Organic azides are well-established as versatile compounds that can act as precursors of different heterocycles (triazoles, triazolines, tetrazoles, etc.) or other nitrogen-containing compounds, such as amines (Staudinger reduction, Curtius rearrangement) or imines (Schmidt rearrangement, aza-Wittig reaction).1 Besides the ubiquitous copper-catalysed azide–alkyne cycloaddition reaction,2 two applications of organic azides have recently attracted the interest of the synthetic community: 1) the preparation of aziridines through the generation of nitrenes3 and 2) the synthesis of nitriles. We were particularly interested in the latter application, owing to the importance of the cyano group in industry,4 as well as its utility as an organic synthon. Traditional cyanation methods suffer from serious drawbacks, such as the use of highly toxic reagents, the generation of stoichiometric quantities of metal waste, the need for harsh conditions, and poor functional-group tolerance.
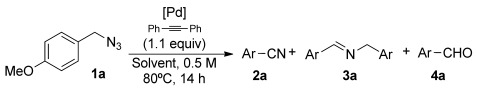
Azides allow the cyanide-free preparation of nitriles with no elongation of the skeletal carbon chain under several conditions: strong stoichiometric oxidants (such as BF3,5a 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ),5b,c or KI/tert-butyl hydroperoxide),5d CuSO4/phenyliodonium diacetate,6 CuI/tert-butyl hydroperoxide,7 or a supported Ru–OH catalyst.8 An earlier report, which employed palladium on charcoal with an alkyne as a hydrogen acceptor, caught our attention for its neutral conditions and its scope, which was not restricted to the formation of benzonitrile derivatives [Eq. (1)].9
| (1) |
In contrast to the other methods, this Pd system required the rigorous exclusion of moisture and oxygen for any reaction to take place at reflux in benzene or diethylamine. However, despite our efforts, we could not reproduce the reported results and only conversions of <20 % were obtained. Considering that different Pd/C catalysts can lead to adventitious results, we optimised the reaction conditions (Table 1).10 We only obtained sluggish reactions under an inert atmosphere or in organic solvents (either anhydrous or technical-grade), but the complete conversion of compound 1 a was achieved on water or neat (Table 1, entries 5 and 6). The reactions on water displayed poor selectivity, whereas, in the absence of solvent, anisaldehyde 4 a (15 %) was obtained as the only by-product,11 although it was inseparable from compound 2 a.
Table 1.
Optimisation studies[a]
 | ||||||
|---|---|---|---|---|---|---|
| Entry | [Pd] [mol %] | Solvent | Conversion [%] | 2 a | 3 a | 4 a |
| 1[b] | Pd/C (5) | toluene | 18 | 55 | 45 | 0 |
| 2 | Pd/C (5) | toluene | 35 | 59 | 8 | 33 |
| 3 | Pd/C (5) | dioxane | 11 | 41 | 0 | 59 |
| 4 | Pd/C (5) | MeCN | 9 | 33 | 0 | 67 |
| 5 | Pd/C (5) | water | >95 | 36 | 45 | 19 |
| 6 | Pd/C (5) | neat | >95 | 85 | 0 | 15 |
| 7 | [Pd2(dba)3] (5) | neat | >95 | 81 | 14 | 5 |
| 8 | Pd(OAc)2 (5 or 1) | neat | >95 | 45 | 0 | 55 |
| 9 | Pd(OAc)2 (1) | MeCN | >95 | 65 | 35 | 0 |
| 10[c] | Pd(OAc)2 (1) | MeCN | >95 | 84 | 16 | 0 |
[a] 1H NMR conversions are an average of two independent experiments. Pd/C was purchased from Acros Organics. [b] In anhydrous toluene under a N2 atmosphere. [c] The reaction was performed at a 0.12 m concentration, with styrene as a hydrogen acceptor.
