Abstract
The in vitro activities of piperacillin (PIP) against β-lactamase-negative ampicillin (AMP)-resistant (BLNAR) Haemophilus influenzae were compared with those of cefotaxime (CTX) and ceftriaxone (CRO), and the potency of PIP as therapy for meningitis caused by BLNAR is also discussed. PIP showed good activity (MIC at which 90% of strains are inhibited, 0.25 μg/ml) against 69 BLNAR strains, and its activity was comparable to that of CRO and superior to that of CTX. No significant correlation was observed between the MICs of PIP and CTX or CRO or AMP, whereas a high correlation was observed between the MICs of CTX and CRO. In the killing study, PIP showed potent bactericidal activity compared with those of CTX and CRO. By microscopic examination, PIP caused the formation of a spindle and short filamentous cells with bulges and induced cell lysis in BLNAR strains, while treatment with CTX and CRO resulted in the formation of large, spherical cells without any obvious lysis. The affinity of Bocillin FL, a fluorescent penicillin used for determination of the 50% inhibitory concentration (IC50s) for penicillin-binding proteins (PBPs), to PBPs 3a and 3b of BLNAR strains was drastically decreased compared with that to an AMP-susceptible strain (ATCC 33391). In the case of the BLNAR strains, the IC50s for PBPs 1a, 1b, and 2 were similar to those for the PBPs of ATCC 33391. Since the affinity of binding to PBPs 3a and 3b of the BLNAR strains decreased drastically, the second targets among the PBPs were PBP 2 for PIP, PBP1 (1a and 1b) for CTX and CRO. In conclusion, PIP showed excellent activities against BLNAR strains in a manner different from those of cephem antibiotics, suggesting that it could be a candidate therapeutic agent for the treatment of meningitis caused by BLNAR strains.
Haemophilus influenzae is one of the most significant causes of respiratory tract infection, acute otitis media, pneumonia, and purulent meningitis. Meningitis, especially in infants, young children, and the elderly, is the most serious and life-threatening clinical manifestation of invasive disease caused by H. influenzae.
Although the H. influenzae type b vaccine has been introduced in many countries, its use has not been prevalent in Japan, and H. influenzae is still the most common cause of bacterial meningitis in infants in Japan (12). The prevalence of ampicillin (AMP) resistance among H. influenzae strains has been the subject of multinational surveillance studies in several countries, and the overall rate of resistance has been reported to be between 10 and 60% (1, 3, 5, 6, 10, 19, 22). In North America, approximately 20 to 40% of H. influenzae isolates are resistant to AMP by virtue of their ability to produce β-lactamases (7, 9, 10, 15, 23, 24, 28). β-Lactamase-negative AMP-resistant (BLNAR) H. influenzae seems to be relatively uncommon, as shown by recent national and multinational surveillance studies (5, 19).
Recently, the numbers of BLNAR H. influenzae strains have been increasing in some countries (11, 14, 17). It is difficult to compare the prevalence of these strains among studies because of methodological differences among the studies and because the level of AMP resistance is often borderline. However, various reports have indicated that the numbers of BLNAR H. influenzae strains have increased rapidly in Japan (20, 25, 30). Ubukata et al. (30) reported that the proportion of BLNAR clinical isolates (categorized according to mutations in the ftsI gene and β-lactam MICs) had rapidly increased to 28.8%, according to nationwide surveillance studies conducted in 1997 and 1998 in Japan. These surveillance studies also revealed that 35% of the strains from patients with meningitis are BLNAR (30).
Several antibiotics show potent activities against H. influenzae. However, the choice of chemotherapeutic agents for meningitis has been restricted because of the limitation of drug use for infants or strong toxicity against the central nervous system.
AMP, cefotaxime (CTX), ceftriaxone (CRO), chloramphenicol, and their combinations are recommended as the standard therapy for meningitis in Japan. β-Lactam resistance in H. influenzae is now a clinical concern, since β-lactams such as AMP are important agents for chemotherapy for meningitis.
