Abstract
Background:
In patients with acute myocardial infarction or unstable angina undergoing coronary angioplasty, abciximab reduces major adverse cardiac events (MACE). Most clinical trials have studied mainly intravenous administration. Intracoronary (IC) bolus application of abciximab causes very high local drug concentrations and may be more effective in reducing acute and sub-acute stent thrombosis (ST). We studied whether IC bolus administration of abciximab is associated with a reduced ST and target vessels revascularization (TVR); therefore, less MACE rate compared with the standard intravenous IV bolus and infusion application.
Materials and Methods:
This was a single-center observational study conducted between June 2007 and 2009. We studied a total of 447 patients admitted with either acute coronary intervention (PCI) and stenting. Patients with bleeding disorder, recent major surgery and high blood pressure were excluded. Patients were divided into two groups: Group I (n = 199) patient received IC bolus of abciximab (reopro) 0.25 μg/kg during the PCI in cath lab. Group II (n = 248) received the standard dose of reopro-a bolus intravenous 0.25 μg/kg and maintenance dose of 0.125 μg/kg over 12 h.
Results:
There were no differences between the groups with regard to diabetes mellitus, group I (56%) vs. group II (58%), P = 0.613; ACS, group I (38%) vs. group II (44%), P = 0.175; Dietthylstilbestrol Drug eluted stent (DES) in group I (66.5%) vs. (57.6%) group II, P = 0.056; Bare Metal Stent (BMS) in group I (33%) vs. (40.7%) group II, P=0.093; target vessel revascularization (TRV) was seen in 9 patients (4%) in group I vs. 16 patients (6%) in group II. ST elevation was seen in 4 patients (2%) in group I vs. 7 patients (2.8%) in group II, all presented with STEMI.
Conclusion:
In this study, there was a trend toward less ST and TVR in patients who received IC reopro vs. intravenous route both in ACS and stable CAD. The percentage of DM was high in both groups (56%), especially in Saudi patients. In-stent restenosis (ISR) was less in group I than in group II, this was mainly associated with BMS usage. The percentage of BMS was more than 30% in both groups, either due to STEMI cases or large vessel size. Randomized controlled trials are warranted to further assess IC application of abciximab in reducing ST.
Keywords: Abciximab, acute coronary artery disease, intracoronary, intravenous, stent thrombosis, tirofiban
INTRODUCTION
In patients with acute stent thrombosis (ST)-elevation myocardial infarction, primary percutaneous coronary intervention (PCI) is the preferred reperfusion regimen if performed by experienced operators in a timely manner.[1]
In patients with acute myocardial infarction or unstable angina undergoing coronary angioplasty, abciximab reduces major adverse cardiac events (MACE). In Meta-analysis studies, when compared with the control group, adjunctive abciximab for STEMI is associated with a significant reduction in 30-day and long-term mortality in patients treated with primary angioplasty, but not in those receiving fibrinolysis.[2] The lower event rates in patients with U/A receiving abciximab were attributable to a reduction in the rate of myocardial infarction, particularly in patients with elevated troponin T.[3]
Intravenous abciximab administration has been studied in randomized clinical trials. Intracoronary (IC) abciximab bolus administration with very high local platelet inhibitor concentrations may be favorable in dissolution of thrombi and microemboli with subsequent improved myocardial microcirculation and reduced no reflow and infarct size.[4,5] In patients with Acute Myocardial Infarction (AMI), treated with primary PCI, early abciximab administration improves myocardial salvage and left ventricular function recovery probably by starting early recanalization of the infarct-related artery. Currently, clinical experience in the efficacy of IC abciximab administration is limited.[6,7] One of major randomized clinical trial of IC vs. intravenous abciximab administration in patients undergoing primary PCI for ST-elevation myocardial infarction showed a reduction in microvascular obstruction extent and infarct size as assessed by magnetic resonance imaging, which is a state-of-the-art imaging technique. Furthermore, myocardial tissue perfusion assessed by ST-segment resolution was significantly better after IC abciximab bolus administration. This resulted in a trend towards better clinical outcome with respect to a combined clinical end-point. However, in the acute setting after the index event, there was no improvement in the left ventricular function and volumes.[8]
MATERIALS AND METHODS
This was a single-center observational study conducted between June 2007 and 2009. We studied a total of 447 patients admitted with either acute coronary syndrome (ACS) or stable coronary artery disease (CAD) undergoing PCI and stenting patients were divided into two groups. Group I (n = 199) patients received IC bolus of abciximab (reopro) 0.25 μg/kg during the PCI in Catheterization Laboratory. Group II (n = 248) received the standard dose of reopro, an intravenous bolus 0.25 μg/kg and maintenance dose of 0.125 μg/kg over over 12 h. Exclusion were prior fibrinolysis. Patients with bleeding disorders, recent major surgery and high blood pressure were excluded, known allergy to heparin, aspirin, or abciximab; active severe bleeding; pregnancy; history of major surgery <4 weeks; and cardiogenic shock with mechanical ventilation.
