Abstract
Galectin-3, a profibrotic mediator, is linked to the development of renal fibrosis in animal models and inversely correlates with GFR in humans, but whether galectin-3 predicts incident kidney disease is unknown. Here, we assessed renal outcomes for 2450 Framingham Offspring participants who attended examination 6 (1995–1998) and had follow-up data at examination 8 (2005–2008). Renal outcomes of interest included rapid decline in renal function (≥3 ml/min per 1.73 m2 per year decline in estimated GFR [eGFR]), CKD (eGFR < 60 ml/min per 1.73 m2), and albuminuria (albumin-to-creatinine ratio ≥17 mg/g in men or ≥25 mg/g in women). We used multivariable logistic regression models to evaluate associations between galectin-3 with incident renal outcomes at examination 8. During a mean follow-up of 10.1 years, GFR declined rapidly in 241 (9.2%) participants, incident CKD developed in 277 (11.3%), and albuminuria developed in 194 (10.1%). Higher plasma levels of galectin-3 were associated with rapid decline in eGFR (per 1-SD log-galectin-3; adjusted odds ratio [OR], 1.49; 95% confidence interval [CI], 1.28 to 1.73]) and a higher risk of incident CKD (OR, 1.47; 95% CI, 1.27 to 1.71), but not with the risk of incident albuminuria. The addition of galectin-3 to clinical predictors improved the C-statistic (0.837–0.845; P=0.02) but did not reach predefined thresholds for clinically significant improvements to risk prediction based on reclassification indices. In conclusion, elevated levels of plasma galectin-3 are associated with increased risks of rapid GFR decline and of incident CKD in the community, which calls for further study in higher-risk groups.
CKD is a major worldwide public health problem1–6 that may result in progressive deterioration in kidney function,7 substantial morbidity,8–10 and increased mortality from both cardiovascular11 and noncardiovascular causes.12 Because of the lack of early clinical signs or symptoms and the poor sensitivity of currently available biomarkers (serum creatinine and urinary protein), CKD is typically detected at an advanced stage. However, when detected early, kidney function decline may be slowed or even reversed and these secondary complications averted.13–15 Hence, there is a pressing clinical need for novel biomarkers that identify at-risk individuals at the earliest possible stage.
Galectin-3 is a β-galactoside–binding lectin that has emerged as a key regulator of inflammation and fibrosis.16 Galectin-3 is strongly linked to the development of organ fibrosis in rodent models: Galectin-3 knockout mice are resistant to the development of fibrosis, whereas infusion of recombinant galectin-3 induces TGF-β−dependent tissue fibroblast proliferation and collagen deposition.17,18 In addition, upregulation of galectin-3 has been implicated in fibrosis of multiple organs, including the liver,19,20 lung,21 and heart.17 Galectin-3 has also been linked to the development of renal fibrosis,22,23 as well as fibrosis attenuation via matrix remodeling,24 indicating a context-dependent role.
Progressive CKD is characterized by the development of tubulointerstitial fibrosis.25 Galectin-3 expression and secretion by macrophages have previously been shown to promote the development of tubulointerstitial fibrosis in mouse models.23 We believe galectin-3 merits study as a renal biomarker on the basis of this association with renal fibrosis, as well as strong cross-sectional correlations with GFR identified in studies of patients with heart failure and in the general population.26–30 We hypothesized that higher plasma galectin-3 levels would be associated with an increased risk of incident CKD in the general population, defined as estimated GFR (eGFR) < 60 ml/min per 1.73 m2 or incident albuminuria. We also considered whether galectin-3 is associated with rapid decline in kidney function, as assessed by decline in eGFR during the follow-up period. To address these questions, we assessed prospective relations of galectin-3 and these outcomes in unselected participants from the Framingham Heart Study (FHS).
Results
The baseline characteristics of the 2450 participants are presented by sex-specific galectin-3 quartiles in Table 1. Overall, the mean age in our sample was 57 years and 53% of participants were women, with a mean follow-up of 10.1 years. Participants with higher galectin-3 levels tended to be older and have a higher body mass index and lower HDL cholesterol level (P for trend < 0.01 for all).
Table 1.
Baseline characteristics by sex-specific quartile of galectin-3 concentration in the Framingham Heart Study
| Characteristic | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P Valuea |
|---|---|---|---|---|---|
| Galectin-3 cut-points (men/women) (ng/ml) | 3.9/5.0 | 10.9/11.8 | 12.7/13.9 | 15/16.3 | — |
| Patients (men/women), n, (n/n) | 615 (279/336) | 616(296/320) | 608(288/320) | 611(279/332) | — |
| Age (yr) | 53±8 | 56±9 | 58±8 | 60±9 | <0.0001 |
| Women (%) | 54.6 | 51.9 | 52.6 | 54.3 | 0.5 |
| Hypertension (%) | 24.9 | 31.8 | 38.2 | 45.8 | 0.006 |
| Hypertension treatment (%) | 13.5 | 19.8 | 24.5 | 32.9 | 0.001 |
| SBP (mmHg) | 123±18 | 125±17 | 128±18 | 129±18 | 0.06 |
| Body mass index (kg/m2) | 26.6±4.4 | 27.9±5.3 | 28.1±5.1 | 28.5±5.1 | <0.0001 |
| Diabetes (%) | 4.9 | 7.5 | 5.9 | 10.8 | 0.9 |
| Current smoking (%) | 13.5 | 13.5 | 16.2 | 13.9 | 0.009 |
| HDL cholesterol (mg/dl) | 53.6±16 | 51.9±16 | 51.6±16 | 49.5±16 | <0.0001 |
| UACR (IQR) (mg/g) | 5.9 (2.8, 12.6) | 5.0 (2.5, 10.8) | 5.8 (2.3, 12.0) | 7.0 (2.7, 15.7) | 0.05 |
| Baseline eGFR (ml/min per 1.73 m2) | 92.8±14 | 90.4±16 | 88.5±16 | 86.0±15 | 0.01 |
| eGFRa at follow-up (ml/min per 1.73 m2) | 84.6±14 | 81.3±15 | 77.7±15 | 73.3±16 | <0.0001 |
| CKD at follow-up (%) | 4.7 | 7.8 | 12.2 | 20.6 | 0.008 |
| Galectin-3 (IQR) (ng/ml) | 10.1 (9.1, 10.7) | 12.3 (11.7, 12.8) | 14.45 (13.9, 15.1) | 17.3 (16.5, 19.2) | <0.0001 |
Quartiles were derived from untransformed data. Data are presented as means ± SD for normally distributed continuous variables, median and IQR for continuous variables that are not normally distributed, or percentage for categorical data. P value for trend across quartiles. CKD was defined as eGFR < 60 ml/min per 1.73 m2 using the CKD-Epidemiology Collaboration equation. SBP, systolic BP; UACR, urinary albumin-to-creatinine ratio; IQR, interquartile range.