The mechanism of resistance of BLNAR H. influenzae is thought to be a decreased affinity of AMP to the penicillin-binding proteins (PBPs) involved in peptidoglycan synthesis (17). If the reduced affinity of AMP to PBPs influences the activities of other β-lactams, as shown in methicillin-resistant Staphylococcus aureus or penicillin-resistant Streptococcus pneumoniae, it was thought that the reduced affinities of AMP to PBPs resulted in cross-resistance to cephems. However, it was reported that there was no or little reduction in the antibacterial activities of some β-lactams, including piperacillin (PIP), against BLNAR H. influenzae strains compared to those of the expanded-spectrum cephalosporins (16).
In the present study, we report on the in vitro antibacterial activities of PIP compared with those of CTX and CRO, both of which are standard agents for the treatment of meningitis, and also discuss the potency of PIP for the treatment of meningitis caused by BLNAR H. influenzae strains.
MATERIALS AND METHODS
Bacteria and antimicrobials.
Sixty-nine BLNAR H. influenzae strains for which AMP and AMP-sulbactam MICs were ≥2 μg/ml were used in this study. All strains were defined as β-lactamase negative by the use of β-lactamase nitrocefin identification sticks (Oxoid Ltd., Hampshire, United Kingdom). The BLNAR strains were isolated from clinical specimens from 1999 to 2001 and were stored at −130°C in skim milk (Difco Laboratories, Detroit, Mich.) until use.
The following commercially available antibacterial agents were used: AMP (Sigma, St. Louis, Mo.), PIP (Toyama Chemical Co., Ltd, Tokyo, Japan), CTX (Nippon Hoechst Marion Roussel, Tokyo, Japan), and CRO (Roche Japan, Tokyo, Japan).
Susceptibility testing.
The MICs were determined by the broth microdilution method in Mueller-Hinton broth supplemented with 15 μg of hematin and β-NAD per ml (HTM broth), as recommended by the National Committee for Clinical Laboratory Standards (NCCLS) (18). H. influenzae ATCC 49247 and ATCC 49766 were used as control organisms. Since there are no NCCLS quality control ranges for the testing of PIP with H. influenzae, approved quality control ranges for PIP-tazobactam were used instead to verify the potency of PIP. Cells from overnight HTM agar plates were suspended in sterile saline to achieve a turbidity equivalent to that of a 0.5 McFarland standard. The wells were inoculated with approximately 5 × 105 CFU/ml, and the plates were incubated in ambient air at 37°C for 24 h. The lowest drug concentration showing no growth was read as the MIC. To evaluate the cross-resistance of the 69 BLNAR strains, the correlation coefficient (r) of the MICs between two drugs was examined by the Spearman rank correlation.
Bactericidal activity.
Time-kill studies with AMP-susceptible H. influenzae ATCC 33391 and a typical BLNAR strain (strain I-1053) were performed with eight times the MICs of PIP, CTX, and CRO. The MICs of AMP, PIP, CTX, and CRO were 0.25, 0.0313, 0.0078, and 0.002 μg/ml, respectively, for H. influenzae ATCC 33391 and 8, 0.0625, 2, and 0.25 μg/ml, respectively, for BLNAR strain I-1053. Microtiter plates containing 100 μl of HTM broth (freshly prepared, as described above) in each well with eight times the MIC of each antibiotic were inoculated with approximately 106 CFU/ml, and the plates were incubated at 37°C. Three wells (each containing 100 μl of broth) were prepared for each sampling point, and 40 μl of the culture from each well was serially diluted with saline and plated on an HTM agar plate to determine the viable cell counts at selected times (6, 12, and 24 h after inoculation). Recovery plates were incubated for up to 24 h.
The detection limit of the colony counts was 25 CFU/ml. The antibiotics were considered bactericidal when they reduced the original inoculum by >3 log10 CFU/ml (99.9%) at each time point, and the antibiotics were considered bacteriostatic when the inoculum was reduced by 0 to 3 log10 CFU/ml.
