Table 1.
Optimisation studies[a]
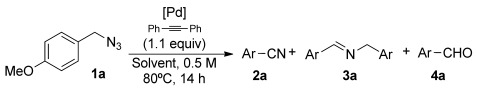 | ||||||
|---|---|---|---|---|---|---|
| Entry | [Pd] [mol %] | Solvent | Conversion [%] | 2 a | 3 a | 4 a |
| 1[b] | Pd/C (5) | toluene | 18 | 55 | 45 | 0 |
| 2 | Pd/C (5) | toluene | 35 | 59 | 8 | 33 |
| 3 | Pd/C (5) | dioxane | 11 | 41 | 0 | 59 |
| 4 | Pd/C (5) | MeCN | 9 | 33 | 0 | 67 |
| 5 | Pd/C (5) | water | >95 | 36 | 45 | 19 |
| 6 | Pd/C (5) | neat | >95 | 85 | 0 | 15 |
| 7 | [Pd2(dba)3] (5) | neat | >95 | 81 | 14 | 5 |
| 8 | Pd(OAc)2 (5 or 1) | neat | >95 | 45 | 0 | 55 |
| 9 | Pd(OAc)2 (1) | MeCN | >95 | 65 | 35 | 0 |
| 10[c] | Pd(OAc)2 (1) | MeCN | >95 | 84 | 16 | 0 |
[a] 1H NMR conversions are an average of two independent experiments. Pd/C was purchased from Acros Organics. [b] In anhydrous toluene under a N2 atmosphere. [c] The reaction was performed at a 0.12 m concentration, with styrene as a hydrogen acceptor.