Trend test P value adjusted for age and sex.
Clinical Correlates of Galectin-3
We determined Pearson correlation coefficients because galectin-3 appeared strongly associated with several CKD risk factors at baseline. We observed a modest inverse correlation between galectin-3 and baseline eGFR (Pearson partial correlation r=−0.15; P≤0.0001) (Table 2), which was substantially attenuated after adjustment for age and sex. The unadjusted correlation between galectin-3 and baseline albuminuria was weak (r= 0.07; P=0.001) and attenuated after adjustment for age and sex (P=0.07) (Table 2).
Table 2.
Correlation coefficients for log galectin-3 in the Framingham Heart Study
| Variable | SBP | HDL | Glucose | eGFR | Log UACR |
|---|---|---|---|---|---|
| Unadjusted | |||||
| r | 0.11 | −0.02 | 0.04 | −0.15 | 0.07 |
| P value | <0.0001 | 0.4 | 0.04 | <0.0001 | 0.001 |
| Age- and sex-adjusted | |||||
| r | 0.02 | −0.10 | 0.01 | −0.05 | 0.04 |
| P value | 0.3 | <0.0001 | 0.6 | 0.02 | 0.07 |
| Age-, sex-, and eGFR-adjusted | |||||
| r | 0.02 | −0.10 | 0.02 | – | 0.04 |
| P value | 0.3 | <0.0001 | 0.4 | – | 0.06 |
Data presented as Pearson correlation coefficient above with associated P value below for each variable at baseline. SBP, systolic BP; UACR, urinary albumin-to-creatinine ratio
Galectin-3 and Rapid Decline in Renal Function
Of 2613 participants assessed, 241 (9.2%) experienced a rapid decline in renal function during follow-up. Galectin-3 was associated with rapid decline in eGFR in age- and sex-adjusted (odds ratio [OR], 1.38 per 1-SD increase in log-galectin-3; 95% confidence interval [CI], 1.21 to 1.58; P<0.0001) and multivariable-adjusted (OR, 1.49; 95% CI, 1.27 to 1.73; P<0.0001) analyses (Table 3).
Table 3.
Odds of kidney disease per 1-SD increase of log galectin-3 (ng/ml) in the Framingham Heart Study
| Variable | OR (95% CI) | P Value |
|---|---|---|
| Incident CKD | ||
| Age- and sex-adjusted | 1.56 (1.35 to 1.80) | <0.0001 |
| Multivariable-adjusteda | 1.47 (1.27 to 1.71) | <0.0001 |
| Multivariable-adjusteda + homocysteine + aldosterone | 1.38 (1.18 to 1.61) | <0.0001 |
| Rapid eGFR decline | ||
| Age- and sex-adjusted | 1.38 (1.21 to 1.58) | <0.0001 |
| Multivariable-adjusteda | 1.49 (1.27 to 1.73) | <0.0001 |
| Incident albuminuria | ||
| Age- and sex-adjusted | 1.05 (0.89 to 1.23) | 0.6 |
| Multivariable-adjustedb | 1.04 (0.88 to 1.23) | 0.6 |
| Multivariable-adjustedb + homocysteine, aldosterone, and BNP | 0.99 (0.83 to 1.18) | 0.9 |
Adjusted for age, sex, diabetes, hypertension, dipstick proteinuria, and baseline eGFR
Adjusted for age, sex, diabetes, hypertension, body mass index, smoking, HDL cholesterol, and baseline log urinary albumin-to-creatinine ratio.
