Abstract
Injury to the adult kidney induces a number of developmental genes thought to regulate repair, including Wnt4. During kidney development, early nephron precursors and medullary stroma both express Wnt4, where it regulates epithelialization and controls smooth muscle fate, respectively. Expression patterns and roles for Wnt4 in the adult kidney, however, remain unclear. In this study, we used reporters, lineage analysis, and conditional knockout or activation of the Wnt/β-catenin pathway to investigate Wnt4 in the adult kidney. Proliferating, medullary, interstitial myofibroblasts strongly expressed Wnt4 during renal fibrosis, whereas tubule epithelia, except for the collecting duct, did not. Exogenous Wnt4 drove myofibroblast differentiation of a pericyte-like cell line, suggesting that Wnt4 might regulate pericyte-to-myofibroblast transition through autocrine signaling. However, conditional deletion of Wnt4 in interstitial cells did not reduce myofibroblast proliferation, cell number, or myofibroblast gene expression during fibrosis. Because the injured kidney expresses multiple Wnt ligands that might compensate for the absence of Wnt4, we generated a mouse model with constitutive activation of canonical Wnt/β-catenin signaling in interstitial pericytes and fibroblasts. Kidneys from these mice exhibited spontaneous myofibroblast differentiation in the absence of injury. Taken together, Wnt4 expression in renal fibrosis defines a population of proliferating medullary myofibroblasts. Although Wnt4 may be dispensable for myofibroblast transformation, canonical Wnt signaling through β-catenin stabilization is sufficient to drive spontaneous myofibroblast differentiation in interstitial pericytes and fibroblasts, emphasizing the importance of this pathway in renal fibrosis.
Wnt4 is a member of a highly conserved family of 19 secreted morphogenic glycoproteins known to regulate a variety of developmental processes and tissue homeostasis in adult organisms.1,2 In kidney development, Wnt4 is expressed on the ventral side of cap metanephric mesenchyme at embryonic day 10.5 and continues to be expressed until postnatal day 2 in pretubular aggregates and early stages of epithelial nephron precursors.3–9 Wnt4 is necessary and sufficient for the transition of the condensed metanephric mesenchyme cells to an epithelial fate and is required for tubulogenesis. Metanephric development in Wnt4−/− mice arrests before the formation of renal vesicles and mice die shortly after birth due to nonfunctioning kidneys.3,4
The similarities between nephrogenesis and epithelial regeneration in adult led to the hypothesis that kidney regeneration recapitulates aspects of kidney development.1,10–13 Although Wnt4 is not expressed in terminally differentiated cap mesenchyme-derived epithelia, it is reported to be re-expressed in proliferating proximal tubule epithelial cells during the repair phase of unilateral ischemia reperfusion injury (U-IRI), which would be consistent with a model in which epithelial injury triggered dedifferentiation to a mesenchymal state.14
Wnt4 has also been shown to be reactivated in chronic kidney fibrosis.15–17 Surendran et al. observed by in situ hybridization that Wnt4 transcripts are expressed in papilla in uninjured adult kidney, and reported that Wnt4 is induced in both the renal interstitium and collecting duct (CD) epithelium after unilateral ureteral obstruction (UUO), in contrast with the strictly tubular Wnt4 localization previously reported.14 Other groups have confirmed Wnt4 upregulation in CKD models at the mRNA or protein level, suggesting that Wnt pathway activation may underlie epithelial to mesenchymal transition, although no further cell localization data for Wnt4 exists.15–17 Wnt4 has also been shown to be expressed in the medullary stroma of embryonic kidney, indicating that re-expression in interstitium of injured adult kidney may represent a recapitulation of developmental signaling mechanisms.18
We find that Wnt4 is only expressed in principal cells of papillary CDs and urothelium under basal conditions. Outside of the papilla, a tubule cell was never observed to be positive for Wnt4 at any stage, under any condition in the adult mouse kidney. Following two different injury models, U-IRI and UUO, we show that Wnt4 is specifically expressed in interstitial myofibroblasts located in the medulla, but not cortical myofibroblasts or epithelium. These Wnt4-positive cells proliferate during fibrotic injury, and although Wnt4 itself is dispensable for myofibroblast proliferation and differentiation, stabilization of β-catenin in these cells was sufficient to drive spontaneous myofibroblast activation in the absence of injury.
Results
Wnt4 Expression Is Restricted to Papillary Principal Cells and Urothelium in Adult Kidney
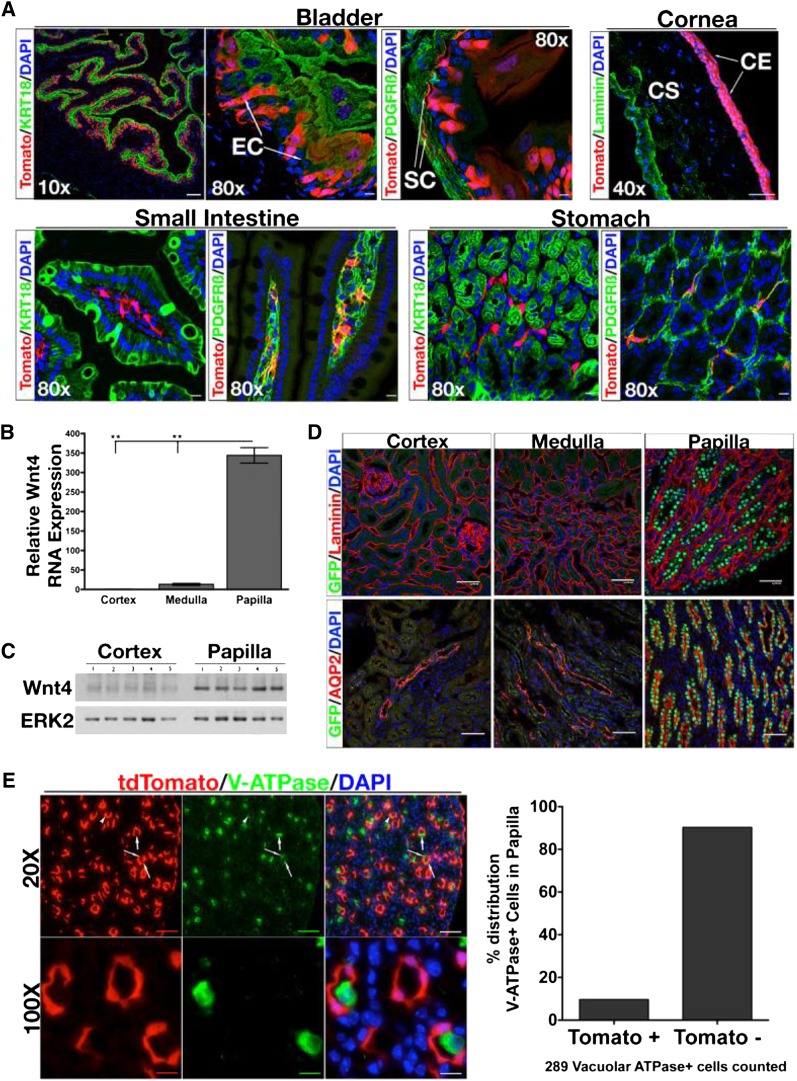
We initially generated and examined bi-genic Wnt4GCE/+;R26tdTomato/+ reporter mice (described below and in Supplemental Figure 1) to determine Wnt4 expression patterns across various tissues including kidney. It is known that Wnt4 is expressed in the epithelial and stromal layers of mouse bladder19 and we show that the tdTomato reporter is expressed within the cytokeratin-18+ and PDGF receptor β+ (PDGFRβ+) cell layers of the bladder (Figure 1A). We detect strong expression of the Tomato fluorophore in the corneal epithelium of mouse eye, where Wnt4 expression has also been reported.20 In addition, Tomato+ cells were observed in the stromal cells of intestine and stomach (Figure 1A). Finally, we used Wnt4GCE/+;R26tdTomato/+ to detect Tomato+ cells in choroid plexus, hepatic stellate cells, epithelial and stromal cells of the ureter, and ovaries21 (Supplemental Figure 2 and data not shown). These data indicate that Wnt4GCE/+;R26tdTomato/+ represent endogenous Wnt4 expression and can be used to study Wnt4 in a variety of tissues.
Figure 1.
