Abstract
Background
Improving neurocognitive abilities is a treatment priority in schizophrenia, however, pharmacological efforts to enhance deficits after illness onset have resulted in quite modest results that are of questionable clinical meaningfulness. Individuals at clinical risk for psychosis demonstrate neurocognitive impairments intermediate to the level of deficits observed in schizophrenia and normative performance, suggesting that a similar magnitude of improvement might result in more clinically meaningful change. In this study, we examined neurocognitive changes after six months of treatment in adolescents with clinical signs of risk for psychosis.
Methods
Adolescents who were referred to the Recognition and Prevention program, which is focused on treatment and research for individuals at a clinical high risk for psychosis, were followed in a naturalistic treatment design. At study entry and approximately six months after starting treatment, we examined neuropsychological functioning and clinical symptoms for patients who remained off medications (OFF; N=27), started selective serotonin reuptake inhibitor antidepressant medication (AD; N=15), or started a second-generation antipsychotic medication (AP; N=11) within three months of study entry. We also included a locally recruited healthy comparison group (HC; N=17).
Results
The clinical groups were not significantly different on baseline demographic, neurocognitive, or clinical symptom measures. Linear mixed models were used to examine cognitive changes, with time between assessments, depressive symptom severity, and attenuated positive symptom severity as random effects. Group by time effects were observed in sustained attention and verbal learning, with the AD group showing a more favorable response than the AP group. The AD group’s improvements were not significantly different from the HC or OFF group.
Conclusion
Early intervention for those at clinical high risk for psychosis may result in neurocognitive improvements. These improvements were observed for those prescribed antidepressant, but not antipsychotic medications even though the groups did not differ in clinical symptom severity or treatment response.
Keywords: Schizophrenia, Prodrome, High risk, Neurocognition, Pharmacological treatment
1. Introduction
As a core feature and the strongest predictor of disability in schizophrenia (Green, 1996; Bowie et al., 2006), neurocognitive impairments have emerged as one of the most important treatment candidates for the illness. Demonstrable, though quite modest, improvements in several functionally-relevant neurocognitive domains, such as verbal learning and memory, attention, language skills, working memory, and executive functions have been observed in schizophrenia patients following treatment with second-generation antipsychotic medications (for reviews, see Harvey and Keefe, 2001; Woodward et al., 2005). Although these studies found statistically significant neurocognitive improvements, the clinical and functional meaningfulness of the results are questionable, since chronic schizophrenia is associated with a degree of neurocognitive impairment that would require much larger effects to bring functioning to within the normal range. The CATIE study, a large-scale and methodologically rigorous comparison of several second-generation antipsychotic medications with an older “typical” antipsychotic medication, failed to support a distinct cognitive advantage for the newer medications (Keefe et al., 2007). Indeed, it has been argued that the magnitude of cognitive improvement observed with second-generation antipsychotic medications is consistent with a mere practice effect (Goldberg et al., 2007). To truly normalize the neurocognitive dysfunction associated with schizophrenia, treatment effects will need to be much larger or addressed during a phase where impairment is less substantial. Accordingly, several large-scale programs designed to study and treat children and adolescents who are considered at clinical or genetic high-risk for psychosis have a strong emphasis on the identification and treatment of neurocognitive impairments during this phase of the illness, with the hope that early intervention may improve outcomes. This period represents a critical developmental window where opportunities to acquire social and adaptive skills depend on the individuals’ neurocognitive abilities, particularly in the early stages of psychosis (Nuechterlein et al., 2011).
Neurocognitive impairments have long been recognized in children and adolescents who are at high-risk for the development of psychosis. Studies of individuals considered to be at genetic high-risk for schizophrenia, by virtue of having a first degree relative with the illness, reveal widespread neurocognitive impairment of mild to moderate severity, most markedly in attention, working memory, executive functions, and verbal declarative memory (Asarnow et al., 1977; Harvey et al., 1981; Winters et al., 1981; Nuechterlein, 1983; Cornblatt and Erlenmeyer-Kimling, 1985; Wolf et al., 2002; Seidman et al., 2006). Prospective studies of adolescents considered to be at clinical high risk, by virtue of having attenuated symptoms of psychosis, have revealed a similar profile of impairment (Hambrecht et al., 2002; Gschwandtner et al., 2003; Hawkins et al., 2004; Gschwandtner et al., 2005; Lencz et al., 2006; Seidman et al., 2010; Woodberry et al., 2010; Carrión et al., 2011). It is important to note that although individuals in high-risk samples share a pattern of neurocognitive deficits with adults with schizophrenia, they differ in the magnitude of impairment; findings consistently place performance during this period midway between the healthy control mean score and scores observed at or after the first episode of psychosis, at which point they appear to be relatively stable (Bilder et al., 2000; Keefe et al., 2006; Hawkins et al., 2008; LePage et al., 2008; Jahshan et al., 2010). That is, impairments across cognitive measures in high-risk individuals tend to be between 0.5 and 1.5 standard deviation scores below normative performance, rather than the deficit of 1.5 to 3 standard deviations observed after illness onset.
This milder profile of deficits in those at risk for psychosis is promising for treatment in two ways. First, there is evidence that neurocognitive and symptom improvement may be more likely in individuals whose impairments are minimal, rather than severe or profound (Fiszdon et al., 2005; Medalia and Richardson, 2005). Second, if the magnitude of response to treatment for individuals at high risk resembles that of chronic schizophrenia patients, which is often approximately 0.5 standard deviation units (Harvey and Keefe, 2001), the milder baseline profile in the high-risk individuals makes it likely that post-treatment functioning would be closer to normal performance. This holds promise for delivering a clinically meaningful neurocognitive improvement during the high-risk phase by implementing interventions such as pharmacological treatments.
The aim of the current study was to examine change in neurocognitive functioning in a sample of clinical high-risk individuals when assessed at baseline and after the initiation of pharmacologic treatment. Individuals who were prescribed antipsychotic or antidepressant medication were compared to those who did not initiate pharmacologic treatment and to a healthy control sample recruited from the same catchment area.
