Abstract
It is unknown whether regular patient-doctor contact (PDC) contributes to better outcomes for patients undergoing hemodialysis. Here, we analyzed the associations between frequency and duration of PDC during hemodialysis treatments with clinical outcomes among 24,498 patients from 778 facilities in the international Dialysis Outcomes and Practice Patterns Study (DOPPS). The typical facility PDC frequency, estimated by facility personnel, was high (more than once per week) for 55% of facilities, intermediate (once per week) for 24%, and low (less than once per week) for 21%. The mean ± SD estimated duration of a typical interaction between patient and physician was 7.7±5.6 minutes. PDC frequency and duration varied across DOPPS phases and countries; the proportion of facilities with high PDC frequency was 17% in the United States and 73% across the other countries. Compared with high PDC frequency, the adjusted hazard ratio (HR) for all-cause mortality was 1.06 (95% confidence interval [CI], 0.96 to 1.17) for intermediate PDC frequency and 1.11 (95% CI, 1.01 to 1.23) for low PDC frequency (P=0.03 for trend). Furthermore, each 5-minutes-shorter duration of PDC was associated with a 5% higher risk for death, on average (HR, 1.05; 95% CI, 1.01 to 1.09), adjusted for PDC frequency and other covariates. Multivariable analyses also suggested modest inverse associations between both PDC frequency and duration with hospitalization but not with kidney transplantation. Taken together, these results suggest that policies supporting more frequent and longer duration of PDC may improve patient outcomes in hemodialysis.
Although maintenance hemodialysis (HD) saves lives, survival of patients with ESRD remains poor and is much worse than for the general population.1 HD facilities differ with respect to provision of important clinical practices;2,3 among these, differences in patterns of dialysis unit staffing might influence mortality.4,5 HD patients usually receive thrice-weekly dialysis provided by a multidisciplinary team of health care professionals (doctors, nurses, technicians, dietitians, and social workers). As part of this team, the physician’s role in improving the quality of chronic disease care is considered crucial.6,7
Many health care providers and researchers believe that more frequent and longer patient-doctor contact (PDC) in HD care may improve patient outcomes because it provides physicians with greater opportunity to monitor treatments; enhance communication and build trust with the patient; and detect, prevent, and treat new medical problems.2,5,8 However, the actual frequency and duration of PDC for HD care have not been reported in many countries, and there is little direct evidence that more frequent and longer PDC contributes to better patient health outcomes. Previous studies from the United States showed that less frequent PDC was associated with lower patient satisfaction, lower patient adherence, lower patient achievement of clinical performance targets, and higher hospitalization, but more frequent PDC was not necessarily related to longer patient survival.8–10 A recent study based on data from the U.S. Renal Data System (USRDS) also reported no difference in survival for PDC frequency of <4 times per month compared with 4 times per month.10 However, the study was limited to one country and was unable to evaluate differences in outcomes between 4 times per month and >4 times per month because of limitations of the billing codes and relatively low proportion of high PDC frequency in the United States.
This study examined the estimated typical frequency and duration of PDC that occurs at the time of HD treatments and its associations with all-cause mortality as a primary outcome among participants in the Dialysis Outcomes and Practice Patterns Study (DOPPS), an international prospective cohort study of HD patients and facilities. PDC was studied at the facility level, reducing the opportunity for patient-level confounding by indication in this international cohort. Among such patients, a high PDC frequency (>4 times per month) is much more common outside of than in the United States. We also examined the associations of PDC frequency and duration with first hospitalization and kidney transplantation as secondary outcomes. A better understanding of the effect of PDC intensity could have implications for health policy in addition to improving health care delivery and HD patient outcomes.
Results
Frequency and Duration of PDC across Country and Study Phase
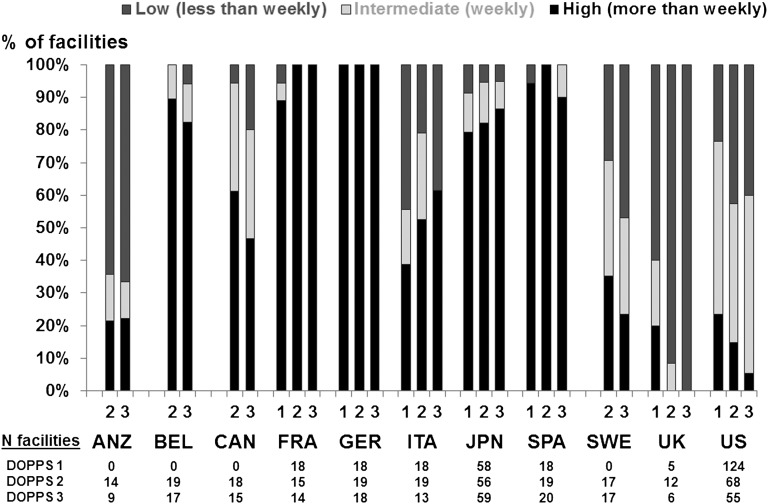
Figure 1 shows the distribution of estimated typical PDC frequency at baseline among facilities in each country by study phase (DOPPS 1–3). Of 778 facilities in 12 countries, PDC frequency was high in 55%, intermediate in 24%, and low in 21%, although the frequency varied enormously across countries. In Belgium, France, Germany, Spain, and Japan, >75% of HD facilities had high PDC frequency in all phases, and >90% had high or intermediate frequency. On the other hand, in Australia and New Zealand, Sweden, the United Kingdom, and the United States, <30% of facilities had high PDC frequency, and in the latter three countries high PDC declined across study phases (from 35% to 24% in Sweden, from 20% to 0% in the United Kingdom, and from 23% to 5% in the United States). In the United Kingdom, intermediate PDC frequency was also low and declined across phases (from 20% to 0%). The proportions of facilities with a high or intermediate PDC frequency were 68% in the United States and 84% in the other countries.
Figure 1.
International variation of patient-doctor contact (PDC) frequency. Percentage of facilities is shown according to the estimated typical frequency of PDC, by DOPPS phase and country. PDC frequency was classified into three categories: high frequency (every, or almost every, dialysis session), intermediate frequency (weekly), and low frequency (less than once per week). ANZ, Australia/New Zealand; BEL, Belgium; CAN, Canada; FRA, France; GER, Germany; ITA, Italy; JPN, Japan; SPA, Spain; SWE, Sweden; UK, United Kingdom; US, United States. ANZ, BEL, CAN, and SWE did not participate in phase 1.
