Abstract
Tracheal rupture is an infrequent, severe complication of endotracheal intubation, which can be difficult to diagnose. Post-intubation tracheal rupture (PiTR) is distinct from non-iatrogenic causes of tracheobronchial trauma and often requires different treatment. The increasing adoption of pre-hospital emergency services increases the likelihood of such complications from emergency intubations. Effective management strategies for PiTR outside specialist cardiothoracic units are possible. Two cases of severe PiTR, successfully managed non-operatively on a general medical-surgical intensive care unit, illustrate a modified approach to current standards. The evidence base for PiTR is reviewed and a pragmatic management algorithm presented.
Keywords: Bronchoscopy, cluster care, post intubation tracheal rupture, tracheal rupture
Introduction
Tracheal rupture (TR) occurs in approximately 1:20,000 intubations.[1] The diagnosis requires a high index of suspicion and is often delayed; nearly 15% of iatrogenic tracheal injuries from emergency intubations are identified at post-mortem.[2] Management guidance of TR is based on case series. Traditionally, TR was managed surgically, with a high perioperative mortality.[3] This was largely tracheobronchial injuries caused by blunt trauma. The recognition that post intubation tracheal rupture (PiTR) has a distinct etiology, characteristic pattern of injury and a high perioperative risk particularly in the critically ill, has led to a more conservative management approach, and two cases illustrate this.
Case Reports
Case 1
In the emergency department, a 76-year-old woman, intubated out of hospital, following a prolonged seizure, developed acute airway obstruction, which resolved on endotracheal suctioning of mucosal tissue debris. Cardiac arrest with pulseless electrical activity ensued. Bilateral tension pneumothoraces, apparently related to jugular central venous catheterization, were relieved by thoracocentesis and tube thoracostomy. Spontaneous cardiac output and oxygenation were restored. However, progressive abdominal distension developed. A plain chest radiograph (CXR) [Figure 1] demonstrated subphrenic and subcutaneous air. Computed tomography [Figures 2a and b] confirmed extensive subcutaneous air, residual pneumothoraces, pneumomediastinum and tension pneumoperitoneum. Gastrograffin contrast imaging excluded oesophageal perforation.
Figure 1.
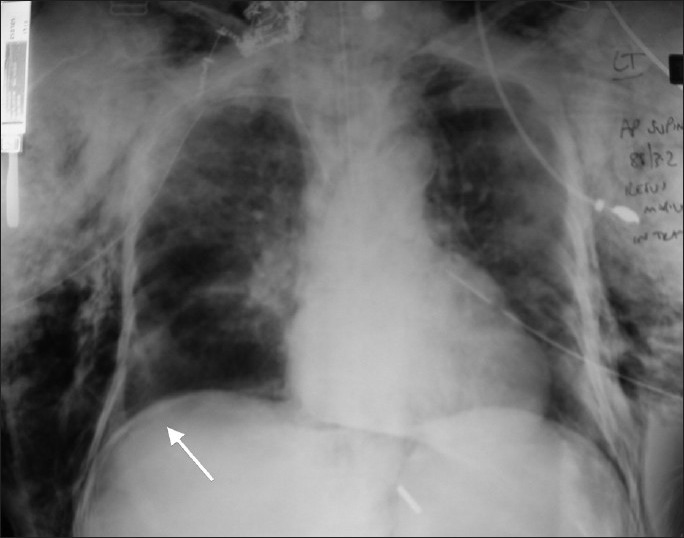
Plain chest radiograph demonstrating endotracheal tube in situ, bilateral tube thoracostomies, extensive subcutaneous emphysema, and subphrenic air (arrow)
Figure 2a.