Validation of Wnt4GCE/+;R26tdTomato/+ reporter mice. (A) tdTomato reflects Wnt4 expression. tdTomato+ cells are seen in epithelial KRT18+ cells, and stromal PDGFRβ+ cells of the bladder. tdTomato is strongly detected in corneal epithelium. We also report tdTomato+ cells in tissues that have not been previously reported to express Wnt4. In the small intestine and stomach, tdTomato+ cells colocalize with PDGFRβ+ cells. (B) Cortex, medulla, and papilla are dissected from wild-type C57BL/6N mice, RNA is extracted, and SybrGreen-based qPCR is performed to detect Wnt4. Wnt4 transcript is significantly upregulated in papilla versus cortex and medulla. (C) Whole cell protein homogenate from cortical and papillary samples are processed for Western blot to test for Wnt4 protein levels. (D) Confocal images of renal cortex, medulla, and papilla from Wnt4GC/+ reporter mice. In the top row, sections are stained with anti-laminin antibody (red), anti-GFP antibody (green), and DNA marker DAPI (blue). Nuclear GFP represents Wnt4+ cells, which are not detected in cortex and medulla but are detected in papilla tubules. The images in the bottom row are of kidney sections stained with anti-aquaporin 2 antibody (red), anti-GFP antibody (green), and DAPI (blue). GFP+ cells colocalize with aquaporin 2+ cells in the papilla and not in the cortex or medulla, indicating that Wnt4+ cells are located specifically in papillary CD epithelia. (E) Sections of papilla from Wnt4GCE/+;R26tdTomato/+ stained with anti-V-ATPase to identify intercalated cells. Quantification reveals that >90% of V-ATPase+ cells are tdTomato−. In B, n=3 or 4 per group. Data analyzed by one-way ANOVA. **P<0.0001 (Tukey’s multiple comparison test). EC, epithelial cell; SC, stromal cell; CE, corneal epithelium; CS, corneal stroma; DAPI, 4',6-diamidino-2-phenylindole. Scale bars, 100 µM in ×10 images in A; 50 µM in ×40 images in A; 10 µM in ×80 in A.
Within the kidney, Wnt4 mRNA is dramatically increased in the papilla, compared with the medulla or cortex under baseline conditions (Figure 1, B and C). Next, we used a Wnt4 reporter mouse, referred to as Wnt4GC/+, to determine the specific cell type expressing Wnt4.22 Green fluorescent protein–positive (GFP+) cells were located in the papilla and costaining with an anti-laminin antibody indicated that these GFP+ cells were tubular cells (Figure 1D). These cells were aquaporin 2+, confirming their CD identity. Interestingly, all CD epithelia of the medulla and cortex were GFP−, whereas papillary CD cells were GFP+ (Figure 1D).
To identify kidney cells with current Wnt4 expression by a complementary approach, we utilized the Wnt4GCE/+;R26tdTomato/+ mice. To distinguish between Wnt4 expression in principal versus intercalated cell types, sections of tamoxifen-injected Wnt4GCE/+;R26tdTomato/+ kidney were stained with vacuolar-type H+-ATPase (V-ATPase), which labels intercalated cells, and the distribution of V-ATPase+ cells in tdTomato+ and tdTomato− cells was quantified. Most of the 289 V-ATPase+ cells in the papilla analyzed were tdTomato− (Figure 1E) with a small proportion being difficult to categorize.
Lineage Analysis Indicates that Wnt4 Is Never Expressed in Cortical or Medullary CD
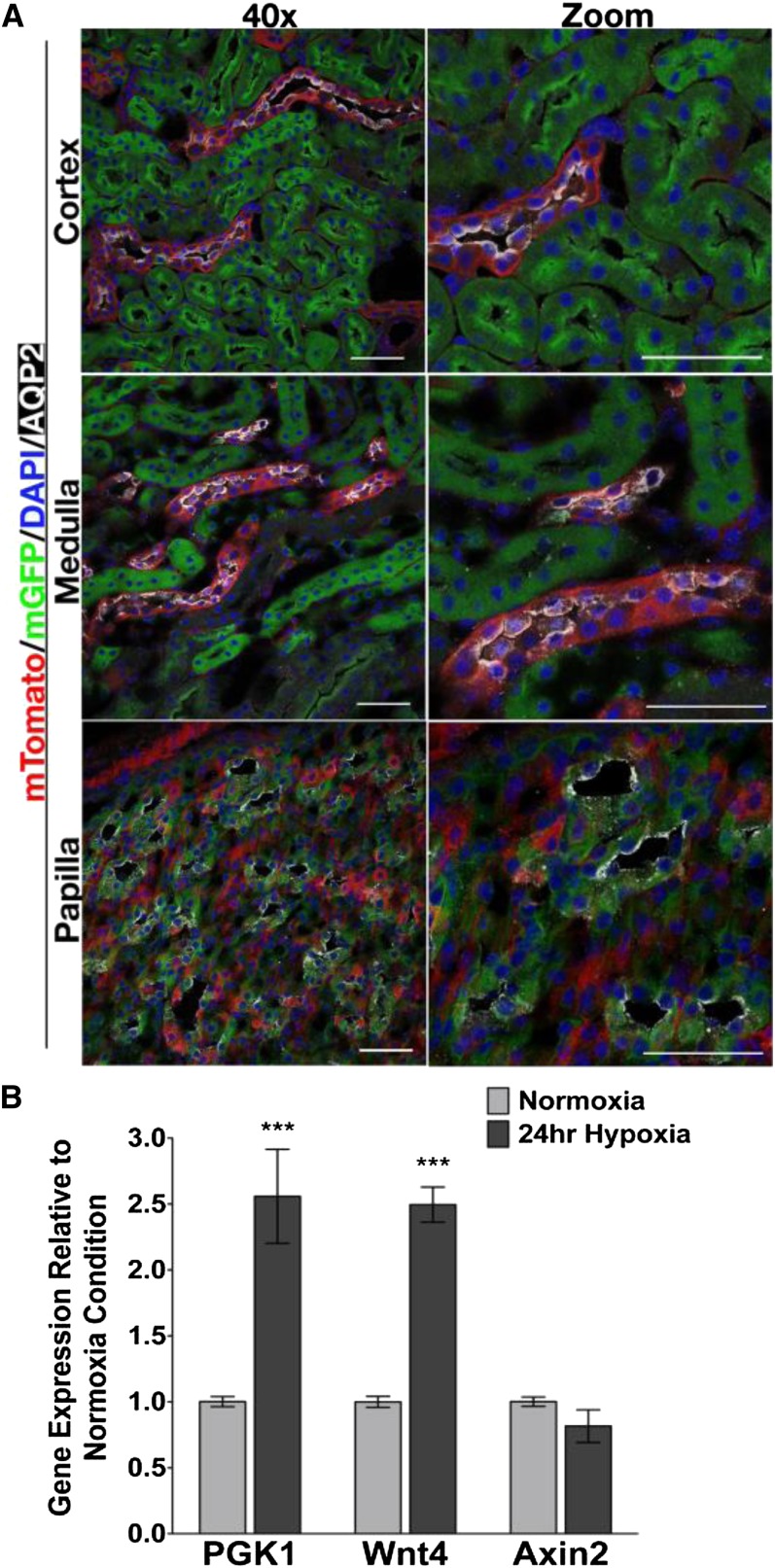
Wnt4 expression in papilla was previously noted in developing kidney23 at E15. It is not known whether Wnt4 is expressed in nonpapillary CDs throughout development. We therefore sought to answer whether cortical and medullary CDs express Wnt4 transiently, but then lose expression, or if Wnt4 is restricted to the papillary segment of CD. We crossed Wnt4GC/+ mice with Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo reporter mice (Supplemental Figure 3).24 These mice express membrane-targeted Tomato before recombination or membrane-targeted GFP after recombination. As expected, all nephron segments in the cortex and medulla proximal to the CD are mGFP+ (Figure 2A), reflecting Wnt4 expression in the condensed metanephric mesenchyme and renal vesicle. However, the ureteric bud–derived CDs in cortex and medulla were mTomato+, indicating that they had never expressed Wnt4. As expected, aquaporin 2+ papillary epithelia were mGFP+ and mTomato− (Figure 2A, third panel row), suggesting continued Wnt4 expression in this region.
Figure 2.
Wnt4 is not expressed in cortical collecting duct during development. (A) Confocal images of cortex, medulla, and papilla taken with a ×40 objective lens in the left column and high-power digital zoom in the right column. Kidney sections are from Wnt4GC/+;R26mTmG/+ mice. Green cells indicate that Wnt4 is expressed in that cell or has been previously expressed in a progenitor of that cell. Red cells never expressed Wnt4. Red and green fluorescence is epifluorescence, blue is DAPI, and white is AQP2. In cortex and medulla, aquaporin 2+ CD epithelia are in red cells. In papilla, AQP2+ cells are in green (Wnt4 expressing or descendant) cells. (B) Primary IMCD cells are grown in normoxic (20% O2) or transiently exposed to hypoxic (3% O2) for 24 hours. Gene expression as measured by qPCR indicates that the hypoxia-responsive gene PGK1 is significantly increased in hypoxia exposed IMCD cells. Wnt4 gene expression is also significantly increased in hypoxic IMCD cells compared with normoxic cells, whereas Axin2 gene expression is unchanged. Data are analyzed by two-way ANOVA comparing gene expression between hypoxic and normoxic conditions (Bonferroni post test). ***P<0.001. Scale bars, 50 µM in A. DAPI, 4',6-diamidino-2-phenylindole; AQP2, aquaporin-2; IMCD, inner medullary collecting duct.