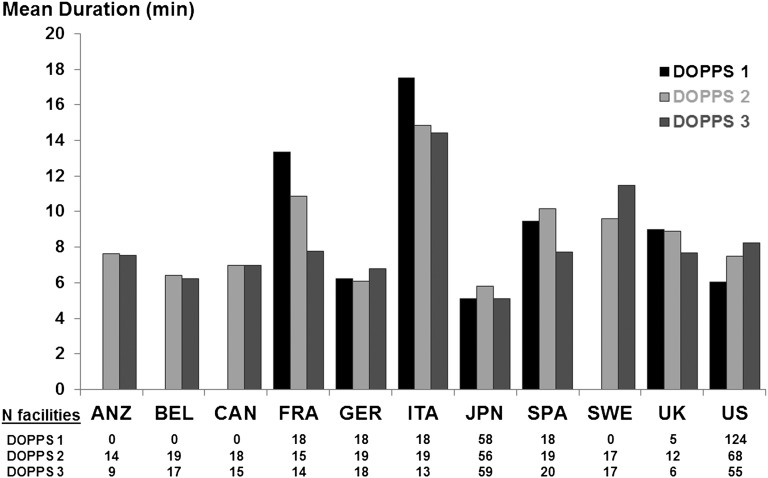
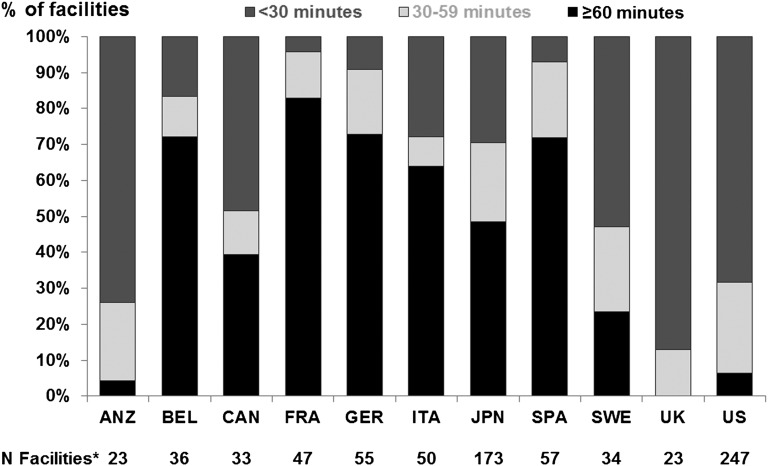
Figure 2 shows mean facility PDC duration (defined as the duration of a typical interaction between the patient and physician) at baseline in each country by DOPPS phase. The overall mean PDC duration ± SD was 7.7±5.6 minutes. The duration of PDC varied greatly across countries (from a mean of 5.3 minutes in Japan to 15.7 minutes in Italy), but it was not necessarily longer in countries with lower PDC frequency. Little correlation existed between the percentage with high PDC frequency and mean PDC duration of each country (Pearson r = −0.13; P=0.70). The proportion of facilities with an average total PDC time (frequency × duration) of ≥60 minutes per patient per month ranged from 0% in the United Kingdom to 83% in France (Figure 3) across all facilities from phases 1 to 3.
Figure 2.
International variation of patient-doctor contact (PDC) duration. Average facility duration of PDC is shown by DOPPS phase and country. PDC duration represents the estimated duration of a typical interaction between the patient and physician. ANZ, Australia/New Zealand; BEL, Belgium; CAN, Canada; FRA, France; GER, Germany; ITA, Italy; JPN, Japan; SPA, Spain; SWE, Sweden; UK, United Kingdom; US, United States. ANZ, BEL, CAN, and SWE did not participate in phase 1.
Figure 3.
International variation of the total patient-doctor contact (PDC) time per month. Percentage of facilities by country is shown according to the total PDC time, calculated as the estimated typical frequency of PDC multiplied by the estimated duration of a typical interaction between the patient and physician. *N represents the total number of facilities across DOPPS phases 1–3. ANZ, Australia/New Zealand; BEL, Belgium; CAN, Canada; FRA, France; GER, Germany; ITA, Italy; JPN, Japan; SPA, Spain; SWE, Sweden; UK, United Kingdom; US, United States.
Patient and Facility Characteristics
Table 1 shows baseline patient characteristics by facility PDC frequency. The high-frequency group had fewer black patients, more patients with a fistula than with a graft or catheter, and more employed and married patients than did the intermediate- and low-frequency groups. Patients in the high-frequency group had fewer comorbid conditions, such as coronary artery disease, diabetes mellitus, and hypertension, than did those in the other groups. Table 1 also shows baseline characteristics of all included patients in the analyses compared with patients excluded because of missing or unknown facility PDC data. Differences between the two groups were generally minor.
Table 1.