Study medication protocol
As per protocol, the abciximab bolus administration was during PCI, after crossing the lesions either in stable CAD with PCI guide wire or after thrombus penetration in ACS patients to achieve very high local concentration. As per protocol, the abciximab bolus administration was during PCI. In the IC group I, in ACS (STEMI) patients, bolus administration was recommended after infarct-related artery recanalization by the PCI wire before balloon dilatation to allow high abciximab concentration in the target region. The bolus was administered directly through the guiding catheter. Filtering was performed before the abciximab solution was pulled into the syringe. Group I (n = 199) patients received IC bolus of abciximab (reopro) 0.25 μg/kg during the PCI in Catheterization Laboratory, group II (n = 248) received the standard dose of reopro, a bolus intravenous 0.25 μg/kg, and maintenance dose of 0.125 μg/kg over 12 h. All patients received 500 mg aspirin and heparin (60 U/kg body weight) intravenously before PCI. Stenting of the culprit lesion was recommended unless the vessel had a diameter <2.0 mm. Clopidogrel (600 mg, orally during PCI, if not administered before, followed by 75 mg/day for at least 12 months) was mandatory. Group II (n = 248) received the standard dose of reopro, a bolus intravenous 0.25 μg/kg and maintenance dose of 0.125 μg/kg over 12 h [Figure 1].
Figure 1.
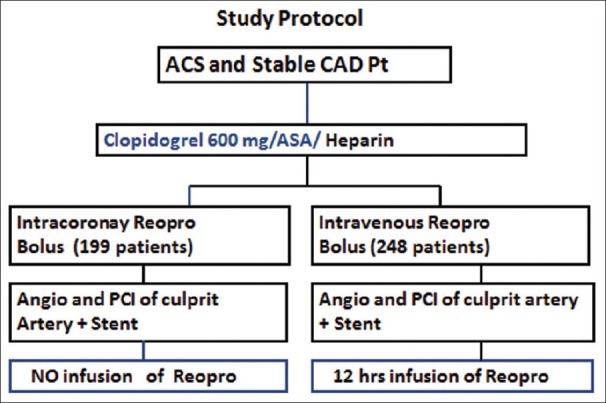
Study protocol
Angiographic analysis
Coronary angiography of the target lesion was performed before and after PCI with the same projections. Angiographic projections used were those that allowed optimal evaluation of the lesion. Angiographic analysis included initial and final lesion assessment including thrombus formation and in-stent restenosis (ISR). Visual assessments were performed off-line by two blinded interventional cardiologists.
Clinical outcome
At the 30-day follow-up, there were two cardiac deaths (1.0%) in the IC (group I) and three deaths (1.1%) in the intravenous abciximab (group II).
Statistical analysis
Differences between group means were assessed with the two-tailed, Student's t-test. Chi-square analysis or Fisher's exact test were used to evaluate differences between proportions. Statistical significance was defined by a P value of less than 0.05.
RESULTS
Patient's demographic are presented in Table 1. The mean age in group I and in group II were 55 and 52 years, respectively, and the majority of study patients were men (73%). Diabetes mellitus (DM) was the most prevalent risk factor for CAD in our study population. There were no significant differences between the groups with regards to DM, group I (56%) vs. group II (58%), P = 0.613. Hypertension was the second most prevalent risk factor.