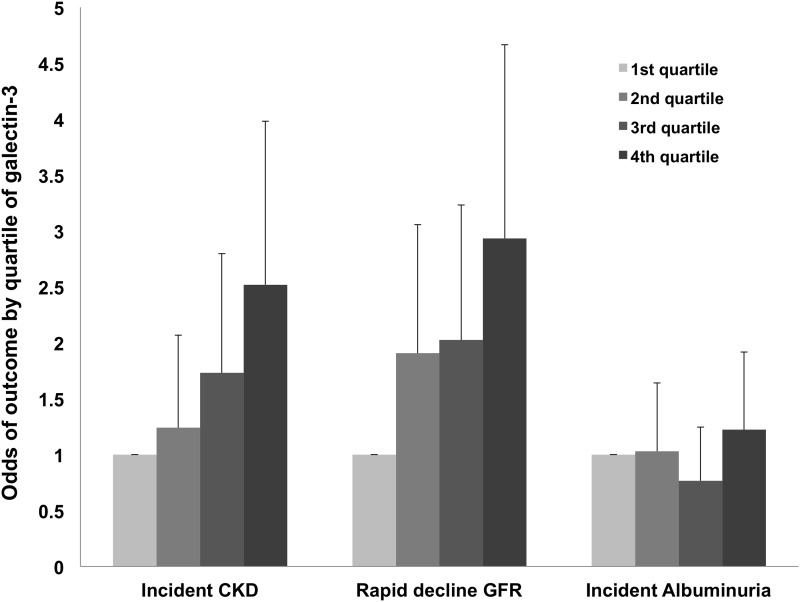
Similarly, the risk for rapid loss of renal function increased by galectin-3 quartile in both age- and sex-adjusted (OR for Q4 versus Q1 2.61; 95% CI, 1.70 to 4.01; P<0.0001) and multivariable-adjusted (OR for Q4 versus Q1, 2.93; 95% CI, 1.84 to 4.67; P<0.0001) analyses (Figure 1).
Figure 1.
Risk for incident CKD and rapid decline in GFR, but not incident albuminuria, increases by galectin-3 quartile. Error bars represent upper 95% CI for the OR estimate. Incident CKD and rapid decline in GFR analyses adjusted for age, sex, diabetes, hypertension, dipstick proteinuria, and baseline eGFR. Incident albuminuria analysis adjusted for age, sex, diabetes, hypertension, body mass index, smoking, HDL cholesterol, and baseline log urinary albumin-to-creatinine ratio.
Galectin-3 and Incident CKD
Incident CKD developed in 277 (11.3%) participants during follow-up. There was an increased risk of incident CKD per 1-SD increase in log-galectin-3 in age- and sex-adjusted analyses (OR, 1.56; 95% CI, 1.35 to 1.80; P<0.0001) (Table 3). Results were similar after multivariable adjustment (OR, 1.47; 95% CI, 1.27 to 1.71; P<0.0001), and additional adjustment for aldosterone and homocysteine did not materially affect the results (OR, 1.38; 95% CI, 1.18 to 1.61; P<0.0001). Similarly, additional adjustment for body mass index, which is correlated with galectin-3, minimally altered the results (OR, 1.46; 95% CI, 1.26 to 1.70; P<0.0001). The association remained significant after adjustment for cystatin C at interim examination 7 (OR, 1.20; 95% CI, 1.02 to 1.42; P=0.03).
The risk of incident CKD also increased by baseline quartile of galectin-3 (age- and sex-adjusted OR for Q4 versus Q1, 2.98; 95% CI, 1.91 to 4.64; P<0.0001) (Figure 1), and there was minimal attenuation with multivariable adjustment (OR for Q4 versus Q1, 2.5; 95% CI, 2.59 to 3.98; P<0.0001) or multivariable plus multimarker (homocysteine and aldosterone) adjustment (OR for Q4 versus Q1, 2.32; 95% CI, 1.42 to 3.77; P=0.0007).
Galectin-3 and Incident Albuminuria
There were 194 (10.1%) new cases of albuminuria at follow-up. There was no evidence of an increased risk of incident albuminuria in age- and sex-adjusted (OR, 1.05; 95% CI, 0.89 to 1.23; P=0.6) or multivariable-adjusted (OR, 1.04; 95% CI, 0.88 to 1.23; P=0.6) (Table 3) analyses per 1-SD increase in log-galectin-3, nor did the risk of incident albuminuria increase by galectin-3 quartile (P>0.1 for all comparisons) (Figure 1).
C-Statistic and Reclassification Analyses
The C statistic for the basic CKD clinical prediction model, comprising age, sex, diabetes, hypertension, dipstick proteinuria, and baseline eGFR, was 0.837. The addition of galectin-3 improved the C statistic to 0.845 (P=0.02).
Galectin-3 improved categorical reclassification of CKD by 3.5% when added to the clinical model (95% CI, 0.4 to 6.7), which did not reach the prespecified threshold for clinical significance of 5%. The category-free net reclassification improvement (NRI) and integrated discrimination improvement (IDI) for the addition of galectin-3 in predicting CKD similarly did not reach the predefined threshold for clinical significance (NRI, 0.35 [95% CI, 0.23 to 0.48]; IDI, 0.01 [95% CI, 0.005 to 0.02]).
Discussion
Principal Findings
The findings of the present study are four-fold. First, higher levels of galectin-3 were associated with an increased risk of incident CKD in the general population. These findings were robust in continuous and categorical analyses after adjustment for known clinical predictors of CKD, as well as circulating biomarkers known to enhance CKD prediction. Second, galectin-3 showed similarly strong associations with rapid loss of renal function over 10 years of follow-up. Third, there was no association between galectin-3 and albuminuria in any analysis. Finally, although improvements in the C-statistic and reclassification indices for the addition of galectin-3 were statistically significant, they did not exceed our predefined thresholds for clinical significance. Taken together, these data suggest that galectin-3 may identify individuals at risk for the development of CKD many years before clinical onset and, more important, suggest an important role for fibrosis early in the pathogenesis of CKD.
In the Context of the Current Literature
Higher galectin-3 levels have been linked to an increased all-cause mortality risk in the general population.31 Furthermore, galectin-3 predicts both acute and chronic heart failure and is a predictor of prognosis in that setting.28,32–35 Higher plasma galectin-3 levels were cross-sectionally associated with lower GFR in 133 patients with chronic systolic heart failure.26 Our study extends upon these observations by demonstrating robust associations with de novo CKD and rapid loss of renal function in prospective analyses of apparently healthy members of the general population.