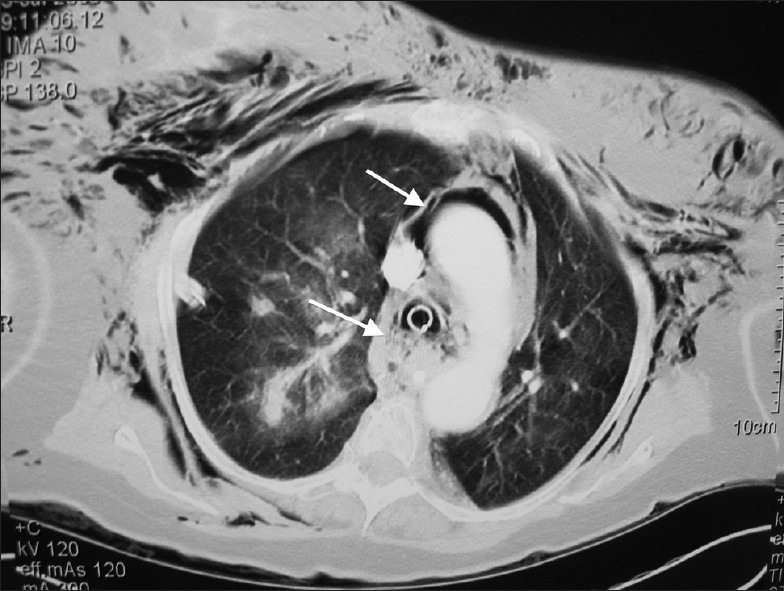
Computed tomography of the thorax. Marked subcutaneous emphysema and pneumomediastinum (thin arrow). Pockets of air around the paratracheal soft-tissue (thick arrow) raise the suspicion of a tracheal defect near the endotracheal tube
Figure 2b.
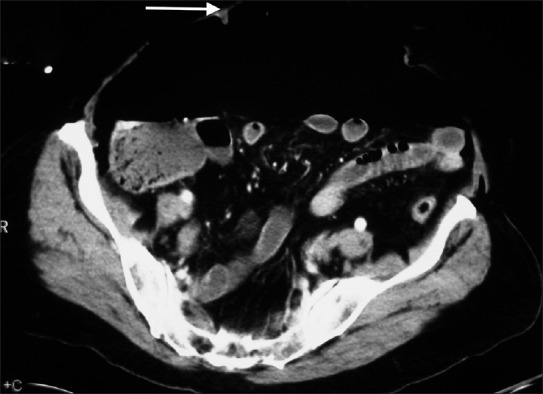
Tension pneumoperitoneum. Computed tomography of the abdomen demonstrates intra-abdominal free gas compressing underlying bowel. The arrow signifies the anterior abdominal wall
In the intensive care unit (ICU), she was stable. The abdomen was distended, but soft. There was no acidosis, normal lactate, and negative diagnostic peritoneal lavage. Diagnostic laparotomy was deferred and fiberoptic bronchoscopy was performed.
Bronchoscopy demonstrated a 4 cm long full thickness defect in the posterior membranous trachea, 1 cm proximal to the carina [Figure 3a]. Following consultation, thoracic surgical repair of the tracheal tear or complex stenting were ruled out due to the high operative risk. A conservative approach was necessary. Bridging the defect by double lumen endotracheal tube (ETT) beyond the distal end of the tear was not possible due to the proximity of the main carina, and risk of the herniation of the ETT cuff into the posterior wall defect, Therefore, a cuffed ETT was placed proximal to the defect, and a low volume lung ventilation strategy was adopted, to allow the defect to recover without over-distension and exacerbating pneumomediastinum. Mechanical ventilation was as follows: Volume (5-6 ml/kg), frequency (25/min), pressure limited (mean airway pressure <25 cm H2O) ventilation with permissive Hypercapnia. Tazobactam-piperacillin was commenced empirically for suspected mediastinitis and pneumonia. As part of a cluster care strategy, blind tracheal suctioning, and patient rotation were avoided to prevent ETT migration. Post-pyloric feeding was established after confirming an intact esophagus and twice-daily bronchoscopy for airway clearance, and correct ETT position above the proximal defect.
Figure 3a.