Table 1.
Patients baseline characteristics in both groups, data presented as n (%)
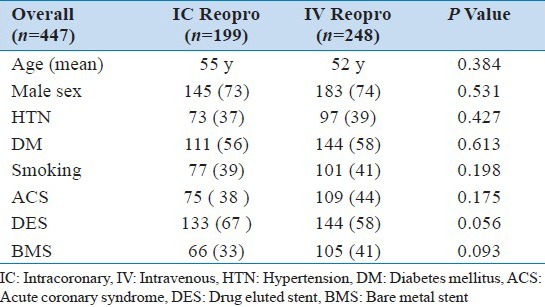
The incidence of ACS in group I was 38% and that in group II was 44% (P = 0.175). The usage of diethylstilbestrol drug eluted stent (DES) in group I was 66.5% vs. 57.6% in group II (P = 0.056). BMS in group I was 33% vs. 40.7% in group II (P = 0.093). Target vessels revascularization (TVR) was seen in 9 patients (4%) in group I vs. 16 patients (6%) in group II (P = 0.41) [Figure 2]. ST was seen in 4 patients (2%) in group I vs. 7 patients (2.8%) in group II (P = 0.76) [Figure 3]; all ST patients presented with STEMI.
Figure 2.
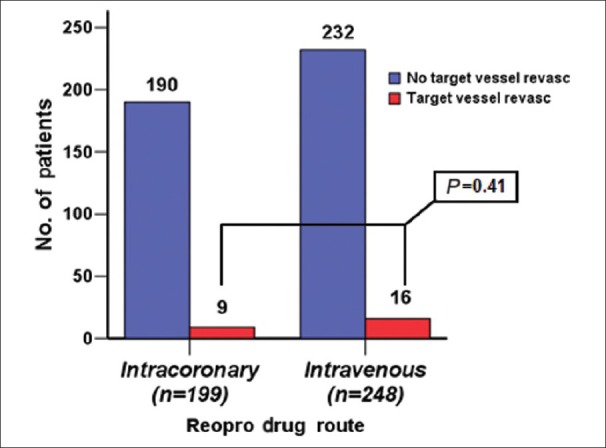
TVR Number of patients who had target vessel revascularization in each group
Figure 3.
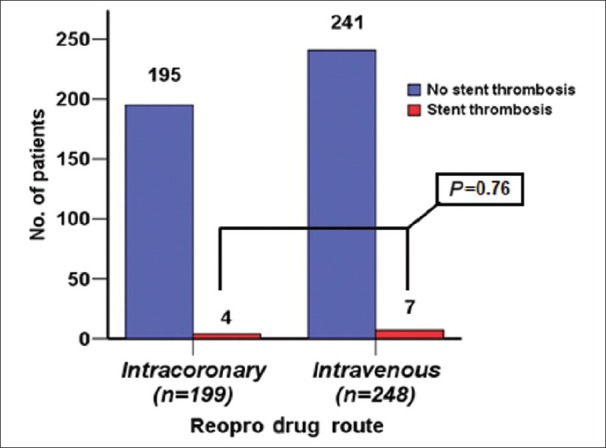
Number of patients who had stent thrombosis in each group
ISR was seen in 5 patients (2%) in group I vs. 16 patients (7%) in group II (P = 0.07) [Figure 4]. The sub-analysis of ISR according to the type of stent, clearly showed more ISR in BMS with 2 patients (3%) in group I vs. 11 patients (11%) in group II (P = 0.14) [Figure 5].
Figure 4.