2. Methods
2.1. Subjects
Study participants (N=70) were a subset of prospectively collected participants enrolled in the larger Recognition and Prevention (RAP) program at The Zucker Hillside Hospital in Glen Oaks, New York. The RAP program is a research clinic serving treatment-seeking adolescents and young adults who have clinical symptoms that place them at high-risk for the development of psychosis. Study inclusion criteria were: English speaking and between 12 and 22 years of age. Exclusion criteria were diagnosis of a schizophreniaspectrum illness or delusional disorder, a medical or neurological disorder that could affect brain functioning, and estimated IQ below 70. Although the RAP program has been enrolling participants since 1998, the participants in the current analyses were consecutive admissions to the clinic between February 2003 and November of 2009, spanning both Phases I and II of the RAP program. Of the 104 subjects that completed both baseline and 6-month retest during the study period, 20 were eliminated due to being on medication at the time of entry. One was prescribed stimulants and 4 were prescribed a combination of stimulants or antidepressant and antipsychotic medications following study entry, while 9 did not return for follow up within the time frame allotted for this study, leaving a final sample of 70 for the current analyses.
The RAP program maintains a naturalistic treatment approach within its specialty research clinic. Upon completion of a research intake, patients are offered a medication consultation with a RAP program psychiatrist, who along with the patient and parent, assess the need for pharmacologic intervention. As indicated above, none of the subjects selected for these analyses was taking psychotropic medication at study entry. Based on their psychiatrist’s clinical decision, participants remained off medication (OFF; N=27), started selective serotonin reuptake inhibitor antidepressant medication (AD; N=15), or started a second-generation antipsychotic medication (AP; N=11) within three months of study entry. We included healthy comparison subjects (HC; N=17) who were assessed at a similar test–retest interval. Patient referrals were made to the RAP Program by affiliated outpatient and inpatient psychiatry departments, local mental health providers, school psychologists or counselors, or were self-referred. Subjects in the control group were recruited through announcements at the medical center and flyers posted at numerous locations within the catchment area of the patients. HC subjects were excluded if they had a first-degree relative with a diagnosed Axis I psychotic disorder in addition to the other exclusions listed above.
All participants provided written informed consent, or assent along with parental consent if under age 18. Research procedures were approved by the Institutional Review Board at the North Shore–Long Island Jewish Health System, the parent organization for Zucker Hillside Hospital.
2.2. Procedures
At study entry, all RAP program participants receive a comprehensive, standardized neuropsychological battery and clinical symptom ratings. Participants were given an abbreviated battery of neuropsychological instruments and symptom ratings again at a follow-up assessment, which was targeted for six month post-baseline assessment, but varied as a function of subject availability. The mean follow up period was 6.00 months (SD, 2.43; median, 6.09 months).
2.3. Measures
2.3.1. Clinical instruments
All participants in the current study met clinical high-risk criteria based upon the level of attenuated positive or negative symptoms present in the month prior to baseline. Attenuated positive and negative symptoms were assessed with the Structured Interview for Prodromal Syndromes (SIPS) and companion Scale of Prodromal Symptoms (SOPS; Miller et al., 1999, 2002, 2003). The SOPS consists of four subscales measuring positive (5 items), negative (6 items), disorganized (4 items), and general (4 items) symptoms. Items are rated on a 7 point anchored scale ranging from absent (0) to psychotic/extreme (6) level of severity. To meet clinical high-risk positive (CHR+) criteria in the RAP program a participant must have at least one attenuated positive symptom (i.e., unusual thought content, suspiciousness, grandiosity, perceptual abnormalities, or conceptual disorganization) at a moderate (3) to severe (5) level. Participants with a psychotic (6) level symptom were excluded. To meet clinical high-risk negative (CHR−) criteria in the RAP program a participant must have a least one attenuated negative symptom (i.e., social anhedonia, avolition, decreased expression of emotion, decreased experience of emotion and self, decreased ideational richness, and decline in occupational functioning) at a moderate (3) to extreme (6) level and no attenuated positive symptoms at a moderate or above level. Both the CHR− and CHR+ groups are considered high-risk based on the RAP program neurodevelopmental model described in detail elsewhere (Cornblatt et al., 2003). Interviewers were trained master’s or Ph.D. level clinicians with documented good reliability for the SIPS/SOPS (Lencz et al., 2004).
Affective symptoms were assessed with the 21-item Beck Depression Inventory (BDI; Beck et al., 1988) for participants age 16 and older and the 27-item Children’s Depression Inventory (CDI, Kovacs, 1985) for participants younger than 16. Anxiety was assessed with the 21-item Beck Anxiety Inventory (BAI; Beck et al., 1988). To ensure comparability of the CDI and BDI, the two age-dependent measures for depression across the entire sample, a total depression score was calculated as a percentage of the maximum possible score on each of these scales. To allow easy comparison of mean levels of anxiety with the mean level of depression, a total anxiety score was also calculated as a percentage of the maximum possible score on the BAI. Axis I diagnoses were assessed by the Schedule for Affective Disorders and Schizophrenia for School-Age Children, Epidemiologic Version (K-SADS-E; Orvaschel and Puig-Antich, 1994).
2.3.2. Neurocognitive assessment
For the purposes of this naturalistic follow-up study, an abbreviated neuropsychological battery was selected based on the relationship of the measures to critical functional outcomes (Green, 1996) and because they are known to be impaired in those at high risk for developing a psychotic disorder and across the lifespan for those with a schizophrenia spectrum illness. All cognitive variables were transformed to z-scores (mean=0, SD=1) based on normative data from an age- and demographically-matched sample (N=80) of healthy subjects recruited at Zucker Hillside Hospital.
2.3.2.1. Verbal learning
The five learning trials of the California Verbal Learning Test (CVLT) were administered as an assessment of verbal learning. The CVLT includes 16 semantically related words that are read by the examiner at a rate of one per two seconds. After each of five trials, the subject is asked to recall the words. For this study, the dependent variable was the total number of correct responses over the five trials.
2.3.2.2. Verbal fluency
The Controlled Oral Word Association Test (COWAT; Benton et al., 1983) is a verbal fluency test that requires the subject to generate as many words that start with a specific letter as possible in one minute. Three trials are presented with the letters PRW or CFL at baseline and follow-up, respectively. The dependent variable was the total number of correct and unique responses over all three trials.
2.3.2.3. Visual motor speed and conceptual shifting
Parts A and B of the Trail Making Test (TMT; Reitan, 1992) were used to assess visual-motor speed and conceptual reasoning. In part A, the subject is asked to use a pencil to connect encircled numbers in consecutive order. Part B is a more cognitively complex task that requires alternating numbers and letters in ascending and alphabetical order. The dependent variable is the total time to complete the visual-motor task (Part A) and the conceptual shifting task (Part B). The Trail Making Test z-scores (with longer time to completion indicating poorer performance) were reversed so that, like other variables, lower scores represent more impairment.