Baseline patient characteristics by PDC frequency
| Characteristic | High Frequency | Intermediate Frequency | Low Frequency | Included Facilities | Excluded Facilities |
|---|---|---|---|---|---|
| Facilities (n) | 429 | 186 | 163 | 778 | 152 |
| Patients (n) | 13,749 | 5778 | 4971 | 24,498 | 4651 |
| Age (yr) | 62.0±14.2 | 61.3±15.2 | 61.6±14.9 | 61.7±14.6 | 60.9±15.5 |
| Male (%) | 58.2 | 55.6 | 58.1 | 57.6 | 57.0 |
| Black (%) | 5.3 | 26.0 | 17.1 | 12.6 | 10.9 |
| Body mass index (kg/m2) | 23.4±4.9 | 25.4±6.1 | 25.5±6.2 | 24.3±5.6 | 25.0±5.7 |
| Dialysis vintage (yr) | 5.6±6.1 | 4.1±4.5 | 4.3±4.9 | 5.0±5.6 | 4.7±5.4 |
| Single pool Kt/V | 1.39±0.30 | 1.46±0.30 | 1.45±0.31 | 1.42±0.30 | 1.47±0.30 |
| Dialysis time (min) | 236±36 | 224±33 | 229±37 | 232±36 | 234±38 |
| Albumin (g/dl) | 3.8±0.5 | 3.7±0.5 | 3.7±0.5 | 3.8±0.5 | 3.8±0.5 |
| Calcium (mg/dl) | 9.2±0.9 | 9.2±0.9 | 9.3±0.9 | 9.3±0.9 | 9.3±0.9 |
| Phosphorus (mg/dl) | 5.5±1.8 | 5.7±1.8 | 5.5±1.8 | 5.6±1.8 | 5.5±1.8 |
| Hemoglobin (g/dl) | 10.9±1.6 | 11.2±1.6 | 11.4±1.6 | 11.1±1.6 | 11.2±1.6 |
| Vascular access (%) | |||||
| Fistula | 71.9 | 42.7 | 52.6 | 61.1 | 59.7 |
| Graft | 12.5 | 32.8 | 23.4 | 19.5 | 17.9 |
| Catheter | 10.6 | 20.1 | 20.4 | 14.8 | 16.2 |
| Employment (%) | 17.7 | 12.7 | 13.0 | 15.6 | 14.1 |
| Education (high school or more) (%) | 34.3 | 46.1 | 44.7 | 39.2 | 39.5 |
| Married (%) | 61.5 | 52.7 | 54.7 | 58.0 | 55.5 |
| Living status (%) | |||||
| Family | 79.9 | 75.1 | 76.7 | 78.1 | 76.0 |
| Nursing Home | 4.1 | 6.5 | 5.0 | 4.9 | 4.4 |
| Alone | 15.2 | 17.3 | 17.4 | 16.2 | 18.8 |
| Comorbid conditions (%) | |||||
| Coronary artery disease | 39.2 | 53.6 | 52.1 | 45.2 | 46.5 |
| Cancer | 10.6 | 11.6 | 11.1 | 10.9 | 12.1 |
| Other cardiovascular disease | 36.9 | 37.0 | 35.5 | 36.7 | 32.3 |
| Cerebrovascular disease | 15.5 | 19.3 | 18.3 | 17.0 | 16.6 |
| Congestive heart failure | 29.5 | 41.5 | 37.2 | 33.9 | 31.9 |
| Diabetes mellitus | 31.0 | 44.2 | 40.5 | 36.0 | 34.2 |
| Gastrointestinal bleeding | 5.1 | 7.9 | 7.4 | 6.2 | 5.6 |
| HIV/AIDS | 0.6 | 1.6 | 0.8 | 0.9 | 0.6 |
| Hypertension | 74.6 | 83.5 | 82.7 | 78.3 | 78.4 |
| Lung disease | 9.2 | 14.0 | 12.0 | 10.9 | 11.0 |
| Neurologic disorder | 9.2 | 13.3 | 11.9 | 10.7 | 9.9 |
| Psychiatric disorder | 13.1 | 22.7 | 22.2 | 17.2 | 16.2 |
| Peripheral vascular disease | 24.1 | 27.4 | 28.2 | 25.7 | 24.6 |
| Recurrent cellulitis | 6.9 | 10.6 | 9.1 | 8.2 | 8.1 |
Values expressed with a plus/minus sign are the mean ± SD.
Table 2 shows baseline facility characteristics according to PDC frequency. We observed a trend toward more patients in facilities with higher PDC frequency. The mean ± SD typical PDC durations were 7.2±5.3, 6.7±4.4, and 10.1±6.9 minutes in the high-, intermediate-, and low-frequency groups, respectively. Consistent with the country level, there was only a weak inverse correlation (r = −0.17; P<0.001) at the facility level between PDC duration and PDC frequency.
Table 2.
Baseline facility characteristics by PDC frequency
| Frequency of PDC | High Frequency | Intermediate Frequency | Low Frequency |
|---|---|---|---|
| Facilities (n) | 429 | 186 | 163 |
| Mean typical PDC duration (min) | 7.2±5.3 | 6.7±4.4 | 10.1±6.9 |
| Mean patients in a facility (n) | 85.5 | 81.8 | 75.0 |
| Mean doctors in a facility (n) | 5.2 | 5.8 | 5.6 |
| Patient/staff ratio for 1 dialysis session | 4.5 | 3.4 | 3.7 |
| Hospital-based facilities (% yes) | 49.7 | 38.7 | 42.3 |
Facility characteristics were reported by each unit nurse manager in the DOPPS unit practice survey. One hundred fifty-two facilities excluded because of missing UPS questionnaire or missing PDC data. Values expressed with a plus/minus sign are the mean ± SD.
PDC and Outcomes (All-Cause Mortality, First Hospitalization, Kidney Transplantation)
Median follow-up time was 22 months (interquartile range, 11–28 months). More frequent PDC was associated with lower all-cause mortality: After adjustment for demographic characteristics, comorbid conditions, other patient and facility characteristics, and PDC duration, the hazard ratios (HRs) for all-cause mortality for intermediate and low PDC frequency compared with high PDC frequency were 1.06 (95% confidence interval [CI], 0.96 to 1.17) and 1.11 (95% CI, 1.01 to 1.23), respectively (P for trend = 0.03) (Table 3, model 4a). In an analysis dichotomizing facilities into two groups, the adjusted HRs for mortality among facilities with PDC frequency <4 times per month were 1.08 (95% CI, 0.99 to 1.18) compared with facilities with PDC frequency ≥4 times per month in all DOPPS countries and 1.08 (95% CI, 0.96 to 1.22) when analyses were restricted to facilities in the United States.
Table 3.
Association (HR and 95% CI) of PDC Frequency and Duration with All-Cause Mortality, after Various Levels of Adjustment
| All-Cause Mortality | Model 0 | Model 1 | Model 2 | Model 3 | Model 4 | Model 4a | Model 4b |
|---|---|---|---|---|---|---|---|
| PDC frequency | |||||||
| Low frequency (less than weekly) | 1.08 (0.99 to 1.19) | 1.11 (1.01 to 1.22) | 1.11 (1.01 to 1.23) | 1.11 (1.01 to 1.22) | 1.08 (0.98 to 1.19) | 1.11 (1.01 to 1.23) | |
| Intermediate frequency (weekly) | 1.10 (1.00 to 1.21) | 1.07 (0.97 to 1.19) | 1.08 (0.97 to 1.19) | 1.07 (0.97 to 1.18) | 1.06 (0.96 to 1.16) | 1.06 (0.96 to 1.17) | |
| High frequency (more than weekly) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| P for trend across categories | 0.07 | 0.04 | 0.02 | 0.04 | 0.11 | 0.03 | |
| PDC duration | |||||||
| Per 5 min shorter duration | 1.08 (1.04 to 1.12) | 1.04 (1.00 to 1.08) | 1.04 (1.00 to 1.08) | 1.05 (1.01 to 1.09) | 1.04 (1.01 to 1.08) | 1.05 (1.01 to 1.09) | |
| P for duration as continuous variable | <0.001 | 0.03 | 0.03 | 0.02 | 0.03 | 0.01 | |
| Categorical analysis | |||||||
| Short duration (≤5 min) | 1.23 (1.10 to 1.38) | 1.11 (1.00 to 1.24) | 1.11 (0.99 to 1.24) | 1.12 (1.00 to 1.25) | 1.11 (0.99 to 1.24) | 1.13 (1.01 to 1.27) | |
| Intermediate duration (6–10 min) | 1.17 (1.03 to 1.32) | 1.10 (0.98 to 1.24) | 1.10 (0.98 to 1.24) | 1.09 (0.97 to 1.23) | 1.11 (0.99 to 1.25) | 1.13 (1.00 to 1.28) | |
| Long duration (>10 min) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
Model 0: stratified by phase and region and accounted for facility clustering effects; model 1: model 0 + age, sex, race, body mass index, dialysis vintage, and 14 comorbid conditions; model 2: model 1 + education, employment and living status, marital status; model 3: model 2 + patient-doctor ratio, patient-staff ratio, and hospital-based units; model 4: model 3 + Kt/V, treatment time, serum albumin, calcium, phosphorus, hemoglobin, and vascular access; model 4a: model 4 + PDC duration (continuous); model 4b: model 4 + PDC frequency (categories).
