydig cell tumor (LCT). The later has a significantly different behavior with up to 10% of being malignant. Nowadays, due to advances in diagnosing and treating CAH, mass-forming TART is rarely encountered. As a result, there is the paucity in the medical literature regarding its features from pathological perspective. We herein present a case of mass-forming TART and we discuss the clinical, radiological, and morphological features as well as the major differential diagnosis of this rare lesion.
Keywords: Leydig cell tumor, testicular adrenal rest tumor, testicular mass
INTRODUCTION
Testicular adrenal rest tumors (TARTs) rarely present as a clinically palpable mass and usually related to lack of medical therapy response.[1] They are almost always benign but because of their presentation as a mass lesion, tissue biopsy and even surgical removal may be performed to exclude a malignant disease.
Congenital adrenal hyperplasia (CAH) patients with testicular enlargement present a clinical and pathological diagnostic challenge. TART, Leydig cell tumors (LCTs), and Leydig cell hyperplasia are the primary etiologic considerations.[2] Distinction is extremely important as the therapeutic approach as well as the prognosis differ.
We report a case of bilateral synchronous TART. The case was a diagnostic dilemma in which the distinction between LCT and TART was extremely difficult.
CASE REPORT
A 15-year-old male known to have the CAH salt-wasting type diagnosed earlier in childhood. He presented with synchronous and progressive enlargement of bilateral testicular masses during substitutive medical therapy.
On examination, he had normal stature (body mass index: 21.2 kg/m2). Genital examination demonstrated firm painless 8 cm mass involving the right testis and 10 cm mass involving the left testis.
Semen analyses revealed azoospermia. Serum hormonal screening showed high 17-OH progesterone; 24.1 nmol/L (1.5-6.4 nmol/L).
Serum tumor markers were low; α-fetoprotein was 1 ng/mL (0-44 ng/mL); β-human chorionic gonadotrophin was <12 IU/L (<5 IU/L).
Testicular ultrasound examination confirmed the presence of bilateral hyperechogenic hypervascularized lesion; right: 8.6 cm × 3.5 cm; left: 8.4 cm × 5.5 cm [Figure 1]. These features although non-specific, raised the possibility of a testicular neoplasm.
Figure 1.
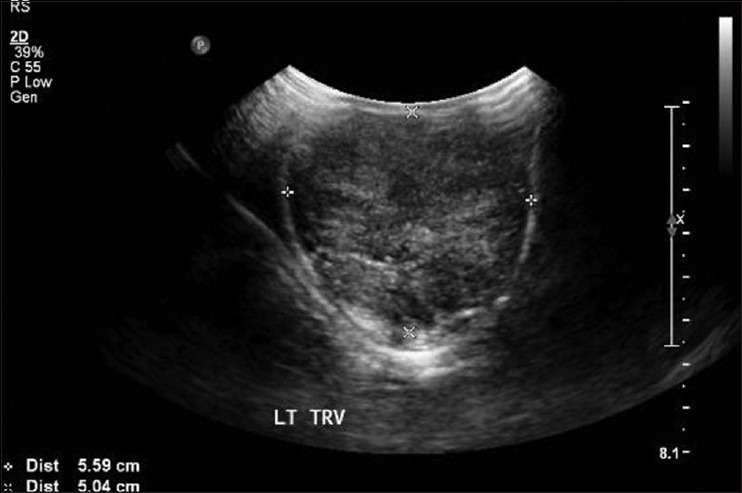
Testicular ultrasound: The testis is large in size with heterogeneous echogenicity and poorly visualized outline
Considering the possibility of malignant testicular neoplasms, a surgical intervention was scheduled.
At surgery, bilateral large firm, lobulated masses were replacing the testicular tissue. The patient underwent bilateral testicular incisional biopsies and the specimens were sent to our department for intra-operative consultation.
Macroscopically, both specimens (2.5 cm from the right testis and 1.8 cm from the left testis) appeared light brown with lobular rubbery cut surface. Frozen sections from each were examined. Microscopic examination showed a complete replacement of the normal testicular tissue by sheets and nests of large round and polygonal cells with defined cell borders, abundant eosinophilic cytoplasm and round central nuclei. Nests of cells were separated by dense fibrous tissue [Figure 2]. The morphological features were of “LCT-like” lesion! Nevertheless, Reinke crystals were not seen. At this point, the differential diagnosis was either LCT or mass-forming TART.
Figure 2.