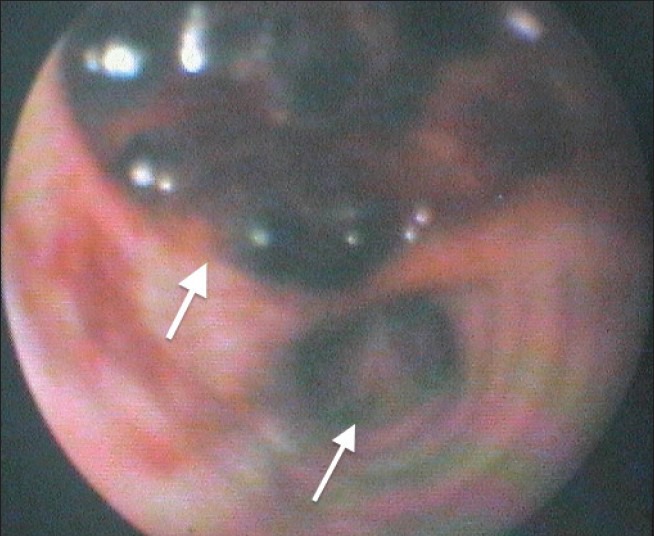
Bronchoscopic view of the trachea beyond the distal end of the endotracheal tube at Day 1. Just proximal to the carina (thin arrow) is the lower edge of a large full thickness defect in the posterior tracheal wall (thick arrow). Beyond the visible clot was the mediastinum and anterior aspect of the esophagus
The patient was weaned with percutaneous tracheostomy and discharged from ICU after 27 days. Follow-up bronchoscopy [Figure 3b] revealed complete linear mucosal healing.
Figure 3b.
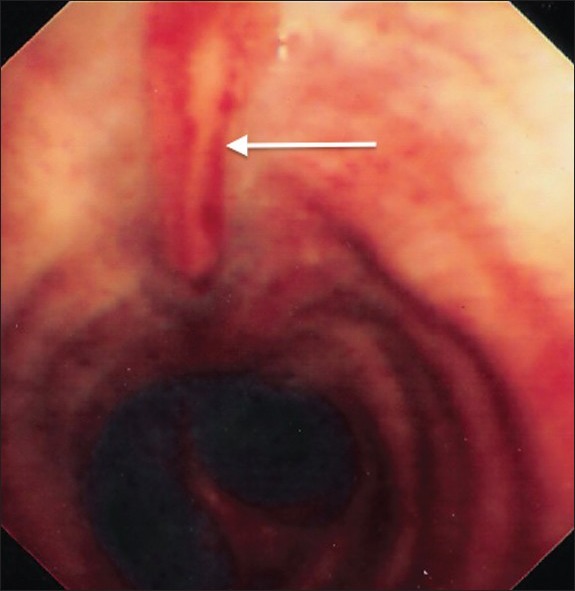
Bronchoscopic view of the trachea at Day 32, 1 week after discharge from intensive care. Complete spontaneous healing of the tracheal defect had occurred (arrow)
Case 2
A 69-year-old female was admitted with coma following a mixed overdose of benzodiazepines and narcotics. She was intubated, using an ETT with stylet.
Persistent leak around her ETT, despite cuff inflation and tube changes initiated bronchoscopy. A 7 cm posterior tracheal tear extending to 2 rings above the carina contained large thrombus and mucopus. The ETT was withdrawn to above the proximal extent of the defect and meropenem for suspected mediastinitis (penicillin allergy).
Thoracic surgical consultation was sought due to the size and length of the defect, the potential for failure of bridging, and the chance of mediastinitis. Conservative management was advised, unless active endobronchial bleeding or progressive ventilatory failure ensued. A protective ventilation strategy, avoidance of blind tracheal suctioning and cluster care to avoid excessive ETT movement on patient repositioning were adopted. Bronchoscopy allowed airway clearance and monitoring of the defect. Spontaneous healing occurred, allowing tracheostomy, weaning, and discharge at 1 month.
Management
In both cases, air leaks that might interfere with ventilation were drained. The ETT was secured proximal to the defect's upper extent, with low volume ventilation, so reducing strain on the tear. There was insufficient intact trachea to allow conventional placement of ETT distal to the tear. Therefore, an approach directed at reducing factors that might prevent or delay healing was implemented. Cluster care was central to this. This approach can easily be applied in non-specialist ICUs for large full thickness PiTR near the carina.