The expression of Wnt4 in papillary CD suggested that it might reflect an adaptation to the hypoxic papillary environment. To test this, primary inner medullary CD epithelial cultures were exposed to hypoxia (3% O2 for 24 hours). This stimulus triggered significant upregulation of PGK1, a hypoxia-responsive gene, which served as a positive control for hypoxia.25 It also activated Wnt4 expression in these cells, providing evidence supporting the notion that papillary hypoxia induces Wnt4 expression (Figure 2B).
Wnt4 Is Upregulated in Medullary Interstitial Myofibroblasts during Fibrosis
In both the U-IRI and UUO injury models, Wnt4 transcript levels were significantly increased in medullary samples of injured kidneys (Figure 3, A and C). Wnt4 expression in cortex was minimal, and no changes in papillary Wnt4 expression after injury were found (data not shown). The temporal increases in Wnt4 correlated with fibrotic marker gene expression including α smooth muscle actin (αSMA), fibronectin, and collagen 1α1 transcript expression (Figure 3, B and D).
Figure 3.
Wnt4 is expressed in medullary myofibroblasts during fibrosis. (A) After U-IRI, mice are euthanized at indicated time points and cortex and medulla are dissected out of injured kidney. Transcript expression analysis with qPCR indicates that Wnt4 expression is significantly higher than sham controls 7, 14, and 28 days after injury. Cortical Wnt4 expression is significantly lower than medullary expression at days 7, 14, and 28. Cortical Wnt4 expression is significantly higher than sham at day 14 only. (B) Analysis of fibrotic markers αSMA, fibronectin, and collagen 1α1 by qPCR show significant increase at days 3, 7, 14, and 28 after U-IRI. (C) Kidney cortex and medulla samples are prepared from UUO at 5 and 10 days after surgery and processed for qPCR. Wnt4 transcript is significantly higher than sham in the medulla and cortex 5 days and 10 days after UUO. Medulla Wnt4 expression is significantly higher than cortex expression on both day 5 and day 10. (D) Transcripts for fibrotic genes αSMA, fibronectin, and collagen 1α1 are significantly higher on days 5 and 10 after UUO compared with sham controls. (E) Seven days after U-IRI, anti-GFP antibody immunofluorescent colabeling of outer medulla from CLK and U-IRI kidney sections along with anti-laminin (basement membrane marker), anti-αSMA, and anti-PDGFRβ (myofibroblast markers) antibodies. Nuclear GFP appears outside of all tubules and colocalizes with cells positive for αSMA and PDGFRβ. (F) GFP and CD31 (endothelial cell marker) do not colocalize. (G) GFP and F480 (macrophage marker) do not colocalize. (H) GFP does not colocalize with neutrophils and (I) GFP does not colocalize with CD3 (pan T cell marker). Asterisks in A-D indicate analysis by one-way ANOVA comparing groups to respective sham controls (post test, Tukey’s multiple comparison test). Hash marks in A and C indicate analysis by two-way ANOVA comparing between sample types within the same time-point (post test, Bonferroni). *#P<0.05; **##P<0.01; ***###P<0.001. n=3–4 per group for U-IRI and 4–5 per group for UUO. col1α1, collagen 1α1; DAPI, 4',6-diamidino-2-phenylindole. Scale bars, 50 µM.
To determine the cell type responsible for increased Wnt4 expression during injury, Wnt4GC/+ mice were subject to U-IRI and UUO. We verified that GFP expression accurately reports Wnt4 expression by quantitative PCR (qPCR) (Supplemental Figure 4) and stained kidney sections from injured and contralateral, uninjured kidney (CLK) controls with anti-GFP and a panel of other antibodies (Figure 3). Costaining of GFP and laminin in Wnt4GC/+ mice shows that Wnt4 is expressed in the medullary interstitium in injured kidneys and was never observed in tubule epithelia (Figure 3E and Supplemental Figure 5A). These interstitial cells were positive for αSMA and PDGFRβ indicating that Wnt4+ cells are myofibroblasts (Figure 3E and Supplemental Figure 5A). In confirmation of our qPCR data, GFP+ cells were not observed in the cortex of kidneys subject to injury by U-IRI or UUO, indicating that Wnt4 expression defines medullary myofibroblasts in distinction with cortical myofibroblasts (Supplemental Figure 5B). To examine other interstitial cell types, colabeling experiments with anti-GFP were conducted with anti-CD31, anti-F480, anti-neutrophil, and anti-CD3 (Figure 3, F–I). The GFP signal did not colocalize with any of these antigens.
Genetic Labeling of Wnt4+ Cells after UUO Highlights Specific Wnt4 Expression in Medullary Myofibroblasts and Reveals Population of Proliferating Myofibroblasts
We next asked whether the Wnt4+ cells we identified expand or migrate during fibrosis using genetic lineage analysis. To genetically label Wnt4+ cells after injury, we used a mouse with a tamoxifen-inducible GFP-Cre-ERT2 cassette knocked-in to the Wnt4 locus, called the Wnt4GCE/+ mouse.26 These mice were bred with Gt(ROSA)26Sortm9(CAG-tdTomato), generating bi-genic Wnt4GCE/+;R26tdTomato/+ mice (Supplemental Figure 1).27–29 To validate this new model in kidney, tamoxifen was administered on day 6 after UUO and mice were euthanized on day 14 after UUO.30 Confocal images of the uninjured kidney show specific labeling in the papillary CDs as well as strong labeling of urothelium (Figure 4, A and C). Images of the injured kidney show expansion of labeled cells exclusively within the medulla. tdTomato+ cells colocalized with PDGFRβ and αSMA (Figure 4, B and C).31 As expected, PDGFRβ+ and αSMA cells in the cortex were negative for the Tomato label (Figure 4C). To further characterize the Wnt4 expression domain, Wnt4 expression was compared with kidney injury molecule-1 expression, revealing nonoverlapping expression domains. Kidney injury molecule-1 strongly stained cortical and outer medullary proximal tubule cells, whereas Wnt4 was expressed in a complementary fashion in the inner medulla (Figure 4C). In addition, because tamoxifen has been shown to affect fibrosis, we show that our tamoxifen treatment paradigm did not effect fibrotic response (Supplemental Figure 6).32
Figure 4.
Wnt4 expression in papillary collecting duct and medullary myofibroblasts during fibrosis. (A) CLK kidney of Wnt4GCE/+;R26tdTomato/+ mouse injected with tamoxifen and euthanized 8 days later. tdTomato signal seen in papilla CDs and urothelia surrounding papilla. Green represents αSMA expression in surrounding ureter. (B) High-power confocal image of a Wnt4+ myofibroblast fate labeled with tdTomato colocalized with PDGFRβ. (C) Anti-PDGFRβ and anti-αSMA staining indicates widespread myofibroblast expression in medulla and cortex, whereas tdTomato epifluorescence only colabels medullary myofibroblast cells. Anti-KIM1 staining highlights the lack of tdTomato fate labeled cells expressed in cortical cells in fibrotic kidney. DAPI, 4',6-diamidino-2-phenylindole; KIM1, kidney injury molecule-1. Scale bars, 100 µM for ×20 and 10 µM for ×60 in A; 10µM in B; 100 µM in C.
