Abstract
Borna disease virus (BDV) causes neurological diseases in a variety of warm-blooded animal species, possibly including humans. To date, there is no effective treatment against BDV infection. Recently, we reported on the antiviral activity of 1-β-d-arabinofuranosylcytosine (Ara-C). However, Ara-C's cytotoxic side effects are a major obstacle for its therapeutic use. Herein, we demonstrate that the nucleoside analog 2′-fluoro-2′-deoxycytidine (2′-FdC) exhibits potent antiviral activity against BDV. Importantly, 2′-FdC-associated cytotoxicity is negligible, indicating 2′-FdC as an excellent candidate for the development of antiviral therapy against BDV.
Borna disease virus (BDV) is a nonsegmented, negative-stranded neurotropic RNA virus (16). BDV is the causative agent of Borna disease, a neurological disorder of horses, sheep, and other farm animals (17). Recent evidence suggests that BDV is distributed worldwide, with a principal focus in Central Europe (25). Natural infections with BDV have been reported in a large variety of warm-blooded animal species, among which are dogs (27) and cats (19). There is considerable evidence that BDV also infects humans (20), making it a possible zoonotic agent. Human BDV infection has been claimed to be associated with certain neuropsychiatric disorders (3, 20), although the epidemiology and the clinical consequences of human infection remain controversial (5, 20).
The importance of BDV infection in veterinary medicine, along with its possible association with human neuropsychiatric disorders, has inspired many groups to search for antiviral drugs against BDV. To date, there is no effective treatment against BDV. Amantadine was initially reported to have some antiviral activity against BDV (2) and has been used in clinical trials of BDV-positive patients with neuropsychiatric disorders (7-9). However, the antiviral activity could not be confirmed in other studies (6, 12, 26). It was suggested that the positive effect of amantadine on clinical parameters in these trials might be attributable instead to the described pharmacological activity of amantadine as a noncompetitive N-methyl-d-aspartate receptor antagonist (13). Several previous studies reported on the inhibitory effect of ribavirin on BDV replication (14, 18), but the effects were modest in vitro and in vivo (24). Recently, we have reported that the nucleoside analog 1-β-d-arabinofuranosylcytosine (Ara-C) possesses potent antiviral activity against BDV (1). However, the cytotoxic side effects of Ara-C impede its therapeutic use against BDV infection. This obstacle inspired us to search for other compounds with anti-BDV activity but with less associated cytotoxicity. We selected two Ara-C-related cytosine nucleosides, 2′-fluoro-2′-deoxycytidine (2′-FdC) and 2′,2′-difluoro-2′-deoxycytidine (gemcitabine), which have one or two fluorine atoms at the 2′ position of the ribose ring (where Ara-C has a hydroxyl group) (Fig. 1). 2′-FdC is a fluoronucleoside for which a moderate antiviral activity toward human immunodeficiency virus and herpesviruses has been demonstrated previously (23, 28). Antiviral activity of 2′-FdC has never been demonstrated toward negative-stranded RNA viruses. It is interesting that previous studies have highlighted the minimal toxicity of 2′-FdC, in particular when compared to that of Ara-C. More detailed studies demonstrated that long-term treatment (90 days) of rats and woodchucks with dosages of up to 500 mg of 2′-FdC/kg of body weight/day was relatively nontoxic (21) in spite of the incorporation of 2′-FdC in liver DNA (22).
FIG. 1.
Molecular structures of Ara-C, 2′-FdC, and gemcitabine.