Morphological changes in the cells.
Cells treated with eight times the MICs of PIP, CTX, and CRO for 6 h at 37°C in HTM broth were examined microscopically with a differential interference-contrast microscope (NTF2; Nikon, Tokyo, Japan).
Detection of PBPs and IC50 determinations.
The detection of PBPs in H. influenzae was carried out by a nonradioactive method with commercially available Bocillin FL, a fluorescent penicillin, as a labeling reagent for the detection and study of PBPs (32). H. influenzae ATCC 33391 (AMP susceptible) and BLNAR strain I-1053 were used for the preparation of membranes for the detection of PBPs. Both strains were grown in HTM broth at 37°C overnight. The overnight cultures (2 ml each) were inoculated into 200 ml of fresh HTM broth, allowed to grow to logarithmic growth phase, and harvested by centrifugation at 6,000 × g for 15 min. The cells were washed once with 1/15 M potassium phosphate (pH 7.0), resuspended in the same buffer, and disrupted with an ultrasonic disrupter (model UR-200P; Tomy Seiko Co., Ltd., Tokyo, Japan). The resulting cell lysates were centrifuged at 15,000 × g for 30 min. The supernatant fractions were collected and centrifuged at 100,000 × g for 30 min. The pellets were collected, washed once, and resuspended in the same phosphate buffer. The resulting suspensions were designated membrane preparations and were used for the fluorescent Bocillin FL binding assays. The protein concentrations of the membrane preparations were determined by a protein assay (Bio-Rad Laboratories, Hercules, Calif.) with bovine serum albumin as the standard and were adjusted to 5 mg/ml.
For detection of the 50% inhibitory concentrations (IC50s) for the PBPs, the reaction mixtures (60 μl each), which contained 35 μl of each membrane preparation (≈200 μg of protein), 20 μl of 50 μM (final concentration) Bocillin FL, and 5 μl of various amounts of antibiotics, were incubated at 37°C for 30 min. Then, 2 μl of 20% sodium sarcosine, including 180 mg of penicillin G per ml, was added to the reaction mixture and the mixture was centrifuged at 10,000 × g for 30 min. Twenty microliters of each of the resulting supernatants was denatured with 20 μl of sodium dodecyl sulfate denaturing solution at 100°C for 3 min. Then, 10 μl of each reaction mixture was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis (10% polyacrylamide gel; Daiichi Pure Chemicals Co., Ltd., Tokyo, Japan). After electrophoresis the gels were rinsed with water for 1 h. To visualize the labeled PBPs, the gels were directly scanned with a LAS-1000 plus Lite scanner (Fuji Film Co., Ltd., Tokyo, Japan). The fluorescence intensity of each band was quantified by using an Image Gauge (Fuji Film Co., Ltd.). The affinities of the β-lactams to the PBPs were expressed as IC50s, which represent the β-lactam concentration needed to cause 50% inhibition of Bocillin FL binding.
RESULTS
In vitro susceptibility.
Table 1 shows the MIC ranges, the MICs at which 50% of isolates are inhibited (MIC50s), and the MIC90s of the β-lactams for 69 BLNAR strains. The range of AMP MICs was narrow (2 to 8 μg/ml), whereas those of PIP, CTX, and CRO were wide and ranged from 0.0156 to 0.25 μg/ml for PIP, 0.0313 to 2 μg/ml for CTX, and 0.0078 to 0.25 μg/ml for CRO. When the correlation between the MICs of each drug for BLNAR strains were examined by the Spearman rank correlation, no significant correlation was observed between the MICs of AMP and PIP, CTX, or CRO (r < 0.5). In a similar manner, the MICs of PIP for BLNAR strains correlated poorly with those of CTX and CRO (r < 0.5). In contrast, the CTX MICs for BLNAR strains increased notably with the increase in the CRO MICs and showed a high correlation to those of CRO. The correlation coefficient was 0.86 (i.e., r > 0.5) (Table 2).
TABLE 1.