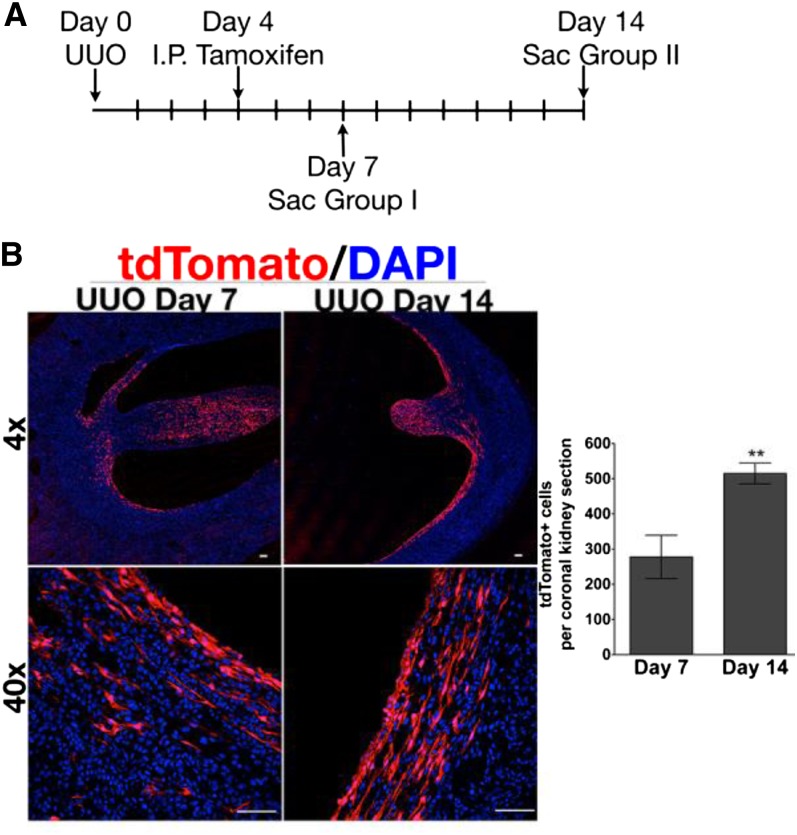
To fate trace Wnt4 expressing cells, we measured baseline recombination 3 days after tamoxifen administration, to be sure that no further recombination would take place.33,34 Wnt4GCE/+;R26tdTomato/+ mice were injected with tamoxifen 4 days after UUO injury and euthanized on day 7 and day 14. tdTomato+ cells were counted in coronal kidney sections of five mice at day 7 after UUO, which reflects the Wnt4+ cells present during days 4–7 after UUO (Figure 5A and Supplemental Figure 7). tdTomato+ cells were then counted from five mice at day 14 after UUO (Figure 5B). We found that the number of tdTomato+ myofibroblasts essentially doubled in UUO injured kidney from day 7 to day 14 (Figure 5B). This may be an underestimation because cell proliferation likely occurred between the time of tamoxifen injection (day 4) and the time of baseline measurement (day 7).
Figure 5.
Wnt4+ myofibroblasts proliferate during fibrosis but do not migrate. (A) Three milligrams of tamoxifen are administered by intraperitoneal injection 4 days after UUO to 10 Wnt4GCE/+;R26tdTomato/+ mice. Five mice are euthanized on day 7 after UUO and five mice are euthanized on day 14 after UUO. (B) Low-power ×4 confocal images of tamoxifen-treated mice 7 days after UUO and 14 days after UUO. Representative ×40 confocal images of tamoxifen-treated mice. The number of tdTomato+ cells per coronal section significantly increases 14 days after UUO compared with 7 days after UUO. In B, n = 5 in each group, and data are analyzed by t test. **P=0.01. DAPI, 4',6-diamidino-2-phenylindole.
Exogenous Wnt4 Induces Myofibroblast Differentiation In Vitro
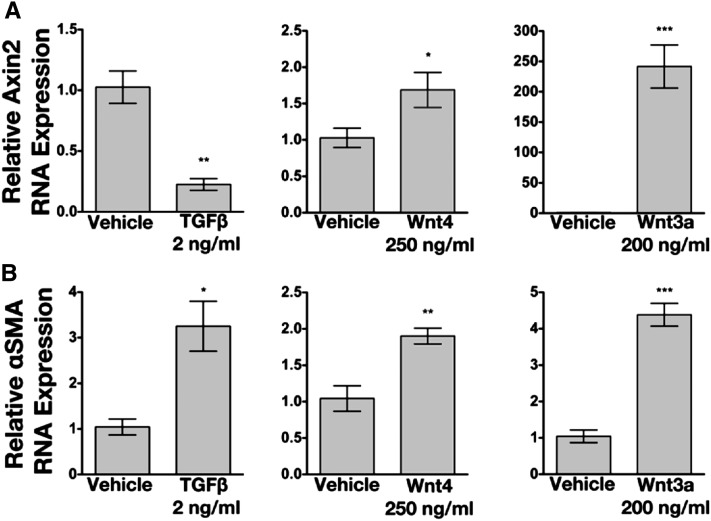
We next asked whether Wnt4 was sufficient to induce αSMA expression in vitro using the 10T1/2 mesenchymal cell line.35–37 We used this line because, similar to kidney pericytes, these cells do not express αSMA but increase its expression upon differentiation into myofibroblasts.31 Treatment of 10T1/2 cells with the prototypical canonical Wnt pathway ligand, Wnt3a, induced robust expression of canonical Wnt target gene Axin2 (Figure 6A). Wnt4 induces a much lower level of Axin2 (<2-fold compared with 250-fold), raising the possibility of noncanonical signaling by Wnt4. Surprisingly, TGF-β produced a consistent and significant downregulation of Axin2 expression in this cell type. Treatment with TGF-β is known to increase expression of αSMA in these cells.36 We typically observed 3- to 5-fold upregulation of αSMA transcript after treatment with 2 ng/ml of TGF-β (Figure 6B). Interestingly, treatment with Wnt4 and Wnt3a produced an upregulation of αSMA in these cells that was comparable to TGF-β, indicating that Wnt ligands can promote the expression of this fibrogenic gene in vitro. 10T1/2 cells were also treated with glycogen synthase kinase 3β inhibitor CHIR99021, which is known to activate canonical β-catenin signaling.38 This compound significantly increased αSMA transcript compared with vehicle controls and at levels comparable to TGF-β treatment (Supplemental Figure 8).
Figure 6.
Treatment of 10T1/2 cells with Wnt ligands increases αSMA expression. (A) Axin2 transcript expression decreases after treatment of cells with TGF-β and increases after treatment with Wnt4 or Wnt3a. (B) Wnt ligands can increase expression of αSMA in 10T1/2 cells. Analyzed by t test. *P<0.05; **P<0.01; ***P<0.001. Samples done in triplicate and repeated twice.
Conditional Deletion of Wnt4 in Interstitium
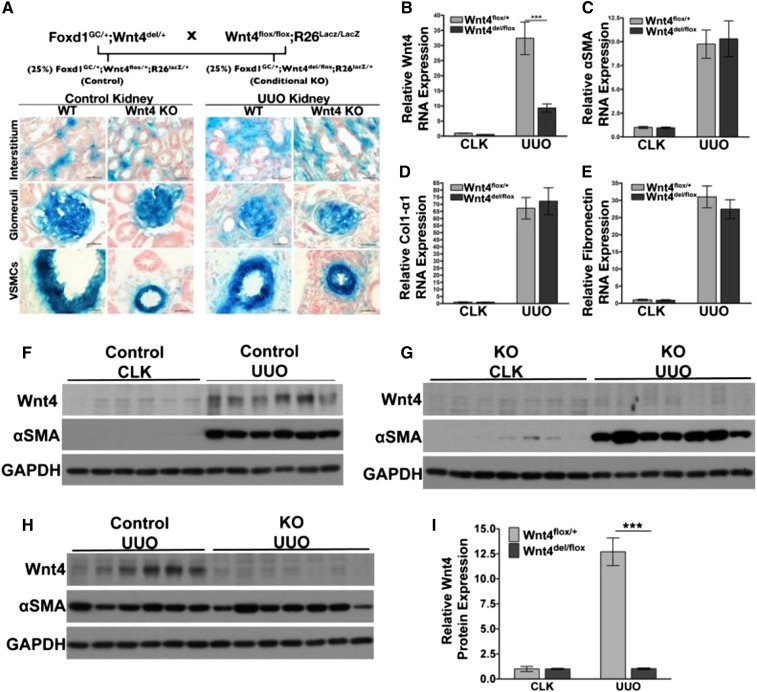
To determine whether Wnt4 is necessary for myofibroblast differentiation and interstitial fibrosis in medulla, we generated mouse model to knock out Wnt4 specifically in interstitial fibroblasts/pericytes, the cells that eventually become myofibroblasts.31,39 FoxD1-GFPCre mice were crossed with Wnt4del/+ mice to create bi-genic Foxd1GC/+;Wnt4del/+ mice.22,31,40,41 These were crossed with Wnt4flox/flox;R26lacZ/lacZ mice (Figure 7A and Supplemental Figure 9).40,42 The resulting offspring yielded 25% Foxd1GC/+;Wnt4del/flox;R26lacZ/+, which are called Wnt4del/flox hereafter, and 25% Foxd1GC/+;Wnt4flox/+;R26lacZ/+, which are called Wnt4flox/+ hereafter. Wnt4del/flox mice have Wnt4 knocked out of all kidney stromal cells, and additionally express lacZ in those Wnt4-negative cells (Supplemental Figure 9). The Wnt4flox/+ mice are controls, in that they still carry a wild-type Wnt4 allele. Mice were born at the expected Mendelian frequency and had no kidney phenotype. UUO was carried out on Wnt4flox/+ and Wnt4del/flox mice and lacZ expression patterns were visualized in sections of the contralateral and injured kidney. Sections from the contralateral (uninjured) and UUO kidney of control and knockout mice show that interstitial cells, mesangial cells, and vascular smooth muscle cells (VSMCs) are all positive for lacZ, confirming Cre activity in these cells (Figure 7A).