Facility PDC duration was also inversely associated with mortality; after multivariable adjustment for potential confounders, the HR was 1.05 per 5 minutes shorter PDC (95% CI, 1.01 to 1.09; P=0.01) (Table 3, model 4b). The estimated HR for PDC duration did not differ appreciably across categories of PDC frequency (P=0.33). Results of analyses treating PDC duration as categorical also appear in Table 3 and were consistent with the continuous PDC duration model. After adjustment for potential confounders, the HR was 1.13 (95% CI, 1.00 to 1.28) for patients in facilities with intermediate PDC duration and 1.13 (95% CI, 1.01 to 1.27) for patients in facilities with short duration compared with patients in facilities with long duration (Table 3, model 4b).
Patients in facilities providing more frequent and longer PDC also had a lower rate of first hospitalization during follow-up. Compared with patients with high PDC frequency, the adjusted HR for first hospitalization was 1.14 (95% CI, 1.04 to 1.25) for intermediate PDC frequency and 1.22 (95% CI, 1.11 to 1.34) for low PDC frequency (P for trend < 0.001), adjusting for demographic characteristics, comorbid conditions, and other patient and facility characteristics. PDC duration was also inversely associated with the rate of first hospitalization (HR per 5 minutes shorter, 1.06; 95% CI, 1.02 to 1.10), adjusting for PDC frequency and the same covariates as above. Estimated region-specific effects of PDC frequency and duration are shown in Table 4 (mortality and first hospitalization).
Table 4.
Association (HR and 95% CI) of patient-doctor contact frequency with all-cause mortality and first hospitalization, by region
| Variable | Overall | North America | Europe/ANZ | Japan |
|---|---|---|---|---|
| All-cause mortality | ||||
| PDC frequency | ||||
| Low frequency (less than weekly) | 1.11 (1.01 to 1.23) | 1.15 (0.97 to 1.35) | 1.06 (0.93 to 1.21) | 0.97 (0.68 to 1.39) |
| Intermediate frequency (weekly) | 1.06 (0.96 to 1.17) | 1.06 (0.92 to 1.22) | 1.04 (0.88 to 1.23) | 1.43 (1.10 to 1.85) |
| High frequency (more than weekly) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| PDC duration | ||||
| Per 5 min shorter duration | 1.05 (1.01 to 1.09) | 1.03 (0.96 to 1.10) | 1.07 (1.02 to 1.12) | 1.01 (0.85 to 1.20) |
| First hospitalization | ||||
| PDC frequency | ||||
| Low frequency (less than weekly) | 1.22 (1.11 to 1.34) | 1.30 (1.09 to 1.55) | 1.20 (1.05 to 1.37) | 1.11 (0.87 to 1.43) |
| Intermediate frequency (weekly) | 1.14 (1.04 to 1.25) | 1.23 (1.07 to 1.41) | 1.08 (0.90 to 1.29) | 1.03 (0.81 to 1.31) |
| High frequency (more than weekly) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| PDC duration | ||||
| Per 5 min shorter duration | 1.06 (1.02 to 1.10) | 1.06 (0.99 to 1.14) | 1.06 (1.01 to 1.11) | 0.98 (0.86 to 1.13) |
Associations between PDC and both mortality and first hospitalizations were assessed in Cox models (1) overall, stratified by region, and (2) including interaction terms for PDC frequency × region and PDC duration × region to provide region-specific estimates. All Cox models stratified by study phase; accounted for facility clustering effects; and adjusted for age, sex, race, body mass index, 14 comorbid conditions, education, employment and living status, marital status, patient-doctor ratio, patient-staff ratio, hospital-based unit, Kt/V, treatment time, serum albumin, calcium, phosphorus, hemoglobin, and vascular access. Overall model additionally stratified by region. PDC frequency models adjusted for continuous PDC duration, and PDC duration models adjusted for PDC frequency categories. ANZ, Australia/New Zealand.
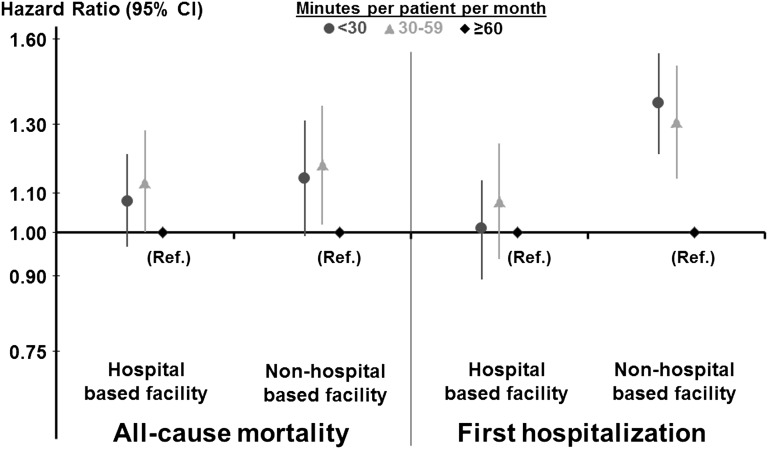
Total PDC time per month (estimated typical frequency of PDC multiplied by the estimated duration of a typical interaction between the patient and physician) was also inversely associated with all-cause mortality. Compared with patients in facilities with an average total PDC time of at least 60 minutes per patient per month, the adjusted HRs were 1.15 (95% CI, 1.04 to 1.27) for total PDC time of 30–60 minutes per patient per month and 1.11 (95% CI, 1.01 to 1.21) for total PDC time <30 minutes per patient per month. This association of total PDC time per month with mortality did not differ much for hospital-based and non–hospital-based facilities (Figure 4). In contrast, total PDC time was inversely associated with the rate of first hospitalization in non–hospital-based facilities but not in hospital-based facilities.
