Abstract
A histopathological classification system for ANCA-associated vasculitis was recently published, but whether this system predicts renal outcome requires validation. Here, we analyzed data from 164 consecutive patients with biopsy-proven renal involvement of ANCA-associated vasculitis. The ANCA-associated GN (AGN) classification categorizes patients as having focal, mixed, crescentic, or sclerotic GN. Five-year renal survival rates by categories of the AGN classification scheme were 91% for focal, 69% for mixed, and 64% for crescentic (log-rank P<0.0001). Only one patient was classified as sclerotic. Furthermore, the percentage of normal glomeruli found on biopsy estimated renal survival with the same precision as did the AGN classification scheme. Patients classified as crescentic or mixed, however, had worse survival when the percentage of normal glomeruli was <25%. In conclusion, the AGN classification for renal biopsy specimens is a practical and informative scheme with which to categorize patients with ANCA-associated vasculitis, but adding the percentage of normal glomeruli to the system seems to improve its predictive value.
Necrotizing crescentic GN is a common feature in ANCA-associated vasculitis (AAV).1 Histologically, renal lesions in AAV are characterized by cellular crescents, fibrinoid necrosis, and interstitial inflammation. Recently, an international vasculitis working group proposed a histopathologic classification of GN in patients with AAV to assess its predictive value for renal survival.2
To validate the ANCA-associated GN classification system (AGN classification), we scored all AAV renal biopsy specimens from patients with AAV who participated in the Limburg Renal Registry, a prospective renal biopsy study on glomerular diseases.3,4 The database was searched for patients with pauci-immune necrotizing crescentic GN.5
Two hundred twenty-one consecutive patients who underwent renal biopsy between January 1, 1979, and August 31, 2011, in the province of Limburg, The Netherlands, were identified as having pauci-immune necrotizing crescentic GN. Eight of these patients were excluded for concomitant renal disease (six with diabetic nephropathy and two with thin glomerular basement membrane nephropathy). Forty-nine patients were excluded because <10 glomeruli were found in the renal biopsy specimen.2
Thus, 164 patients with a mean age ± SD of 61.0±14.6 years were included (113 men and 52 women) with a mean follow-up of 8.5 years (range, 1 day–33 years). Eighty-three patients were positive for proteinase-3 ANCA and 81 were positive for myeloperoxidase (MPO) ANCA. Mean baseline serum creatinine was 349.7 ± 242.6 µmol/L, and median baseline proteinuria was 1.3 g per 24 hours (range, 0–11).
Before 2000, patients received corticosteroids in combination with oral cyclophosphamide. Since 2000, all patients have been treated according to the European League Against Rheumatism (EULAR) guidelines:5 induction therapy with steroids and oral cyclophosphamide, 2 mg/kg per day, over 3–6 months and maintenance therapy with azathioprine and low-dose corticosteroids.6 Since 2009, induction therapy consisted of corticosteroids with intravenous cyclophosphamide at a dose of 15 mg/kg per cycle over three to six pulses with 2-week intervals, or with oral cyclophosphamide.7 Patients with a serum creatinine >500 μmol/L or alveolar lung hemorrhage at the time of renal biopsy were considered to have severe or life-threatening vasculitis and received 1000 mg of prednisolone per day for 3 days or plasma exchange in addition to the standard treatment as described above.5
Eighty-one (49.4%) biopsy specimens were classified as focal, 43 (26.2%) as crescentic, and 39 (23.8%) as mixed. Only one biopsy specimen was classified as sclerotic (i.e., >50% sclerotic glomeruli). Baseline characteristics (at the time of renal biopsy) are presented in Table 1.
Table 1.
Baseline characteristics according to ANCA-associated glomerulonephritis (AGN) histologic classification
| Characteristic at Time of Renal Biopsy | Focal (n=81) | Crescentic (n=43) | Mixed (n=39) | Sclerotic (n=1) | All (n=164) |
|---|---|---|---|---|---|
| Age | 60.1±15.6 | 62.0±14.4 | 61.5±13.1 | 75.9 | 60.9±14.6 |
| Men/women (n/n) | 54/27 | 33/10 | 25/14 | 1 (male) | 113/51 |
| Histologic features (%) | – | – | – | – | – |
| Normal | 72.9±15.6 | 17.6±11.9 | 29.7±13.6 | 33.3 | 48.0±28.9 |
| Cellular crescents | 17.2±10.2 | 66.7±29.5 | 30.6±13.4 | 16.7 | 33.5±20.9 |
| Obliterated | 4.6±7.0 | 7.5±10.2 | 15.0±13.6 | 50 | 7.9±10.6 |
| Serum creatinine | 269.8±210.3 | 487.0±249.5 | 363.4±235.3 | 375.0 | 349.5±243.3 |
| eGFR | 39.3±29.4 | 16.8±14.7 | 24.3±19.5 | 14.6 | 29.7±25.8 |
| Proteinuria | 0.7 (0.1–10.5) | 1.3 (0.1–8.7) | 2.0 (0.2–11.0) | 2.4 | 1.3 (0–11) |
| MPO/PR3 ANCA | 49/32 | 17/26 | 16/23 | 0/1 | 81/83 |
Values expressed with a plus/minus sign are the mean ± SD. PR3, proteinase-3.