Activities of antibacterial agents against 69 clinical isolates of BLNAR H. influenzae
| Antimicrobial agent | MIC (μg/ml)
|
||
|---|---|---|---|
| Range | 50% | 90% | |
| AMP | 2-8 | 2 | 4 |
| PIP | 0.0156-0.25 | 0.0625 | 0.25 |
| CTX | 0.0313-2 | 0.5 | 1 |
| CRO | 0.0078-0.25 | 0.125 | 0.25 |
TABLE 2.
Spearman rank correlation coefficients between the MICs of AMP, PIP, CTX, and CRO for 69 BLNAR strains
| Antimicrobial agent | Correlation coefficient
|
|||
|---|---|---|---|---|
| AMP | PIP | CTX | CRO | |
| AMP | 1.00 | −0.043 | 0.21 | 0.20 |
| PIP | 1.00 | 0.39 | 0.29 | |
| CTX | 1.00 | 0.86 | ||
| CRO | 1.00 | |||
Time-kill study and morphological changes of the cells.
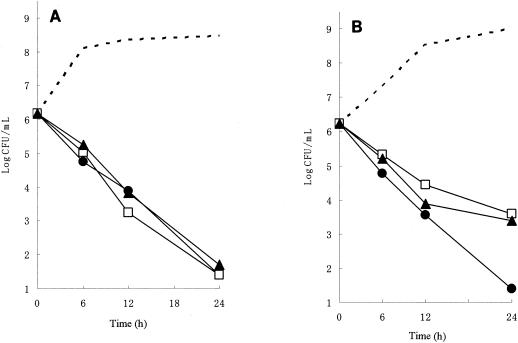
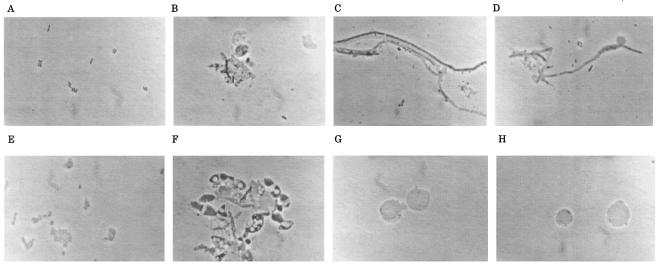
To investigate the differences in the modes of action of PIP, CTX, and CRO against H. influenzae, we performed time-kill studies and observed by microscopy cells treated with these agents. Figure 1 shows the time-kill patterns of PIP, CTX, and CRO at eight times the MICs for AMP-susceptible strain ATCC 33391 and BLNAR strain I-1053. Photomicrographs of H. influenzae cells treated with the same concentration for 6 h are shown in Fig. 2. In drug-free medium, the counts of both bacterial strains increased logarithmically from approximately 106 to 108 CFU/ml after 12 h of incubation. For strain ATCC 33391, all antibiotics achieved 99% killing after 12 h and 99.9% killing after 24 h. No significant differences in bactericidal activity were seen among these agents. By microscopic examination, PIP caused the formation of a spindle and short filamentous cells with bulges and induced cell lysis (Fig. 2B). On the other hand, filamentous cells were predominantly observed in cultures treated with CTX and CRO, and no cells were obviously lysed, as shown in Fig. 2C and D, respectively. For strain I-1053, PIP achieved 99% killing after 12 h and 99.9% killing after 24 h, similar to the case for strain ATCC 33391. PIP caused the formation of a spindle and short filamentous cells with bulges, similar to the case for strain ATCC 33391 (Fig. 2F). On the other hand, although CTX and CRO killed strain I-1053, with an approximately 99% reduction of the original inoculum after 12 h, the decrease in viable counts slowed and did not reach a 99.9% reduction even at 24 h after inoculation. Treatment with CTX and CRO resulted in the formation of large, spherical cells without any obvious lysis (Fig. 2G and H, respectively).
FIG. 1.