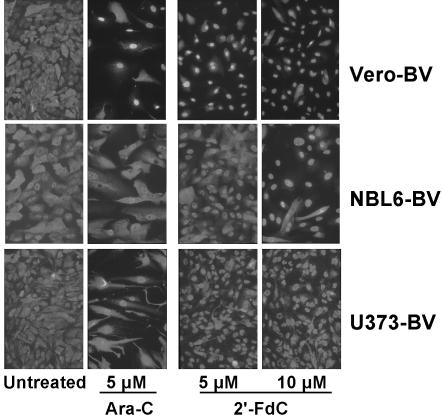
The antiviral activity of 2′-FdC was assessed by using different in vitro tests. Previously, we showed that inhibition of BDV replication by Ara-C was associated with a nuclear retention of BDV antigens in infected cells (1). We took advantage of this characteristic to rapidly screen 2′-FdC and gemcitabine for their potential to inhibit BDV replication in Vero cells that were persistently infected with BDV (i.e., Vero-BV). Using immunofluorescence analysis of BDV nucleoprotein expression, we observed that of these two compounds, only 2′-FdC caused nuclear retention of BDV antigens as Ara-C does (Fig. 2). In addition, we tested Ara-C and 2′-FdC on two other cell lines persistently infected with BDV, equine dermis cells (NBL6-BV) and human astrocytoma cells (U373-BV). To our surprise, treatment of those cell lines with Ara-C did not result in a nuclear retention of viral proteins as robust as that seen in Vero-BV cells, whereas the cytotoxic side effects of Ara-C treatment (reduced cell density and altered shape of the cells) were clearly visible (Fig. 2). In contrast, the characteristic nuclear accumulation of BDV antigen was readily detectable upon 2′-FdC treatment (Fig. 2), suggesting enhanced sensitivity of these cell lines to 2′-FdC treatment compared to treatment with Ara-C. Thus, the ability of 2′-FdC to inhibit BDV dissemination was confirmed in a cell line of equine origin (NBL6), the natural BDV host for which antiviral therapy is most sought. In addition, we also showed that 2′-FdC was a potent inhibitor of BDV infection and spread in primary hippocampal rat neurons (>98% inhibition, data not shown). This finding is especially relevant since in vivo, BDV replicates and persists predominantly in neurons of the limbic system (11).
FIG. 2.
2′-FdC treatment results in nuclear retention of BDV nucleocapsid protein (N) in Vero-BV cells (top), NBL6-BV cells (middle), and U373-BV cells (bottom). Cells were treated or not for 5 days and stained with anti-N antibody followed by fluorescein isothiocyanate-conjugated anti-rabbit antibody. Original magnification, ×150.
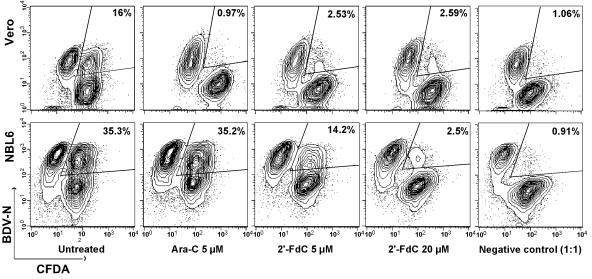
Since BDV infectivity is tightly cell associated (10) and efficient transmission of BDV is likely to require cell-to-cell contact, we next used a sensitive assay to analyze cell-to-cell spread of BDV (1). Confluent layers of Vero or NBL6 cells were labeled with 5 (and 6)-carboxyfluorescein diacetate (CFDA) and subsequently cocultivated for 5 days with unlabeled Vero-BV or NBL6-BV cells. Cocultivation took place under daily treatment with different doses of Ara-C, 2′-FdC, or gemcitabine. Both Ara-C and 2′-FdC exerted potent anti-BDV activity on Vero-BV cells (Fig. 3), whereas gemcitabine did not show anti-BDV activity (data not shown). In order to correct for the antimitotic effects of Ara-C on the different cell populations in the dissemination assay, we calculated viral spread indices between Vero-BV and Vero cells treated with Ara-C or 2′-FdC (Table 1). As shown in Table 1, treatment with 2′-FdC resulted in similar inhibition of BDV spread compared to that of Ara-C. For NBL6-BV cells, gemcitabine had no effect on BDV dissemination, and activity of Ara-C was limited. In contrast, 2′-FdC was as active as it was on Vero-BV cells (Fig. 3).
FIG. 3.
