Abstract
Background:
Accurate diagnosis of acute kidney injury (AKI) is problematic especially in critically-ill patients in whom renal function is in an unsteady state.
Aim:
Our aim was to evaluate the role of serum (S.) cystatin C as an early biomarker of AKI in critically-ill children.
Subjects and Methods:
S. creatinine and S. cystatin C were measured in 32 critically-ill children who were at risk for developing AKI. AKI was defined by both: Risk,-injury,-failure,-loss, and-endstage renal disease (RIFLE) classification and glomerular filtration rate (GFR) <80 ml/min/1.73 m2. GFR was estimated by both Schwartz formula and S. cystatin C-based equation.
Results:
S. cystatin C was not statistically higher in AKI patients compared with non-AKI by RIFLE classification (median 1.48 mg/l vs. 1.16 mg/l, P = 0.1) while S. creatinine was significantly higher (median 0.8 mg/dl vs. 0.4 mg/dl, P = 0.001). On estimating GFR by the two equations we found, a lag between rise of S. cystatin C and creatinine denoted by lower GFR by Schwartz formula in four patients, on other hand, six patients had elevated S. cystatin C with low GFR despite normal creatinine and GFR, denoting poor concordance between the two equations and the two markers. The ability of S. creatinine in predicting AKI was superior to S. cystatin with area under the curve (AUC) 0.95 with sensitivity and specificity (100% and 84.6%, respectively) using the RIFLE classification. The same findings were found when using Schwartz formula.
Conclusion:
S. cystatin C is a poor biomarker for diagnosing AKI in critically-ill children.
Keywords: Cystatin C, risk-injury-failure-loss-end stage renal disease criteria, schwartz formula
Introduction
Acute kidney injury (AKI) is a common complication of critical illness and carries high mortality despite significant advances in medical care.[1,2] Early detection of renal function impairment in pediatric intensive care would be of great value, allowing accurate treatment, adjustment of drug dose, and prevention of more severe renal damage.[3,4,5]
Glomerular filtration rate (GFR), which can be measured by determining the clearance of various substances, is the gold standard for monitoring renal function. The ideal endogenous marker would be characterized by stable production rate, stable circulating levels (unaffected by pathological changes), lack of protein binding, free glomerular filtration, and lack of reabsorption or secretion; to date, no such marker has been identified.[6] Some substances such as creatinine, urea, β2 -microglobulin, and retinol-binding protein have been used as endogenous markers of GFR, by measuring either their plasma levels or their renal clearance.[7,8]
Among them, the most useful markers for assessing GFR are S.creatinine and renal creatinine clearance. This is secondary to their correlations with the renal clearance of some exogenous substances (inulin, creatinine-Ethylenediaminetetraacetic acid (EDTA), iothalamate) that are considered “gold standards” for determining GFR.[9,10]
However, creatinine production changes significantly according to the muscle mass of the body and dietetic factors. It is filtered by the glomeruli, but it is also secreted by the renal tubules. This tubular secretion contributes to approximately 20% of the total creatinine excretion by the kidney, and it can increase as GFR decreases. S. creatinine could not detect renal failure until GFR decreases by more than 50%.[11,12]
S. cystatin C is a low-molecular weight protein freely filtered by the glomerulus; its production in the body is a stable process that is not influenced by renal conditions, increased protein catabolism, or dietetic factors.[13,14] Although S. cystatin C is less influenced by age, sex, and muscle mass compared with S. creatinine level, it still can be affected by these patient variables.[15]
Previous studies evaluated S. cystatin C as a biomarker for early detection of AKI, yet results are controversial. Some studies demonstrated the superiority of S. cystatin C compared with S.creatinine in the evaluation of GFR, especially when there is a minor reduction in GFR.[16,17,18,19] They found that S. cystatin C could detect renal dysfunction 1-2 days before S.creatinine.[20] However, other studies showed that S. cystatin C did not rise earlier than S. creatinine and was a poor biomarker for AKI.[21,22,23,24] So, the aim of this work was to re-evaluate the role of S. cystatin C as a biomarker for early detection of AKI in critically-ill children.
Subjects and Methods
This is a prospective observational study that includes 32 patients, 17 males, and 15 females aged from 1 month to 168 months (median 7 months) who were admitted to the pediatrics intensive care unit, Specialized Children Hospital Cairo University between March and October 2011.