2.3.2.4. Working memory
The MATRICS version of the letter–number span test (Nuechterlein et al., 2008) is a test of verbal working memory in which subjects are presented with a string of interspersed letters and numbers and have to recall the numbers first, in numerical order, followed by the letters alphabetically. The length of the items increases until a subject fails all four items at a given span. The dependent variable is the total number of correct items.
2.3.2.5. Sustained attention
The Continuous Performance Test — Identical Pairs version (Cornblatt et al., 1988) is a computerized measure of sustained attention that requires the subject to respond when a stimulus is identical to the immediately preceding stimulus. In this longitudinal study, some subjects performed the original version of the task, for which the required response to a target was a lift off of the mouse button, while others performed the MATRICS version, which required a finger press on the mouse button. Separate z-score calculations were computed from health comparison subjects that were tested from the respective version. In this study, two conditions of the test were of interest. In the four-digit condition, the subject is to respond when a string of four digits is identical to the previous string of four digits. In the shapes condition, the subject is to respond when identical shapes are presented sequentially. The variable of interest for each condition was d-prime, which is a measure of signal detection representing the ratio of correct hits to false positives.
2.4. Statistical analyses
All analyses were conducted using SPSS 19.0 (SPSS Inc., Chicago, Illinois). Group comparisons of demographic and clinical variables were performed using Pearson Chi-Square tests for categorical variables and one-way analysis of variance (ANOVA) for continuous variables. Significant ANOVAs were followed by post hoc Games–Howell tests (p<.05), which take into account unequal variances and sample sizes (Kirk, 1995).
Bivariate correlations were conducted to examine the relationships of neurocognitive domains with clinical symptoms at baseline, with the alpha level adjusted using the Bonferroni procedure. We examined the correlations of change scores (endpoint score–baseline score) for both neurocognitive domains and clinical symptoms with bivariate correlations, again with the Bonferroni adjustment for the number of tests in this analysis. For both correlational approaches, we selected only the clinical groups for the analyses because the HC group is not expected to manifest clinical symptoms and is not of interest for examining these relationships.
Changes in positive and depressive symptoms were examined with repeated measures analysis of variance tests. Only the three clinical groups were selected for these analyses. Group was entered as the between subjects factor and time (baseline and endpoint) was entered as the repeated measure.
Changes in neurocognition between groups from baseline to endpoint were examined with linear mixed models. Assessment (baseline and follow-up) was the repeated measure and participant identification code was the subjects’ variable. Covariance type was selected through an iterative process in which the default (Diagonal) type was first run, followed by subsequent analyses examining the covariance structure and fit statistics. Subject group (HC, OFF, AD, AP) was the between-subjects factor. Time between assessments, change in depressive symptoms, and change in attenuated positive symptoms were entered as random factors in the model with variance components as the covariance type and the intercept included. Estimated marginal means with standard error are presented at both assessments for all groups. Significant interaction effects were followed with post-hoc pairwise comparisons using the same parameters. For significant Group×Visit interactions, Cohen’s d was computed to illustrate the effect size for cognitive change at retest (Cohen, 1988). Cohen’s d was calculated as the mean difference from the raw scores divided by the pooled standard deviation (d=(Mean Visit 2—Mean Visit 1)/σ pooled) and can be interpreted using the following categories (Cohen, 1988): small =.20, medium=.50, and large=.80.
3. Results
3.1. Baseline comparisons
Demographic variables and clinical characteristics are presented in Table 1. At baseline, no significant differences in symptoms were observed among the clinical groups; as expected, the healthy comparison group had lower levels of symptom severity. In addition, there were no differences among the four groups in age, premorbid IQ, current IQ, years of education, handedness, race, ethnicity or gender.
Table 1.
Baseline demographics and clinical characteristics.
| Characteristic | HC (N=17) |
OFF/OFF (N=27) |
OFF/AD (N=15) |
OFF/AP (N=11) |
Test statistic | p value |
Post-hoc contrasts |
|---|---|---|---|---|---|---|---|
| Age, y, mean (SD) | 16.41 (2.26) | 16.40 (2.00) | 15.52 (1.94) | 16.44 (1.52) | F3,66=.80 | .50 | – |
| Estimated premorbid IQ, mean (SD) | 110.41 (10.14) | 106.7 (10.21) | 103.73 (12.92) | 108.18 (7.31) | F3,66=1.14 | .34 | – |
| Estimated current IQ, mean (SD) | 108.29 (18.75) | 108 (16.93) | 101.73 (10.22) | 95.09 (13.93) | F3,66=2.22 | .09 | – |
| Years of education, mean (SD) | 10.65 (2.26) | 9.89 (2.03) | 9.33 (1.99) | 10.27 (1.74) | F3,66=1.20 | .32 | – |
| Gender, no. (%) | |||||||
| Female | 8 (47.1) | 6 (22.2) | 5 (33.3) | 2 (18.2) | .27 | – | |
| Male | 9 (52.9) | 21 (77.8) | 10 (66.7) | 9 (81.8) | |||
| Handedness, right, no. (%) | 14 (82.4) | 23 (85.2) | 13 (86.7) | 10 (90.9) | .94 | – | |
| Race | |||||||
| White, no. (%) | 11 (64.7) | 18 (66.7) | 8 (53.3) | 7 (63.6) | .86 | – | |
| Ethnic origin | |||||||
| Hispanic, no. (%) | 2 (11.8) | 7 (25.9) | 2 (13.3) | 0 (0.0) | 22 | – | |
| Anxiety score, mean (SD)a | 0.09 (0.11) | 0.19 (0.14) | 0.19 (0.20) | 0.13 (0.09) | F3,62=1.91 | .14 | – |
| Depression score, mean (SD)b | 0.08 (0.08) | 0.24 (0.15) | 0.27 (0.14) | 0.19 (0.10) | F3,60=6.22 | b.01 | NC<OFF/OFF, OFF/AD, OFF/AP |
| SIPS score, mean (SD) | |||||||
| Positive | 1.00 (1.87) | 7.52 (4.56) | 6.53 (4.41) | 6.27 (3.29) | F3,66=10.74 | <.001 | NC<OFF/OFF, OFF/AD, OFF/AP |
| Negative | 1.88 (1.69) | 13.19 (4.81) | 16.6 (6.05) | 13.91 (7.4) | F3,66=27.08 | <.001 | NC<OFF/OFF, OFF/AD, OFF/AP |
| Disorganized | 0.76 (1.52) | 4.33 (3.11) | 6.2 (4.16) | 4.09 (3.14) | F3,66=8.78 | <.001 | NC<OFF/OFF, OFF/AD, OFF/AP |
| General | 0.88 (1.62) | 7.85 (4.97) | 8.33 (3.18) | 6.73 (3.77) | F3,66=14.19 | <.001 | NC<OFF/OFF, OFF/AD, OFF/AP |
| DSM-IV diagnoses, no. (%) | |||||||
| Moodc | – | 15 (55.6) | 9 (60.0) | 5 (45.5) | .76 | – | |
| Anxietyd | – | 16 (59.3) | 11 (73.3) | 7 (63.6) | .66 | – | |
| Substancee | – | 3 (11.1) | 0 (0.0) | 2 (18.2) | .27 | – | |
| Time to retest, months, mean (SD) | 5.27 (3.27) | 6.82 (2.27) | 4.88 (1.19) | 6.67 (1.78) | F3,66=3.12 | .03 | OFF/AD<OFF/OFF |
Note: SIPS, structured interview for prodromal syndromes; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition.