Figure 4.
Association between total patient-doctor contact (PDC) time per month and outcomes: Interaction with hospital-based facilities. Association of total PDC time per month with all-cause mortality and first hospitalization is shown separately for hospital-based and non–hospital-based facilities. Total PDC time was calculated as the estimated typical frequency of PDC multiplied by the estimated duration of a typical interaction between the patient and physician. Among 24,498 DOPPS 1–3 patients, multivariable Cox models were stratified by region and study phase and adjusted for age, sex, race, body mass index, years with ESRD, 14 comorbid conditions, living arrangements, socioeconomic status, serum albumin, hemoglobin, serum phosphorus, serum calcium, Kt/V, duration of treatment, vascular access, and facility staff deployment. The interaction of PDC by facility type was much stronger for hospitalization (P=0.001) than for mortality (P=0.80).
Among patients who did not die, the overall proportion of patients who left the study because of kidney transplantation was 8%. The proportions were 10%, 9%, and 8% among patients with low, intermediate, and high PDC frequency, respectively, and 7%, 10%, and 10% among patients with short, intermediate, and long PDC duration. After adjustment for country, kidney transplantation was minimally associated with PDC frequency (P=0.84) and duration (P=0.93).
Discussion
Prior studies have shown that a variety of practice patterns of HD vary appreciably across countries,2,3 but to the best of our knowledge this study is the first to describe international variations in the frequency and duration of PDC in HD facilities and their associations with mortality. If the findings are valid, they raise the possibility that increasing PDC time by raising frequency and/or duration of PDC might lower the rate of adverse patient outcomes. Longer duration of the typical interaction at dialysis units between the patient and physician was consistently associated with lower mortality, regardless of the frequency of PDC. Similarly, the apparent benefit of more frequent PDC was consistent, regardless of the duration of the contact. We also tested the total PDC time (minutes per patient per month), and results were consistent: Mortality was lowest in facilities that provided the most total PDC time. Although the associations observed between PDC and mortality were relatively weak, this is a modifiable treatment factor and the potential impact might be clinically important. Results in North America, Europe, and Australia/New Zealand were qualitatively consistent with the overall estimates (Table 4). Results in Japan were more divergent and imprecise, probably reflecting both a limited number of events and minimal within-country variation. PDC at Japanese facilities was typically identified as high frequency and short duration (Figures 1 and 2).
Variation in PDC frequency and duration across countries may be due to a variety of factors, including differences in health care delivery, variations in physician reimbursement, and cultural differences. In the United States, for example, until recently, physicians received a fixed, capitated payment per month, but now payment reflects visit frequency in a month, independent of the duration or the quality of the contact. The fact that PDC frequency was lower in facilities that treated patients with more comorbid conditions suggests that the organization of PDC may not necessarily be determined by patients’ clinical needs. Use of nonphysician practitioners may also account for the variations in PDC across countries. In the United Kingdom and United States, nurse practitioners or physician assistants, who have completed advanced education and training in the diagnosis and management of dialysis patients, serve as physician extenders in the dialysis units.11,12 Additionally, the balance between human resources and geographic factors may influence PDC patterns. In Australia and New Zealand, for instance, many renal centers provide health care services at associated satellite HD facilities in vast outlying areas of the country, and it may be difficult to constantly allocate or frequently dispatch physicians to these remote HD units.13
Results from previous research have suggested that dialysis unit staffing patterns might influence patient mortality.4,5 Earlier studies also examined the relation between PDC and outcomes, but data used in those analyses focused on frequency, not duration, of PDC.8–10 We provide new information extending results of previous studies and demonstrate that both PDC frequency and duration, as well as total PDC time, are associated with all-cause mortality. Assuming our findings are valid, they suggest that more face-to-face encounters with physicians, whether more frequently or longer, may contribute to better patient outcomes. Therefore, our findings may have implications for both health policy and day-to-day management of dialysis patients. More frequent and longer PDC can allow for greater engagement of physicians with patients, additional opportunities for timely detection of new medical problems, closer monitoring of clinical and laboratory data, greater opportunity for optimal disease management, and health education, with resultant potential for greater patient satisfaction with care. HD patients usually receive renal replacement therapy thrice weekly, and nephrologists or dialysis doctors at HD units may serve as primary care physicians for patients with ESRD.14 Despite advances in medical technology and evidence-based guidelines, many efforts to improve health require significant change in patient behavior. These changes in behavior might involve reduction or elimination of destructive behaviors, promotion of healthier lifestyles, and adherence to medical regimens intended to treat acute or chronic illness.15 Continuity in the provision of PDC, a basic tenet of high-quality care, may increase the probability of successful changes in behavior.
A previous study showed that more frequent PDC was positively associated with the achievement of clinical performance targets in HD care, focusing on serum albumin, serum calcium phosphate product, dialysis dose (Kt/V), vascular access type, and hemoglobin.9 The achievement of these targets may directly or indirectly influence long-term outcomes; therefore, these findings are consistent with our result that more PDC was associated with better patient survival. These factors may be intermediate variables in the hypothesized causal pathway between PDC and outcome.
On the other hand, an earlier study from the United States reported that more frequent PDC was not associated with lower mortality, although more frequent PDC was related to higher patient satisfaction.8 These analyses were based on data from 735 patients (versus 24,498 patients in the present study), and the statistical power may have been too low to detect a moderate effect of PDC on mortality. In fact, when we analyzed the associations between PDC and mortality in each of the 12 countries, our effect estimates were imprecise for certain countries because of the limited sample size and, in some cases, minimal within-country variation in PDC (results not shown). A recent study based on analysis of data from the USRDS (n=130,892) also showed that PDC frequency, although associated with lower hospitalization, was not consistently associated with mortality.10 One potential explanation for this unexpected result could be that the study targeted only one country, which may have less variability in practice patterns: Forty percent of dialysis patients were seen <4 times per month. In the DOPPS, the proportions of facilities with PDC frequency <4 times per month were 32% in the United States and 16% in other countries (ranging from 0% in Germany to 87% in the United Kingdom). Additionally, in that previous study, the investigators were unable to distinguish between frequencies of 4 times per month and >4 times per month (the intermediate and high groups in our study) because of limitations of the billing codes used as the data source.10
The association between PDC frequency and mortality in our study was monotonic (Table 3, model 4a). By dichotomizing the exposure variable and limiting the sample to patients in the United States to approximate the methods of the previous study,10 we observed that the adjusted HR for mortality associated with a frequency <4 times per month was 1.08 (95% CI, 0.96 to 1.22) compared with ≥4 times per month; inversely, the HR for mortality was 0.92 (95% CI, 0.82 to 1.04) in association with a frequency ≥4 times per month compared with <4 times per month in the patients from the DOPPS in the United States. Slinin et al. reported a HR of 0.98 (95% CI, 0.96 to 1.01) for ≥4 times per month compared with <4 times per month.10 According to worldwide DOPPS data, however, the adjusted mortality HR for <4 times per month was 1.08 (95% CI, 0.99 to 1.18) compared with ≥4 times per month, which was consistent with the result of our main analysis using three PDC categories.