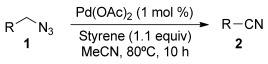
If [Pd2(dba)3] was used as the catalyst (Table 1, entry 7), 1,5-diphenyl-3-pentanone was also isolated.12 This compound was generated by the hydrogenation of the dibenzylideneacetone (dba) ligands that were originally on the palladium centre. After screening several Pd0 and PdII sources, Pd(OAc)2 was found to be the best candidate because it suppressed the undesired formation of compound 4 a (imine 3 a was readily separable from compound 2 a) and it was active in technical-grade MeCN at a concentration of only 1 mol % (Table 1, entries 6 and 10). Moreover, we also changed the hydrogen acceptor to styrene, because it is cheaper and both styrene and its reduced form can be easily removed from the crude products under reduced pressure. Notably, azide 1 a was completely converted in the absence of styrene as well, but higher proportions of imine 3 a and the formation of unknown by-products were observed.
Pleasantly, imines 3 were only observed as minor products in the formation of benzonitriles 2 a–2 c and a diverse range of nitriles (2) were isolated in good-to-excellent yields. One exception was phthalimide 2 f, which was isolated together with a bisphthalimide by-product.12 Also, no reaction was observed with cinnamyl azide.13 Terminal alkenes or carboxylic acids did not react under the optimised conditions, despite the generation of H2 (Table 2, entries 9 and 10). The reactions were allowed to proceed for 10 h, but shorter reaction times were sufficient in the cases of, for instance, nitriles 2 g, 2 h, and 2 l.14
Table 2.
Pd(OAc)2-catalysed synthesis of nitriles from azides
 | |||
|---|---|---|---|
| Entry | Nitrile | Yield [%][a] | |
| 1 | 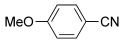 |
2 a | 75 |
| 2 | 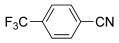 |
2 b | 61 |
| 3 | 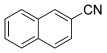 |
2 c | 71 |
| 4 | 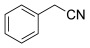 |
2 d | 91 |
| 5 | 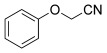 |
2 e | 79 |
| 6 | 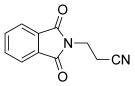 |
2 f | 61[b] |
| 7 | 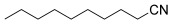 |
2 g | 92 |
| 8 |  |
2 h | 89 |
| 9 | 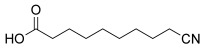 |
2 i | 90 |
| 10 | 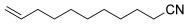 |
2 j | 89 |
| 11 | 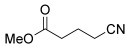 |
2 k | 72 |
| 12 |  |
2 l | 84[c] |
[a] Yield of the isolated product; the values are an average of two independent experiments. Reactions were performed at a 0.12 m concentration for the formation of benzonitriles and at a 0.5 m concentration for all other substrates. [b] Isolated with N,N′-propylenebisphthalimide. [c] From 7-azidoheptanitrile.
Although organic azides are generally safe and stable towards water and oxygen,1 those of low molecular weight can be particularly dangerous and difficult to handle.15 Gratifyingly, azides 1 could be generated in situ for the one-pot formation of nitriles from their corresponding bromides and NaN3, thereby avoiding the need to isolate any intermediate azides (Table 3). A slightly higher palladium loading (2 mol %) and a concentration of 1 m of the starting bromide maximised the reaction conversion for a range of nitriles that contained different functional groups (Table 3).
Table 3.
Pd(OAc)2-catalysed synthesis of nitriles from in situ generated azides[a]
 | |||
|---|---|---|---|
| Entry | Nitrile | Conversion [%][a] | |
| 1 |  |
2 c | 64[b] |
| 2 |  |
2 d | 83 |
| 3 |  |
2 g | >95[c] |
| 4 |  |
2 h | >95 |
| 5 |  |
2 i | 81 |
| 6 |  |
2 j | >95 |
| 7 |  |
2 l | >95[d] |
[a] 1H NMR conversions are an average of two independent experiments. [b] Imine 3 a was also formed (36 % conversion). [c] Yield of isolated product. [d] From 7-bromoheptanitrile.