Percentage of the maximum possible score on BDI/CDI.
Percentage of the maximum possible score on BAI.
DSM-IV defined diagnosis of major depressive disorder, dysthymic disorder, or depressive disorder, not otherwise specified (NOS).
DSM-IV defined diagnosis of panic disorder, posttraumatic stress disorder, obsessive–compulsive disorder, generalized anxiety disorder, anxiety disorder NOS, or phobias including simple phobia and social phobia.
DSM-IV defined diagnosis of alcohol, amphetamine, cannabis, cocaine, hallucinogen, opioid, or polysubstance abuse.
3.2. Bivariate correlations
Bivariate correlations revealed baseline associations between neurocognitive and psychopathology measures that were small in magnitude and inconsistent in the direction of the relationships, suggesting relative independence of cognitive and symptom domains at baseline (see Table 2). None of the correlations was significant at the Bonferroni-adjusted level of .0014. Similarly, neurocognitive change scores from time one to time two were weakly and not significantly correlated with clinical symptom change scores (see Table 2) after Bonferroni correction.
Table 2.
Correlations (Pearson R, (p-value)) among neurocognitive and psychopathological dimensions at baseline (top) and change scores for cognition and psychopathological dimensions from baseline to endpoint (bottom).
| Positive | Negative | Disorganized | Depression | Anxiety | ||
|---|---|---|---|---|---|---|
| CVLT total learning |
Baseline | .08 | −.32* | −.16 | .42** | .27* |
| Change scores |
−.18 | .01 | .11 | −.28 | −.01 | |
| CPT shapes | Baseline | −.01 | −.07 | −.17 | −.04 | .05 |
| Change scores |
−.03 | −.36* | −.16 | .07 | −.04 | |
| CPT digits | Baseline | −.01 | −.06 | −.35** | .16 | .27 |
| Change scores |
−.03 | −.26 | −.03 | −.05 | −.04 | |
| Verbal fluency |
Baseline | .13 | −.03 | −.16 | .39** | .14 |
| Change scores |
.05 | .08 | .15 | −.04 | .008 | |
| TMT A | Baseline | .02 | −.34** | −.24 | .14 | .12 |
| Change scores |
.07 | −.16 | −.41** | −.05 | −.11 | |
| TMT B | Baseline | .10 | −.30* | −.11 | −.05 | −.15 |
| Change scores |
.19 | .01 | −.16 | .10 | .25 | |
| LNS | Baseline | .13 | −.06 | .04 | .03 | .10 |
| Change scores |
−.13 | .02 | .22 | .33 | .08 |
Note: p<.05.
p<.01.
3.3. Symptom changes
Repeated measures analysis of variance tests were used to examine time and group by time interactions for the three clinical groups. A significant main effect for time [F(1,50)=5.7, p=.02], but not the interaction [F(2,50)=0.2, p=.97] was found for positive symptoms. Significant main effects for time [F(1,50)=8.9, p=.004], but not the interaction [F(2,50)=0.12, p=.88] were found for depressive symptoms.
3.4. Linear mixed models
Results for the linear mixed models are presented in Table 3. The linear mixed models revealed significant main effects for visit (baseline to endpoint) on CPT digits, CPT shapes, and Letter Number Sequencing. Significant visit by group interactions were observed for CVLT Total Learning and CPT digits. Post hoc comparisons revealed an improvement in CVLT Total Learning for the AD group compared to the AP group, (F(1,26)=5.6, p=.027) (Fig. 1). Post hoc comparisons for CPT digits revealed improvement in the AD group relative to the AP group (F(1,21)=11.0, p=.003) and the OFF group compared to the AP group (F(1,29)=10.4, p=.003) (Fig. 2).
Table 3.
Results of linear mixed models analyses.
| Cognitive test | Mean Z-score (SE) |
Effects |
Fit statistics | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Endpoint | F (df) | p-value | |||||
| CVLT total learning | HC | .11 (.31) | .47 (.37) | Group | 6.2 (3,70) | .001 | −2LL | 396.3 |
| Off/Off | −.58 (.25) | −.19 (.22) | Visit | 3.2 (1,61) | .079 | AIC | 408.3 | |
| Off/AD | −.56 (.34) | .43 (.29) | Group×Visit | 2.9 (3,59) | .040 | BIC | 425.0 | |
| Off/AP | −1.11 (.39) | −1.71 (.33) | Contrasts: AD>AP | |||||
| CPT 4-digit D’ | HC | .17 (.21) | .55 (.22) | Group | 1.2 (3,65) | .29 | −2LL | 331.7 |
| Off/Off | −.37 (.19) | .28 (.20) | Visit | 14.1 (1,60) | <.001 | AIC | 343.7 | |
| Off/AD | −.48 (.24) | .22 (.25) | Group×Visit | 3.8 (3,59) | .014 | BIC | 360.6 | |
| Off/AP | −.01 (.29) | −.22 (.20) | Contrasts: AD, OFF>AP | |||||
| CPT shapes | HC | .05 (.23) | .72 (.26) | Group | 1.1 (3,56) | .375 | −2LL | 307.5 |
| Off/Off | .44 (.19) | .98 (.21) | Visit | 14.1 (1,50) | <.001 | AIC | 319.5 | |
| Off/AD | .02 (.25) | .40 (.26) | Group×Visit | 0.7 (3,50) | .531 | BIC | 335.8 | |
| Off/AP | .28 (.29) | .37 (.31) | ||||||
| COWAT | HC | −.37 (.24) | −.06 (.29) | Group | 0.9 (3,52) | .403 | −2LL | 398.7 |
| Off/Off | −.45 (.23) | −.10 (.24) | Visit | 1.7 (1,60) | .196 | AIC | 410.7 | |
| Off/AD | −.14 (.29) | .11 (.31) | Group×Visit | 0.7 (3,59) | .514 | BIC | 427.9 | |
| Off/AP | −.62 (.34) | −.72 (.38) | ||||||
| LNS | HC | −.37 (.27) | .47 (.29) | Group | 0.6 (3,61) | .600 | −2LL | 349.0 |
| Off/Off | −.46 (.21) | −.17 (.20) | Visit | 12.8 (1,58) | .001 | AIC | 361.0 | |
| Off/AD | −.38 (.29) | −.17 (.27) | Group×Visit | 1.5 (3,58) | .232 | BIC | 377.8 | |
| Off/AP | −.59 (.33) | −.26 (.31) | ||||||
| Trails A | HC | −.18 (.26) | .43 (.17) | Group | 1.7 (3,88) | .17 | −2LL | 384.5 |
| Off/Off | −.06 (.23) | .16 (.17) | Visit | 12.9 (1,69) | .001 | AIC | 396.5 | |
| Off/AD | −.67 (.29) | −.09 (.19) | Group×Visit | 0.6 (3,69) | .574 | BIC | 413.8 | |
| Off/AP | −.65 (.37) | −.12 (.26) | ||||||
| Trails B | HC | .10 (.27) | .25 (.19) | Group | 1.6 (3,59) | .18 | −2LL | 361.2 |
| Off/Off | −.37 (.22) | −.25 (.16) | Visit | 3.9 (1,62) | .051 | AIC | 373.2 | |
| Off/AD | −.55 (.29) | −.21 (.20) | Group×Visit | 0.2 (3,62) | .846 | BIC | 390.4 | |
| Off/AP | −.61 (.36) | −.23 (.25) | ||||||
Note: Z-scores are calculated from an age- and demographically-matched sample (N=80) of healthy subjects recruited locally.