Literature Review
PiTR is an uncommon, but potentially catastrophic consequence of endotracheal intubation.
Increased risk of PiTR is associated with female gender, short stature, difficult airway anatomy, steroid use, chronic obstructive pulmonary disease and underlying connective tissue disease.[3,4] Mechanical factors are also implicated; use of rigid stylets, incorrectly sized ETTs, cuff over-inflation, head movement or vigorous coughing poor prognosticators are diagnosis outside the operating theatre (delayed diagnosis), necessary operative intervention, and emergency intubation.[1]
PiTR typically causes longitudinal lacerations of the posterior membranous trachea, rarely extending into the main bronchi.[5] The injury occurs from the ETT tip catching a flaccid portion of the trachea or from radial traction due to cuff over-distension. In mechanically ventilated patients, the resulting air leak into surrounding tissues may lead to tension pneumothorax and pneumomediastinum.[1]
The most common presenting features of PiTR are subcutaneous air, pneumomediastinum, and pneumothorax.[1,3,5] Pneumoperitoneum and hemoptysis may occur. In ventilated patients, a persistent air leak may be the only sign, whilst self-ventilating patients may exhibit respiratory distress.
In the first case, abdominal distension and subsequent tension pneumoperitoneum occurred after relief of acute airway obstruction owing to a tracheal membranous flap being released. The high positive airway pressure through the PiTR likely maintained an air pressure gradient between the mediastinum and peritoneum across the central diaphragmatic crura.[6]
Plain CXR may show subcutaneous air, pneumomediastinum, pneumoperitoneum or pneuomthoraces[7] Other signs are a displaced ETT, an eccentric cuff or dilated trachea. CT provides diagnostic suspicion and detailed assessment of TR.[8]
Diagnosis is confirmed by tracheobronchoscopy, allowing precise delineation of location, extent, and depth of the PiTR, and accurate repositioning of ETT.
The management of PiTR is guided by whether the patient is self or mechanically ventilated, and whether an operative approach is required. Determinants include location and, length of defect, time to diagnosis and sequela (e.g., mediastinitis, bleeding or progressive respiratory failure).[5,9,10] Surgery is suggested when bridging of the TR is not feasible.[11] Yet, the decision for surgery or not is most challenging in critically-ill mechanically ventilated patients, with TR. Here, conservative management is felt most likely to fail, but conversely, these patients pose the highest perioperative risk for surgical repair.
Criteria for conservative management and its increasing trend in selected cases include cardiovascular stability, the absence of sequela such as progressive air leaks or sepsis, and no esophageal injury.[10,12]
Risk stratification of patients according to their ventilatory status, underlying pathology and nature of the PiTR (<4 cm long), allowed 17 of 18 patients to be treated conservatively. Consideration for surgical repair were rapid progression of air leaks, mediastinitis, and ventilatory deterioration.[13]
In the largest case series, (n = 30) a conservative approach for PiTR was independent of length of defect.[5] Recommendations were drainage of symptomatic air collections, early extubation if possible, perioperative risk stratification and “bridging” of the tracheal defect This is “simple bridging” by passing a single lumen tube beyond the PiTR or by selective endo-bronchial intubation using two endobronchial tubes passed through a large tracheostomy.
Non-invasive ventilation was used successfully in a subgroup with respiratory compromise due to protrusion of the esophagus into the trachea. Surgery was considered due to unsuccessful “bridging” of the tear.
Two main surgical approaches for repair of TR are right thoracotomy for middle/lower third defects or left cervical approach for upper third tears.
Airway stents have been used for temporary bridging of PiTR.[14] They may be placed by flexible or rigid bronchoscopy. Their use in benign central airways disease, including PiTR, must be balanced against potential complications including misplacement, migration, and airway stenosis.[15] Stenting is considered where conservative treatment fails and the risk of surgical repair is deemed too high.