Figure 7.
Myofibroblast-specific Wnt4 knockout does not impact renal fibrosis. (A) Breeding strategy results in 25% control mice and 25% KO mice. lacZ recombination is evident in interstitial pericytes, mesangial cells, and VSMCs in both Wnt4flox/+ and Wnt4del/flox mice. As expected, there is evidence of expansion of lacZ+ interstitial cells, whereas labeling of mesangial cells and VSMCs remains unchanged. (B) Ten days after UUO, Wnt4 transcript is increased approximately 30-fold in control mice over CLK. Wnt4 transcript levels are significantly decreased in UUO samples from Wnt4del/flox (knockouts) compared with control mice. (C–E) Transcript levels of fibrotic marker genes αSMA, collagen 1α1, and fibronectin are all increased 10 days after UUO in control and KO mice compared with CLK, however, are not different from each other. (F–H) Western blots comparing expression of Wnt4 and αSMA within genotypes and across genotypes in the 10 day UUO and CLK samples. (I) Quantification of integrated densities of Wnt4 protein bands indicates a significant increase in Wnt4 protein in control mice after UUO. Comparison of relative Wnt4 protein levels between control and KO samples after UUO shows an approximately 93%–94% decrease of Wnt4 in the KO kidneys. Wnt4 protein levels in UUO kidneys of KO mice are similar to Wnt4 protein levels in CLK kidneys. Data analyzed by two-way ANOVA comparing between genotypes in CLK and UUO conditions. In F–H, blots are analyzed by comparing integrated optical densities of bands and normalizing with GAPDH bands followed by two-way ANOVA (Bonferroni’s post test). ***P<0.001. Scale bars, 25 µM in A. KO, knockout; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Decreased Wnt4 Expression in Conditional Knockouts with Normal Fibrotic Response
To analyze the contribution of myofibroblast-derived Wnt4 in fibrosis, UUO was performed on Wnt4flox/+ (n=6) and Wnt4del/flox (n=8) mice that were euthanized on day 10. Wnt4 expression in the injured kidneys of Wnt4flox/+ mice increased approximately 30-fold over the contralateral kidney. We observed a significant 75%–80% decrease in Wnt4 transcript expression in injured Wnt4del/flox kidneys compared with Wnt4flox/+ controls (Figure 7B), validating our cell-specific knockout strategy. Wnt4 protein expression was also analyzed in lysates obtained from tissue samples. As expected, Wnt4 protein was increased in the Wnt4flox/+ UUO kidney compared with contralateral kidney, and there was no detectable increase of Wnt4 protein in UUO Wnt4del/flox kidneys compared with contralateral kidneys (Figure 7, F–H). Quantification of immunoblots comparing relative Wnt4 protein expression in Wnt4flox/+ UUO mice versus Wnt4del/flox UUO mice shows an approximately 93% decrease in Wnt4 protein levels in the knockout (Figure 7I). Wnt4 protein expression in Wnt4del/flox UUO kidneys are comparable to protein expression in CLK kidneys. Expression of αSMA, collagen 1α1, and fibronectin transcripts were all increased after UUO in controls; however, there was no change in these markers in Wnt4del/flox mice (Figure 7, C–E). In sections, levels of αSMA and F480 were quantified by digitally assessing the average number of positive pixels per section and comparing Wnt4flox/+ and Wnt4del/flox mice. The number of positive Ki67+ cells were counted and also compared between genotypes. We did not observe any difference between groups in these assays (Supplemental Figure 10).
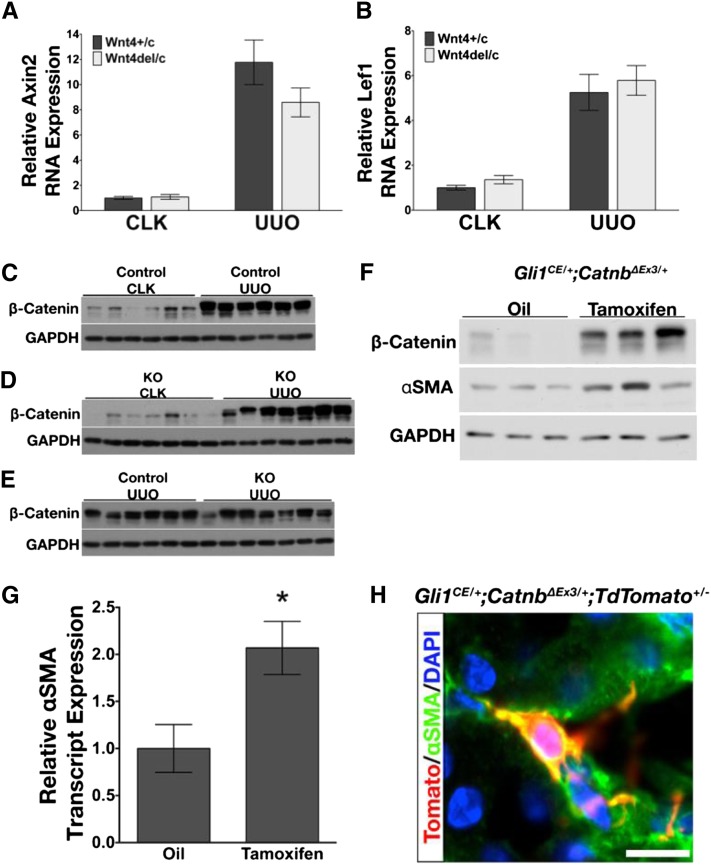
To determine whether conditional knockdown of Wnt4 modulated canonical Wnt signaling, transcript levels of Axin2 and Lef1 in Wnt4flox/+ and Wnt4del/flox mice were analyzed. Although both genes displayed a significant increase over their respective control kidney samples, they were not significantly different after UUO (Figure 8, A and B). β-catenin protein was also analyzed by Western blot in control and UUO kidneys of Wnt4flox/+ and Wnt4del/flox mice. Although an increase in stabilized β-catenin protein was apparent in UUO samples compared with control, comparison between genotypes did not show a significant difference after UUO (Figure 8, C–E, and data not shown). It has also been shown that a variety of the 19 different Wnt ligands significantly increase in kidney after UUO.17 Analysis of transcripts of Wnt3a, Wnt5a, Wnt6, Wnt7b, Wnt10a, and Wnt16 showed that they were all significantly upregulated in UUO kidney compared with CLK of both genotypes (Supplemental Figure 11).
Figure 8.
Stabilization of β-catenin in a subset of pericytes and fibroblasts is sufficient to drive myofibroblast transformation. (A and B) Transcripts of canonical Wnt pathway target genes Axin2 and Lef1 are analyzed by qPCR and found to be unchanged after UUO in Wnt4flox/+ and Wnt4del/flox mice. (C–E) Western blot comparing levels of stabilized β-catenin protein between CLK and UUO samples from Wnt4flox/+ mice, CLK and UUO from Wnt4del/flox mice, and UUO between both genotypes. β-catenin levels are increased after UUO in both genotypes compared with CLK and do not differ from each other after UUO (quantitation not shown). (F) Gli1CE/+;CatnbΔEx3/+ mice are treated with corn oil or tamoxifen at 1 mg for 5 days. Two weeks after the last injection, lysates are probed for expression of β-catenin and αSMA. An increase in β-catenin and αSMA protein is observed in the tamoxifen-treated mice. (G) αSMA transcript is significantly increased in the tamoxifen-treated group. (H) A tdTomato+ interstitial cell in tri-genic Gli1CE/+;CatnbΔEx3/+;tdTomato+/− mouse expressing αSMA. In A and B, data are analyzed by two-way ANOVA comparing between genotypes in each condition. In G, data are analyzed by the t test. DAPI, 4',6-diamidino-2-phenylindole.