All patients who were at risk for developing AKI (hemodynamically unstable patients on high inotropic support and/or individuals receiving high number and/or doses of nephrotoxic drugs including frusemide; bolus or infusion) and septic patients but without any previous elevation in their S. creatinine were included in the study. Patients receiving high doses of corticosteroid therapy were excluded. Demographic data including sex and gender were recorded. Clinical condition on admission was recorded; diagnosis on admission, evidence of sepsis defined as systemic inflammatory response syndrome plus proven bacterial culture according to international pediatric sepsis consensus conference,[25] organ failure assessment defined according to previously published criteria,[25,26] severity of illness assessed by pediatric risk of mortality (PRISM III) score during the first 24 h,[27] post-cardiopulmonary resuscitation event, blood pressure, number of nephrotoxic drugs, number of inotropic drugs, and need for mechanical ventilation. Laboratory data, duration of stay in the intensive care unit and outcome were recorded. Thirty healthy subjects matched for age and sex served as controls.
Patients were classified to have AKI according to the risk,-injury,-failure,-loss-, end stage renal disease (RIFLE) classification.[28] The RIFLE classification was based on two important parameters: (1) Increase in S. creatinine level ≥50% from baseline and (2) urine output at specific time points.
For the same patients, AKI was defined also as GFR < 80 ml/min/1.73 m2 and was estimated by the following equations:
Eq. 1 based upon S. creatinine (Schwartz equation):
GFR (ml/min/1.73 m2) = Height (cm) × k/S. creatinine (mg/dl), where k = 0.44 for children under 2 years old and 0.55 for children over 2 years.[29]
Eq. 2 based upon S. cystatin C:[30]
GFR (ml/min/1.73 m2) = 91.62×(S. cystatin C [mg/l])−1.123.
Parents of the patients and the healthy subjects gave informed consent to participate in this study which was approved by the National Research Centre Ethical Committee.
Ultrasonagraphy of the kidneys, ureter and bladder was done on all AKI patients.
The blood samples were collected from at risk children for developing AKI after assessment of urine output for 24 h to measure S. creatinine and S. cystatin C. About 5 ml of venous blood was withdrawn from patients and controls in plain blood-collection tubes. The obtained samples were centrifuged at 3000 g for 15 min, and serum was stored at −20°C until analysis. Samples were thawed and mixed thoroughly just prior to the assay to avoid erroneous results of repeated freeze/thaw cycles. Hemolyzed and lipemic samples were discarded.
S. cystatin C was assayed according to the manufacturer's protocol using Human cystatin-C Enzyme linked immunosorbent assay (ELISA) enzyme immunoassay (BioVendor – Laboratorní medicína a.s.Catalogue. No.: RD191009100, European Union: In vitro diagnostic medical device Rest of the world) for the quantitative measurement of human cystatin C. Standards, quality controls and serum samples were incubated in microtitrate plate-wells pre-coated with polyclonal anti-human cystatin C antibody. After incubation and washing, polyclonal anti-human cystatin C antibody, conjugated with horseradish peroxidase was added to the wells and incubated. Following a second washing step, a substrate solution tetramethylbenzidine (TMB) was added. The reaction was stopped by an acidic solution and absorbance of the resulting yellow product measurement was proportional to the concentration of cystatin C. A standard curve was plotted between the absorbance values and concentrations of cystatin C standards, and concentrations of unknown samples were determined using this standard curve. Reference ranges were 520-900 ng/ml for males and 560-980 ng/ml for females. The upper limit of the assay was 10,000 ng/ml.[31] While S. creatinine levels were measured by using the kinetic spectrophotometric method according to Jaffé method without deproteinization.[32] This is a compensated method based on manufacturer's instructions (Roche Diagnostics GmbH, Mannheim, Germany).
Statistical analysis
Data were analyzed using Statistical Package for Special Science software computer program version 16.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as median, minimum, and maximum. Categorical variables were expressed as number (n), percent (%), and were compared using the Chi-square test or Fischer's exact tests, as indicated. Kappa, which is a measure of agreement between the equations, was used, where a value of 0.5 or more means strong agreement. Continuous variables were compared using Mann–Whitney test. The associations between variables were assessed by Spearman rank order correlation analysis. In order to measure the sensitivity and specificity of S. cystatin C and S. creatinine for the prediction of AKI by Schwartz formula and RIFLE classification, receiver operating characteristic (ROC) curve was generated and the area under the curve (AUC) was calculated. An AUC of 0.5 is no better than expected by chance, whereas a value of 1.0 signifies a perfect biomarker.[33] P < 0.05 was considered statistically significant.