CVLT=California Verbal Learning Test; HC=Healthy Control; AD=Antidepressant; AP=Second-Generation Antipsychotic; CPT=Continuous Performance Test—Identical Pairs version; COWAT=Controlled Oral Word Association Test; LNS=Letter–Number Span;−2LL=−2 Restricted Log Likelihood; AIC=Akaike Information Criteria; BIC=Schwarz’s Bayesian Criterion.
Fig. 1.
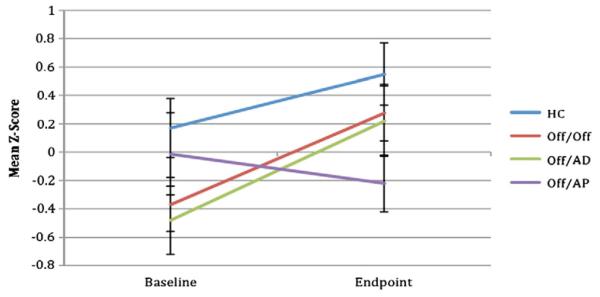
Six-month change in Continuous Performance Test — Identical Pairs, 4-digit condition by group (estimated marginal means, standard error bars). HC = healthy comparison group (N=17), Off/Off = clinical high risk for psychosis subjects off of medication at both time points (N=27), Off/AD = clinical high risk for psychosis subjects off medication at baseline and on an antidepressant at follow-up (N=15), Off/AP = clinical high risk for psychosis subjects off medication at baseline and on a second generation antipsychotic at follow-up (N=11).
Fig. 2.
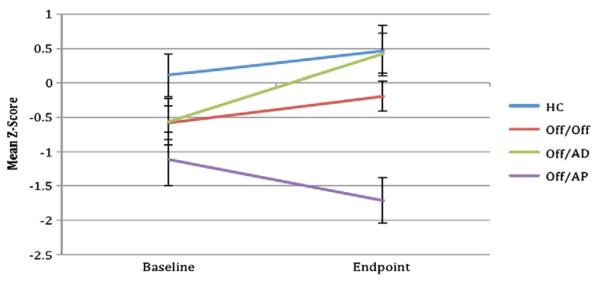
Six-month change in CVLT total learning by group (estimated marginal means, standard error bars). HC = healthy comparison group (N=17), Off/Off = clinical high risk for psychosis subjects off of medication at both time points (N=27), Off/AD = clinical high risk for psychosis subjects off medication at baseline and on an antidepressant at follow-up (N=15), Off/AP = clinical high risk for psychosis subjects off medication at baseline and on a second generation antipsychotic at follow-up (N=11).
Effect size improvements for raw scores were moderate for the healthy comparison group on the CVLT Total Learning (d=0.53) and CPT (d=0.45). The group that remained off medication had small effect size changes in CVLT Total Learning (d=0.39) and large on the CPT digits (d=0.80). The OFF/AD had moderate to large effect size improvements on the CVLT Total Learning (d=0.63) and CPT digits (d=1.02). In contrast, the OFF/AP group had small effects indicating worsening of performance on the CVLT Total Learning (d=−0.33) and CPT digits (d=−0.39).
4. Discussion
The results of this naturalistic study of neurocognitive change following pharmacological treatment in subjects who are considered to be at clinical high risk for psychosis support evidence for differential changes in neurocognition as a function of the class of psychotropic medication. Treatment with antidepressant medications over a six-month period was associated with improvements in verbal learning and sustained attention, but treatment with second-generation antipsychotic medications resulted in a worsening on those cognitive domains. These statistically significant improvements could be described as clinically meaningful given their effect size and when considering the importance of verbal memory (Brewer et al., 2005; Lencz et al., 2006) and sustained attention (Keefe et al., 2006; Correll et al., 2008) deficits as predictors of conversion from the prodrome to psychosis. These cognitive findings extend a previous report, which included some of the current subjects, that demonstrated greater adherence to medication and less likelihood of converting to psychosis in at-risk adolescents who were treated with antidepressants when compared to those treated with second-generation antipsychotics (Cornblatt et al., 2007).
In the present sample, the degree of baseline impairment would be considered mild to moderate in the domains of verbal learning, working memory, sustained attention, processing speed, and verbal productivity. This magnitude of impairment is consistent with those found in other samples of clinical high-risk participants (Seidman et al., 2010; Woodberry et al., 2010). The magnitude of improvement for the clinical subjects following treatment resulted in cognitive abilities that were in the normative range for the group, with the exception of the group treated with second-generation antipsychotic medications on domains of sustained attention, verbal learning, and verbal productivity.
Clinical symptoms were inconsistently and only modestly associated with neurocognitive deficits at baseline. Further, the observed changes in neurocognition were weakly associated with the small changes following treatment in the attenuated positive, attenuated negative, disorganized, depressive, and anxiety symptoms. Consistent with previous reports in adults with schizophrenia (Harvey et al., 2006), psychopathological symptoms and neurocognition appear to be distinct features in individuals at high clinical risk for the schizophrenia spectrum disorders.