Supportive measures for PiTR aim to avoid sequlae such as mediastinitis and delayed healing of the rupture, whilst early broad-spectrum antibiotics are indicated where mediastinitis is suspected. Regular bronchoscopy for microbiological sampling, airway clearance and surveillance of the TR. Cluster care nursing procedures performed 1-2 times/day and ETT external fixators will minimize possible disruption to the tracheal tear.
There is no consensus on specific modes of ventilation, but high ventilatory pressures should be avoided. The use of high frequency oscillatory ventilation would seem intuitive in this setting; although, there are no published reports. To facilitate low pressure ventilation and avoid coughing and straining, appropriate sedo-analgesic regimens should be employed. Some authors have recommended avoiding NG tubes in the presence of full thickness tracheal tears as they make contribute to the formation of tracheoesophageal fistulas. Feeding should only be introduced after excluding esophageal injury.
A systematic review of 182 cases of PiTR reported a female preponderance (85.7).[1] Overall mortality rate was 22%. A total of 111 patients had surgical management and 71 conservative. All those diagnosed intraoperatively (n = 31) were managed surgically at the time. Those diagnosed later, who required surgical management had a two fold increase in mortality.[1]
In summary, a multidisciplinary approach to PiTR in critically ill patients, with risk stratification (including triggers for surgical intervention or airway stenting) can allow conservative management, even in large, and non-bridgeable tears. A combination of “protective” ventilation, cluster care, and regular bronchoscopy allow spontaneous repair. A pragmatic management algorithm modified from Wurtz et al. illustrates this [Figure 4].[11]
Figure 4.
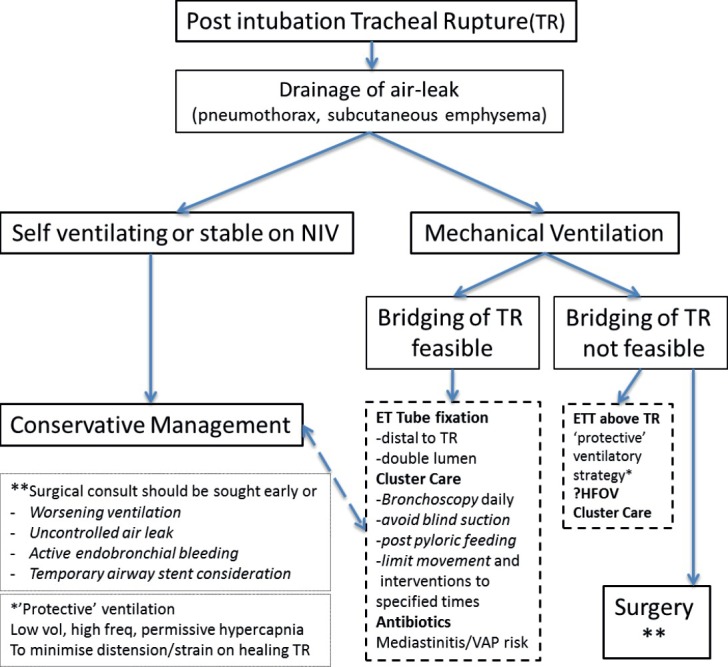
A management algorithm for post intubation tracheal rupture, modified from Wurtz et al.[11] Conservative management represents elements of non-respiratory support, irrespective of self-ventilation, non-invasive ventilation or mechanical ventilation. Where possible, a surgical consult is advised early on
Acknowledgments
We thank our colleagues and staff in the Intensive Care Unit, for their dedicated involvement, the patients for their consent to discuss their cases, and Dr. N. Soni for reviewing the manuscript.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Miñambres E, Burón J, Ballesteros MA, Llorca J, Muñoz P, González-Castro A. Tracheal rupture after endotracheal intubation: A literature systematic review. Eur J Cardiothorac Surg. 2009;35:1056–62. doi: 10.1016/j.ejcts.2009.01.053. [DOI] [PubMed] [Google Scholar]
- 2.Richardson JD. Outcome of tracheobronchial injuries: A long-term perspective. J Trauma. 2004;56:30–6. doi: 10.1097/01.TA.0000108631.72315.78. [DOI] [PubMed] [Google Scholar]
- 3.Hofmann HS, Rettig G, Radke J, Neef H, Silber RE. Iatrogenic ruptures of the tracheobronchial tree. Eur J Cardiothorac Surg. 2002;21:649–52. doi: 10.1016/s1010-7940(02)00037-4. [DOI] [PubMed] [Google Scholar]