Results
A total of 32 patients, 17 males and 15 females, aged between 1 and 168 months (median 7 months) were included in the study. The demographic, clinical, and laboratory data of the patients are shown in Table 1.
Table 1.
Demographic characteristics, clinical, and laboratory data of the critically-ill children
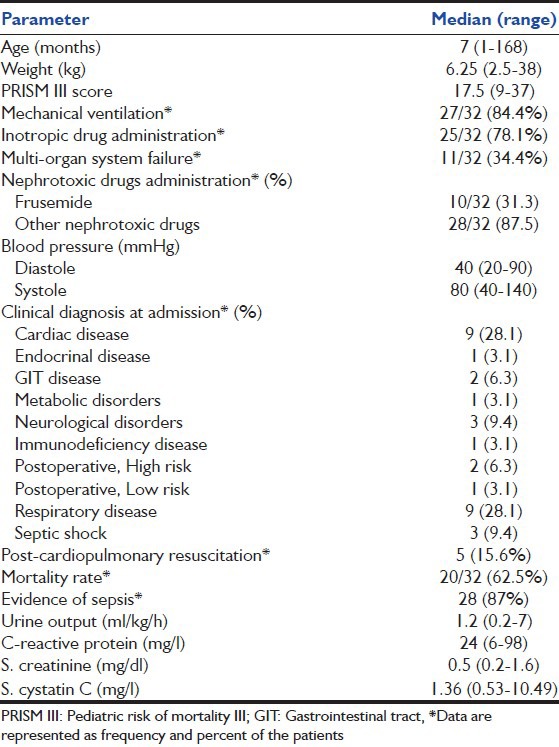
Critically-ill children had significantly higher median S. cystatin C compared with healthy controls (median 1.36 [0.53-10.49] vs. median 0.68 [0.32-0.9] P = 0.001) whereas no significant difference was found between patients and controls as regards S. creatinine level.
The correlations of S. cystatin C and S. creatinine with different risk factors were assessed [Table 2].
Table 2.
The correlation of S.cystatin C and S. creatinine with different risk factors
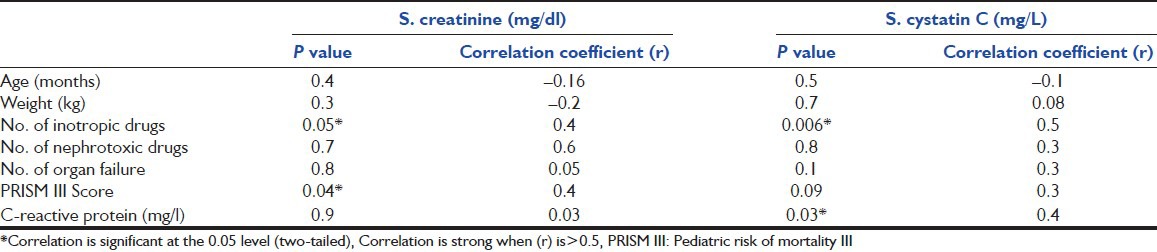
There was no significant correlation between the levels of S. cystatin C and S. creatinine (r = 0.3, P = 0.06).
According to the definition of AKI by RIFLE classification, we found that 13/32 (40.6%) had AKI (six patients had creatinine ≥50%, 1 by oliguria only and six had both). By ultrasonagraphy, we found that 7/13 presented with renal cause while 6/13 patients presented with prerenal cause.
There was no significant difference in S. cystatin C levels between AKI and non-AKI patients (median 1.48 [0.75-10.49] mg/l vs. median 1.16 mg/l [0.53-1.84], P = 0.1) while S. creatinine was significantly higher in AKI patients (median 0.8 [0.4-1.6] mg/dl vs. median 0.4 [0.2-0.6] mg/dl, P = 0.001).