We also investigated the effect of these practices on hospitalization outcomes. This study suggests that, assuming our PDC effects are valid, increasing PDC time by increasing frequency and/or duration of PDC may decrease the rate of first hospitalization. By reducing hospitalization, more frequent and longer PDC therefore has the potential to lower the total cost of care. At non–hospital-based facilities, total PDC time was inversely associated with the rate of first hospitalization; however, this was not the case at hospital-based facilities. One possible explanation is that patients at hospital-based facilities may have been sicker than those at non–hospital-based facilities; another is that PDC may have less effect on survival at hospital-based facilities because patients have more contact with other health care providers. The observation of contact time may also be related to the geographic difference between hospital-based and non–hospital-based facilities because hospital-based facilities may dialyze inpatients in those facilities whereas non–hospital-based facilities cannot. Other facility organization types might also play an important role for PDC associated with patient outcomes. Profit status (not-for-profit versus for-profit), for example, was reported to affect mortality.16 According to data from the DOPPS phase 3 (not available in phase 1 and 2), however, PDC frequency and duration were similar regardless of profit status in facilities in the United States and those in other countries (data not shown).
We also examined the effect of PDC on kidney transplantation, but only trivial associations of PDC frequency and duration with higher kidney transplantation rate were found. Kidney transplantation may improve the quality of life and reduce the mortality risk for most patients with ESRD compared with maintenance dialysis.17 However, the incidence of kidney transplantation, as a treatment of choice for ESRD, largely depends on the cultural background and the social support system in each country. Additionally, there are a shortage of organs and a growing waiting list for kidney transplantation worldwide. These factors may explain why more frequent and longer PDC was not associated with a higher kidney transplantation rate.
Despite its strengths—large sample size, international prospective cohort study, robust multivariable analyses to minimize confounding by indication, scientifically plausible and potentially important results—key limitations of this work should be recognized. First, data on PDC frequency and duration were based on the DOPPS Unit Practice Survey (UPS), which was not completed in all DOPPS facilities (excluded facilities shown in Table 1). The UPS was completed by the facility nurse managers, and PDC frequency and duration reflected practice patterns at the facility in general and not the experience of individual study participants. Not only could those measures have been inaccurate assessments of typical PDC frequency and duration in the facilities, but they also do not capture the heterogeneity within facilities at the patient level. However, the UPS data were reported not only at baseline but also during the study phase in order to assess whether facility practice patterns changed during the phase. Additionally, frequency and duration of PDC were also reported in the Medical Director Survey by medical doctors as well as in the UPS so as to validate the accuracy of the reported data. The level of agreement was very good in both cases, as described in the Concise Methods section. Nevertheless, there still may have been error in measuring average facility PDC, and the unknown degree of within-facility heterogeneity represents additional measurement error at the patient level. Thus, our estimates of PDC effects may have been biased. The direction of that bias is difficult to predict; however, we would expect to underestimate the effect of PDC if the error was random and independent of other variables.
Second, our data did not detail the actions doctors actually took during PDC, such as extent of rapport, methods of patient counseling, thoroughness of physical examinations, and any treatment modifications (e.g., in prescribed dialysis dose, treatment time, medications, quality of fluid, and BP management). The quality of PDC may be more relevant than its frequency or duration for patient outcomes. In this regard, it may be important whether each dialysis patient is seen mostly by a single physician or rotating physicians, which can influence the quality of PDC. Although PDC in this study refers mainly to visits occurring during HD treatments, physicians’ work also includes non–face-to-face activities before and after the visits, such as treatment planning, care coordination among other health care professionals, medical record documentation, and communication with patient families. Variation in time spent on these non–face-to-face activities could also affect quality of care and outcomes. In countries with low PDC time, such as Australia, New Zealand, and the United Kingdom, regular PDC with a nephrologist may also occur in a clinic setting separately from the HD treatment. Furthermore, patients may have substantial contact with their primary care physicians away from the dialysis facility. Variation in PDC time spent outside of the dialysis facility may also have an effect on clinical outcomes of dialysis patients. Future studies are needed to clarify the nature of PDC (both direct and indirect) and what components of PDC are associated with improved quality of care and patient outcomes in HD units.
Third, this research focused on doctors’ contact with HD patients but did not consider the contribution of other health care professionals. Nonphysician practitioners, such as nurse practitioners, advance practice nurses, dietitians, and social workers, might visit patients more frequently in addition to, or in lieu of, the physician visits, and they could influence patient outcomes. Previous research has suggested that staffing patterns and multidisciplinary rounds may influence patient survival.18,19 In our analyses, we adjusted for patient-doctor ratio and the patient–dialysis staff ratio at facilities, but not for the quality of care by nonphysician staff. The quality of contacts between patients and staff may also be more relevant than its quantity. Future studies should delineate how contact with other health care professionals contributes to patient outcomes in the dialysis unit.
Finally, because this is an observational study, our ability to make causal inferences is also limited by the lack of randomization. Therefore, residual confounding due to unmeasured or mismeasured confounders is a concern. It may be, for example, that doctors see healthier patients more frequently or spend more time with them than they do with patients in poorer health, or that unmeasured quality-of-care practices may have been associated with average facility patient-doctor contact. However, randomized controlled trials cannot be undertaken in all situations where evidence is needed to guide care,20 and well-designed observational studies will continue to remain invaluable tools for comparative effectiveness research in the real-world setting.21 Despite the limitations, this study provides new and detailed information that is useful for dialysis practice because randomized trials are difficult to design and execute.