The 5-year renal survival rates (censored for death) per classification group were 91% for the focal group, 64% for the crescentic group, and 69% for the mixed group (log-rank analysis P<0.0001) (Figure 1). Renal survival did not significantly differ between the crescentic and the mixed groups (P=0.64).
Figure 1.
Renal survival, as shown by AGN classification, is best in the focal group (log rank analysis P<0.0001). The sclerotic group was left out because it consisted of only one patient.
Data on renal function during follow-up were available from 96 patients who had not died (n=37), were not dependent on renal replacement therapy (n=16), and were not lost to follow-up (n=14). At 1-year follow-up, mean estimated GFRs (eGFRs) were 54.5±20.9 ml/min per 1.73 m2 in the focal group (n=56), 41.0±21.1 ml/min per 1.73 m2 in the crescentic group (n=17), and 36.7±18.6 ml/min per 1.73 m2 in the mixed group (n=23) (focal versus crescentic, P=0.02; focal versus mixed, P=0.007; crescentic versus mixed, P=0.41). eGFR data at 2 years of follow-up were available from 83 patients: 53.5±20.8 ml/min per 1.73 m2 in the focal group (n=54) , 38.8±22.3 ml/min per 1.73 m2 in the crescentic group (n=12), and 38.3±16.0 ml/min per 1.73 m2 in the mixed group (n=17) (focal versus crescentic, P=0.03; focal versus mixed, P=0.007; crescentic versus mixed, P=0.95).
The 1- and 5-year patient survival rates were 82.9% and 73.1% for the focal group, 61.5% and 52.3% for the crescentic group, and 87.8% and 68.3% for the mixed group (P=0.06).
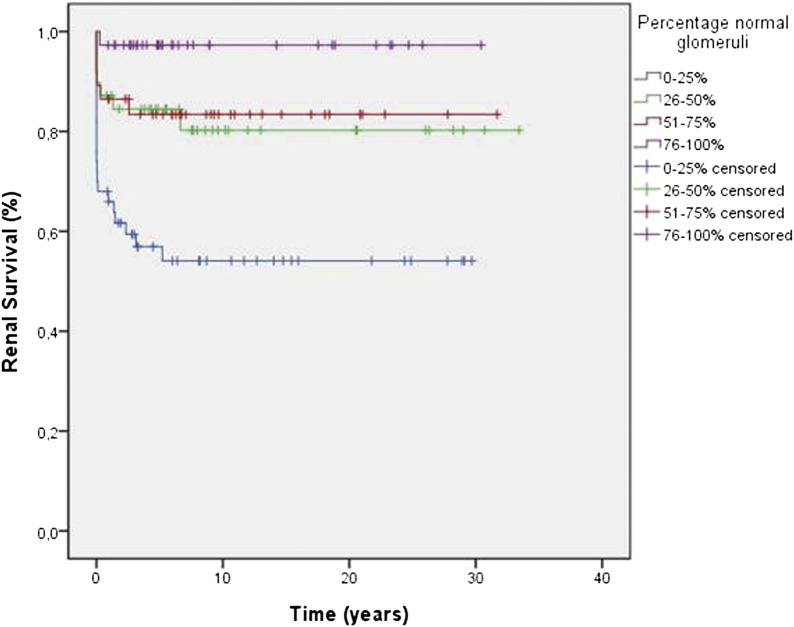
When renal biopsy specimens were grouped according to the percentage of normal glomeruli, we found 5-year renal survival rates of 93.2% for the group with >75% normal glomeruli, 81.0% for the group with 50%–75% normal glomeruli, 80.7% for patients with 25%–50% normal glomeruli, and 57.8% for the group with <25% normal glomeruli (log rank analysis P<0.0001) (Figure 2). Importantly, renal survival was significantly worse in patients classified as crescentic or mixed when the percentage of normal glomeruli in the renal biopsy was <25% (P=0.04) (Figure 3).