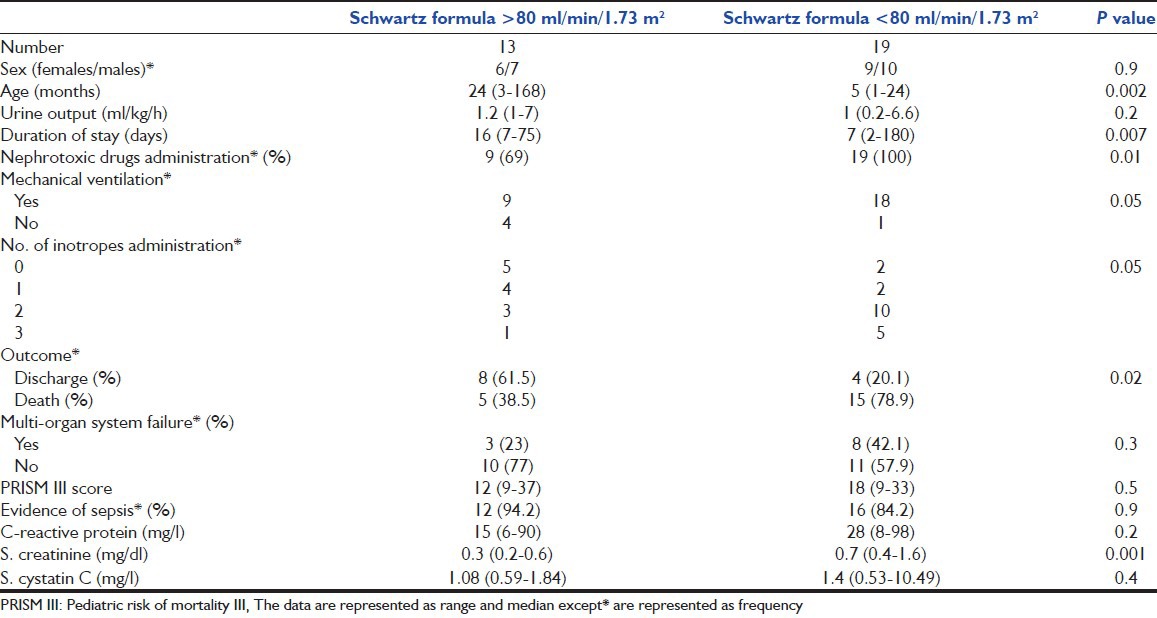
On the other hand, 19/32 (59.3%) patients were classified as having AKI by Schwartz formula (Eq. 1). The comparison between the two groups is summarized in Table 3.
Table 3.
Comparison of two groups based on creatinine clearance lower and higher than 80 ml/min/1.73 m2 as estimated by Schwartz formula
Moreover, we found that 21/32 (66%) patients had GFR <80 ml/min/1.73 m2 by estimation of GFR based on S. cystatin C (Eq. 2).
Assessing the agreement between the Schwartz formula (Eq. 1) and cystatin C-based equation (Eq. 2) is shown in Table 4. The agreement was weak between the two equations (P = 0.05 and Kappa = 0.336).
Table 4.
The agreement between Schwartz formula and serum cystatin C-based equation (EQ2)

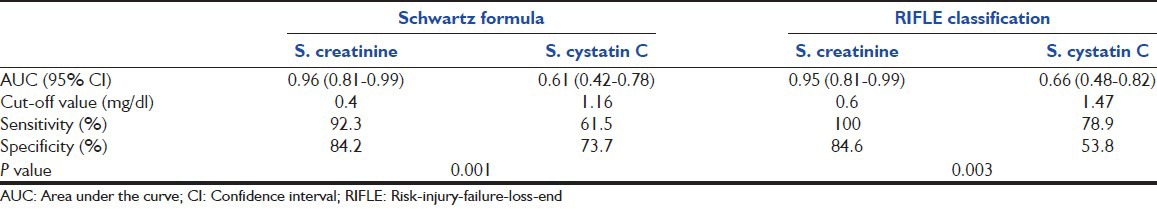
The ROC curves for diagnosis of AKI by RIFLE classification and Schwartz formula are demonstrated in Figure 1, while Table 5 shows the efficacy of both S. creatinine and S. cystatin C to detect early renal dysfunction by RIFLE classification and Schwartz formula.
Figure 1.