Next, two competition experiments were performed. First, we reacted cinnamyl azide 1 o in the presence of compound 1 g and recovered the former compound unreacted, together with 1-cyanodecane (2 g, Scheme 1 A). All of the previously reported catalytic systems for this transformation converted compound 1 o into its corresponding nitrile.5b,c, 7–9 Furthermore, secondary azides have been reported to produce their corresponding ketones under the same reaction conditions as for the formation of nitriles.11 However, possibly owing to the absence of a strong oxidant, Pd(OAc)2 did not react with secondary azide 1 p, whereas compound 1 l was fully converted (Scheme 1 B).
Scheme 1.
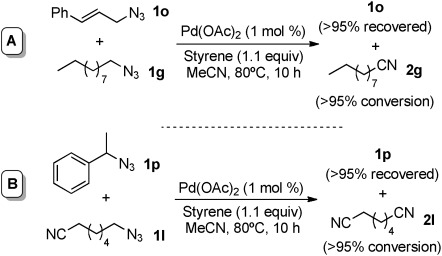
Competition experiments: A) Allyl versus alkyl azides; B) Secondary versus primary azides.
Regarding the imine formation, Pd(OAc)2 was also the only palladium source that was found to yield compound 3 a as major product if the model reaction was performed under neat conditions.10 This result might not seem surprising, owing to the bimolecular nature of this transformation, but several Pd/C catalysts, as well as [Pd2(dba)3], actually led to the formation of nitrile 1 a as the major product, even in the absence of solvent. Imines are often observed as minor products in the synthesis of nitriles from azides,16 but, to the best of our knowledge, only a molybdenum-based catalyst has been reported to produce them preferentially.17 As a consequence, we prepared several of these derivatives from different benzylic azides.18 In all cases, imines 3 were formed as the major products and they could be separated from their corresponding nitriles (2) by recrystallization or sublimation (Scheme 2).
Scheme 2.
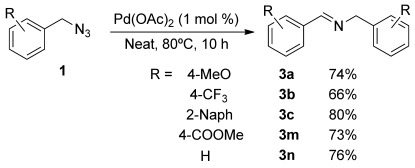
Pd(OAc)2-catalysed synthesis of imines from benzyl azides.
No styrene was used in the synthesis of imines because it lessened the formation of compound 3 a, thus indicating that dihydrogen might be necessary for the imine formation. Also, nitrile 2 a was integrally recovered after heating for 24 h at 80 °C in the presence of Pd(OAc)2. The reactions shown in Scheme 2 proceeded with evolution of gas within the first five minutes of stirring and the formation of ammonia was indirectly evidenced with wet pH paper. These facts lead us to propose that, in the case of aromatic nitriles, the palladium species in the reaction mixture can hydrogenate compounds 2 to generate a mixture of the imino and amino derivatives that would react together to generate compounds 3 and a molecule of ammonia (Scheme 3).19 Further mechanistic studies on this system are underway.
Scheme 3.
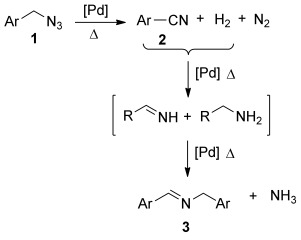
Proposed imine-formation pathway.
In conclusion, Pd(OAc)2 is an exceptional catalyst for the preparation of nitriles and imines from primary azides (or their corresponding bromides, thus avoiding the need to isolate the intermediary azide). The reactions proceeded under neutral conditions in air and showed unprecedented selectivities in the selected cases shown. The versatility of both azides and nitriles is expected to lead to the widespread application of this system in organic synthesis.
Acknowledgments
Imperial College London is gratefully acknowledged for their financial support of this work, as well as Johnson Matthey Plc. for the loan of palladium salts. L.M.S. thanks the Universidad de Valencia and Bancaja (Spain) for an Erasmus Scholarship.