Figure 2.
Renal survival, as shown by percentage of normal glomeruli in the renal biopsy specimen, is best in patients with ≥75% and worst in patients with ≤25% normal glomeruli in the renal biopsy specimen (log rank analysis P<0.0001).
Figure 3.
Renal survival of patients classified as crescentic and mixed by the AGN classification is worse in patients with <25% normal glomeruli (log rank analysis P=0.04).
We confirmed the study by Berden et al. by showing that patients with a renal biopsy specimen classified as focal GN had the best renal survival. Several important differences between our study and that of Berden et al.2 were observed.
First, only one patient could be classified in the sclerotic group. For all other biopsy specimens, the percentage of sclerotic glomeruli was <50%. In contrast, Berden et al. classified 13 of their 100 patients (13%) in the sclerotic group. In our study, we sought to make an early diagnosis of GN in patients with erythrocyturia and proteinuria,3,4 possibly resulting in fewer sclerotic glomeruli.
Of note, however, our patient population was similar to the patients in Berden and colleagues' study in terms of age and distribution of proteinase-3 ANCA versus MPO ANCA.2
Second, patients who were classified in the crescentic group had a similar renal survival compared with patients in the mixed group. In contrast, Berden et al. found a better renal survival in patients in the crescentic group than in the mixed group. Our finding that mixed and crescentic patients had similar renal outcomes was true not only for patients treated before 2000, when EULAR guidelines for treatment were not yet available, but also for patients treated after 2000, when plasma exchange, in addition to cyclophosphamide and oral steroids, was introduced in Limburg for the most severely affected patients.5 Recently, Chang et al. also found that renal outcome of mixed patients and crescentic patients was similar.8 Most patients in their study were MPO ANCA positive. This finding differs from the study of Berden et al.2 and our study, demonstrating that the AGN classification system has predictive value irrespective of the ANCA phenotype.
As shown in the past, the percentage of normal glomeruli strongly predicts renal survival.9 Indeed, in our patients who were classified in the crescentic group, a somewhat lower percentage of normal glomeruli was found compared with the mixed group: 17.6% normal glomeruli and 29.7%, respectively. This probably explains the difference between our study and the one by Berden et al.
Most important, patients classified as crescentic and mixed in our study had a significantly worse renal survival when the percentage of normal glomeruli was <25%. Therefore, we suggest that renal pathologists mention the specific percentage of normal glomeruli found in the renal biopsy specimen in addition to classification into one of the four AGN categories. A biopsy sample would then, for example, be described as follows: “crescentic, 30% normal glomeruli.”
The AGN classification is based on glomerular features only. Interstitial features, however, have been included in earlier histopathologic classifications.9 Recently, Berden et al.10 showed that tubular atrophy and tubulitis predict eGFR at 12 months in patients with ANCA-associated GN treated with a rituximab-based regimen. This finding indicates that interstitial changes in the renal biopsy specimen may have predictive value in addition to glomerular features.
In summary, we confirmed that the AGN classification system is a useful tool with a good predictive value for renal survival. Importantly, the nephropathologist can optimize the system by mentioning the specific percentage of normal glomeruli in the biopsy specimen.
Concise Methods
Baseline criteria from patients were proteinuria, serum creatinine levels, and ANCA status,11 measured at the time of renal biopsy. ANCA was measured by immunofluorescence and by ELISA.11 ANCA was measured retrospectively in patients included before 1989, when routine measurement of ANCA was introduced in our center. An exclusion criterion was the presence of <10 glomeruli per slide.2 Patients were also excluded if they had another renal disease in addition to ANCA-associated GN. Renal biopsy specimens were processed for light microscopy and immunofluorescence as previously described.3,4 Biopsy specimens were termed pauci-immune when immunofluorescence was 2+ or less (on a scale of 0–4+) for any immunoglobulin or complement.12
Renal biopsy specimens were scored independently and blinded from clinical information by two observers (P.V.B.V. and M.H.). When no agreement was reached between the two observers, interpretation was resolved with a third observer (P.V.P.). Per biopsy, at least five levels of (2-μm) section were analyzed. Biopsy specimens were classified using the AGN classification into four groups: focal (>50% glomeruli on the slide appear normal), crescentic (>50% of glomeruli contain cellular crescents), sclerotic (>50% of glomeruli are sclerotic), and mixed (specimen does not fit in the other groups).2
Disclosures
None.