These findings extend the literature on novel biomarkers of CKD risk. We have previously shown that both serum aldosterone and homocysteine are associated with incident CKD independent of traditional risk factors and result in a small but significant reclassification improvement.36 Similar observations were made for a higher aldosterone-to-renin ratio and incident CKD in an Asian community-based cohort.37 Our study extends the literature in this regard by identifying a novel biomarker of de novo kidney disease that is independent of both traditional risk factors and these novel predictors of renal risk. Furthermore, unlike aldosterone and homocysteine,38–42 galectin-3 has a very modest baseline correlation with GFR (r=−0.05; P=0.02).
Potential Mechanisms
Galectin-3 is a functionally diverse 32- to 35-kDa member of the galectin family of β-galactoside–binding lectins, a group functionally characterized by the presence of a carbohydrate recognition domain.43 It is expressed both intracellularly, where it regulates proliferation and apoptosis via carbohydrate-independent mechanisms, and on the cell surface and extracellular space, where it modulates cell-cell interactions (including cell adhesion, activation, and chemoattraction) and regulates cell growth, differentiation, and inflammation via its carbohydrate-binding functions.43–45 In the kidney, galectin-3 plays a complex role that is context-dependent.23,46 In development, it appears to promote normal nephrogenesis, being strongly expressed in the ureteric bud and its derivatives,47,48 with subsequently lower expression levels in the mature tubule.24
Galectin-3 plays a pro-resolution role in inflammation and repair. It is intensely upregulated in response to ischemic and nephrotoxic AKI,49 preventing chronic tubular injury by limiting apoptosis, enhancing matrix remodeling and attenuating fibrosis.24 The accelerated development of diabetic nephropathy in galectin-3 −/− mice is further evidence of renoprotective properties.50 However, when tissue injury is persistent or repetitive, galectin-3 may modulate the transition to chronic inflammation and fibrosis.51 It is a potent activator of fibroblasts in a range of tissues, including the kidney,52 liver,53 gut,54 and myocardium.17 Kidney fibrosis in human transplant recipients also depends on galectin-3.22 As such, galectin-3 may have proresolution or profibrotic effects along the continuum from acute inflammation to chronic inflammation and ultimately fibrosis.
Although galectin-3 is believed to play a causal role in the development of fibrosis of certain human organs, such as heart and liver,17,19 we cannot address whether a similar causal relationship might explain our results. Other explanations are possible. For example, the significant unadjusted inverse correlation between galectin-3 and baseline GFR suggests that galectin-3 might be handled by the kidney and thus be a surrogate for kidney function. We believe this is unlikely to be the sole explanation for our findings for three reasons. First, the clearance of galectin-3 appears to be primarily hepatic in origin on the basis of prior studies.55 Second, participants in our study were free of CKD at the time of assay, so patients with elevations in galectin-3 due to CKD were excluded. Finally, the correlation between galectin-3 and baseline GFR was not significant after adjustment for age and sex. Another possibility is that elevations in galectin-3 might occur as a response to renal injury but have no direct involvement in CKD progression.
Implications
As well as enhancing our ability to identify individuals at risk for CKD, our study gives insight into potential underlying biologic mechanisms. For example, it suggests that subclinical tubulointerstitial fibrosis may be important in the early stages of CKD, whereas the lack of association with albuminuria argues against glomerular injury or foot process effacement as a mechanism. Furthermore, because elevations in galectin-3 were identified years before overt kidney disease, they may offer a window of opportunity to initiate early, preventive treatment aimed at preventing disease occurrence. This is important because efforts to attenuate the profibrotic effects of galectin-3 in a mouse model of AKI have shown promise.56 A nontoxic pectin derivative found in citrus fruit, modified citrus pectin, decreased circulating levels of galectin-3 by binding the carbohydrate recognition domain and resulted in significant attenuation of renal fibrosis and inflammation in mouse models.57 Further research is necessary to determine whether galectin-3 is along the causal pathway for the development of CKD and whether targeting galectin-3 can reduce CKD development.
Finally, although statistically significant, improvements in the C-statistic and reclassification indices did not exceed our predefined thresholds for clinical significance. Nonetheless, the weak signal observed in this healthy, community-based cohort raises the possibility of a stronger effect in higher-risk groups, which is an interesting avenue for future study.
Strengths and Limitations
The strengths of our study are our well characterized sample, prospective cohort design, and an adequate number of CKD and albuminuria events. Some limitations also require mention. Cystatin C was not available at the baseline examination, and although we adjusted for its effects at an interim time point, this may have introduced a survival bias. Nonetheless, results were consistent with the primary analyses. We do not have a gold standard measure of GFR because this is not feasible in a large epidemiologic study. The large number of missing urinary albumin measures at both baseline and follow-up reduced the sample size for that analysis. The lack of repeated measures of galectin-3 over time is also a limitation. Finally, the sample was elderly and white, limiting generalizability to other groups.
Conclusions
Higher circulating galectin-3 levels are associated with increased risk of incident CKD and rapid loss of renal function over time in the community. Our findings suggest that galectin-3 may detect kidney injury years before the clinical onset of CKD, potentially affording an opportunity to institute early, targeted treatment aimed at disease prevention.
Concise Methods
Participants
The design and methods of the Framingham Offspring Study are described elsewhere.58 Each examination comprises a standardized medical history, routine questionnaires, physical examination, anthropometry, and blood testing. The present study includes participants assessed during the sixth examination cycle of the Offspring Study (1995–1998), at which time galectin-3 was assayed, who also returned for follow-up at examination 8. All participants provided written informed consent, and the institutional review boards of the Boston University Medical Center approved the study.