Because other upregulated Wnt ligands might compensate for the absence of Wnt4, we next asked whether activation of canonical Wnt signaling might spontaneously induce pericyte/fibroblast to myofibroblast transition. It was previously shown in the kidney and in other organs that β-catenin signaling plays a major role in the development of fibrosis43–51; however, our results suggested that β-catenin remained active in the Wnt4 mutants. Therefore, rather than deleting Wnt pathway components and assessing the effect on fibrosis, we sought to activate β-catenin signaling pathway in uninjured cells, and ask whether this led to spontaneous myofibroblast activation. Therefore, we crossed Gli1CE/+ with CatnbΔEx3/ΔEx3 to obtain Gli1CE/+; CatnbΔEx3/+ mice. CatnbΔEx3/ΔEx3 mice contain LoxP sequences flanking exon 3 in the β-catenin coding sequence and when exon 3 is excised by Cre mediated recombination, a truncated, stable and constitutively active form of β-catenin is produced.44,52 We, and others, previously showed that Gli1 is expressed in kidney interstitial fibroblasts.1,53 We treated adult Gli1CE/+;CatnbΔEx3/+ mice with tamoxifen, and show in Figure 8F that β-catenin protein is upregulated after 14 days, compared with corn oil controls in uninjured kidney lysates. Importantly, mice with increased interstitial β-catenin expression show increased αSMA protein and mRNA transcript expression compared with controls (Figure 8, F and G). Because CreERt2 is expressed in only a fraction of interstitial stromal cells in Gli1CE/+ mice, the induction of αSMA was much smaller than seen in UUO, reflecting β-catenin stabilization in only a small fraction of total kidney pericytes and fibroblasts. To identify cells that had undergone recombination, we produced a tri-genic mouse (Gli1CE/+; CatnbΔEx3/+;tdTomato+/−) in which cells with stabilized β-catenin would also coexpress tdTomato. There were no tdTomato+/αSMA+ kidney cells in corn oil–treated mice but Figure 8H depicts an example of a tdTomato+/αSMA+ interstitial cell in uninjured mouse kidney, demonstrating that β-catenin stabilization is sufficient to drive αSMA expression. Overall, these data provide definitive evidence that canonical Wnt signaling is sufficient to drive myofibroblast differentiation in the absence of an injury stimulus.
Discussion
In this study we comprehensively defined the regulated expression of Wnt4 during adult kidney homeostasis and CKD. Previous evidence implicated Wnt4 re-expression after acute injury in epithelial cells, although other reports indicated both interstitial and epithelial expression after chronic injury. Because Wnt4 is required for mesenchymal to epithelial transition in development, we reasoned that Wnt4 re-expression after injury could play a role in the injury response by driving the re-differentiation of injured tubular epithelia. Such a response could promote repair by antagonizing an epithelial to mesenchymal transition (EMT) program and promoting epithelial differentiation. However, we observed Wnt4 induction solely in interstitial myofibroblasts during two chronic injury models and never in tubules.
This result adds complexity to the current hypothesis that adult kidney injury recapitulates developmental signaling paradigms. There are several examples of developmental signaling pathways that are reactivated in the adult descendants of progenitor cells during injury. Fibroblast growth factor-7,54 the notch pathway,55 and Gli1 re-expression1 are examples of developmental pathways being reactivated during injury in the adult. Wnt4 is expressed in epithelial progenitors during development and is required for tubulogenesis, and Wnt4 is also expressed developmentally in medullary stroma.18 Although a requirement for Wnt4 in stromal differentiation is less clear, Itäranta et al. suggest that Wnt4 is necessary for control of smooth muscle cell fate in the kidney.18 We find that Wnt4 is reactivated in adult stroma—pericytes and fibroblasts—but it is not reactivated in tubule epithelium. The reason for this latter finding is not entirely clear, but one possibility is that Wnt4 is required so early in tubulogenesis, in the pretubular aggregate, that epithelial dedifferentiation after injury does not revert to this early developmental stage.
The expression of Wnt4 in medullary myofibroblasts during kidney fibrosis also emphasizes the regional heterogeneity of stromal cells in adult kidney—an area that has not received much investigation. A recent review by Boor and Floege describes the dearth of molecular data available to accurately describe renal fibroblasts and myofibroblasts.56 These cell types are clearly part of a heterogeneous group and our report identifies Wnt4 as a specific marker of medullary myofibroblasts. By contrast, CD73 (ecto-5′-nucleotidase) is expressed exclusively in cortical, but not medullary, interstitial cells of uninjured rat kidney and serves as another example of regional specificity of stromal cells. Although it is increasingly clear that medullary and cortical stroma are not equivalent, the functional differences between pericytes and fibroblasts from these separate regions are largely unexplored.57 To our knowledge, Wnt4 is the first marker that distinguishes medullary αSMA+ kidney myofibroblasts from cortical myofibroblasts.
Cell-specific Wnt4 knockout in kidney stroma had no effect on fibrosis or myofibroblast proliferation, despite the ability of Wnt4 to drive myofibroblast differentiation in vitro. Several possible explanations can be envisioned. Because Wnt4 can diffuse, it is possible that we did not achieve sufficient knockout of Wnt4 to prevent signaling from the fraction of cells that escaped recombination. Although this is unlikely based on the near complete absence of Wnt4 protein after UUO in medullary lysates (Figure 7I), it cannot be ruled out based on the observation that Wnt4 mRNA was only reduced by 75%. Conceivably, Wnt4 protein derived from CD could also compensate. In addition, Wnt4 may be delivered from infiltrating cells, such as macrophages, which would be detected in RNA extracted from injured kidney.58 Finally, it is well known that different Wnt ligands with redundant and overlapping functions are upregulated in kidney fibrosis. Despite Wnt4 knockout, stabilized β-catenin was still significantly increased in the fibrotic kidney, and canonical Wnt target genes were significantly upregulated (Figure 8 and Supplemental Figure 11). This raises the most likely explanation that other upregulated Wnt ligands compensate for the absence of Wnt4. Our finding that β-catenin stabilization in interstitial stromal cells drives myofibroblast differentiation provides direct evidence for the importance of this pathway in kidney fibrosis, and is consistent with the notion that Wnt4 knockout had no effect on fibrosis due compensatory canonical Wnt signaling by other upregulated Wnt ligands. The ability of other Wnt ligands to recapitulate the role of Wnt4 as an inducer of mesenchymal to epithelial transition (MET) during nephrogenesis is also consistent with functional redundancy.4
Although deletion of Wnt4 does not modify fibrosis, we show an important role for the canonical Wnt pathway in myofibroblast differentiation because stabilization of β-catenin in interstitial stromal cells was sufficient to drive expression of the myofibroblast marker αSMA spontaneously, in the absence of an injury stimulus (Figure 8). This observation provides definitive evidence that canonical Wnt signaling is important in myofibroblast transition, consistent with the ability of canonical pathway inhibitors dickkopf-1, dickkopf-2, or secreted frizzled-related protein-4 to ameliorate fibrosis in various tissue types.17,43,58–60 These results are also consistent with an important role for β-catenin signaling in the development of fibrosis in other organs.43–51 As a consequence, efforts to develop antifibrotic therapies should not target a single Wnt ligand but rather should block either multiple Wnt ligands or alternatively inhibit downstream effectors of the entire pathway.
Concise Methods
Animal Experiments
All mouse experiments were performed according to the animal experimental guidelines issued by the Animal Care and Use Committee at Harvard University. Wild-type mice were 8- to 12-week-old C57Bl/6N mice from Charles River Laboratories. Wnt4GC/+ mice were generated as described by Mugford et al.22 Wnt4GCE/+ were generated as described by Kobayashi et al.26 Foxd1GC/+ mice were described by Humphreys et al. and full details of their generation will be described elsewhere (A.K. and A.P.M., unpublished reagent).31 Wnt4del/+ were described by Stark et al.3 and Wnt4flox/flox were generated by Akio Kobayashi and described Kobayashi et al.40 The reporter mice R26lacZ, R26mTmG, Gli1CreERt2, and R26tdTomato were obtained from the Jackson Laboratory with stock numbers 9427, 7576, 7913, and 7914, respectively.24,28,42 CatnbΔEx3/ΔEx3 mice were described by Harada et al.52 All genetic mouse models were maintained on a mixed C57Bl6/129sv background.
For surgical models, mice aged 8–12 weeks were anesthetized with pentobarbital sodium (60 mg/kg body weight) before surgery, and body temperatures were controlled at 36.5°C–37.5°C throughout all procedures. For UUO, the left kidney was exposed through a flank incision and the left ureter tied off at the level of the lower pole with two 3.0 silk ties. For U-IRI, the left kidney was exposed through a flank incision, and the renal pedicle was clamped with nontraumatic microaneurysm clamps (Roboz), which were removed after 28 minutes. Reperfusion was visually verified. Two hours after surgery, 1 ml of 0.9% NaCl intraperitoneally was administered. Tamoxifen was diluted in corn oil and 3% ethanol to 20 mg/ml. Three milligrams of tamoxifen was injected into Wnt4GCE/+;R26tdTomato/+ mice 4 days after UUO and mice were euthanized 7 days or 14 days after UUO.