2′-FdC inhibits BDV cell-to-cell spread. Confluent Vero (top row) or NBL6 (bottom row) cells were labeled with CFDA and subsequently cocultivated for 5 days at a ratio of 1:1 with unlabeled Vero-BV or NBL6-BV cells. Cocultivation took place under daily treatment with 5 μM Ara-C or with 5 or 20 μM 2′-FdC. Thereafter, cells were analyzed by flow cytometry. The percentages shown in the figure indicate the double-positive population within the population of viable cells, which is indicative of the amount of viral spread. The negative control consisted of a 1:1 mixture of CFDA-labeled Vero or NBL6 cells with Vero-BV or NBL6-BV cells, respectively, which were fixed directly after mixing.
TABLE 1.
Normalized viral spread indices (SI) and percent inhibition of viral spread of 2′-FdC and Ara-C treatments measured in dissemination assays between Vero-BV and Vero cellsa
| Treatment (concn) | Normalized SIb | Inhibition (%) |
|---|---|---|
| Untreated | 100 | 0 |
| 2′-FdC (5 μM) | 12.21 ± 0.39 | 87.8 ± 0.4 |
| 2′-FdC (10 μM) | 7.79 ± 2.11 | 92.2 ± 2.1 |
| 2′-FdC (20 μM) | 9.44 ± 2.03 | 90.6 ± 2 |
| Ara-C (5 μM) | 3.77 ± 0.82 | 96.2 ± 0.8 |
Values reflect the means of four independent experiments (± standard deviation).
Viral spread index for viral spread was calculated using the following formula: [(number of double-positive cells/total number of cells positive for CFDA)/number of cells single positive for BDV-N antigen] × 100. Thereafter, spread indices were normalized to the value obtained in the untreated cell control for each experiment.
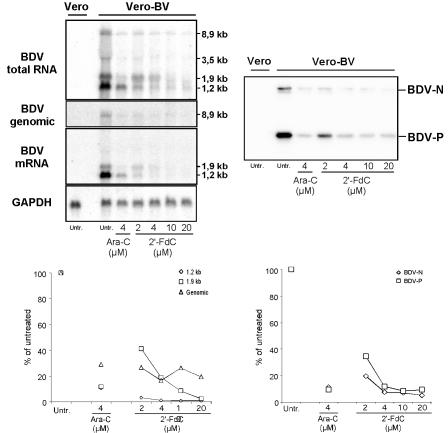
We next assessed the effects of 2′-FdC treatment on viral RNA levels in Vero-BV cells by analyzing total cellular RNA by Northern blotting. We used strand-specific oligonucleotide probes for BDV mRNA and BDV genomic RNA, and we used random-primed probes for BDV total RNA and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; a host cell housekeeping gene) mRNA (Fig. 4). We observed a dose-dependent inhibition of both BDV genomic RNA (up to 84%) and BDV mRNA (up to 99% inhibition of the 1.2-kb transcript coding for BDV-N) upon treatment with 2′-FdC. In addition, Western blotting showed that the expression levels of BDV-N and phosphoprotein (P) were decreased upon 2′-FdC treatment (Fig. 4). Quantification of the signal showed up to 95% inhibition of BDV-N levels compared to expression levels in untreated cells and showed up to 90% inhibition for BDV-P (Fig. 4). At a comparable dose (4 μM), 2′-FdC was as effective as Ara-C in inhibiting BDV RNA and protein levels.
FIG. 4.
BDV RNA and protein expression levels are inhibited by 2′-FdC treatment. Total cellular RNA and proteins from Vero-BV cells were extracted at day 5 of treatment and analyzed. (Upper left) Northern blot analysis of BDV total RNA, genomic RNA, and mRNA levels using a random-primed cDNA probe and strand-specific oligonucleotide probes. The expected sizes of the different BDV mRNAs and genomic RNA are indicated. (Lower left) Quantification of BDV mRNA (1.2 and 1.9 kb) and genomic BDV RNA levels was done by phosphorimager analysis and was thereafter standardized to GAPDH levels. Results are expressed as the percentages of untreated control levels, which were set to 100%. (Upper right) Western blot analysis. Equal amounts of protein were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a Hybond membrane, and reacted with anti-N and anti-P antibodies (as indicated). (Lower right) Quantification of BDV protein levels was done by using a LAS-1000+ Imager. Untr., untreated.