Of 3448 eligible participants, 5 were excluded for having extreme galectin-3 measures (>5 log-SDs above or below the log-transformed mean). Of the remaining 3443 participants, 2450 were included in the incident CKD analysis after 993 exclusions (lack of follow-up, n=699; prevalent CKD, n=163; missing baseline creatinine, n=12; missing follow-up creatinine, n=99; and missing covariates, n=20). There were 2613 participants in the analysis for rapid decline in eGFR after 830 exclusions (lack of follow-up, n=699; missing baseline creatinine, n=12; missing follow-up creatinine, n=99; and missing covariates, n=20). Finally, there were 1919 participants in the incident albuminuria analysis after 1524 exclusions (lack of follow-up, n=577; prevalent albuminuria, n=339; missing baseline urinary albumin, n=502; missing follow-up urinary albumin, n=104; and missing covariates, n=2). Participants who did not attend follow-up tended to be older (63 versus 57 years), male, and in poorer health (more likely to smoke or to have diabetes, hypertension, proteinuria, and/or a lower mean eGFR).
Outcome Definitions
Rapid decline in GFR was defined as the dichotomous outcome of eGFR loss of ≥3 ml/min per 1.73 m2 per year based on two measures (baseline and follow-up eGFR) using the CKD-Epidemiology Collaboration equation.59This outcome has previously been linked to cardiovascular disease and all-cause mortality risk in people with and without CKD.60,61
CKD was defined as an eGFR < 60 ml/min per 1.73 m2. Serum creatinine was measured using the modified Jaffé method and calibrated as previously described.62
Albuminuria was defined as a urinary albumin-to-creatinine ratio ≥25 mg/g in women and ≥17 mg/g in men).63 Spot urine samples collected at the baseline examination (1995–1998) were stored at −20°C and then transitioned to −80°C. The urinary albumin concentration was measured using immunoturbidimetry (www.roche.com), and urinary creatinine levels were measured using the Jaffé method.64 Proteinuria was assessed by dipstick assay (Ames Labstix, Elkhardt, IN). Spot morning urine samples assessed by urine dipstick were briefly dipped during the clinic visit and read after 1 minute.65
Plasma Galectin-3 Measurement
Blood samples were collected after an overnight fast and immediately centrifuged and stored at −80°C until assayed. Plasma concentrations of galectin-3 were measured using an ELISA (BG Medicine, Waltham, MA).66 The lower detection limit was 1.32 ng/ml with an upper detection limit of 96.6 ng/ml. The within-run and total precision are reported between 2.1%–5.7% and 4.2%–12.0%, respectively.66
Covariate Assessment
Participants underwent blood testing and were assessed for CKD risk factors. HDL cholesterol and blood glucose were measured on morning blood samples while fasting. Diabetes was defined as fasting blood glucose level of ≥126 mg/dl (7 mmol/L) or use of medication for the treatment of diabetes. Systolic and diastolic BP measurements were taken as the mean of two physician readings using a mercury sphygmomanometer. Hypertension was defined as a systolic BP ≥140 mmHg or a diastolic BP ≥90 mmHg or self-reported use of medication for hypertension. Body mass index was defined as an individual’s weight in kilograms divided by height in meters squared. Current smoking status was defined by self-report. Plasma brain natriuretic peptide was measured with high-sensitivity immunoradiometric assays (Shionogi, Japan; coefficient of variation [CV], 12.2%). Serum aldosterone was measured by radioimmunoassay (Quest Diagnostics; CV, 3.8%–6.0%). Plasma total homocysteine was measured by HPLC with fluorometric detection (CV, 9%). Brain natriuretic peptide, aldosterone, and homocysteine were assayed at baseline examination 6. Serum cystatin C was measured using particle-enhanced immunonephelometry (Dade Behring BN 100; CV, 2.4%). Cystatin C was measured only at interim examination 7.
Statistical Analyses
Because of sex differences in galectin-3 distribution, sex-specific quartiles of galectin-3 were used to present baseline descriptive data. Galectin-3 was log-transformed for all subsequent analyses. Baseline clinical characteristics were summarized by quartiles of log-galectin-3, and age-adjusted trends in means across quartiles were compared by ANOVA. We used sex-standardized log-galectin-3 for all correlation and regression analyses. The correlation of galectin-3 with CKD risk factors was examined using age- and sex-adjusted and age-, sex-, and eGFR-adjusted Pearson partial correlation coefficients.
Multivariable logistic regression was used to assess relations of galectin-3 to the three outcomes: incident CKD, rapid decline in kidney function, and albuminuria. In these analyses, galectin-3 was modeled both as a continuous variable (odds of outcome per 1-SD increase of log plasma galectin-3) and a categorical variable (odds of renal outcomes by quartiles of log plasma galectin-3). Models were adjusted for (1) age and sex and (2) CKD covariates: age, sex, diabetes, hypertension, dipstick proteinuria, and baseline eGFR. The multivariable model for incident albuminuria was adjusted for age, sex, diabetes, hypertension, body mass index, smoking, HDL cholesterol, and baseline log urinary albumin-to-creatinine ratio. Covariates for these models are derived from prior work.67,68 Participants with baseline CKD or albuminuria were excluded from the respective incident analyses. No participants were excluded from the rapid decline in kidney function analysis, and the multivariable model applied the same covariates as for the incident CKD analysis.
In a secondary analysis, we adjusted for biomarkers previously shown to enhance risk prediction of kidney disease beyond clinical factors: serum homocysteine and aldosterone both for incident CKD and aldosterone, brain natriuretic peptide, and homocysteine for incident albuminuria.36 We also additionally adjusted for cystatin C in a secondary analysis.