There are several interpretations of possible mechanisms underlying pro-cognitive change following antidepressant treatment. The group by time interactions that were observed in verbal memory and sustained attention following AD treatment were relative to the AP group, but not greater than the OFF or HC groups, suggesting that the AD group might have demonstrated a relatively greater capacity for practice effects and that the antipsychotic medication might have resulted in poorer cognitive outcomes. An alternative hypothesis is the possibility of true cognitive improvements. Phencyclidine-induced cognitive deficits are proposed to have psychotomimetic effects; cognitive impairments induced by phencyclidine in mice are attenuated following administration of fluvoxamine, a serotonin inhibitor (Hashimoto et al., 2007). It is possible that treatment with SSRIs produced pro-cognitive effects in the adolescents at high risk for psychosis, a finding supported by studies with other clinical populations (Levkovitz et al., 2002; Bremner and Vermetten, 2004). Conversely, in healthy adults SSRIs have produced impairment in memory (Wadsworth et al., 2005) and sustained attention (Schmitt et al., 2002), though these impairments have been postulated to result from additional inhibition of dopamine transporters (Schmitt et al., 2002), an interesting hypothesis in light of our findings of reduced verbal memory and sustained attention performance in those prescribed antipsychotic medications, which have strong anti-dopaminergic effects. Possible mechanisms of these effects, however, can only be speculated. It is possible that SSRIs and/or the reduction of mood symptoms with their treatment are associated with increases in brain-derived neurotrophic factor (BDNF; Deltheil et al., 2008), which is associated with life stress (Savitz et al., 2007), is a predictor of clinical and neurocognitive (Vinogradov et al., 2009) treatment response, and is a major factor in neuronal plasticity (Kapczinski et al., 2008). More detailed work in the future might shed light on whether antidepressant treatment interacts with neurotrophins to produce increased brain plasticity and examine interactive effects on reduced life stressors. Additionally, it is important to consider whether observed performance improvements were pseudospecific; that is, whether they were a function of improved clinical symptoms. This seems unlikely though, because symptoms were not severe and symptom changes were similar across groups.
Given the naturalistic design of this study, these data must be considered hypothesis generating and interpreted with caution. Given the short re-test interval, changes in the positive direction might reflect practice effects, which can be considered a type of learning. However, we observed changes in different directions across tests as a function of treatment group, which suggests that changes were not simply an artifact of exposure. Studies with longer follow-up periods might result in more robust findings and/or minimize practice effects. We did not adjust the analyses for baseline symptoms, because there were no significant differences on the ratings scales. However, it is possible that signs and symptoms not explicitly assessed in this study, but that were detected by the clinician, or emerged after the time of assessment, influenced a decision to treat with an antidepressant medication, an antipsychotic medication, or without medication. It is also important to consider the prognostic limitations of the clinical high-risk design; it is possible that some individuals might not truly be in the prodromal phase for schizophrenia and they might be more likely to be assigned a different treatment regimen.
Following several modest findings from early examinations of pro-cognitive effects of antipsychotic and adjunctive pharmacological medications, the MATRICS project (Green and Nuechterlein, 2004) underscored the importance of using methodologically rigorous assessment strategies. In treatment studies with children and adolescents at risk, who may not develop a full-blown psychiatric condition, randomization to psychotropic medications presents an ethical dilemma. The present findings from a naturalistic design suggest a possible worsening in verbal memory and sustained attention following antipsychotic treatment, a finding dissimilar to what has been observed in samples that included people with schizophrenia subsequent to illness onset (Harvey et al., 2003); as such, they warrant more rigorous examination of the potential for different classes of medication to affect neurocognition in the individuals at high-risk for the development of psychosis. Whether the improvements effects we observed following antidepressants is a true pro-cognitive effect will need to be confirmed with larger and more rigorously designed studies. Additionally, it would be sensible to adapt behavioral strategies for enhancing neurocognition to the prodrome. These treatments appear to have larger cognitive effects than medications (McGurk et al., 2007) and eliminate the cost/benefit ratio of treatment/side effects. Finally, it is necessary to determine if improving neurocognition reduces the risk for conversion to psychosis or is at least associated with better functional prognosis.
Acknowledgments
The authors thank Ruth Olsen, Emma Bassett and Kaelen Boyd for organizing literature reviews and formatting.
Role of funding source Funding for this study was provided by a NARSAD Young Investigator Award (Dr. Bowie) and supported by grants MH61523-02 and MH61523-8 from the National Institute of Mental Health (Dr. Cornblatt) and the Zucker Hillside Hospital NIMH Advanced Center for Intervention and Services Research for the Study of Schizophrenia MH 074543-01 (John M Kane, M.D.). NARSAD and NIMH had no role in study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Footnotes
Contributors Author 1 designed the study with Author 5 as the mentor of the Award. Author 1 wrote the protocol, first draft of the manuscript and undertook the statistical analysis. Authors 2 and 3 assisted with analysis and interpretation of data and contributed to manuscript writing. Author 4 contributed to data collection and manuscript writing. Author 5 contributed to the writing of the manuscript and study design. All authors have contributed to and have approved the final manuscript.
Conflict of interest Dr. Bowie has served on a scientific advisory board for Abbott. Dr. Cornblatt was the original developer of the CPT-IP and has been an advisor for Merck. All other authors report no disclosures.