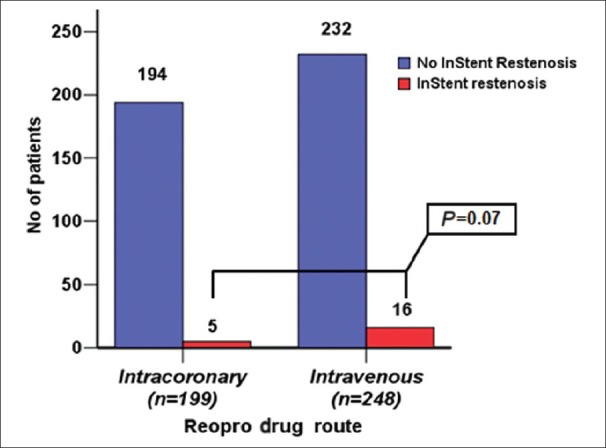
ISR Number of patients who had in-stent re-stenosis in each group
Figure 5.
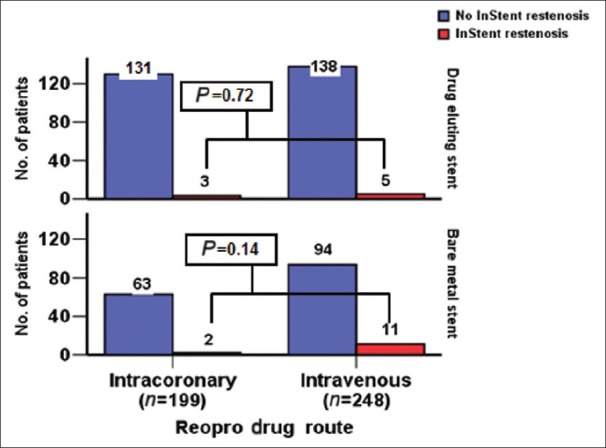
Number of patients comparing in-stent re-stenosis in both DES and BMS in each group
Definitions of ACS
The diagnosis of the different types of ACS was based on the definitions of the Joint Committee of the European Society of Cardiology/American College of Cardiology (ACC). Serum cardiac bio-markers, including troponin I, was done to assist in the diagnosis of myocardial injury in our patients.[9]
Definition of ST
Angiographic confirmation of a thrombus that originates in the stent or in the segment 5 mm proximal or distal to the stent, with or without vessel occlusion, which is associated with acute onset of ischemic symptoms at rest or electrocardiography (ECG) signs of acute ischemia or typical rise and fall in cardiac biomarkers within 48 h of angiography or pathologic confirmation of ST determined from tissue obtained following thrombectomy.
Primary end points
Target vessel revascularization (TVR)
Stent thrombosis (definite thrombus inside stent during angiography)
In-stent restenosis (ISR).
DISCUSSION
This study was conducted in a single center, comparing to the standard practice of IV administration of abciximab (reopro), a glycoprotein IIb/IIIa inhibitor to IC use during PCI in the Catheterization Laboratory. The rationale for this study was derived from the hypothesis that high local concentrations of drug facilitates the diffusion of the antibody to platelets inside the flow-limiting thrombus. Ideally, this would result in improved dissolution of thrombi and microemboli at the ruptured plaque and further downstream through the microcirculation.[10]
Another potential mechanism by which high local concentrations of abciximab may exert a beneficial effect relates to its anti-inflammatory properties. Abciximab also binds to the vitronectin receptor on endothelial, smooth muscle and inflammatory cells and to an activated conformation of the aMb2 receptor on leukocytes. Such cross-reactivity raises the possibility that clinical benefits derived from its use may not be exclusively due to its anti-thrombotic effect, but may be related to the suppression of inflammatory pathways involving platelets, white blood cells and the vascular endothelium. Based on this, the local administration of abciximab at the site of coronary thrombosis may enhance the effect by increasing its local concentration, the binding to both platelet, and endothelium receptors.[11] The results of several angiographic studies assessing the effect of IC abciximab administration support on clinical grounds its adoption in patients with fresh coronary thrombosis. Indeed, better post-angioplasty coronary flow, greater degree of myocardial salvage, and a better left ventricular function recovery have been achieved with a bolus compared to intravenous and systemic administration.[12]
These clinical considerations are supported by experimental data showing a dose-dependent platelet disaggregation.[13]
One of the observations of this study is that our cardiac Saudi patients were younger than Western patients, and the mean age was 55 years in group I and 52 years in group II; this could be explained by different factors such as younger populations in developing countries and poor control of CAD risk factors. The second observation is that a very high prevalence of DM in both groups were >56% and there are possible factors that can contribute to this such a obesity, sedentary lifestyle, and poor diet. Similar observations were reported in our national SPACE registry.[14]
TVR
The first finding was TVR. In this particular study, no significant difference in both study groups were seen. Nine patients out of 199 in group I had TRV vs. 16 patients out of 248 in group II (P = 0.41).