To assess the ability of galectin-3 to improve CKD prediction beyond clinical measures, we compared C-statistics for the addition of galectin-3 to a CKD clinical prediction model comprising age, sex, diabetes, hypertension, dipstick proteinuria, and baseline eGFR.67 We also estimated the category-based and category-free NRI metrics for the addition of galectin-3 to fully adjusted models.69,70 We defined cut-points for risk groups for the category-based NRI using tertiles of predicted risk from the model (<3%, 3%–6%, >6%).36 We also estimated the IDI, which may be interpreted as a continuous version of the NRI with probability differences used instead of categories.
A type I error threshold of 0.05 was used to indicate statistical significance. All statistical analyses were performed using SAS software, version 9.2 (http://www.sas.com/).
Disclosures
None of the authors report any significant financial disclosures. Galectin-3 assays were provided by BG Medicine Inc. (Waltham, MA). This company did not have access to study data and had no input into the data analyses, interpretation, or preparation of the manuscript for submission.
Acknowledgments
The Framingham Heart Study is supported by the National Heart, Lung, and Blood Institute (N01-HC-25195). J.E.H. is supported by an American Heart Association Clinical Research Program award.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Galectin-3 and New-Onset CKD: Marker or Mediator?,” on pages 1342–1344.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Zhang QL, Rothenbacher D: Prevalence of chronic kidney disease in population-based studies: Systematic review. BMC Public Health 8: 117, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chadban SJ, Briganti EM, Kerr PG, Dunstan DW, Welborn TA, Zimmet PZ, Atkins RC: Prevalence of kidney damage in Australian adults: The AusDiab kidney study. J Am Soc Nephrol 14[Suppl 2]: S131–S138, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Wildman RP, Gu D, Kusek JW, Spruill M, Reynolds K, Liu D, Hamm LL, Whelton PK, He J: Prevalence of decreased kidney function in Chinese adults aged 35 to 74 years. Kidney Int 68: 2837–2845, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Perkovic V, Cass A, Patel AA, Suriyawongpaisal P, Barzi F, Chadban S, Macmahon S, Neal B, InterASIA Collaborative Group : High prevalence of chronic kidney disease in Thailand. Kidney Int 73: 473–479, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Tanaka H, Shiohira Y, Uezu Y, Higa A, Iseki K: Metabolic syndrome and chronic kidney disease in Okinawa, Japan. Kidney Int 69: 369–374, 2006 [DOI] [PubMed] [Google Scholar]
- 7.McClellan WM, Flanders WD: Risk factors for progressive chronic kidney disease. J Am Soc Nephrol 14[Suppl 2]: S65–S70, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Muntner P, Vupputuri S, Coresh J, Uribarri J, Fox CS: Metabolic abnormalities are present in adults with elevated serum cystatin C. Kidney Int 76: 81–88, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu CY, McCulloch CE, Curhan GC: Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: Results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol 13: 504–510, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Ensrud KE, Lui LY, Taylor BC, Ishani A, Shlipak MG, Stone KL, Cauley JA, Jamal SA, Antoniucci DM, Cummings SR, Osteoporotic Fractures Research Group : Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med 167: 133–139, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Fried LF, Shlipak MG, Crump C, Bleyer AJ, Gottdiener JS, Kronmal RA, Kuller LH, Newman AB: Renal insufficiency as a predictor of cardiovascular outcomes and mortality in elderly individuals. J Am Coll Cardiol 41: 1364–1372, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ: Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studies. J Am Soc Nephrol 15: 1307–1315, 2004 [DOI] [PubMed] [Google Scholar]
- 13.de Zeeuw D, Ramjit D, Zhang Z, Ribeiro AB, Kurokawa K, Lash JP, Chan J, Remuzzi G, Brenner BM, Shahinfar S: Renal risk and renoprotection among ethnic groups with type 2 diabetic nephropathy: A post hoc analysis of RENAAL. Kidney Int 69: 1675–1682, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Ruggenenti P, Perna A, Gherardi G, Garini G, Zoccali C, Salvadori M, Scolari F, Schena FP, Remuzzi G: Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 354: 359–364, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Makino H, Nakamura Y, Wada J: Remission and regression of diabetic nephropathy. Hypertens Res 26: 515–519, 2003 [DOI] [PubMed] [Google Scholar]
- 16.de Boer RA, Yu L, van Veldhuisen DJ: Galectin-3 in cardiac remodeling and heart failure. Curr Heart Fail Rep 7: 1–8, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JP, Schroen B, André S, Crijns HJ, Gabius HJ, Maessen J, Pinto YM: Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 110: 3121–3128, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Liu YH, D’Ambrosio M, Liao TD, Peng H, Rhaleb NE, Sharma U, André S, Gabius HJ, Carretero OA: N-acetyl-seryl-aspartyl-lysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. Am J Physiol Heart Circ Physiol 296: H404–H412, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu DK, Dowling CA, Jeng KC, Chen JT, Yang RY, Liu FT: Galectin-3 expression is induced in cirrhotic liver and hepatocellular carcinoma. Int J Cancer 81: 519–526, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Henderson NC, Mackinnon AC, Farnworth SL, Poirier F, Russo FP, Iredale JP, Haslett C, Simpson KJ, Sethi T: Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci U S A 103: 5060–5065, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishi Y, Sano H, Kawashima T, Okada