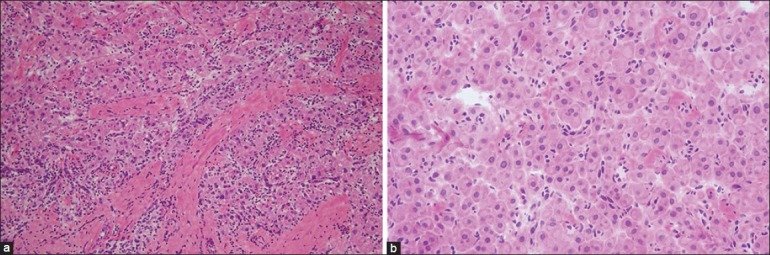
Frozen sections: (a: left) The lesion was composed of sheets and nests separated by dense fibrous tissue (H and E, ×200), (b: right) The individual cells were large round and polygonal cells with defined cell borders, abundant eosinophilic cytoplasm and round central nuclei (H and E, ×400)
The clinical picture of CAH and the bilaterality of the lesions were more compatible with the TART. However, rapid enlargement and the destruction of the testicular parenchyma implying irreversible damage together with worrisome atypical ultrasound features were in keeping with LCT. Two known TART characteristic features if present could strengthen its probability: Hilar location and evidence of response to suppressive therapy. In our case, the lesions occupied the entire testis without definitive hilar location. In addition, no suppressive therapy was tried; the patient was on substitutive therapy when operated.
Being indistinguishable from TART on H and E sections, LCT was difficult to exclude. The pathologic frozen section diagnosis was of benign neoplasm.
A decision of bilateral orchiectomy was made and the specimen was sent to our department for permanent examination.
Macroscopically, the lesions were bilateral involving almost the entire testes separated into lobules with prominent fibrous bands [Figure 3].
Figure 3.
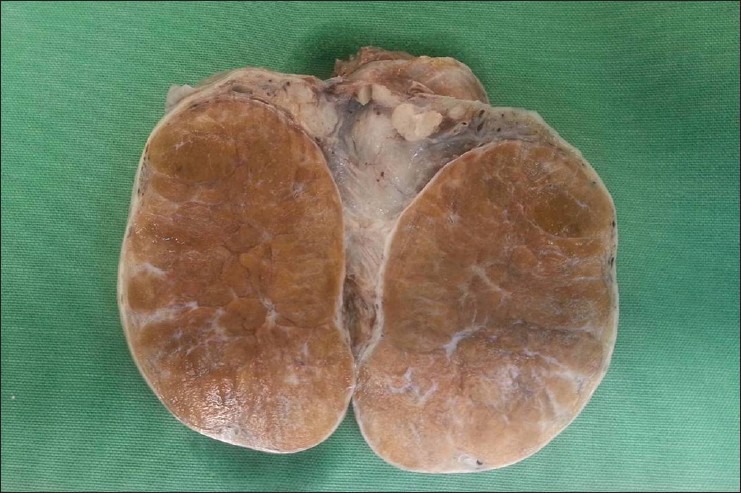
Gross evaluation of orchiectomy specimen. Cut surface revealing a well-circumscribed, non-capsulated, solid, and lobulated brown lesion
Microscopically, no normal testicular tissue was identified. The lesions were composed of sheets, nests and cords of polygonal cells with abundant eosinophilic cytoplasm. Few of these cells contained lipochrome pigments, but Reinke's crystals were absent. There was mild nuclear pleomorphism without mitotic activity. Some lymphoid aggregates were seen [Figure 4]. Immunohistochemical analyses were performed [Figure 4]. The polygonal cells showed positivity to four markers known to be shared between LCT and TART namely inhibin alpha, melan A, vimentin, and calretinin. However, the cells showed a distinguishing immuno-profile of TART: Intense and widespread staining for CD56, patchy yet strong staining for synaptophysin and non-reactivity to CD10.
Figure 4.