Time-kill studies with PIP (circles), CTX (squares), and CRO (triangles) against H. influenzae ATCC 33391 (A) and I-1053 (B). Viable cell counts were determined by the colony-counting method. Each drug was added to the culture at eight times the MIC at time zero. The viable cell counts at 0, 6, 12, and 24 h are represented by dashed lines without symbols.
FIG. 2.
Photomicrographs of H. influenzae ATCC 33391 (A to D) and I-1053 (E to H) treated with PIP (B and F), CTX (C and G), and CRO (D and H) at eight times the MIC for 6 h or not treated (A and E). Magnifications, ×1,000.
PBP binding.
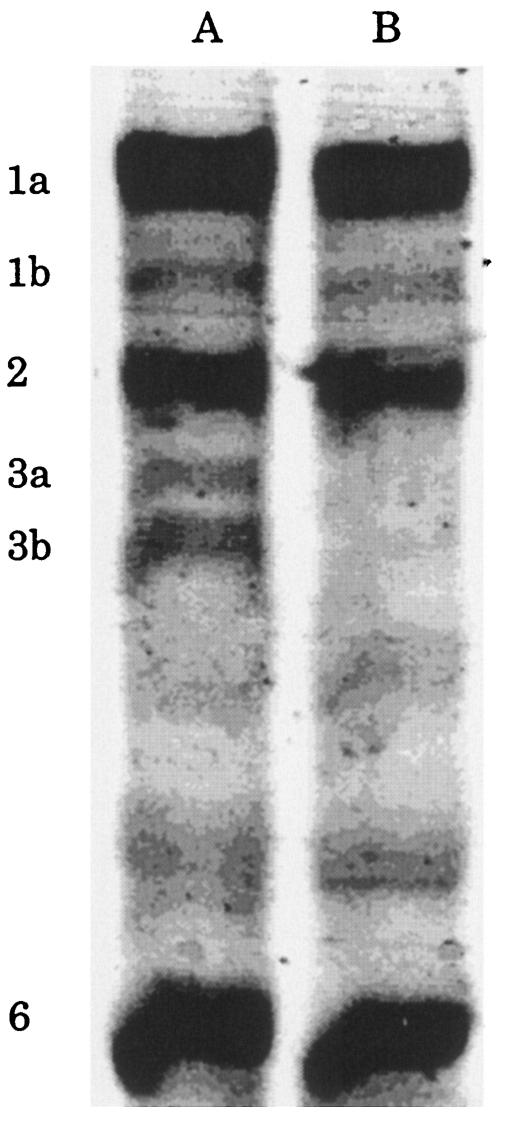
We then examined the relationship between the antimicrobial activities of these β-lactams and their affinities to PBPs. Figure 3 shows the PBP profiles of H. influenzae ATCC 33391 and BLNAR strain I-1053. PBPs 1a, 1b, 2, 3a, 3b, and 6 were detected by Bocillin FL in ATCC 33391; however, PBPs 4 and 5 were not. In the case of BLNAR strain I-1053, the affinity of Bocillin FL to PBPs 3a and 3b decreased drastically compared with those to the PBPs of ATCC 33391. The IC50s for these two PBPs of strain I-1053 could not be measured since the affinity of Bocillin FL to PBP 3a and PBP 3b was too weak to detect.
FIG. 3.
PBP profiles of H. influenzae ATCC 33391(lane A) and BLNAR I-1053 (lane B).
Table 3 shows the MICs and the IC50s of PIP, CTX, and CRO for the PBPs of H. influenzae ATCC 33391 and BLNAR strain I-1053. Among the PBPs of H. influenzae ATCC 33391, CTX and CRO were preferentially bound to PBP 3a (IC50s, 0.08 to 0.15 μM) and PBP 3b (IC50s, 0.04 to 0.19 μM) but showed a poor affinity for PBP 1b (IC50s, 10 to 11 μM), PBP 1a (IC50s, 33 to 80 μM), and PBP 2 (IC50s, >100 μM), in the indicated order of potency. PIP also showed a potent affinity of binding to PBP 3b (IC50, 0.18 μM) and PBP 3a (IC50, 0.34 μM), comparable to those of CTX and CRO, but it showed a high affinity of binding to PBP 2 (IC50, 2.3 μM). The affinities of PIP binding to PBP 1a (>100 μM) and PBP 1b (47 μM) were lower than those of CTX and CRO. In the case of BLNAR strain I-1053, the IC50s of each drug for PBPs 1a, 1b, and 2 were similar to those for the PBPs of ATCC 33391. Therefore, the PBPs of the BLNAR strain with the highest susceptibilities to the drugs were PBP 2 for PIP, PBP1 (1a and 1b) for CTX and CRO.