ST
The second finding is ST, this occurred in 4 patients (2%) out of 199 in group I vs. 7 patients (2.8%) out of 248 in group II (P = 0.76). These findings showed no difference in both the groups, which might explain the effect of anti-platelet regardless of the route of administration. Some studies showed less ST in patients who received abciximab, the rate was 1%, while it was 4.3% in patients not receiving abciximab treatment.[15]
ISR
Third finding, the ISR in 5 patients out of 199 in group I vs. 16 patients out of 248 in group II (P = 0.07). This showed no significant differences, but there was a trend toward less restenosis in group II. One study tested whether the dual β3 integrin blocking agent abciximab, administered for 12 or 24 h at the same intravenous dose as that shown to reduce adverse clinical events (death, infarction, and revascularization) after angioplasty, would reduce restenosis tissue volume, as measured by intravascular ultrasound (IVUS) at 6 months. Of the 215 patients who received stents and study drug, 191 (88.8%) returned for late (≥4 months) coronary evaluation. Tissue volume, expressed as a percentage of stent volume did not differ for the patients in the placebo and the 12- and 24-h abciximab groups (25 ± 15%, 27 ± 15%, and 29 ± 14%, respectively). Lack of abciximab benefit was confirmed by quantitative coronary angiography (dichotomous restenosis: 11.6%, 18.9%, and 19.4%; loss index: 0.33, 0.52, and 0.47, respectively, P = NS).[16]
BMS vs DES
The fourth finding was when we compared bare metal stent (BMS) with DES, there was more ISR in patients who received BMS 11 in 105 in group II vs. 2 in 65 in DES (P = 0.14). This difference was not significant in DES patients, and this is related to the benefit of DES in preventing intimal proliferation and ISR, as published in previous studies.
Early thrombosis (acute or sub-acute) after primary PCI has a very high rate of MACE, including death and re-infarction, and usually require repeat coronary angioplasty or surgery for management. Complex baseline angiographic morphology and smaller maximal balloon diameter are predictors of early thrombosis after primary PCI for AMI. The incidence of early thrombosis after primary angioplasty and stenting is decreased by abciximab use, and this was shown in CADILLAC trial that was done on STEMI patients.[17]
In Capture trial, more frequent thrombus resolution was observed and a higher angiographic success rate was achieved in patients treated with abciximab before and during angioplasty as compared with placebo. Patients with complex lesions as the underlying pathology reached fewer end-points if treated with abciximab before and during angioplasty.[18]
In the ISAR-REACT 2 trial, abciximab was compared with placebo in high-risk patients with an NSTE-ACS undergoing PCI after pre-treatment with 600 mg clopidogrel. The main finding is that the beneficial effects of abciximab observed at 30 days are maintained 1 year after the PCI procedure. The 1-year composite of death, myocardial infarction or TRV was reduced by 20% among patients who received abciximab when compared with patients who received placebo.[19]
An important factor is timing of glycoprotein IIb/IIIa inhibitor administration. Clinical trials have shown that earlier administration results in higher pre-interventional TIMI flow with subsequent improved perfusion after PCI.[20,21] However, in a pooled analysis and a recently published trial, there was no effect on mortality or clinical end points.[22]
CONCLUSION
In this study, there was a trend toward less ST and TVR in patients who received IC reopro vs. intravenous route both in ACS and stable CAD. The percentage of DM was high in both groups as reported before in Saudi patients. ISR was less in group I than in group II and this was mainly related to the use of BMS. BMS was >30% in both groups and this was either due to STEMI cases or large vessel size. a randomize trial is warranted to further assess IC application of abciximab in reducing ST.
Limitation of the study
This study was a single-center study. The patient number is medium size on comparing both groups. Abciximab bolus was given in both the groups but there was no infusion dose given in group I (IC).