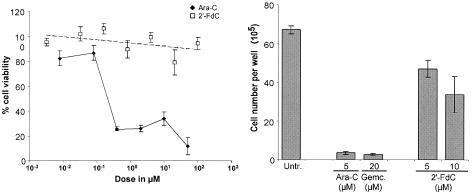
Treatment with nucleoside analogs is typically accompanied by cytotoxic side effects. To assess the potential cytotoxic effects of treatment with 2′-FdC, Vero cells were treated with different concentrations of 2′-FdC or Ara-C, and cell viability was measured by using the Uptiblue viable cell counting assay (Interchim), which is based on the detection of metabolic activity of the cells. Ara-C treatment produced a sharp, concentration-dependent reduction in cell viability (74%). In contrast, treatment with up to 100 μM 2′-FdC did not result in a concentration-dependent loss of cell viability. Reduction of cell viability was limited to a maximum of 21% at a concentration of 20 μM (Fig. 5, left). Treatment with concentrations of 2′-FdC that inhibit BDV replication (5 to 10 μM) did not result in a loss of cell viability. In order to test for potential cytostatic effects, we compared the number of cells after 5 days of daily treatment with Ara-C, 2′-FdC, gemcitabine, or no treatment. Treatment with 5 μM Ara-C resulted in a 20-fold reduction of cell numbers (Fig. 5, right). This reduction was comparable to the reduction of cell numbers after treatment with 20 μM gemcitabine, which is well known for its cytostatic potential (4). In contrast, treatment with 10 μM 2′-FdC resulted in an only twofold reduction of total cell numbers compared to that of untreated cells (Fig. 5, right).
FIG. 5.
2′-FdC is not cytotoxic at concentrations that inhibit BDV replication. (Left) Cell viability analysis. Cell metabolic activity was measured quantitatively by using the Uptiblue viable cell counting assay. Subsequently, cell viability of treated Vero cells was expressed relative to that of untreated Vero cells. (Right) Analysis of cellular division. Vero cells were seeded at the same density in six-well plates; treated daily with 2′-FdC, Ara-C, or gemcitabine for 5 days; collected; and counted. Untr., untreated.
In this study, we describe for the first time that the nucleoside analog 2′-FdC exhibits antiviral activity against BDV. Its activity is comparable to that of Ara-C. In addition, some cell lines exhibit enhanced susceptibility to the antiviral action of 2′-FdC when compared to that of Ara-C. This difference in sensitivity of the tested cell lines to 2′-FdC might be accounted for by variations in intracellular content of the cellular enzyme deoxycytidine kinase (dCK). Once in the cell, dCK converts cytidine/deoxycytidine (its natural substrates), 2′-FdC, and Ara-C into their monophosphorylated metabolites. These are in turn converted into triphosphorylated metabolites by nucleoside monophosphate kinase and nucleoside diphosphate kinase, respectively. During this process, the phosphorylation by dCK is the rate-limiting step. As a consequence, the antiviral activities of 2′-FdC and Ara-C are directly dependent on the availability of dCK in the cell. Since the affinity of dCK for 2′-FdC is much stronger than its affinity for Ara-C (15), 2′-FdC might be more effective than Ara-C in cells with a relatively low dCK content. Finally, since 2′-FdC is not cytotoxic, even at concentrations that exceed the effective dose by 5- to 10-fold, it represents the most attractive candidate to date for the development of effective antiviral therapy against BDV.
Acknowledgments
This work was supported by grants from the Institut Pasteur, the Pasteur-Weizmann council, the CNRS, and INSERM Avenir. J.J.B. is a recipient of a Marie Curie fellowship of the European Community program “Improving Human Research Potential and the Socio-Economic Knowledge Base” under contract number HPMF-CT-2000-01088. R.V. is sponsored by a grant from the French Ministry of Research.
We thank M. Brahic, S. B. Geutskens, A. Hans, and L. Sartorius for critically reading the manuscript.