TABLE 3.
Affinities of PIP, CTX, and CRO to H. influenzae ATCC 33391 and BLNAR I-1053 PBPs
| Strain | Antimicrobial agent | MICa (μg/ml) | IC50 (μM) for PBP:
|
||||
|---|---|---|---|---|---|---|---|
| 1a | 1b | 2 | 3a | 3b | |||
| ATCC 33391 | PIP | 0.0313 | >100 | 47 | 2.3 | 0.34 | 0.18 |
| CTX | 0.0078 | 80 | 11 | >100 | 0.15 | 0.19 | |
| CRO | 0.002 | 33 | 10 | >100 | 0.08 | 0.04 | |
| I-1053 | PIP | 0.0625 | >100 | 71 | 3.4 | NDb | ND |
| CTX | 2 | 86 | 20 | >100 | ND | ND | |
| CRO | 0.25 | 16 | 19 | 97 | ND | ND | |
MICs were determined by the broth microdilution method.
ND, not detected.
DISCUSSION
In this study, the activities of PIP against clinical isolates of BLNAR H. influenzae and its bactericidal profiles were compared with those of CTX and CRO, which are standard therapeutic agents for meningitis.
PIP showed good activity (MIC90, 0.25 μg/ml) against BLNAR strains, and its activity was comparable to that of CRO and superior to that of CTX. Similarly, it has been reported in a surveillance study that the MIC90 of PIP was 0.25 μg/ml for BLNAR strains isolated from patients with respiratory tract infections in 1998 to 1999 in Japan and that PIP showed antibacterial activity superior to those of AMP-sulbactam, cefazolin, cefotiam, cefmetazole, cefozopran, flomoxef, and imipenem (16). Interestingly, a high correlation between susceptibility to CTX and CRO was observed, but no such correlation between susceptibility to AMP and those to PIP, CTX, and CRO or susceptibility to PIP and those to CTX and CRO was observed. Similar results indicating a small correlation between susceptibility to carbapenem and those to AMP, cephalosporins, and aztreonam have been reported (21).
It has been reported that inhibition of PBP 1a and/or PBP 1b in Escherichia coli is associated with rapid cell lysis (27, 31). However, there is some evidence that PBPs 2 and 3 have important roles in the cell lysis process (13, 29), suggesting that the mechanism of bacterial lysis induced by β-lactam antibiotics is uncertain. It has been reported that the mechanism of resistance of BLNAR H. influenzae strains is alterations of PBPs, especially decreased affinities to PBP 3a and PBP 3b (17, 30), which correspond to PBP 3 of E. coli. Our results also show that the main targets of CTX and CRO are PBPs 3a and 3b and that the reduced susceptibilities of BLNAR strains to these two drugs are related to the decreased affinities of the drugs to these PBPs.
In addition, the formation of L-form-like cells, that is, large, spherical cells, was observed in BLNAR H. influenzae strains after CTX and CRO treatment. Similarly, an osmotically stable L form of H. influenzae emerged with AMP, cephalothin, and penicillin treatment (2), suggesting that the emergence of L-form-like cells in BLNAR H. influenzae strains treated with CTX and CRO would be also caused by the inhibition of specific PBPs. It can easily be predicted that BLNAR H. influenzae cells were converted to the L form without death by the inhibition of peptidoglycan synthesis caused by the binding of CTX and CRO to PBPs 1a and 1b in a short period, and thereafter, killing of these cells was hardly affected by these agents. On the other hand, PIP preferentially bound to PBPs 3a and 3b, similar to the case for CTX and CRO, and also bound to PBP 2 at a concentration of eight times the MIC, unlike the two cephalosporins. The formation of the spindle and short filamentous cells with bulges after treatment with PIP could be caused by the inhibition of PBP 2 in both the AMP-susceptible strain and the BLNAR strain.