Real-Time PCR Experiments
Cortex, medulla, and papilla tissue samples were subdissected out of mouse kidney and snap-frozen in liquid nitrogen. RNA was extracted using the Qiagen RNeasy Mini Kit and 600 ng of total RNA was reversed transcribed with iScript (BioRad). Real-time qPCR was performed using iQ-SYBR Green supermix (BioRad) and the BioRad CFX96 Real-Time System with the C1000 Touch Thermal Cycler. Cycling conditions were 95°C for 3 minutes then 40 cycles of 95°C for 15 seconds and 60°C for 1 minute, followed by 1 cycle of 95°C for 10 seconds. Glyceraldehyde-3-phosphate dehydrogenase was used as the loading control. See Supplemental Figure 12 for primer sequences used.
Tissue Preparation and Histology
Mice were anesthetized with isoflurane and immediately perfused via the left ventricle with ice-cold PBS for 1 minute. Kidneys were fixed in 4% paraformaldehyde (PFA) on ice for 2 hours, and then incubated in 30% sucrose in PBS at 4°C overnight. Optimum cutting temperature–embedded (Sakura Finetek) kidneys were cryosectioned into 7-µm sections and mounted on Superfrost slides (Fisher). For immunofluorescence studies, sections were washed in 1× PBS, blocked in 10% normal goat serum (Vector Labs), and incubated with various primary antibodies, including the following: chicken anti-GFP at 1:2000 (category no. 14-1402; Aves Labs), FITC-conjugated anti-αSMA at 1:1000 (category no. F3777; Sigma ) rat anti-F4/80 1:500 (category no. ab6640; Abcam), rat anti-CD31 1:500 (category no. 14-0311; eBioscience), rabbit anti-aquaporin 2 at 1:200 (category no. 15116; Abcam), rabbit anti-CD3 1:500 (category no. VP-RM01;Vector Labs), rabbit anti-laminin at 1:1000 (category no. L9393; Sigma), rat anti-mouse neutrophil at 1:500 (category no. sc-71674; Santa Cruz Biotechnology), rabbit anti-V-ATPase B1/2 at 1:150 (category no. sc20943; Santa Cruz Biotechnology), and rabbit anti-Ki-67 at 1:200 (category no. VP-RM04; Vector Labs). Secondary antibodies were FITC-, Cy3-, or Cy5-conjugated (Jackson ImmunoResearch). Sections were then stained with 4',6-diamidino-2-phenylindole counterstain and mounted in Prolong Gold (Invitrogen).
To identify lacZ activity in kidney sections, PFA fixed frozen sections were incubated in standard 5-bromo-4-chloro-3-indolyl-β-d-galactoside for 24 hours counterstained with nuclear Fast Red and mounted.
Quantification of tdTomato+ after UUO was done on five mice in each group. Pictures were taken across entire section in order to capture all positive cells in a coronal kidney section. Positive cells were counted manually. V-ATPase–positive cells were counted from the papilla of three different mice and colocalization was determined. All images were obtained by confocal or standard microscopy (C1 Eclipse or Eclipse 90i, respectively; both from Nikon).
Western Blot Analyses
Kidneys were dissected and snap-frozen in liquid nitrogen and stored at −80°C. Tissue samples were homogenized in lysis buffer containing 10 mM HEPES, pH 7.4, 0.32 M sucrose, 2 mM EDTA, 1 mM dithiothreitol, 1 mM PMSF and 1 protease inhibitor tablet per 10 ml of lysis buffer (category no. 11836153001; Roche). Samples were sonicated and protein concentration was determined by the Bradford assay. Ten to twenty micrograms of protein from lysates was loaded on a 10% polyacrylamide gel and separated by SDS electrophoresis. Proteins were transferred to polyvinylidene difluoride membrane, blocked in 5% milk in PBSTw, and probed overnight at 4°C with various primary antibodies diluted in blocking solution including the following: goat anti-Wnt4 at 1:1000 (category no. AF475; R&D Systems), mouse anti-αSMA at 1:5000 (category no. A2547; Sigma), mouse anti-β-catenin at 1:2000 (category no. 610154; BD Transduction Laboratories), and rabbit anti-glyceraldehyde-3-phosphate dehydrogenase at 1:4000 (category no. A300–641A; Bethyl Laboratories). After incubation with primary antibody, blots were washed, probed with respective horseradish peroxidase–conjugated secondary antibodies at 1:5000 (category no. P0447, 0448, 0449; Dako) for 1 hour at room temperature, and then visualized using the Western Lightning enhanced chemiluminescence (ECL) kit from PerkinElmer (category no. NEL100001EA).
Cell Culture
We cultured 10T1/2 cells (American Type Culture Collection) in basal Eagle’s medium (Gibco; Invitrogen) supplemented with 10% FBS, penicillin and streptomycin, and 2 mmol/L glutamine. Cells were starved overnight by replacing media with 0.5% FBS and subsequently treated with recombinant Wnt4 at 250 ng/ml (category no. 475-WN-005; R&D Systems), Wnt3a at 200 ng/ml (category no. 1324-WN-010, R&D Systems), or TGF-β at 2 ng/ml (category no. 100–21; PeproTech) for 24 hours. RNA was subsequently extracted and real-time qPCR was performed as above.
Primary inner medullary CD epithelial cells were generated from pooling 12 dissected papillae from 6 mice. Papillae were dissected form mice perfused with sterile PBS, finely minced with a razor, and incubated at 37°C for 40 minutes in a 1 mg/ml collagenase type 2 (Worthington, LS-004176) solution diluted in DMEM. One half volume of 10% FBS was added to the collagenase solution to terminate enzyme reaction. The digest was filtered with a 100 µM sieve, the filtrate was centrifuged, and the supernatant was removed. Pellets were then resuspended in sterile PBS and centrifuged, the supernatant was removed, and pellets were resuspended in 10 ml of RenaLife growth media with RenaLife Life Factors (LL-0025). Cells were plated in six-well cell culture dishes that had been coated in 2% gelatin.
Statistical Analyses
Statistical analyses were performed using Prism 5.0 (GraphPad Software Inc.). Comparison of two groups was performed by two-tailed t test. Comparison of more than two groups was performed by one-way or two-way ANOVA followed by Tukey’s post test. A P value <0.05 was considered significant. The results are presented as means and error bars indicate ±SEM.
Disclosures
None.
Acknowledgments
We thank Dr. Richard Behringer for providing the Wnt4flox/flox mice.
This work was supported by grants from the Harvard Stem Cell Institute and from the National Institutes of Health (DK088923 to B.D.H. and DK054364 to A.P.M.), by a research fellowship from the National Kidney Foundation (2011-D000691 to D.P.D.) and by the March of Dimes, Harvard Stem Cell Institute, American Society of Nephrology, and National Kidney Foundation serving New England (to A.K.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012050512/-/DCSupplemental.