ACKNOWLEDGMENT
We would like to acknowledge the support of the Emergency Department and Catheterization Laboratory of KACC for helping us in recruiting those patients who fit the criteria of the study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: Executive summary. Circulation. 2004;110:588–636. doi: 10.1161/01.CIR.0000134791.68010.FA. [DOI] [PubMed] [Google Scholar]
- 2.De Luca G, Suryapranata H, Stone GW, Antoniucci D, Tcheng JE, Neumann FJ, et al. Abciximab as adjunctive therapy to reperfusion in acute ST-segment elevation myocardial infarction: A meta-analysis of randomized trials. JAMA. 2005;293:1759–65. doi: 10.1001/jama.293.14.1759. [DOI] [PubMed] [Google Scholar]
- 3.Hamm CW, Heeschen C, Goldmann B, Vahanian A, Adgey J, Miguel CM, et al. Benefit of abciximab in patients with refractory unstable angina in relation to serum troponin T levels. c7E3 Fab Antiplatelet Therapy in Unstable Refractory Angina (CAPTURE) Study Investigators. N Engl J Med. 1999;340:1623–9. doi: 10.1056/NEJM199905273402103. [DOI] [PubMed] [Google Scholar]
- 4.Barsness GW, Buller C, Ohman EM, Schechter E, Pucillo A, Taylor MA, et al. Reduced thrombus burden with abciximab delivered locally before percutaneous intervention in saphenous vein grafts. Am Heart J. 2000;139:824–9. doi: 10.1016/s0002-8703(00)90014-0. [DOI] [PubMed] [Google Scholar]
- 5.Thuraisingham S, Tan KH. Dissolution of thrombus formed during direct coronary angioplasty with a single 10 mg intracoronary bolus dose of abciximab. Int J Clin Pract. 1999;53:604–7. [PubMed] [Google Scholar]
- 6.Bellandi F, Maioli M, Gallopin M, Toso A, Dabizzi RP. Increase of myocardial salvage and left ventricular function recovery with intracoronary abciximab downstream of the coronary occlusion in patients with acute myocardial infarction treated with primary coronary intervention. Catheter Cardiovasc Interv. 2004;62:186–92. doi: 10.1002/ccd.20041. [DOI] [PubMed] [Google Scholar]
- 7.Galache Osuna JG, Sánchez-Rubio J, Calvo I, Diarte JA, Lukic A, Placer LJ. Does intracoronary abciximab improve the outcome of percutaneous coronary interventions? A randomized controlled trial. Rev Esp Cardiol. 2006;59:567–74. [PubMed] [Google Scholar]
- 8.Thiele H, Schindler K, Friedenberger J, Eitel I, Fürnau G, Grebe E, et al. Intracoronary compared with intravenous bolus abciximab application in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention: The randomized Leipzig immediate percutaneous coronary intervention abciximab IV versus IC in ST-elevation myocardial infarction trial. Circulation. 2008;118:49–57. doi: 10.1161/CIRCULATIONAHA.107.747642. [DOI] [PubMed] [Google Scholar]
- 9.Cannon CP, Battler A, Brindis RG, Cox JL, Ellis SG, Every NR, et al. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes. A report of the American college of cardiology task force on clinical data standards (Acute Coronary Syndromes Writing Committee) J Am Coll Cardiol. 2001;38:2114–30. doi: 10.1016/s0735-1097(01)01702-8. [DOI] [PubMed] [Google Scholar]
- 10.Mascelli MA, Lance ET, Damaraju L, Wagner CL, Weisman HF, Jordan RE. Pharmacodynamic profile of short-term abciximab treatment demonstrates prolonged platelet inhibition with gradual recovery from GPIIb/IIIa receptor blockade. Circulation. 1998;97:1680–8. doi: 10.1161/01.cir.97.17.1680. [DOI] [PubMed] [Google Scholar]