T, Kuroda T, Kikkawa K, Kawashima S, Tanabe M, Goto T, Matsuzawa Y, Matsumura R, Tomioka H, Liu FT, Shirai K: Role of galectin-3 in human pulmonary fibrosis. Allergol Int 56: 57–65, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Dang Z, MacKinnon A, Marson LP, Sethi T: Tubular atrophy and interstitial fibrosis after renal transplantation is dependent on galectin-3. Transplantation 93: 477–484, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Henderson NC, Mackinnon AC, Farnworth SL, Kipari T, Haslett C, Iredale JP, Liu FT, Hughes J, Sethi T: Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol 172: 288–298, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okamura DM, Pasichnyk K, Lopez-Guisa JM, Collins S, Hsu DK, Liu FT, Eddy AA: Galectin-3 preserves renal tubules and modulates extracellular matrix remodeling in progressive fibrosis. Am J Physiol Renal Physiol 300: F245–F253, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodríguez-Iturbe B, Johnson RJ, Herrera-Acosta J: Tubulointerstitial damage and progression of renal failure. Kidney Int Suppl S82–S86, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Tang WH, Shrestha K, Shao Z, Borowski AG, Troughton RW, Thomas JD, Klein AL: Usefulness of plasma galectin-3 levels in systolic heart failure to predict renal insufficiency and survival. Am J Cardiol 108: 385–390, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, Larson MG, Levy D: Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol 60: 1249–1256, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lok DJ, Van Der Meer P, de la Porte PW, Lipsic E, Van Wijngaarden J, Hillege HL, van Veldhuisen DJ: Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: data from the DEAL-HF study. Clin Res Cardiol 99: 323–328, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Boer RA, van Veldhuisen DJ, Gansevoort RT, Muller Kobold AC, van Gilst WH, Hillege HL, Bakker SJ, van der Harst P: The fibrosis marker galectin-3 and outcome in the general population. J Intern Med 272: 55–64, 2012 [DOI] [PubMed] [Google Scholar]
- 30.de Boer RA, Lok DJ, Jaarsma T, van der Meer P, Voors AA, Hillege HL, van Veldhuisen DJ: Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann Med 43: 60–68, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Boer RA, van Veldhuisen DJ, Gansevoort RT, Muller Kobold AC, van Gilst WH, Hillege HL, Bakker SJ, van der Harst P: The fibrosis marker galectin-3 and outcome in the general population: data from PREVEND. J Intern Med 272: 55–64, 2012 [DOI] [PubMed] [Google Scholar]
- 32.van Kimmenade RR, Januzzi JL, Jr, Ellinor PT, Sharma UC, Bakker JA, Low AF, Martinez A, Crijns HJ, MacRae CA, Menheere PP, Pinto YM: Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J Am Coll Cardiol 48: 1217–1224, 2006 [DOI] [PubMed] [Google Scholar]
- 33.de Filippi C, Christenson RH, Shah R, Bhardwaj A, Januzzi JL: Clinical validation of a novel assay for galectin-3 for risk assessment in acutely destabilized heart failure [Abstract]. J Cardiac Fail 15(Suppl): S9, 2009 [Google Scholar]
- 34.Shah RV, Chen-Tournoux AA, Picard MH, van Kimmenade RR, Januzzi JL: Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure. Eur J Heart Fail 12: 826–832, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ueland T, Aukrust P, Broch K, Aakhus S, Skårdal R, Muntendam P, Gullestad L: Galectin-3 in heart failure: High levels are associated with all-cause mortality. Int J Cardiol 150: 361–364, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Fox CS, Gona P, Larson MG, Selhub J, Tofler G, Hwang SJ, Meigs JB, Levy D, Wang TJ, Jacques PF, Benjamin EJ, Vasan RS: A multi-marker approach to predict incident CKD and microalbuminuria. J Am Soc Nephrol 21: 2143–2149, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terata S, Kikuya M, Satoh M, Ohkubo T, Hashimoto T, Hara A, Hirose T, Obara T, Metoki H, Inoue R, Asayama K, Kanno A, Totsune K, Hoshi H, Satoh H, Sato H, Imai Y: Plasma renin activity and the aldosterone-to-renin ratio are associated with the development of chronic kidney disease: the Ohasama Study. J Hypertens 30: 1632–1638, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Wollesen F, Brattström L, Refsum H, Ueland PM, Berglund L, Berne C: Plasma total homocysteine and cysteine in relation to glomerular filtration rate in diabetes mellitus. Kidney Int 55: 1028–1035, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Veldman BA, Vervoort G, Blom H, Smits P: Reduced plasma total homocysteine concentrations in type 1 diabetes mellitus is determined by increased renal clearance. Diabet Med 22: 301–305, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Wesson DE, Simoni J, Broglio K, Sheather S: Acid retention accompanies reduced GFR in humans and increases plasma levels of endothelin and aldosterone. Am J Physiol Renal Physiol 300: F830–F837, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Winocour PH, Waldek S, Baker R: Relationships between renin, aldosterone, blood pressure and renal function in hypertensive insulin-dependent diabetes mellitus. Diabetes Res Clin Pract 7: 99–108, 1989 [DOI] [PubMed] [Google Scholar]