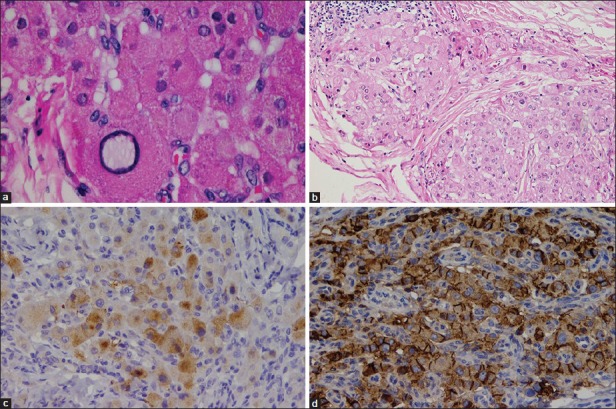
Permanent histopathological and immunohistochemical evaluation: (a: upper left) Cells contain lipochrome pigments and show mild nuclear pleomorphism with absence of Reinke crystals and lack of mitotic activity (H and E, ×400), (b: upper right) Fibrosis surrounding the eosinophilic polygonal cells and lymphoid aggregates (H and E, ×200), (c: lower left) Patchy reactivity for synaptophysin (IHC, ×200), (d: lower right) Positive reactivity to CD56 (IHC, ×200)
These immunohistochemical features with the absence of Reinke crystals leaded to the final diagnosis of TART and implied a better prognosis.
DISCUSSION
TARTs were first described by Wilkins et al. in 1940.[3] He coined the term “testicular tumors of the adrenogenital syndrome”-Adrenogenital syndrome is a former name for CAH. Later, they were also called testicular adrenal rest,[4] or testicular tumors derived from the ectopic adrenal tissue.[5]
To date, the etiology of TART is not completely understood. Possible origins that have been considered include: Adrenal rests, interstitial cells, and pluripotential cells of the testicular stroma stimulated by elevated levels of ACTH.[6,7,8,9]
The reviewed prevalence of the TART surprisingly varies between 0% and 94%.[9] This wide range is attributed to variations between the studies in patient selection and detection methods.
In most cases, the clinical diagnosis of CAH is made before detection of the anticipated TART. Lesions are palpable or ultrasound-detectable. If present, TART usually disappears with optimized suppressive medical therapy.
Although TART is a detectable and medically treatable lesion, it could be misdiagnosed as LCT in two situations. The first is milder CAH forms in which the testicular lesion may be the first presentation without a previous diagnosis of CAH or a concomitant clinical suspicion.[10] Another situation where CAH is diagnosed, however, the testicular lesions are resistant to medical therapy. In similar scenarios, male patients with CAH had undergone unnecessary testicular surgery. Bilateral orchiectomy for TART that could not be differentiated from a malignant tumor reported by Unuvar et al.[11] in a case of 2-year-old boy.
Facing a real challenge to make the correct diagnosis of TART in the aforementioned situations is solved by complete clinical, radiological and the histopathological evaluation. While both LCT and TART occur in early adulthood,[12] TART is typically associated with CAH. Biochemical assessment of hormonal profile (17-OH progesterone, 11-desoxycortisol, dehydroepiandrosterone and androstenedione) is helpful to confirm CAH. Furthermore, TARTs are bilateral in more than 80%, whereas LCTs are bilateral in only 3% of the cases.[9] Ultrasound is good but a non-specific method for detection and monitoring of TART. In our case, there was unusual hypervascularity, which turned out to be due to coexisting bilateral varicocele.
The typical location of TARTs is within the rete testis and could grow to occupy the entire testis. They form single or multiple dense nodules, or irregular nodular masses. In section, they form multi-lobular merging light brown foci separated by narrow greyish bands of fibrous tissue.[13] Claahsen-van der Grinten et al.[9] proposed a five-stage classification for the development and growth of TARTs. In stage one adrenal rest cells are confined within the rete testis and are non-detectable. In non-CAH subjects the rest cells undergo involution during early infancy. In stage two, the adrenal rest cells undergo hypertrophy and hyperplasia in response to growth stimulating factors and may become ultrasound-detectable. In stage three, the cumulative exposure to ACTH and further growth of the adrenal rest cells will compress the rete testis. This mechanical effect together with paracrine effect on the surrounding tissue[14] cause infertility problems that pubertal or post-pubertal CAH patients present with at this stage.[15] In stages two and three medical suppressive therapy by high dosage glucocorticooids can reverse the TART adverse effects and preserve the fertility. In stage four, further hypertrophy and hyperplasia of the adrenal rest cells lead to induction of fibrosis and focal lymphocytic infiltration. Several small rests confluate to lobulated lesion sharply separated from the gonadal parenchyma by fibrous strands.[16] In this stage, early testicular damage occur and medical therapy probably no longer effective because the adrenal rest cells lose their ACTH dependency. Conservative surgery should be considered at this stage or even earlier at stage three. In the advanced stage five, chronic obstruction leads to irreversible damage of testicular parenchyma. The lesion may be replaced partially by adipose tissue. Neither medical therapy nor surgery could preserve the fertility at this stage. Prior to Claahsen-van der Grinten et al.[9] review article, TART has been described in several papers, mainly as case reports, and most of them, in the English literature, focused on early detection and fertility preservation. Although biopsy of the lesion is not routinely recommended[9] from pathological point of view, it is essential to know the morphological characteristics of TART and its differential diagnosis. This is important because there is direct proportion between TART stage level, the role of surgical intervention and the possibility of misdiagnosis with LCT. This situation could only be solved at the histological basis.