In conclusion, results of this study demonstrated that both frequency and duration of PDC at the time of hemodialysis sessions varied appreciably across the 12 countries in the DOPPS. More frequent and longer PDC, as estimated at the facility level for a typical patient, was inversely associated with all-cause mortality and time to first hospitalization, adjusting for an extensive set of patient and facility characteristics. The physician’s role in the multidisciplinary team of health care professionals in the dialysis facility is crucial for improving the quality of HD care, and policies supporting more frequent and longer duration of PDC may be an important means of improving HD patient outcomes.
Concise Methods
Data Source
This study was based on analyses of data from the DOPPS phase 1 (1996–2001), phase 2 (2002–2004), and phase 3 (2005–2008). DOPPS is a prospective cohort study of HD practice patterns and outcomes in 12 countries (Australia, Belgium, Canada, France, Germany, Italy, Japan, New Zealand, Spain, Sweden, United Kingdom, and United States).22 A nationally representative random sample of dialysis facilities was selected and enrolled from each country. The overall design of the DOPPS has been published previously.23 Consent to collect anonymous patient information was obtained as needed from the local or national ethics committees or institutional review boards. Patient data were obtained for the DOPPS by medical record abstraction. For these analyses, data on demographic factors, comorbid conditions, and laboratory values were collected at study entry (baseline) of each study phase.
Our analysis was based on data obtained from 24,498 patients from 778 facilities in the 12 countries. All eligible patients were at least 18 years of age and receiving maintenance HD for >90 days (“prevalent cases”) at study entry. The typical frequency and duration of PDC and other facility characteristics were reported by each DOPPS facility, usually by a unit nurse manager, in the DOPPS UPS. They were reported not only at baseline but also during the study phase in order to assess whether facility practice patterns changed during the phase. Among the 430 facilities in phases 1–2 in which PDC frequency was reported during follow-up, approximately 1 year after baseline, the level of agreement was very good (weighted κ = 0.77). Additionally, frequency and duration of PDC were reported in the Medical Director Survey by medical doctors as well as in the UPS to validate the accuracy of the reported data. Among the 670 facilities in which PDC frequency was reported by both the nurse manager and the medical doctor, the level of agreement was very good (weighted κ = 0.75). Facility and patient data were excluded from the present study if the facility did not report both frequency and duration of PDC at baseline. Among 930 facilities in DOPPS phases 1–3 (41,226 patients with follow-up data), 886 (with 39,769 patients) completed a UPS. Fifty-four of these facilities had missing or unknown PDC frequency, and 71 of these facilities had missing or unknown PDC duration (17 had no information on either), leaving 778 facilities with 34,841 patients with complete PDC information. We finally restricted the analysis to 24,498 patients in these 778 facilities with dialysis vintage >90 days.
The frequency of PDC was classified into three groups: (1) high frequency (more than once per week), (2) intermediate frequency (once per week), and (3) low frequency (less than once per week). Duration of PDC (i.e., the duration of a typical interaction between the patient and physician in each facility) was treated as a continuous variable and as a categorical variable classified into three categories: (1) long duration (>10 minutes), (2) intermediate duration (6–10 minutes), and (3) short duration (≤5 minutes). Total PDC time (minutes per patient per month) was estimated, not directly measured, as the duration of a typical contact multiplied by the approximated number of contacts per month and classified into three categories: (1) ≥60 minutes, (2) 30–59 minutes, and (3) <30 minutes. To compare our results with the recently published analysis of USRDS data,10 PDC frequency was also dichotomized as 4 or more visits per month (≥4 times per month) versus <4 visits per month (<4 times per month). This classification was equivalent to a comparison of our high- and intermediate-frequency groups with our low-frequency group.
Statistical Analyses
Standard descriptive statistics were used to describe PDC frequency and duration at baseline among prevalent HD patients and their distributions across facilities. Patients enrolled in each phase were followed until the end of the phase. Data on facility practice patterns, including physician visits, were collected not only at baseline but also during the follow-up period of the phase. Because the practice changes were minimal, we used the baseline PDC as the exposure in this study rather than a time-varying covariate approach. The association between the percentage of high PDC frequency and the average typical PDC duration in each country was assessed by Pearson correlation.
PDC was investigated as a primary predictor of all-cause mortality and time to first hospitalization for any reason during follow-up, using three different measures: (1) PDC frequency, (2) PDC duration, and (3) total PDC time. Cox regression was used to estimate the HR and 95% CI, comparing patients with different amounts of PDC. Patients were at risk from study entry until death (or first hospitalization), kidney transplantation, 7 days after departure from the facility, loss to follow-up, or the end of follow-up (whichever came first). Cox models were stratified by region and study phase, and PDC effects were adjusted for both patient and facility characteristics. Patient characteristics were age, sex, race, body mass index, years with ESRD, 14 summary comorbid conditions present at study entry (listed in Table 1), living arrangements (living alone, with family, at nursing home), socioeconomic status (education, employment, and marital status), serum albumin, hemoglobin, serum phosphorus, serum calcium, single pool Kt/V, duration of treatment, and vascular access. Facility characteristics were staff deployment (patient-doctor ratio in a facility, patient–dialysis staff ratio in a typical dialysis patient shift), and facility type (hospital-based facility or non–hospital-based facility). Facility clustering effects were taken into account with the robust sandwich variance estimator.24,25 Region-specific effects of PDC frequency and duration were additionally estimated in a Cox model, including PDC frequency × region and PDC duration × region interaction terms. Logistic regression using generalized estimating equations to account for facility clustering was used to assess the association between PDC and kidney transplantation, adjusted for country.
Using similarly adjusted Cox models, we assessed the association between dichotomized PDC frequency and mortality, both overall and restricted to DOPPS data from the United States. We also examined the interaction between frequency and duration of PDC (using three categories of frequency while modeling duration as a continuous variable) to detect departure from multiplicative effects (i.e., heterogeneity of the HR for each variable over levels of the other variable). Interactions were also examined between PDC and type of dialysis facility (hospital-based or non–hospital-based).
Values were missing for several covariates in these analyses; the proportion missing was <10% for all covariates except for Kt/V (26%) and albumin (13%). Assuming these data were missing at random, missing covariate values were multiply imputed using the chained equation method26 by IVEWARE (IVEware: Imputation and Variance Estimation Software, University of Michigan). Missing values were sequentially updated using a bootstrap or Markov chain Monte Carlo method, based on multiple regression models with other variables as covariates and accounting for the multilevel aspect of the data. This procedure was carried out for 10 iterations, thereby constructing an imputed data set. Results from five such imputed data sets were combined for the final analysis using Rubin’s formula27 that was implemented in PROC MIANALYZE in SAS. All statistical analyses were performed with SAS software, version 9.2 (SAS Institute, Cary, NC).