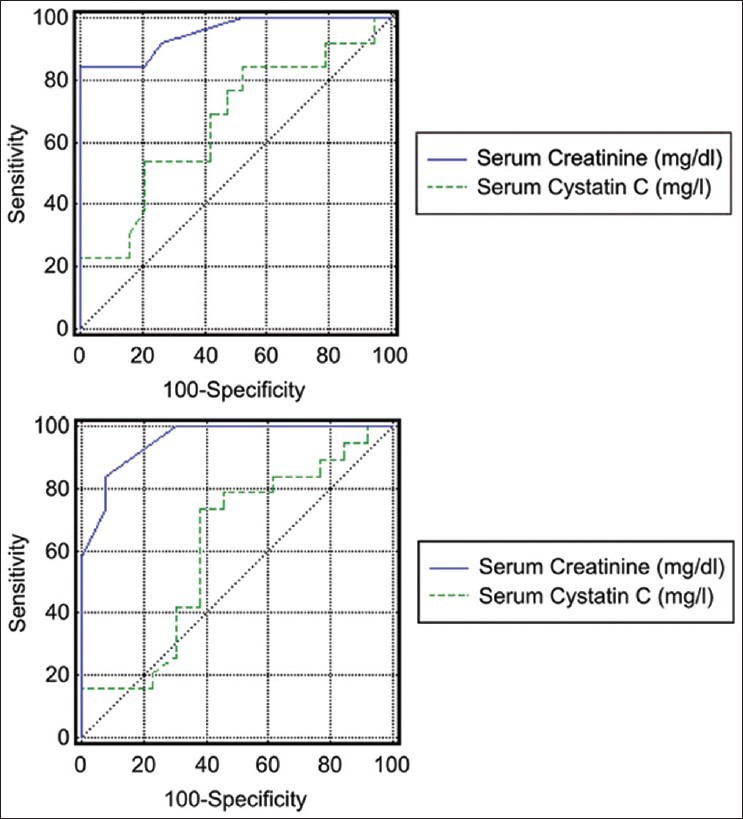
Receiver operating characteristic curve to diagnose efficacy of S. cystatin C and S. creatinine concentrations for prediction of acute kidney injury in critically-ill children by risk-injury-failure-loss-end stage renal disease classification (the upper) and Schwartz formula (the lower)
Table 5.
Diagnostic efficacy values for serum S. creatinine, and S. cystatin C to detect early renal dysfunction by Schwartz formula and risk-injury-failure-loss-end stage renal disease classification
Discussion
Monitoring renal function is extremely important in the management of critically-ill patients.[34] The accurate diagnosis of AKI is especially problematic in critically-ill patients in whom renal function is in an unsteady state.[15] Potentially effective therapeutic interventions for AKI may currently fail because they are applied late in the course of injury.[5] The ideal endogenous marker has not yet been identified.[6]
In our study, we evaluated the accuracy of S. cystatin C in diagnosing early AKI (using RIFLE classification and Schwartz formula) in at risk critically-ill patients. We employed 80 ml/min/1.73 m2 in Schwartz formula as the cutoff point to identify renal dysfunction although lower rates were accepted as normal in our study but we aimed to test the sensitivity of S. cystatin C in diagnosing early and mild renal dysfunction in critically-ill patients. By both classifications, we found elevated S. cystatin C in children of the AKI group compared with the non-AKI group; however, it was not statistically significant; in contrast, S. creatinine was significantly higher in AKI patients. Our results were similar to Wald et al.[35] and Zaffanello et al.[36] who found that S. cystatin C was not a better marker than S. creatinine in being able to identify acute kidney failure earlier.
Estimation of the efficacy of S. cystatin C and S. creatinine by ROC analysis in diagnosing AKI defined by RIFLE criteria, showed that creatinine had high sensitivity and specificity (100% and 84.6%, respectively) to detect AKI with AUC 0.95 versus 0.66 for S. cystatin C. The findings were similar while using the Schwartz formula.
Our findings are in concordance with Royakkers et al.[23] who found that the sensitivity of S. cystatin C was fair two days before developing AKI (AUC 0.72) and poor 1 day before developing AKI (AUC 0.62). Slort et al.[24] found that the maximum relative rise of S.creatinine was significantly higher than S.cystatin C. They concluded that S. cystatin C was not superior to S.creatinine.
Although S. cystatin C is said to be less influenced by age, sex, and muscle mass compared with S. creatinine level, it has been shown to be significantly affected by several patient variables.[15]
Our study showed a positive correlation between C-reactive protein levels and S. cystatin C. Stevens et al.[37] found that S. cystatin C levels can be affected by the markers of inflammation such as white blood cell count and C-reactive protein level which play an important role in critically-ill patients and could affect the reference interval.
Studying the concordance between the cystatin C based equation and Schwartz formula, we found that six patients diagnosed by S. cystatin C based equation as having GFR <80 ml/min/1.73 m2 while having GFR > 80 ml/min/1.73 m2 by Schwartz formula; this finding could be explained by the hypothesis that cystatin C might have been elevated due to non-renal causes.