- 11.Neumann FJ, Blasini R, Schmitt C, Alt E, Dirschinger J, Gawaz M, et al. Effect of glycoprotein IIb/IIIa receptor blockade on recovery of coronary flow and left ventricular function after the placement of coronary-artery stents in acute myocardial infarction. Circulation. 1998;98:2695–701. doi: 10.1161/01.cir.98.24.2695. [DOI] [PubMed] [Google Scholar]
- 12.Romagnoli E, Burzotta F, Trani C, Biondi-Zoccai GG, Giannico F, Crea F. Rationale for intracoronary administration of abciximab. J Thromb Thrombolysis. 2007;23:57–63. doi: 10.1007/s11239-006-9000-0. [DOI] [PubMed] [Google Scholar]
- 13.Marciniak SJ, Mascelli MA, Furman MI, Michelson AD, Jakubowski A, Jordan RE, et al. An additional mechanism of action of abciximab: Dispersal of newly formed platelet aggregates. Thromb Haemost. 2002;87:1020–5. [PubMed] [Google Scholar]
- 14.AlHabib KF, Hersi A, AlFaleh H, Kurdi M, Arafah M, Youssef M, et al. The Saudi project for assessment of coronary events (SPACE) registry: Design and results of a phase I pilot study. Can J Cardiol. 2009;25:255–8. doi: 10.1016/s0828-282x(09)70513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Migliorini A, Valenti R, Moschi G, Parodi G, Cerisano G, Buonamici P, et al. Predictor of stent thrombosis in patients treated with turbostratic carbon-coated stent implantation for acute myocardial infarction. J Interv Cardiol. 2010;23:554–9. doi: 10.1111/j.1540-8183.2010.00564.x. [DOI] [PubMed] [Google Scholar]
- 16.Acute platelet inhibition with abciximab does not reduce in-stent restenosis (ERASER study). The ERASER Investigators. Circulation. 1999;100:799–806. doi: 10.1161/01.cir.100.8.799. [DOI] [PubMed] [Google Scholar]
- 17.Dangas G, Aymong ED, Mehran R, Tcheng JE, Grines CL, Cox DA, et al. Predictors of and outcomes of early thrombosis following balloon angioplasty versus primary stenting in acute myocardial infarction and usefulness of abciximab (the CADILLAC trial) Am J Cardiol. 2004;94:983–8. doi: 10.1016/j.amjcard.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 18.van den Brand M, Laarman GJ, Steg PG, De Scheerder I, Heyndrickx G, Beatt K, et al. Assessment of coronary angiograms prior to and after treatment with abciximab, and the outcome of angioplasty in refractory unstable angina patients. Angiographic results from the CAPTURE trial. Eur Heart J. 1999;20:1572–8. doi: 10.1053/euhj.1999.1662. [DOI] [PubMed] [Google Scholar]
- 19.Ndrepepa G, Kastrati A, Mehilli J, Neumann FJ, Ten Berg J, Bruskina O, et al. One-year clinical outcomes with abciximab vs. placebo in patients with non-ST-segment elevation acute coronary syndromes undergoing percutaneous coronary intervention after pre-treatment with clopidogrel: Results of the ISAR-REACT 2 randomized trial. Eur Heart J. 2008;29:455–61. doi: 10.1093/eurheartj/ehm562. [DOI] [PubMed] [Google Scholar]
- 20.Godicke J, Flather M, Noc M, Gyongyosi M, Arntz HR, Grip L, et al. Early versus periprocedural administration of abciximab for primary angioplasty: A pooled analysis of 6 studies. Am Heart J. 2005;150:1015. doi: 10.1016/j.ahj.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 21.Maioli M, Bellandi F, Leoncini M, Toso A, Dabizzi RP. Randomized early versus late abciximab in acute myocardial infarction treated with primary coronary intervention (RELAx-AMI Trial) J Am Coll Cardiol. 2007;49:1517–24. doi: 10.1016/j.jacc.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 22.Ellis SG, Tendera M, de Belder MA, van Boven AJ, Widimsky P, Janssens L, et al. Facilitated PCI in patients with ST-elevation myocardial infarction. N Engl J Med. 2008;358:2205–17. doi: 10.1056/NEJMoa0706816. [DOI] [PubMed] [Google Scholar]