References
- 1.Fabian SL, Penchev RR, Jacques BS, Rao AN, Sipilä P, West KA, McMahon AP, Humphreys BD: Hedgehog-Gli pathway activation during kidney fibrosis. Am J Pathol 180: 1441–1453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Logan CY, Nusse R: The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20: 781–810, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Stark K, Vainio S, Vassileva G, McMahon AP: Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372: 679–683, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Kispert A, Vainio S, McMahon AP: Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development 125: 4225–4234, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Tanigawa S, Wang H, Yang Y, Sharma N, Tarasova N, Ajima R, Yamaguchi TP, Rodriguez LG, Perantoni AO: Wnt4 induces nephronic tubules in metanephric mesenchyme by a non-canonical mechanism. Dev Biol 352: 58–69, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shan J, Jokela T, Skovorodkin I, Vainio S: Mapping of the fate of cell lineages generated from cells that express the Wnt4 gene by time-lapse during kidney development. Differentiation 79: 57–64, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Carroll TJ, Park J-S, Hayashi S, Majumdar A, McMahon AP: Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell 9: 283–292, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Schedl A: Renal abnormalities and their developmental origin. Nat Rev Genet 8: 791–802, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Boyle SC, Kim M, Valerius MT, McMahon AP, Kopan R: Notch pathway activation can replace the requirement for Wnt4 and Wnt9b in mesenchymal-to-epithelial transition of nephron stem cells. Development 138: 4245–4254, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witzgall R, Brown D, Schwarz C, Bonventre JV: Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest 93: 2175–2188, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabian SL, Humphreys BD: What’s past is prologue: Developmental pathways and chronic allograft dysfunction. Am J Transplant 12: 5–6, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishibe S, Cantley LG: Epithelial-mesenchymal-epithelial cycling in kidney repair. Curr Opin Nephrol Hypertens 17: 379–385, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Villanueva S, Céspedes C, Vio CP: Ischemic acute renal failure induces the expression of a wide range of nephrogenic proteins. Am J Physiol Regul Integr Comp Physiol 290: R861–R870, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Terada Y, Tanaka H, Okado T, Shimamura H, Inoshita S, Kuwahara M, Sasaki S: Expression and function of the developmental gene Wnt-4 during experimental acute renal failure in rats. J Am Soc Nephrol 14: 1223–1233, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Nguyen HT, Thomson AA, Kogan BA, Baskin LS, Cunha GR: Expression of the Wnt gene family during late nephrogenesis and complete ureteral obstruction. Lab Invest 79: 647–658, 1999 [PubMed] [Google Scholar]
- 16.Surendran K, McCaul SP, Simon TC: A role for Wnt-4 in renal fibrosis. Am J Physiol Renal Physiol 282: F431–F441, 2002 [DOI] [PubMed] [Google Scholar]
- 17.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y: Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol 20: 765–776, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itäranta P, Chi L, Seppänen T, Niku M, Tuukkanen J, Peltoketo H, Vainio S: Wnt-4 signaling is involved in the control of smooth muscle cell fate via Bmp-4 in the medullary stroma of the developing kidney. Dev Biol 293: 473–483, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Shin K, Lee J, Guo N, Kim J, Lim A, Qu L, Mysorekar IU, Beachy PA: Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature 472: 110–114, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Figueira EC, Di Girolamo N, Coroneo MT, Wakefield D: The phenotype of limbal epithelial stem cells. Invest Ophthalmol Vis Sci 48: 144–156, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Jääskeläinen M, Prunskaite-Hyyryläinen R, Naillat F, Parviainen H, Anttonen M, Heikinheimo M, Liakka A, Ola R, Vainio S, Vaskivuo TE, Tapanainen JS: WNT4 is expressed in human fetal and adult ovaries and its signaling contributes to ovarian cell survival. Mol Cell Endocrinol 317: 106–111, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Mugford JW, Yu J, Kobayashi A, McMahon AP: High-resolution gene expression analysis of the developing mouse kidney defines novel cellular compartments within the nephron progenitor population. Dev Biol 333: 312–323, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu J, Carroll TJ, Rajagopal J, Kobayashi A, Ren Q, McMahon AP: A Wnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development 136: 161–171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L: A global double-fluorescent Cre reporter mouse. Genesis 45: 593–605, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Mazumdar J, O’Brien WT, Johnson RS, LaManna JC, Chavez JC, Klein PS, Simon MC: O2 regulates stem cells through Wnt/β-catenin signalling. Nat Cell Biol 12: 1007–1013, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP: Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3: 169–181, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grgic I, Brooks CR, Hofmeister AF, Bijol V, Bonventre JV, Humphreys BD: Imaging of podocyte foot processes by fluorescence microscopy. J Am Soc Nephrol 23: 785–791, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H: A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaner NC, Patterson GH, Davidson MW: Advances in fluorescent protein technology. J Cell Sci 120: 4247–4260, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Humphreys BD, Czerniak S, DiRocco DP, Hasnain W, Cheema R, Bonventre JV: Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci U S A 108: 9226–9231, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Humphreys BD, Lin S-L, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS: Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176: 85–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dellê H, Rocha JRC, Cavaglieri RC, Vieira JM, Jr, Malheiros DMAC, Noronha IL: Antifibrotic effect of tamoxifen in a model of progressive renal disease. J Am Soc Nephrol 23: 37–48, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayashi S, McMahon AP: Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: A tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol 244: 305–318, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Monvoisin A, Alva JA, Hofmann JJ, Zovein AC, Lane TF, Iruela-Arispe ML: VE-cadherin-CreERT2 transgenic mouse: A model for inducible recombination in the endothelium. Dev Dyn 235: 3413–3422, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Dhar K, Dhar G, Majumder M, Haque I, Mehta S, Van Veldhuizen PJ, Banerjee SK, Banerjee S: Tumor cell-derived PDGF-B potentiates mouse mesenchymal stem cells-pericytes transition and recruitment through an interaction with NRP-1. Mol Cancer 9: 209, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Darland DC, D’Amore PA: TGF beta is required for the formation of capillary-like structures in three-dimensional cocultures of 10T1/2 and endothelial cells. Angiogenesis 4: 11–20, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Hirschi KK, Rohovsky SA, D’Amore PA: PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol 141: 805–814, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye S, Tan L, Yang R, Fang B, Qu S, Schulze EN, Song H, Ying Q, Li P: Pleiotropy of glycogen synthase kinase-3 inhibition by CHIR99021 promotes self-renewal of embryonic stem cells from refractory mouse strains. PLoS ONE 7: e35892, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grgic I, Duffield JS, Humphreys BD: The origin of interstitial myofibroblasts in chronic kidney disease. Pediatr Nephrol 27: 183–193, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobayashi A, Stewart CA, Wang Y, Fujioka K, Thomas NC, Jamin SP, Behringer RR: β-Catenin is essential for Müllerian duct regression during male sexual differentiation. Development 138: 1967–1975, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV: Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2: 284–291, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Soriano P: Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21: 70–71, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Akhmetshina A, Palumbo K, Dees C, Bergmann C, Venalis P, Zerr P, Horn A, Kireva T, Beyer C, Zwerina J, Schneider H, Sadowski A, Riener M-O, MacDougald OA, Distler O, Schett G, Distler JHW: Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat Commun 3: 735, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beyer C, Schramm A, Akhmetshina A, Dees C, Kireva T, Gelse K, Sonnylal S, de Crombrugghe B, Taketo MM, Distler O, Schett G, Distler JHW: β-catenin is a central mediator of pro-fibrotic Wnt signaling in systemic sclerosis. Ann Rheum Dis 71: 761–767, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA: Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317: 807–810, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Lam AP, Flozak AS, Russell S, Wei J, Jain M, Mutlu GM, Budinger GRS, Feghali-Bostwick CA, Varga J, Gottardi CJ: Nuclear β-catenin is increased in systemic sclerosis pulmonary fibrosis and promotes lung fibroblast migration and proliferation. Am J Respir Cell Mol Biol 45: 915–922, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lam AP, Gottardi CJ: β-catenin signaling: A novel mediator of fibrosis and potential therapeutic target. Curr Opin Rheumatol 23: 562–567, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chilosi M, Poletti V, Zamò A, Lestani M, Montagna L, Piccoli P, Pedron S, Bertaso M, Scarpa A, Murer B, Cancellieri A, Maestro R, Semenzato G, Doglioni C: Aberrant Wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol 162: 1495–1502, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo Y, Xiao L, Sun L, Liu F: Wnt/beta-catenin signaling: A promising new target for fibrosis diseases. Physiol Res 61: 337–346, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Hao S, He W, Li Y, Ding H, Hou Y, Nie J, Hou FF, Kahn M, Liu Y: Targeted inhibition of β-catenin/CBP signaling ameliorates renal interstitial fibrosis. J Am Soc Nephrol 22: 1642–1653, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He W, Kang YS, Dai C, Liu Y: Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol 22: 90–103, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM: Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J 18: 5931–5942, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ding H, Zhou D, Hao S, Zhou L, He W, Nie J, Hou FF, Liu Y: Sonic hedgehog signaling mediates epithelial-mesenchymal communication and promotes renal fibrosis. J Am Soc Nephrol 23: 801–813, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ichimura T, Finch PW, Zhang G, Kan M, Stevens JL: Induction of FGF-7 after kidney damage: A possible paracrine mechanism for tubule repair. Am J Physiol 271: F967–F976, 1996 [DOI] [PubMed] [Google Scholar]
- 55.Gupta S, Li S, Abedin MJ, Wang L, Schneider E, Najafian B, Rosenberg M: Effect of Notch activation on the regenerative response to acute renal failure. Am J Physiol Renal Physiol 298: F209–F215, 2010 [DOI] [PubMed] [Google Scholar]
- 56.Boor P, Floege J: The renal (myo-)fibroblast: A heterogeneous group of cells. Nephrol Dial Transplant 27: 3027–3036, 2012 [DOI] [PubMed] [Google Scholar]
- 57.Gandhi R, Le Hir M, Kaissling B: Immunolocalization of ecto-5′-nucleotidase in the kidney by a monoclonal antibody. Histochemistry 95: 165–174, 1990 [DOI] [PubMed] [Google Scholar]
- 58.Lin S-L, Li B, Rao S, Yeo E-J, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, Pollard JW, McMahon AP, Lang RA, Duffield JS: Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A 107: 4194–4199, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsushima K, Suyama T, Takenaka C, Nishishita N, Ikeda K, Ikada Y, Sawa Y, Jakt LM, Mori H, Kawamata S: Secreted frizzled related protein 4 reduces fibrosis scar size and ameliorates cardiac function after ischemic injury. Tissue Eng Part A 16: 3329–3341, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang B, Zhou KK, Ma J-X: Inhibition of connective tissue growth factor overexpression in diabetic retinopathy by SERPINA3K via blocking the WNT/beta-catenin pathway. Diabetes 59: 1809–1816, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]