Many studies have found that S. cystatin C levels could be influenced by some extra-renal conditions, including diabetic ketosis,[38] insulin,[39] and thyroid dysfunction where S. cystatin C levels might be increased or decreased, respectively, as a result of sub-clinical hypo- or hyperthyroidism.[40] Moreover, administration of methylprednisolone pulses or cyclosporin A could, respectively, over- or under-estimate S. cystatin C levels.[41] These findings could explain the lack of correlation between S. cystatin C and S. creatinine in our study.
On the other hand, four patients had GFR <80 ml/min/1.73 m2 as estimated by Schwartz, while having GFR > 80 ml/min/1.73 m2 as estimated by S. cystatin C-based equation (Eq. 2), which could mean that there was a lag between rise of S. cystatin and S. creatinine.
In contrast to our results, several studies demonstrated superiority of S. cystatin C over S. creatinine in early detection of AKI in critically-ill children.[8,18,42] Liangos et al.[43] commented that these studies were limited and their results were inconsistent; however, comparison among studies is hampered by case mix and heterogeneity in the study designs. Notably, no two studies used identical definitions for AKI, and this definition is critical in biomarker research.
Patients in the present study diagnosed as AKI (GFR < 80 ml/min/1.73 m2) by Schwartz formula were younger, had higher mortality rate, higher PRISM III score values and required mechanical ventilation. Our results were in agreement with the study of Alkandari et al.[44] who found that critically-ill children with AKI had increased mortality rate, were independently associated with longer intensive care unit stay and required mechanical ventilation. However, our AKI patients had shorter duration of stay in ICU, which could be explained by the high mortality rate (78.9%) among them. This finding points to the importance of AKI as a risk factor for poor outcome in critically-ill children.
This study has important limitations. First, is the small size of the study group. Second, this was a short-term study of AKI in critically-ill children. We did not follow-up patients to determine the decline or increase or normalization of cystatin C in comparison with creatinine regarding improvement of AKI or deterioration needing dialysis. This will be an important future study. Third, the definition of AKI was based on elevations in S. creatinine, which may cause a dilemma and affect the performance of S. cystatin C.
Conclusion
The results of this study point to the poor value of S. cystatin C as a biomarker for detection of AKI in critically-ill children. However, a single biomarker for AKI may not provide complete information as all biomarkers have their individual strengths and weaknesses. Simultaneous estimation of more than one biomarker may be more beneficial.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Joannidis M, Metnitz PG. Epidemiology and natural history of acute renal failure in the ICU. Crit Care Clin. 2005;21:239–49. doi: 10.1016/j.ccc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, et al. Continuous renal replacement therapy: A worldwide practice survey. The beginning and ending supportive therapy for the kidney (B.E.S.T. kidney) investigators. Intensive Care Med. 2007;33:1563–70. doi: 10.1007/s00134-007-0754-4. [DOI] [PubMed] [Google Scholar]
- 3.Grubb A, Nyman U, Björk J, Lindström V, Rippe B, Sterner G, et al. Simple cystatin C-based prediction equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the Counahan-Barratt prediction equations for children. Clin Chem. 2005;51:1420–31. doi: 10.1373/clinchem.2005.051557. [DOI] [PubMed] [Google Scholar]
- 4.Hoste EA, Damen J, Vanholder RC, Lameire NH, Delanghe JR, Van den Hauwe K, et al. Assessment of renal function in recently admitted critically ill patients with normal serum creatinine. Nephrol Dial Transplant. 2005;20:747–53. doi: 10.1093/ndt/gfh707. [DOI] [PubMed] [Google Scholar]
- 5.Jo SK, Rosner MH, Okusa MD. Pharmacologic treatment of acute kidney injury: Why drugs haven't worked and what is on the horizon. Clin J Am Soc Nephrol. 2007;2:356–65. doi: 10.2215/CJN.03280906. [DOI] [PubMed] [Google Scholar]