- 4.Massard G, Rougé C, Dabbagh A, Kessler R, Hentz JG, Roeslin N, et al. Tracheobronchial lacerations after intubation and tracheostomy. Ann Thorac Surg. 1996;61:1483–7. doi: 10.1016/0003-4975(96)00083-5. [DOI] [PubMed] [Google Scholar]
- 5.Conti M, Pougeoise M, Wurtz A, Porte H, Fourrier F, Ramon P, et al. Management of postintubation tracheobronchial ruptures. Chest. 2006;130:412–8. doi: 10.1378/chest.130.2.412. [DOI] [PubMed] [Google Scholar]
- 6.Macklin CC. Transport of air along sheaths of pulmonic blood vessels from alveoli to the mediastinum. Arch Intern Med. 1939;64:913–26. [Google Scholar]
- 7.Nishiumi N, Maitani F, Yamada S, Kaga K, Iwasaki M, Inokuchi S, et al. Chest radiography assessment of tracheobronchial disruption associated with blunt chest trauma. J Trauma. 2002;53:372–7. doi: 10.1097/00005373-200208000-00032. [DOI] [PubMed] [Google Scholar]
- 8.Faure A, Floccard B, Pilleul F, Faure F, Badinand B, Mennesson N, et al. Multiplanar reconstruction: A new method for the diagnosis of tracheobronchial rupture? Intensive Care Med. 2007;33:2173–8. doi: 10.1007/s00134-007-0830-9. [DOI] [PubMed] [Google Scholar]
- 9.Carbognani P, Bobbio A, Cattelani L, Internullo E, Caporale D, Rusca M. Management of postintubation membranous tracheal rupture. Ann Thorac Surg. 2004;77:406–9. doi: 10.1016/S0003-4975(03)01344-4. [DOI] [PubMed] [Google Scholar]
- 10.Ross HM, Grant FJ, Wilson RS, Burt ME. Nonoperative management of tracheal laceration during endotracheal intubation. Ann Thorac Surg. 1997;63:240–2. doi: 10.1016/s0003-4975(96)01077-6. [DOI] [PubMed] [Google Scholar]
- 11.Wurtz A, Benhamed L, Hysi I, Conti M. Endoscopic or conservative management of postintubation tracheal membrane laceration. Ann Thorac Surg. 2011;91:1654–5. doi: 10.1016/j.athoracsur.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 12.Jougon J, Ballester M, Choukroun E, Dubrez J, Reboul G, Velly JF. Conservative treatment for postintubation tracheobronchial rupture. Ann Thorac Surg. 2000;69:216–20. doi: 10.1016/s0003-4975(99)01129-7. [DOI] [PubMed] [Google Scholar]
- 13.Gómez-Caro Andrés A, Moradiellos Díez FJ, Ausín Herrero P, Díaz-Hellín Gude V, Larrú Cabrero E, de Miguel Porch E, et al. Successful conservative management in iatrogenic tracheobronchial injury. Ann Thorac Surg. 2005;79:1872–8. doi: 10.1016/j.athoracsur.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Yopp AC, Eckstein JG, Savel RH, Abrol S. Tracheal stenting of iatrogenic tracheal injury: A novel management approach. Ann Thorac Surg. 2007;83:1897–9. doi: 10.1016/j.athoracsur.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 15.Du Rand IA, Barber PV, Goldring J, Lewis RA, Mandal S, Munavvar M, Singh S, et al. British Thoracic Society guideline for advanced diagnostic and therapeutic flexible bronchoscopy in adults. Thorax. 2011;66(Suppl 3):iii1–21. doi: 10.1136/thoraxjnl-2011-200713. [DOI] [PubMed] [Google Scholar]