References
- Asarnow RF, Steffy RA, MacCrimmon DJ, Cleghorn JM. An attentional assessment of foster children at risk for schizophrenia. J. Abnorm. Psychol. 1977;86(3):267–275. doi: 10.1037//0021-843x.86.3.267. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin. Psychol. Rev. 1988;8(1):77–100. [Google Scholar]
- Benton A, Hamsher K, Sivan A. Multilingual Aphasia Examination. 3rd ed AJA Associates; Iowa City, Iowa: 1983. [Google Scholar]
- Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, et al. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am. J. Psychiatry. 2000;157(4):549–559. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Reichenberg A, Patterson TL, Heaton RK, Harvey PD. Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. Am. J. Psychiatry. 2006;163(3):418–425. doi: 10.1176/appi.ajp.163.3.418. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E. Neuroanatomical changes associated with pharmacotherapy in posttraumatic stress disorder. Ann. N. Y. Acad. Sci. 2004;1032:154–157. doi: 10.1196/annals.1314.012. [DOI] [PubMed] [Google Scholar]
- Brewer WJ, Francey SM, Wood SJ, Jackson HJ, Pantelis C, et al. Memory impairments identified in people at ultra-high risk for psychosis who later develop first-episode psychosis. Am. J. Psychiatry. 2005;162(1):71–78. doi: 10.1176/appi.ajp.162.1.71. [DOI] [PubMed] [Google Scholar]
- Carrión RE, Goldberg TE, McLaughlin D, Auther AM, Correll CU, Cornblatt BA. Impact of neurocognition on social and role functioning in individuals at clinical high risk for psychosis. Am. J. Psychiatry. 2011;168(8):806–813. doi: 10.1176/appi.ajp.2011.10081209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. L. Erlbaum Associates; Hillsdale, N.J.: 1988. [Google Scholar]
- Cornblatt BA, Erlenmeyer-Kimling L. Global attentional deviance as a marker of risk for schizophrenia: specificity and predictive validity. J. Abnorm. Psychol. 1985;94(4):470–486. doi: 10.1037//0021-843x.94.4.470. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Risch NJ, Faris G, Friedman D, Erlenmeyer-Kimling L. The Continuous Performance Test, identical pairs version (CPT-IP): I. New findings about sustained attention in normal families. Psychiatry Res. 1988;26(2):223–238. doi: 10.1016/0165-1781(88)90076-5. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Lencz T, Smith CW, Correll CU, Auther AM, Nakayama E. The schizophrenia prodrome revisited: a neurodevelopmental perspective. Schizophr. Bull. 2003;29(4):633–651. doi: 10.1093/oxfordjournals.schbul.a007036. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Lencz T, Smith CW, Olsen R, Auther AM, Nakayama E, et al. Can antidepressants be used to treat the schizophrenia prodrome? Results of a prospective, naturalistic treatment study of adolescents. J. Clin. Psychiatry. 2007;68(4):546–557. doi: 10.4088/jcp.v68n0410. [DOI] [PubMed] [Google Scholar]
- Correll CU, Smith CW, Auther AM, McLaughlin D, Shah M, Foley C, et al. Predictors of remission, schizophrenia, and bipolar disorder in adolescents with brief psychotic disorder or psychotic disorder not otherwise specified considered at very high risk for schizophrenia. J. Child Adolesc. Psychopharmacol. 2008;18(5):475–490. doi: 10.1089/cap.2007.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deltheil T, Guiard BP, Cerdan J, David DJ, Tanaka KF, Repérant C, et al. Behavioral and serotonergic consequences of decreasing or increasing hippocampus brain-derived neurotrophic factor protein levels in mice. Neuropharmacology. 2008;55(6):1006–1014. doi: 10.1016/j.neuropharm.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Fiszdon JM, Cardenas AS, Bryson GJ, Bell MD. Predictors of remediation success on a trained memory task. J. Nerv. Ment. Dis. 2005;193(9):602–608. doi: 10.1097/01.nmd.0000177790.23311.ba. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Goldman RS, Burdick KE, Malhotra AK, Lencz T, Patel RC, et al. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Arch. Gen. Psychiatry. 2007;64(10):1115–1122. doi: 10.1001/archpsyc.64.10.1115. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiatry. 1996;153(3):321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophr. Res. 2004;72(1):1–3. doi: 10.1016/j.schres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Gschwandtner U, Aston J, Borgwardt S, Drewe M, Feinendegen C, Lacher D, et al. Neuropsychological and neurophysiological findings in individuals suspected to be at risk for schizophrenia: preliminary results from the Basel early detection of psychosis study — Früherkennung von Psychosen (FEPSY) Acta Psychiatr. Scand. 2003;108(2):152–155. doi: 10.1034/j.1600-0447.2003.00157.x. [DOI] [PubMed] [Google Scholar]
- Gschwandtner U, Pflüger M, Aston J, Borgwardt S, Drewe M, Stieglitz RD, Riecher-Rössler A. Fine motor function and neuropsychological deficits in individuals at risk for schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2005;256(4):201–206. doi: 10.1007/s00406-005-0626-2. [DOI] [PubMed] [Google Scholar]
- Hambrecht M, Lammertink M, Klosterkötter J, Matuschek E, Pukrop R. Subjective and objective neuropsychological abnormalities in a psychosis prodrome clinic. Br. J. Psychiatry. 2002;(Suppl. 43s):30–37. doi: 10.1192/bjp.181.43.s30. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Keefe RS. Studies of cognitive change in patients with schizophrenia following novel antipsychotic treatment. Am. J. Psychiatry. 2001;158(2):176–184. doi: 10.1176/appi.ajp.158.2.176. [DOI] [PubMed] [Google Scholar]
- Harvey P, Winters K, Weintraub S, Neale JM. Distractibility in children vulnerable to psychopathology. J. Abnorm. Psychol. 1981;90(4):298–304. doi: 10.1037//0021-843x.90.4.298. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Green MF, McGurk SR, Meltzer HY. Changes in cognitive functioning with risperidone and olanzapine treatment: a large-scale, double-blind, randomized study. Psychopharmacology (Berl.) 2003;169:404–411. doi: 10.1007/s00213-002-1342-5. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Koren D, Reichenberg A, Bowie CR. Negative Symptoms and Cognitive Deficits: an examination of the nature of their relationship. Schizophr. Bull. 2006;32:250–258. doi: 10.1093/schbul/sbj011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Fujita Y, Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of fluvoxamine: role of sigma-I receptors. Neuropsychopharmacology. 2007;32:514–521. doi: 10.1038/sj.npp.1301047. [DOI] [PubMed] [Google Scholar]