- 6.Stevens LA, Levey AS. Measurement of kidney function. Med Clin North Am. 2005;89:457–73. doi: 10.1016/j.mcna.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Herget-Rosenthal S, Marggraf G, Hüsing J, Göring F, Pietruck F, Janssen O, et al. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66:1115–22. doi: 10.1111/j.1523-1755.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 8.Villa P, Jiménez M, Soriano MC, Manzanares J, Casasnovas P. Serum cystatin C concentration as a marker of acute renal dysfunction in critically ill patients. Crit Care. 2005;9:R139–43. doi: 10.1186/cc3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouvet Y, Bouissou F, Coulais Y, Séronie-Vivien S, Tafani M, Decramer S, et al. GFR is better estimated by considering both serum cystatin C and creatinine levels. Pediatr Nephrol. 2006;21:1299–306. doi: 10.1007/s00467-006-0145-z. [DOI] [PubMed] [Google Scholar]
- 10.Zappitelli M, Parvex P, Joseph L, Paradis G, Grey V, Lau S, et al. Derivation and validation of cystatin C-based prediction equations for GFR in children. Am J Kidney Dis. 2006;48:221–30. doi: 10.1053/j.ajkd.2006.04.085. [DOI] [PubMed] [Google Scholar]
- 11.Huber AR, Risch L. Recent developments in the evaluation of glomerular filtration rate: Is there a place for beta-trace? Clin Chem. 2005;51:1329–30. doi: 10.1373/clinchem.2005.053389. [DOI] [PubMed] [Google Scholar]
- 12.Bianchi C, Donadio C, Tramonti G, Consani C, Lorusso P, Rossi G. Reappraisal of serum beta2-microglobulin as marker of GFR. Ren Fail. 2001;23:419–29. doi: 10.1081/jdi-100104725. [DOI] [PubMed] [Google Scholar]
- 13.Herget-Rosenthal S, Trabold S, Pietruck F, Holtmann M, Philipp T, Kribben A. Cystatin C: Efficacy as screening test for reduced glomerular filtration rate. Am J Nephrol. 2000;20:97–102. doi: 10.1159/000013564. [DOI] [PubMed] [Google Scholar]
- 14.Finney H, Newman DJ, Price CP. Adult reference ranges for serum cystatin C, creatinine and predicted creatinine clearance. Ann Clin Biochem. 2000;37:49–59. doi: 10.1258/0004563001901524. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein SL. Kidney function assessment in the critically ill child: Is it time to leave creatinine behind? Crit Care. 2007;11:141. doi: 10.1186/cc5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herget-Rosenthal S, Marggraf G, Hüsing J, Göring F, Pietruck F, Janssen O, et al. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66:1115–22. doi: 10.1111/j.1523-1755.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 17.Coll E, Botey A, Alvarez L, Poch E, Quintó L, Saurina A, et al. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis. 2000;36:29–34. doi: 10.1053/ajkd.2000.8237. [DOI] [PubMed] [Google Scholar]
- 18.Filler G, Priem F, Lepage N, Sinha P, Vollmer I, Clark H, et al. Beta-trace protein, cystatin C, beta (2)-microglobulin, and creatinine compared for detecting impaired glomerular filtration rates in children. Clin Chem. 2002;48:729–36. [PubMed] [Google Scholar]
- 19.John GT, Fleming JJ, Talaulikar GS, Selvakumar R, Thomas PP, Jacob CK. Measurement of renal function in kidney donors using serum cystatin C and beta (2)-microglobulin. Ann Clin Biochem. 2003;40:656–8. doi: 10.1258/000456303770367252. [DOI] [PubMed] [Google Scholar]
- 20.Herget-Rosenthal S, Marggraf G, Hüsing J, Göring F, Pietruck F, Janssen O, et al. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66:1115–22. doi: 10.1111/j.1523-1755.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 21.Ahlström A, Tallgren M, Peltonen S, Pettilä V. Evolution and predictive power of serum cystatin C in acute renal failure. Clin Nephrol. 2004;62:344–50. doi: 10.5414/cnp62344. [DOI] [PubMed] [Google Scholar]