In the histopathological picture, TARTs resemble adrenocortical tissue;[17] Large polygonal cells with abundant eosinophilic cytoplasm arranged in strands or cords. Features that are more common to TART compared with LCT include lack of cytological atypia, low mitotic activity, dense fibrous septa, lymphoid aggregates, adipose metaplasia and prominent lipochrome pigment. Reinke crystals, which can be found in 25-40% of LCTs, are absent in TART. Immunohistochemically, TART shows diffuse and strong positivity for CD56, focal or diffuse strong reactivity for synaptophysin and negative reactivity for the androgen receptor. In contrast, LCT displays focal weak to moderate or negative reactivity for CD56 and focal weak or negative reactivity for synaptophysin, but positive reactivity for the androgen receptor.[1,18]
Other testicular lesion that should be differentiated from TART is Leydig cell hyperplasia, which is usually solitary and the distinction is made based on its size (less than 0.5 cm). The nodular hyperplasia is characteristically multifocal. However, it does not destroy surrounding tubules. Hence, the lesion is not exclusively consists of Leydig cells.[19]
It is worth pointing out that rare association of LCT and CAH have been reported in literature and most of them are unilateral.[20] Entezari et al.[21] reported bilateral LCTs in two brothers with CAH. Charfi et al.[2] reported an unusual association of CAH, with coexistence of bilateral TARTs and epididymal LCT. Interestingly, in both reports the CAHs were due to 11 β-hydroxylase deficiency!
CONCLUSION
This patient presented with bilateral synchronous testicular masses of uncertain origin and bilateral orchiectomy was carried out. Differentiating between LCT and TART was extremely difficult from several aspects: Clinically, there was no evidence of therapeutic response; radiologically, the lesions were hypervascular; pathologically, there was no definitive hilar location. Using CD56, synaptophysin and CD10 leaded to diagnosis of TART. Considering lessons obtained from this case, knowledge of histopathological and immunohistochemical features of TART will allow preservation of fertility and avoidance of unnecessary surgical procedures applied in the case of testicular tumors.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Wang Z, Yang S, Shi H, Du H, Xue L, Wang L, et al. Histopathological and immunophenotypic features of testicular tumour of the adrenogenital syndrome. Histopathology. 2011;58:1013–8. doi: 10.1111/j.1365-2559.2011.03861.x. [DOI] [PubMed] [Google Scholar]
- 2.Charfi N, Kamoun M, Feki Mnif M, Mseddi N, Mnif F, Kallel N, et al. Leydig cell tumor associated with testicular adrenal rest tumors in a patient with congenital adrenal hyperplasia due to 11βHydroxylase Deficiency. Case Rep Urol 2012. 2012 doi: 10.1155/2012/648643. 648643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilkins L, Fleischmann W, Howard JE. Macrogenitosomia precox associated with hyperplasia of the androgenic tissue of the adrenal and death from corticoadrenal insufficiency. Endocrinology. 1940;26:385–95. [Google Scholar]
- 4.Lee D, Rodgers SK. Testicular adrenal rests. Ultrasound Q. 2008;24:105–7. doi: 10.1097/RUQ.0b013e31817bdd98. [DOI] [PubMed] [Google Scholar]
- 5.Budzyńska E, Beń-Skowronek I. Testicular adrenal rest tumours in boys with congenital adrenal hyperplasia: Case report and literature review. Pediatr Endocrinol Diabetes Metab. 2011;17:239–42. [PubMed] [Google Scholar]