- Hawkins KA, Addington J, Keefe RS, Christensen B, Perkins DO, Zipurksy R. Neuropsychological status of subjects at high risk for a first episode of psychosis. Schizophr. Res. 2004;67(2–3):115–122. doi: 10.1016/j.schres.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Hawkins KA, Keefe RS, Christensen BK, Addington J, Woods SW, Callahan J, et al. Neuropsychological course in the prodrome and first episode of psychosis: findings from the PRIME North America Double Blind Treatment Study. Schizophr. Res. 2008;105(1–3):1–9. doi: 10.1016/j.schres.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Jahshan C, Heaton RK, Golshan S, Cadenhead KS. Course of neurocognitive deficits in the prodrome and first episode of schizophrenia. Neuropsychology. 2010;24(1):109–120. doi: 10.1037/a0016791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapczinski F, Frey BN, Kauer-Sant’Anna M, Grassi-Oliveira R. Brain-derived neurotrophic factor and neuroplasticity in bipolar disorder. Expert. Rev. Neurother. 2008;8(7):1101–1113. doi: 10.1586/14737175.8.7.1101. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Perkins DO, Gu H, Zipursky RB, Christensen BK, Lieberman JA. A longitudinal study of neurocognitive function in individuals at-risk for psychosis. Schizophr. Res. 2006;88(1–3):26–35. doi: 10.1016/j.schres.2006.06.041. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch. Gen. Psychiatry. 2007;64(6):633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- Kirk RE. Experimental Design: Procedures for the Behavioral Sciences. 3rd ed Brooks/Cole Publishing; New York: 1995. [Google Scholar]
- Kovacs M. The children’s depression inventory (CDI) Psychopharmacol. Bull. 1985;21(4):995–998. [PubMed] [Google Scholar]
- Lencz T, Smith CW, Auther A, Correll CU, Cornblatt B. Nonspecific and attenuated negative symptoms in patients at clinical high-risk for schizophrenia. Schizophr. Res. 2004;68(1):37–48. doi: 10.1016/S0920-9964(03)00214-7. [DOI] [PubMed] [Google Scholar]
- Lencz T, Smith CW, McLaughlin D, Auther A, Nakayama E, Hovey L, Cornblatt BA. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol. Psychiatry. 2006;59(9):863–871. doi: 10.1016/j.biopsych.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Lepage M, Buchy L, Bodnar M, Bertrand MC, Joober R, Malla A. Cognitive insight and verbal memory in first episode psychosis. Eur. Psychiatry. 2008;23(5):368–374. doi: 10.1016/j.eurpsy.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Levkovitz Y, Caftori R, Avital A, Richter-Levin G. The SSRIs drug Fluoxetine, but not the noradrenergic tricyclic drug Desipramine, improves memory performance during acute major depression. Brain Res. Bull. 2002;58(4):345–350. doi: 10.1016/s0361-9230(01)00780-8. [DOI] [PubMed] [Google Scholar]
- McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A meta-analysis of cognitive remediation in schizophrenia. Am. J. Psychiatry. 2007;164(12):1791–1802. doi: 10.1176/appi.ajp.2007.07060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalia A, Richardson R. What predicts a good response to cognitive remediation interventions? Schizophr. Bull. 2005;31(4):942–953. doi: 10.1093/schbul/sbi045. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, Hoffman R, Davidson L. Symptom assessment in schizophrenic prodromal states. Psychiatr. Q. 1999;70(4):273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, Woods SW. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am. J. Psychiatry. 2002;159(5):863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr. Bull. 2003;29(4):703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH. Signal detection in vigilance tasks and behavioral attributes among offspring of schizophrenic mothers and among hyperactive children. J. Abnorm. Psychol. 1983;92(1):4–28. doi: 10.1037//0021-843x.92.1.4. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am. J. Psychiatry. 2008;165(2):203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Subotnik KL, Green MF, Ventura J, Asarnow RF, Gitlin MJ, Yee CM, Gretchen-Doorly D, Mintz J. Neurocognitive predictors of work outcomes in recent-onset schizophrenia. Schizophr. Bull. 2011;37(Suppl. 2):S33–S40. doi: 10.1093/schbul/sbr084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvaschel H, Puig-Antich J. Schedule for Affective Disorders and Schizophrenia for School-Age Children—Epidemiologic Version. Center for Psychological Studies, Nova Southeastern University; Fort Lauderdale, Florida: 1994. [Google Scholar]
- Reitan RM. Trail Making Test: Manual for Administration and Scoring. Neuropsychological Laboratory; Tucson, Arizona: 1992. [Google Scholar]
- Savitz J, van der Merwe L, Stein DJ, Solms M, Ramesar R. Genotype and childhood sexual trauma moderate neurocognitive performance: a possible role for brain-derived neurotrophic factor and apolipoprotein E variants. Biol. Psychiatry. 2007;62(5):391–399. doi: 10.1016/j.biopsych.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Schmitt JA, Ramaekers JG, Kruizinga MJ, van Boxtel MP, Vuurman EF, Riedel WJ. Additional dopamine reuptake inhibition attenuates vigilance impairment induced by serotonin reuptake inhibition in man. J. Psychopharmacol. 2002;16(3):207–214. doi: 10.1177/026988110201600303. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Giuliano AJ, Smith CW, Stone WS, Glatt SJ, Meyer E, et al. Neuropsychological functioning in adolescents and young adults at genetic risk for schizophrenia and affective psychoses: results from the Harvard and Hillside Adolescent High Risk Studies. Schizophr. Bull. 2006;32(3):507–524. doi: 10.1093/schbul/sbj078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Giuliano AJ, Meyer EC, Addington J, Cadenhead KS, Cannon TD, et al. North American Prodrome Longitudinal Study (NAPLS) Group Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Arch. Gen. Psychiatry. 2010;67(6):578–588. doi: 10.1001/archgenpsychiatry.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, Holland C, Shelly W, Wolkowitz O, Mellon SH. Is serum brain-derived neurotrophic factor a biomarker for cognitive enhancement in schizophrenia? Biol. Psychiatry. 2009;66(6):549–553. doi: 10.1016/j.biopsych.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth EJ, Moss SC, Simpson SA, Smith AP. SSRIs and cognitive performance in a working sample. Hum. Psychopharmacol. 2005;20(8):561–572. doi: 10.1002/hup.725. [DOI] [PubMed] [Google Scholar]
- Winters KC, Stone AA, Weintraub S, Neale JM. Cognitive and attentional deficits in children vulnerable to psychopathology. J. Abnorm. Child Psychol. 1981;9(4):435–453. doi: 10.1007/BF00917794. [DOI] [PubMed] [Google Scholar]
- Wolf LE, Cornblatt BA, Roberts SA, Shapiro BM, Erlenmeyer-Kimling L. Wisconsin Card Sorting deficits in the offspring of schizophrenics in the New York High-Risk Project. Schizophr. Res. 2002;57(2–3):173. doi: 10.1016/s0920-9964(01)00301-2. [DOI] [PubMed] [Google Scholar]
- Woodberry KA, Seidman LJ, Giuliano AJ, Verdi MB, Cook WL, McFarlane WR. Neuropsychological profiles in individuals at clinical high risk for psychosis: relationship to psychosis and intelligence. Schizophr. Res. 2010;123(2–3):188–198. doi: 10.1016/j.schres.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Purdon SE, Meltzer HY, Zald DH. A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. Int. J. Neuropsychopharmacol. 2005;8(3):457–472. doi: 10.1017/S146114570500516X. [DOI] [PubMed] [Google Scholar]