Disclosures
B.M.R. has received speaker’s fees from Kyowa Hakko Kirin. R.L.P. has received speaker’s fees from Amgen, Kyowa Hakko Kirin, and Vifor; has served as a consultant for Pursuit Vascular; and has served on an advisory panel for Merck. T.A. has received speaker’s fees and research grant for Kyowa Hakko Kirin. The corresponding author and all other authors have no potential conflicts of interest to declare.
Acknowledgments
The DOPPS is administered by Arbor Research Collaborative for Health and is supported by scientific research grants from Amgen (since 1996), Kyowa Hakko Kirin (since 1999, in Japan), Sanofi Renal (since 2009), AbbVie (since 2009), Baxter (since 2011), and Vifor Fresenius Renal Pharma (since 2011), without restrictions on publications.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Levey AS, Beto JA, Coronado BE, Eknoyan G, Foley RN, Kasiske BL, Klag MJ, Mailloux LU, Manske CL, Meyer KB, Parfrey PS, Pfeffer MA, Wenger NK, Wilson PW, Wright JT, Jr: Controlling the epidemic of cardiovascular disease in chronic renal disease: what do we know? What do we need to learn? Where do we go from here? National Kidney Foundation Task Force on Cardiovascular Disease. Am J Kidney Dis 32: 853–906, 1998 [DOI] [PubMed] [Google Scholar]
- 2.McClellan WM, Soucie JM, Flanders WD: Mortality in end-stage renal disease is associated with facility-to-facility differences in adequacy of hemodialysis. J Am Soc Nephrol 9: 1940–1947, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Goodkin DA, Mapes DL, Held PJ: The dialysis outcomes and practice patterns study (DOPPS): How can we improve the care of hemodialysis patients? Semin Dial 14: 157–159, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Held PJ, Pauly MV, Diamond L: Survival analysis of patients undergoing dialysis. JAMA 257: 645–650, 1987 [PubMed] [Google Scholar]
- 5.Pifer TB, Satayathum S, Dykstra DM, Mapes DL, Goodkin DA, Canaud B, Bommer J, Kurokawa K, Held PJ, Pisoni RL, Wolfe RA, Young EW: Hemodialaysis (HD) staffing and patient outcomes in the Dialysis Outcomes and Practice Patterns Study (DOPPS). [Abstract] J Am Soc Nephrol 13: 425A, 2002 [Google Scholar]
- 6.Lindenfeld S, Vlchek D: Engaging physicians in continuous quality improvement. Adv Ren Replace Ther 8: 120–124, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Kliger AS: The dialysis medical director’s role in quality and safety. Semin Dial 20: 261–264, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Plantinga LC, Fink NE, Sadler JH, Levey AS, Levin NW, Rubin HR, Coresh J, Klag MJ, Powe NR: Frequency of patient-physician contact and patient outcomes in hemodialysis care. J Am Soc Nephrol 15: 210–218, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Plantinga LC, Jaar BG, Fink NE, Sadler JH, Levin NW, Coresh J, Klag MJ, Powe NR: Frequency of patient-physician contact in chronic kidney disease care and achievement of clinical performance targets. Int J Qual Health Care 17: 115–121, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Slinin Y, Guo H, Li S, Liu J, Ensrud K, Gilbertson DT, Collins AJ, Ishani A: Association of provider-patient visit frequency and patient outcomes on hemodialysis. J Am Soc Nephrol 23: 1560–1567, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Easom A, Allbritton G: Advanced practice nurses in nephrology. Adv Ren Replace Ther 7: 247–260, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Headley CM, Wall B: Advanced practice nurses: roles in the hemodialysis unit. Nephrol Nurs J 27: 177–184, quiz 185–186, 2000 [PubMed] [Google Scholar]
- 13.Agar JW: Home hemodialysis in Australia and New Zealand: Practical problems and solutions. Hemodial Int 12[Suppl 1]: S26–S32, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Holley JL: Nephrologists as primary care providers: A review of the issues. Am J Kidney Dis 31: 574–583, 1998 [DOI] [PubMed] [Google Scholar]
- 15.O’Connell D: Behavioral Medicine. A Guide for Clinical Practice, edited by Feldman MD, Christensen JF, 3rd Ed., New York, McGraw-Hill, 2008, pp 141–157 [Google Scholar]
- 16.Garg PP, Frick KD, Diener-West M, Powe NR: Effect of the ownership of dialysis facilities on patients’ survival and referral for transplantation. N Engl J Med 341: 1653–1660, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Plantinga LC, Fink NE, Jaar BG, Sadler JH, Coresh J, Klag MJ, Levey AS, Powe NR: Frequency of sit-down patient care rounds, attainment of clinical performance targets, hospitalization, and mortality in hemodialysis patients. J Am Soc Nephrol 15: 3144–3153, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Halm MA, Gagner S, Goering M, Sabo J, Smith M, Zaccagnini M: Interdisciplinary rounds: Impact on patients, families, and staff. Clin Nurse Spec 17: 133–142, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ: Analysis of observational studies in the presence of treatment selection bias: Effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA 297: 278–285, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stukel TA, Lucas FL, Wennberg DE: Long-term outcomes of regional variations in intensity of invasive vs medical management of Medicare patients with acute myocardial infarction. JAMA 293: 1329–1337, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young EW, Goodkin DA, Mapes DL, Port FK, Keen ML, Chen K, Maroni BL, Wolfe RA, Held PJ: The Dialysis Outcomes and Practice Patterns Study (DOPPS): An international hemodialysis study. Kidney Int 57: S74–S81, 2000 [Google Scholar]
- 23.Pisoni RL, Gillespie BW, Dickinson DM, Chen K, Kutner MH, Wolfe RA: The Dialysis Outcomes and Practice Patterns Study (DOPPS): Design, data elements, and methodology. Am J Kidney Dis 44[Suppl 2]: 7–15, 2004 [DOI] [PubMed] [Google Scholar]
- 24.SAS/STAT User's Guide : Version 8, Vol. 2, Cary, North Carolina, SAS Institute, 2000, p 1452 [Google Scholar]
- 25.Klein J, Moeschberger M: Survival Analysis Techniques for Censored and Truncated Data, New York, Springer, 1997, pp 416–418 [Google Scholar]
- 26.van Buuren S, Boshuizen HC, Knook DL: Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med 18: 681–694, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Little RJA, Rubin DB: Statistical Analysis with Missing Data, New York, Wiley, 1987 [Google Scholar]