- 6.Barwick TD, Malhotra A, Webb JA, Savage MO, Reznek RH. Embryology of the adrenal glands and its relevance to diagnostic imaging. Clin Radiol. 2005;60:953–9. doi: 10.1016/j.crad.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Val P, Jeays Ward K, Swain A. Identification of a novel population of adrenal-like cells in the mammalian testis. Dev Biol. 2006;299:250–6. doi: 10.1016/j.ydbio.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 8.Paner GP, Kristiansen G, McKenney JK, Amin MB. Rete testis associated nodular steroid cell nests: Description of putative pluripotential testicular hilus steroid cells. Am J Surg Pathol. 2011;35:505–11. doi: 10.1097/PAS.0b013e31820f16cb. [DOI] [PubMed] [Google Scholar]
- 9.Claahsen-van der Grinten HL, Otten BJ, Stikkelbroeck MM, Sweep FC, Hermus AR. Testicular adrenal rest tumours in congenital adrenal hyperplasia. Best Pract Res Clin Endocrinol Metab. 2009;23:209–20. doi: 10.1016/j.beem.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Emerson RE, Ulbright TM. Morphological approach to tumours of the testis and paratestis. J Clin Pathol. 2007;60:866–80. doi: 10.1136/jcp.2005.036475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unuvar T, Demir K, Abaci A, Atas A, Cakmakci H, Bober E. A 2-year-old boy with a testicular mass. Diagnosis: Testicular tumor of adrenogenital syndrome due to 11-beta-hydroxylase deficiency. Pediatr Ann. 2010;39:471–4. doi: 10.3928/00904481-20100726-04. [DOI] [PubMed] [Google Scholar]
- 12.Speiser PW, White PC. Congenital adrenal hyperplasia. N Engl J Med. 2003;349:776–88. doi: 10.1056/NEJMra021561. [DOI] [PubMed] [Google Scholar]
- 13.Mirzaei MR, Rezvanian H, Siavash M, Parham M, Mahzouni P. A patient with refractory testicular adrenal rest tumour in the setting of cyp11b1 deficiency congenital adrenal hyperplasia. BMJ Case Rep. 2009:2009. doi: 10.1136/bcr.06.2008.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy H, George C, de Kretser D, Judd S. Successful treatment with ICSI of infertility caused by azoospermia associated with adrenal rests in the testes: Case report. Hum Reprod. 2001;16:263–7. doi: 10.1093/humrep/16.2.263. [DOI] [PubMed] [Google Scholar]
- 15.Claahsen-van der Grinten HL, Otten BJ, Hermus AR, Sweep FC, Hulsbergen-van de Kaa CA. Testicular adrenal rest tumors in patients with congenital adrenal hyperplasia can cause severe testicular damage. Fertil Steril. 2008;89:597–601. doi: 10.1016/j.fertnstert.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 16.Stikkelbroeck NM, Suliman HM, Otten BJ, Hermus AR, Blickman JG, Jager GJ. Testicular adrenal rest tumours in postpubertal males with congenital adrenal hyperplasia: Sonographic and MR features. Eur Radiol. 2003;13:1597–603. doi: 10.1007/s00330-002-1786-3. [DOI] [PubMed] [Google Scholar]
- 17.Claahsen-van der Grinten HL, Otten BJ, Sweep FC, Span PN, Ross HA, Meuleman EJ, et al. Testicular tumors in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency show functional features of adrenocortical tissue. J Clin Endocrinol Metab. 2007;92:3674–80. doi: 10.1210/jc.2007-0337. [DOI] [PubMed] [Google Scholar]
- 18.Ashley RA, McGee SM, Isotaolo PA, Kramer SA, Cheville JC. Clinical and pathological features associated with the testicular tumor of the adrenogenital syndrome. J Urol. 2007;177:546–9. doi: 10.1016/j.juro.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 19.Tazi MF, Mellas S, El Fassi MJ, Farih MH. Leydig cell hyperplasia revealed by gynecomastia. Rev Urol. 2008;10:164–719. [PMC free article] [PubMed] [Google Scholar]
- 20.Santoriello A, Benevento R, Petronella P, Perna G, Canonico S. Congenital adrenal hyperplasia and Leydig cell tumor of testis. Case report and review of literature. Ann Ital Chir. 2010;81:445–8. [PubMed] [Google Scholar]
- 21.Entezari P, Kajbafzadeh AM, Mahjoub F, Vasei M. Leydig cell tumor in two brothers with congenital adrenal hyperplasia due to 11-β hydroxylase deficiency: A case report. Int Urol Nephrol. 2012;44:133–7. doi: 10.1007/s11255-010-9890-9. [DOI] [PubMed] [Google Scholar]