REFERENCES
- 1.Bajramovic, J. J., S. Syan, M. Brahic, J. C. De La Torre, and D. Gonzalez-Dunia. 2002. 1-β-d-Arabinofuranosylcytosine inhibits Borna disease virus replication and spread. J. Virol. 76:6268-6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bode, L., D. E. Dietrich, R. Stoyloff, H. M. Emrich, and H. Ludwig. 1997. Amantadine and human Borna disease virus in vitro and in vivo in an infected patient with bipolar depression. Lancet 349:178-179. [DOI] [PubMed] [Google Scholar]
- 3.Bode, L., W. Zimmermann, R. Ferszt, F. Steinbach, and H. Ludwig. 1995. Borna disease virus genome transcribed and expressed in psychiatric patients. Nat. Med. 1:232-236. [DOI] [PubMed] [Google Scholar]
- 4.Bouffard, D. Y., L. F. Momparler, and R. L. Momparler. 1991. Comparison of antineoplastic activity of 2′,2′-difluorodeoxycytidine and cytosine arabinoside against human myeloid and lymphoid leukemic cells. Anti-Cancer Drugs 2:49-55. [DOI] [PubMed] [Google Scholar]
- 5.Carbone, K. M. 2001. Borna disease virus and human disease. Clin. Microbiol. Rev. 14:513-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cubitt, B., and J. C. de la Torre. 1997. Amantadine does not have antiviral activity against Borna disease virus. Arch. Virol. 142:2035-2042. [DOI] [PubMed] [Google Scholar]
- 7.Dietrich, D. E., L. Bode, C. W. Spannhuth, T. Lau, T. J. Huber, B. Brodhun, H. Ludwig, and H. M. Emrich. 2000. Amantadine in depressive patients with Borna disease virus (BDV) infection: an open trial. Bipolar Disord. 2:65-70. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich, D. E., A. Kleinschmidt, U. Hauser, U. Schneider, C. W. Spannhuth, K. Kipp, T. J. Huber, B. M. Wieringa, H. M. Emrich, and S. Johannes. 2000. Word recognition memory before and after successful treatment of depression. Pharmacopsychiatry 33:221-228. [DOI] [PubMed] [Google Scholar]
- 9.Ferszt, R., K. P. Kuhl, L. Bode, E. W. Severus, B. Winzer, A. Berghofer, G. Beelitz, B. Brodhun, B. Muller-Oerlinghausen, and H. Ludwig. 1999. Amantadine revisited: an open trial of amantadinesulfate treatment in chronically depressed patients with Borna disease virus infection. Pharmacopsychiatry 32:142-147. [DOI] [PubMed] [Google Scholar]
- 10.Gosztonyi, G., B. Dietzschold, M. Kao, C. E. Rupprecht, H. Ludwig, and H. Koprowski. 1993. Rabies and Borna disease. A comparative pathogenetic study of two neurovirulent agents. Lab. Investig. 68:285-295. [PubMed] [Google Scholar]
- 11.Gosztonyi, G., and H. Ludwig. 1995. Borna disease—neuropathology and pathogenesis, p. 39-73. In H. Koprowski and I. Lipkin (ed.), Borna disease. Springer-Verlag KG, Berlin, Germany. [PubMed]
- 12.Hallensleben, W., M. Zocher, and P. Staeheli. 1997. Borna disease virus is not sensitive to amantadine. Arch. Virol. 142:2043-2048. [DOI] [PubMed] [Google Scholar]
- 13.Huber, T. J., D. E. Dietrich, and H. M. Emrich. 1999. Possible use of amantadine in depression. Pharmacopsychiatry 32:47-55. [DOI] [PubMed] [Google Scholar]
- 14.Jordan, I., T. Briese, D. R. Averett, and W. I. Lipkin. 1999. Inhibition of Borna disease virus replication by ribavirin. J. Virol. 73:7903-7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kierdaszuk, B., K. Krawiec, Z. Kazimierczuk, U. Jacobsson, N. G. Johansson, B. Munch-Petersen, S. Eriksson, and D. Shugar. 1999. Substrate/inhibitor properties of human deoxycytidine kinase (dCK) and thymidine kinases (TK1 and TK2) towards the sugar moiety of nucleosides, including O′-alkyl analogues. Nucleosides Nucleotides 18:1883-1903. [DOI] [PubMed] [Google Scholar]
- 16.Kishi, M., K. Tomonaga, P. K. Lai, and J. C. de la Torre. 2002. Borna disease virus molecular virology, p. 23-44. In K. Carbone (ed.), Borna disease virus and its role in neurobehavioral disease. ASM Press, Washington D.C.