- 42.Roldán J, Morillas P, Castillo J, Andrade H, Guillén S, Núñez D, Quiles J, Bertomeu V: Plasma aldosterone and glomerular filtration in hypertensive patients with preserved renal function. Rev Esp Cardiol 63: 103–106, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Dumic J, Dabelic S, Flögel M: Galectin-3: an open-ended story. Biochim Biophys Acta 1760: 616–635, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Nieminen J, Kuno A, Hirabayashi J, Sato S: Visualization of galectin-3 oligomerization on the surface of neutrophils and endothelial cells using fluorescence resonance energy transfer. J Biol Chem 282: 1374–1383, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Ochieng J, Furtak V, Lukyanov P: Extracellular functions of galectin-3. Glycoconj J 19: 527–535, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Iacobini C, Menini S, Oddi G, Ricci C, Amadio L, Pricci F, Olivieri A, Sorcini M, Di Mario U, Pesce C, Pugliese G: Galectin-3/AGE-receptor 3 knockout mice show accelerated AGE-induced glomerular injury: Evidence for a protective role of galectin-3 as an AGE receptor. FASEB J 18: 1773–1775, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Bullock SL, Johnson TM, Bao Q, Hughes RC, Winyard PJ, Woolf AS: Galectin-3 modulates ureteric bud branching in organ culture of the developing mouse kidney. J Am Soc Nephrol 12: 515–523, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Winyard PJ, Bao Q, Hughes RC, Woolf AS: Epithelial galectin-3 during human nephrogenesis and childhood cystic diseases. J Am Soc Nephrol 8: 1647–1657, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Nishiyama J, Kobayashi S, Ishida A, Nakabayashi I, Tajima O, Miura S, Katayama M, Nogami H: Up-regulation of galectin-3 in acute renal failure of the rat. Am J Pathol 157: 815–823, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pugliese G, Pricci F, Iacobini C, Leto G, Amadio L, Barsotti P, Frigeri L, Hsu DK, Vlassara H, Liu FT, Di Mario U: Accelerated diabetic glomerulopathy in galectin-3/AGE receptor 3 knockout mice. FASEB J 15: 2471–2479, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Henderson NC, Sethi T: The regulation of inflammation by galectin-3. Immunol Rev 230: 160–171, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Sasaki S, Bao Q, Hughes RC: Galectin-3 modulates rat mesangial cell proliferation and matrix synthesis during experimental glomerulonephritis induced by anti-Thy1.1 antibodies. J Pathol 187: 481–489, 1999 [DOI] [PubMed] [Google Scholar]
- 53.Maeda N, Kawada N, Seki S, Arakawa T, Ikeda K, Iwao H, Okuyama H, Hirabayashi J, Kasai K, Yoshizato K: Stimulation of proliferation of rat hepatic stellate cells by galectin-1 and galectin-3 through different intracellular signaling pathways. J Biol Chem 278: 18938–18944, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Lippert E, Falk W, Bataille F, Kaehne T, Naumann M, Goeke M, Herfarth H, Schoelmerich J, Rogler G: Soluble galectin-3 is a strong, colonic epithelial-cell-derived, lamina propria fibroblast-stimulating factor. Gut 56: 43–51, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weigert J, Neumeier M, Wanninger J, Bauer S, Farkas S, Scherer MN, Schnitzbauer A, Schäffler A, Aslanidis C, Schölmerich J, Buechler C: Serum galectin-3 is elevated in obesity and negatively correlates with glycosylated hemoglobin in type 2 diabetes. J Clin Endocrinol Metab 95: 1404–1411, 2010 [DOI] [PubMed] [Google Scholar]
- 56.Glinsky VV, Raz A: Modified citrus pectin anti-metastatic properties: One bullet, multiple targets. Carbohydr Res 344: 1788–1791, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kolatsi-Joannou M, Price KL, Winyard PJ, Long DA: Modified citrus pectin reduces galectin-3 expression and disease severity in experimental acute kidney injury. PLoS ONE 6: e18683, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP: An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 110: 281–290, 1979 [DOI] [PubMed] [Google Scholar]
- 59.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rifkin DE, Shlipak MG, Katz R, Fried LF, Siscovick D, Chonchol M, Newman AB, Sarnak MJ: Rapid kidney function decline and mortality risk in older adults. Arch Intern Med 168: 2212–2218, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shlipak MG, Katz R, Kestenbaum B, Siscovick D, Fried L, Newman A, Rifkin D, Sarnak MJ: Rapid decline of kidney function increases cardiovascular risk in the elderly. J Am Soc Nephrol 20: 2625–2630, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D: Predictors of new-onset kidney disease in a community-based population. JAMA 291: 844–850, 2004 [DOI] [PubMed] [Google Scholar]
- 63.National Kidney Foundation : K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 64.Hsu CC, Brancati FL, Astor BC, Kao WH, Steffes MW, Folsom AR, Coresh J: Blood pressure, atherosclerosis, and albuminuria in 10,113 participants in the atherosclerosis risk in communities study. J Hypertens 27: 397–409, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Culleton BF, Larson MG, Parfrey PS, Kannel WB, Levy D: Proteinuria as a risk factor for cardiovascular disease and mortality in older people: A prospective study. Am J Med 109: 1–8, 2000 [DOI] [PubMed] [Google Scholar]
- 66.Christenson RH, Duh SH, Wu AH, Smith A, Abel G, deFilippi CR, Wang S, Adourian A, Adiletto C, Gardiner P: Multi-center determination of galectin-3 assay performance characteristics: Anatomy of a novel assay for use in heart failure. Clin Biochem 43: 683–690, 2010 [DOI] [PubMed] [Google Scholar]
- 67.O’Seaghdha CM, Lyass A, Massaro JM, Meigs JB, Coresh J, D’Agostino RB, Sr, Astor BC, Fox CS: A risk score for chronic kidney disease in the general population. Am J Med 125: 270–277, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Seaghdha CM, Hwang SJ, Upadhyay A, Meigs JB, Fox CS: Predictors of incident albuminuria in the Framingham Offspring cohort. Am J Kidney Dis 56: 852–860, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS: Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med 27: 157–172; discussion 207–112, 2008 [DOI] [PubMed] [Google Scholar]
- 70.Pencina MJ, D’Agostino RB, Sr, Steyerberg EW: Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 30: 11–21, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]