- 22.Perianayagam MC, Seabra VF, Tighiouart H, Liangos O, Jaber BL. Serum cystatin C for prediction of dialysis requirement or death in acute kidney injury: A comparative study. Am J Kidney Dis. 2009;54:1025–33. doi: 10.1053/j.ajkd.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 23.Royakkers AA, Korevaar JC, van Suijlen JD, Hofstra LS, Kuiper MA, Spronk PE, et al. Serum and urine cystatin C are poor biomarkers for acute kidney injury and renal replacement therapy. Intensive Care Med. 2011;37:493–501. doi: 10.1007/s00134-010-2087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slort PR, Ozden N, Pape L, Offner G, Tromp WF, Wilhelm AJ, et al. Comparing cystatin C and creatinine in the diagnosis of pediatric acute renal allograft dysfunction. Pediatr Nephrol. 2012;27:843–9. doi: 10.1007/s00467-011-2073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldstein B, Giroir B, Randolph A International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 26.Proulx F, Fayon M, Farrell CA, Lacroix J, Gauthier M. Epidemiology of sepsis and multiple organ dysfunction syndrome in children. Chest. 1996;109:1033–7. doi: 10.1378/chest.109.4.1033. [DOI] [PubMed] [Google Scholar]
- 27.Pollack MM, Patel KM, Ruttimann UE. PRISM III: An updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24:743–52. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P Acute Dialysis Quality Initiative workgroup. Acute renal failure – Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571–90. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 30.Filler G, Lepage N. Should the Schwartz formula for estimation of GFR be replaced by cystatin C formula? Pediatr Nephrol. 2003;18:981–5. doi: 10.1007/s00467-003-1271-5. [DOI] [PubMed] [Google Scholar]
- 31.Hossain MA, Emara M, El Moselhi H, Shoker A. Comparing measures of cystatin C in human sera by three methods. Am J Nephrol. 2009;29:381–91. doi: 10.1159/000168486. [DOI] [PubMed] [Google Scholar]
- 32.Mazzachi BC, Peake MJ, Ehrhardt V. Reference range and method comparison studies for enzymatic and Jaffé creatinine assays in plasma and serum and early morning urine. Clin Lab. 2000;46:53–5. [PubMed] [Google Scholar]
- 33.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 34.Mak RH. Acute kidney injury in children: The dawn of a new era. Pediatr Nephrol. 2008;23:2147–9. doi: 10.1007/s00467-008-1014-8. [DOI] [PubMed] [Google Scholar]
- 35.Wald R, Liangos O, Perianayagam MC, Kolyada A, Herget-Rosenthal S, Mazer CD, et al. Plasma cystatin C and acute kidney injury after cardiopulmonary bypass. Clin J Am Soc Nephrol. 2010;5:1373–9. doi: 10.2215/CJN.06350909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaffanello M, Franchini M, Fanos V. Is serum cystatin-C a suitable marker of renal function in children? Ann Clin Lab Sci. 2007;37:233–40. [PubMed] [Google Scholar]
- 37.Stevens LA, Schmid CH, Greene T, Li L, Beck GJ, Joffe MM, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75:652–60. doi: 10.1038/ki.2008.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holmquist P, Torffvit O, Sjöblad S. Metabolic status in diabetes mellitus affects markers for glomerular filtration rate. Pediatr Nephrol. 2003;18:536–40. doi: 10.1007/s00467-003-1086-4. [DOI] [PubMed] [Google Scholar]
- 39.Yokoyama H, Inoue T, Node K. Effect of insulin-unstimulated diabetic therapy with miglitol on serum cystatin C level and its clinical significance. Diabetes Res Clin Pract. 2009;83:77–82. doi: 10.1016/j.diabres.2008.09.049. [DOI] [PubMed] [Google Scholar]
- 40.Wiesli P, Schwegler B, Spinas GA, Schmid C. Serum cystatin C is sensitive to small changes in thyroid function. Clin Chim Acta. 2003;338:87–90. doi: 10.1016/j.cccn.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 41.Galteau MM, Guyon M, Gueguen R, Siest G. Determination of serum cystatin C: Biological variation and reference values. Clin Chem Lab Med. 2001;39:850–7. doi: 10.1515/CCLM.2001.141. [DOI] [PubMed] [Google Scholar]
- 42.Herrero-Morin JD, Malaga S, Fernandez N, Rey C, Diéguez MA, Gonzalo S. Cystatin C and β2-microglobulin: markers of glomerular filtration in critically ill children. Crit Care. 2007;11:141–7. doi: 10.1186/cc5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liangos O, Tighiouart H, Perianayagam MC, Kolyada A, Han WK, Wald R, et al. Comparative analysis of urinary biomarkers for early detection of acute kidney injury following cardiopulmonary bypass. Biomarkers. 2009;14:423–31. doi: 10.1080/13547500903067744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alkandari O, Eddington KA, Hyder A, Gauvin F, Ducruet T, Gottesman R, et al. Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: A two-center retrospective cohort study. Crit Care. 2011;15:R146. doi: 10.1186/cc10269. [DOI] [PMC free article] [PubMed] [Google Scholar]