- 17.Ludwig, H., L. Bode, and G. Gosztonyi. 1988. Borna disease: a persistent virus infection of the central nervous system. Prog. Med. Virol. 35:107-151. [PubMed] [Google Scholar]
- 18.Mizutani, T., H. Inagaki, K. Araki, H. Kariwa, J. Arikawa, and I. Takashima. 1998. Inhibition of Borna disease virus replication by ribavirin in persistently infected cells. Arch. Virol. 143:2039-2344. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura, Y., M. Watanabe, W. Kamitani, H. Taniyama, T. Nakaya, Y. Nishimura, H. Tsujimoto, S. Machida, and K. Ikuta. 1999. High prevalence of Borna disease virus in domestic cats with neurological disorders in Japan. Vet. Microbiol. 70:153-169. [DOI] [PubMed] [Google Scholar]
- 20.Planz, O., K. Bechter, and M. Schwemmle. 2002. Human Borna disease virus infection, p. 179-226. In K. Carbone (ed.), Borna disease virus and its role in neurobehavioral disease. ASM Press, Washington D.C.
- 21.Richardson, F. C., B. C. Tennant, D. J. Meyer, K. A. Richardson, P. C. Mann, G. R. McGinty, J. L. Wolf, P. M. Zack, and R. A. Bendele. 1999. An evaluation of the toxicities of 2′-fluorouridine and 2′-fluorocytidine-HCl in F344 rats and woodchucks (Marmota monax). Toxicol. Pathol. 27:607-617. [DOI] [PubMed] [Google Scholar]
- 22.Richardson, F. C., C. Zhang, S. R. Lehrman, H. Koc, J. A. Swenberg, K. A. Richardson, and R. A. Bendele. 2002. Quantification of 2′-fluoro-2′-deoxyuridine and 2′-fluoro-2′-deoxycytidine in DNA and RNA isolated from rats and woodchucks using LC/MS/MS. Chem. Res. Toxicol. 15:922-926. [DOI] [PubMed] [Google Scholar]
- 23.Sato, Y., K. Utsumi, T. Maruyama, T. Kimura, I. Yamamoto, and D. Richman. 1994. Synthesis and hypnotic and anti-human immunodeficiency virus-1 activities of N3-substituted 2′-deoxy-2′-fluorouridines. Chem. Pharm. Bull. 42:595-598. [DOI] [PubMed] [Google Scholar]
- 24.Solbrig, M. V., R. Schlaberg, T. Briese, N. Horscroft, and W. I. Lipkin. 2002. Neuroprotection and reduced proliferation of microglia in ribavirin-treated bornavirus-infected rats. Antimicrob. Agents Chemother. 46:2287-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staeheli, P., C. Sauder, J. Hausmann, F. Ehrensperger, and M. Schwemmle. 2000. Epidemiology of Borna disease virus. J. Gen. Virol. 81:2123-2135. [DOI] [PubMed] [Google Scholar]
- 26.Stitz, L., O. Planz, and T. Bilzer. 1998. Lack of antiviral effect of amantadine in Borna disease virus infection. Med. Microbiol. Immunol. 186:195-200. [DOI] [PubMed] [Google Scholar]
- 27.Weissenbock, H., N. Nowotny, P. Caplazi, J. Kolodziejek, and F. Ehrensperger. 1998. Borna disease in a dog with lethal meningoencephalitis. J. Clin. Microbiol. 36:2127-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wohlrab, F., A. T. Jamieson, J. Hay, R. Mengel, and W. Guschlbauer. 1985. The effect of 2′-fluoro-2′-deoxycytidine on herpes virus growth. Biochim. Biophys. Acta 824:233-242. [DOI] [PubMed] [Google Scholar]