Acknowledgments
We thank H. van Rie, N. Bijnens, and P. Heerings-Rewinkel (Laboratory of Clinical Immunology, Maastricht University Medical Center, Maastricht) for assistance. We also thank all participating nephrologists of the Limburg Renal Registry: F. de Heer, M.M.E. Krekels, G.H. Verseput (Orbis Medisch Centrum, Sittard), S. Boorsma, W. Grave, J. Wirtz, J. Huitema (St. Laurentiusziekenhuis, Roermond), W.E. Boer, L.A.M. Frenken, J. Wolters (Atrium Medisch Centrum, Heerlen).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Bajema IM, Hagen EC, Hansen BE, Hermans J, Noël LH, Waldherr R, Ferrario F, van der Woude FJ, Bruijn JA: The renal histopathology in systemic vasculitis: An international survey study of inter- and intra-observer agreement. Nephrol Dial Transplant 11: 1989–1995, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, Neumann I, Noël LH, Pusey CD, Waldherr R, Bruijn JA, Bajema IM: Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol 21: 1628–1636, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Tiebosch AT, Wolters J, Frederik PF, van der Wiel TW, Zeppenfeldt E, van Breda Vriesman PJ: Epidemiology of idiopathic glomerular disease: a prospective study. Kidney Int 32: 112–116, 1987 [DOI] [PubMed] [Google Scholar]
- 4.van Paassen P, van Breda Vriesman PJ, van Rie H, Tervaert JW: Signs and symptoms of thin basement membrane nephropathy: A prospective regional study on primary glomerular disease — The Limburg Renal Registry. Kidney Int 66: 909–913, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Mukhtyar C, Guillevin L, Cid MC, Dasgupta B, de Groot K, Gross W, Hauser T, Hellmich B, Jayne D, Kallenberg CG, Merkel PA, Raspe H, Salvarani C, Scott DG, Stegeman C, Watts R, Westman K, Witter J, Yazici H, Luqmani R, European Vasculitis Study Group : EULAR recommendations for the management of primary small and medium vessel vasculitis. Ann Rheum Dis 68: 310–317, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Slot MC, Tervaert JW, Franssen CF, Stegeman CA: Renal survival and prognostic factors in patients with PR3-ANCA associated vasculitis with renal involvement. Kidney Int 63: 670–677, 2003 [DOI] [PubMed] [Google Scholar]
- 7.de Groot K, Harper L, Jayne DR, Flores Suarez LF, Gregorini G, Gross WL, Luqmani R, Pusey CD, Rasmussen N, Sinico RA, Tesar V, Vanhille P, Westman K, Savage CO, EUVAS (European Vasculitis Study Group) : Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: A randomized trial. Ann Intern Med 150: 670–680, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Chang DY, Wu LH, Liu G, Chen M, Kallenberg CG, Zhao MH: Re-evaluation of the histopathologic classification of ANCA-associated glomerulonephritis: A study of 121 patients in a single center. Nephrol Dial Transplant 27: 2343–2349, 2012 [DOI] [PubMed] [Google Scholar]
- 9.de Lind van Wijngaarden RA, Hauer HA, Wolterbeek R, Jayne DR, Gaskin G, Rasmussen N, Noël LH, Ferrario F, Waldherr R, Hagen EC, Bruijn JA, Bajema IM: Clinical and histologic determinants of renal outcome in ANCA-associated vasculitis: A prospective analysis of 100 patients with severe renal involvement. J Am Soc Nephrol 17: 2264–2274, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Berden AE, Jones RB, Erasmus DD, Walsh M, Noël LH, Ferrario F, Waldherr R, Bruijn JA, Jayne DR, Bajema IM, European Vasculitis Society : Tubular lesions predict renal outcome in antineutrophil cytoplasmic antibody-associated glomerulonephritis after rituximab therapy. J Am Soc Nephrol 23: 313–321, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Damoiseaux JGMC, Slot MC, Vaessen M, Stegeman CA, Van Paassen P, Tervaert JW: Evaluation of a new fluorescent-enzyme immuno-assay for diagnosis and follow-up of ANCA-associated vasculitis. J Clin Immunol 25: 202–208, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Falk RJ, Jennette JC: ANCA small-vessel vasculitis. J Am Soc Nephrol 8: 314–322, 1997 [DOI] [PubMed] [Google Scholar]