Supplementary material
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- 1a.Patai S, editor. The Chemistry of the Azido Group. Chichester: Interscience; 1971. [Google Scholar]
- 1b.Bräse S, Gil C, Knepper K, Zimmermann V. Angew. Chem. 2005;117:5320–5374. doi: 10.1002/anie.200400657. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2005;44:5188–5240. doi: 10.1002/anie.200400657. [DOI] [PubMed] [Google Scholar]
- 2.Meldal M, Tornøe CW. Chem. Rev. 2008;108:2952–3015. doi: 10.1021/cr0783479. For a comprehensive review, see. [DOI] [PubMed] [Google Scholar]
- 3.Jung N, Bräse S. Angew. Chem. 2012;124:5632–5634. [Google Scholar]
- Angew. Chem. Int. Ed. 2012;51:5538–5540. doi: 10.1002/anie.201200966. For a timely highlight, see: and the references therein. [DOI] [PubMed] [Google Scholar]
- 4.Pollak P, Romeder G, Hagedorn F, Gelbke H-P. Nitriles, Ullmann’s Encyclopedia of Industrial Chemistry. 2000. [Google Scholar]
- 5a.Sasson R, Rozen S. Org. Lett. 2005;7:2177–2179. doi: 10.1021/ol050523h. [DOI] [PubMed] [Google Scholar]
- 5b.Qin C, Jiao N. J. Am. Chem. Soc. 2010;132:15893–15895. doi: 10.1021/ja1070202. [DOI] [PubMed] [Google Scholar]
- 5c.Zhou W, Xu J, Zhang L, Jiao N. Org. Lett. 2010;12:2888–2891. doi: 10.1021/ol101094u. [DOI] [PubMed] [Google Scholar]
- 5d.Lamani M, Devadig P, Prabhu KR. Org. Biomol. Chem. 2012;10:2753–2759. doi: 10.1039/c2ob06949k. [DOI] [PubMed] [Google Scholar]
- 6.Zhou W, Zhang L, Jiao N. Angew. Chem. 2009;121:7228–7231. [Google Scholar]
- Angew. Chem. Int. Ed. 2009;48:7094–7097. doi: 10.1002/anie.200903838. [DOI] [PubMed] [Google Scholar]
- 7.Lamani M, Prabhu KR. Angew. Chem. 2010;122:6772–6775. doi: 10.1002/anie.201002635. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2010;49:6622–6625. doi: 10.1002/anie.201002635. [DOI] [PubMed] [Google Scholar]
- 8.He J, Yamaguchi K, Mizuno N. J. Org. Chem. 2011;76:4606–4610. doi: 10.1021/jo2004956. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi H, Ohno A, Oka S. Bull. Chem. Soc. Jpn. 1976;49:506–509. [Google Scholar]
- 10. Selected reactions are shown in Table 1. For a comprehensive screening, see the Supporting Information.
- 11. The formation of carbonyl compounds as either minor or major products has been reported previously; see Refs. [5b,d, 7, 8]
- 12. For further details, see the Supporting Information.
- 13.Zhou W, Xu J, Zhang L, Jiao N. Synlett. 2011:887–890. For a palladium-mediated Tsuji–Trost/oxidation sequence for the preparation of alkenyl nitriles, see. [Google Scholar]
- 14. Reaction times of 3, 4, and 5 h, respectively.
- 15a.Scriven EFV, Turnbull K. Chem. Rev. 1988;88:297–368. [Google Scholar]
- 15b.Saxon E, Bertozzi CR. Science. 2000;287:2007–2010. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- 16.Risse J, Scopelliti R, Severin K. Organometallics. 2011;30:3412–3418. See also references [8, 9] [Google Scholar]
- 17.Ramesha AR, Bhat S, Chandrasekaran S. J. Org. Chem. 1995;60:7682–7683. [Google Scholar]
- 18. Even though we had no incidents during this study, for safety reasons, we performed the neat reactions of azides in thick-walled, sealed vials.
- 19.Srimani D, Feller M, Ben-David Y, Milstein D. Chem. Commun. 2012;48:11853–11855. doi: 10.1039/c2cc36639h. For a related proposal see. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