Inui et al. (8) reported that the killing and lytic activities caused by PIP were weaker than those caused by aspoxicillin and that H. influenzae cells treated with PIP showed a filamentous shape without the bulge and without lysis when they were treated with the drug at four times the MIC for 2 h, even though PIP showed potent binding affinities to PBPs 2, 3a, and 3b. This finding and our results suggest that PIP inhibits PBPs 3a and 3b mainly at low concentrations over a short time period but inhibits PBP 2 at a high concentration, in addition to its inhibition of PBPs 3a and 3b.
Shishido and Matsumoto (26) reported that the level of PIP in cerebrospinal fluid (CSF) samples ranged from 1.0 to 6.7 μg/ml in one patient given intermittent infusions of 74 mg/kg of body weight at 6-h intervals. In addition, the concentration of PIP in the CSF of patients with acute meningitis was found to reach one-third of the level maintained in serum by continuous infusion (4). From these observations, the concentration of PIP achieved was sufficient to kill the bacteria in CSF, suggesting that PIP would be useful for therapy of meningitis caused by BLNAR H. influenzae strains. At present, although CRO still has excellent activity against BLNAR strains, BLNAR strains resistant to CRO could easily be selected if the rates of resistance to CTX in BLNAR strains increase in the future.
In conclusion, PIP showed excellent activities against BLNAR strains, suggesting that it could be a candidate for the treatment of meningitis caused by BLNAR strains.
REFERENCES
- 1.Bijlmer, H. A., L. van Alphen, B. M. Greenwood, L. G. van den Broek, H. Á. Valkenburg, and J. Dankert. 1994. Antibiotic susceptibility of invasive and non-invasive isolates of Haemophilus influenzae from the Gambia, West Africa. J. Antimicrob. Chemother. 34:275-280. [DOI] [PubMed] [Google Scholar]
- 2.Bottone, E., Z. Brandman, and S. S. Schneierson. 1976. Spheroplasts of Haemophilus influenzae induced by cell wall-active antibiotics and their effect upon the interpretation of susceptibility tests. Antimicrob. Agents Chemother. 9:327-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campos, J., S. Garcia-Tornel, J. M. Gairi, and I. Fabregues. 1986. Multiple resistant Haemophilus influenzae type b causing meningitis: comparative clinical and laboratory study. J. Pediatr. 108:897-902. [DOI] [PubMed] [Google Scholar]
- 4.Dickinson, G. M., D. G. Droller, R. L. Greenman, and T. A. Hoffman. 1981. Clinical evaluation of piperacillin with observations on penetrability into cerebrospinal fluid. Antimicrob. Agents Chemother. 20:481-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doern, G. V., and the Alexander Project Collaborative Group. 1996. Antimicrobial resistance among lower respiratory tract isolates of Haemophilus influenzae: results of a 1992-93 Western Europe and USA collaborative surveillance study. J. Antimicrob. Chemother. 38:59-69. [DOI] [PubMed] [Google Scholar]
- 6.Doern, G. V., A. B. Brueggemann, G. Pierce, H. P. Holley, and A. Rauch, Jr. 1997. Antibiotic resistance among clinical isolates of Haemophilus influenzae in the United States in 1994 and 1995 and detection of β-lactamase-positive strains resistant to amoxicillin-clavulanate: results of a national multicenter surveillance study. Antimicrob. Agents Chemother. 41:292-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doern, G. V., R. N. Jones, M. A. Pfaller, and K. Kugler. 1999. Haemophilus influenzae and Moraxella catarrhalis from patients with community-acquired respiratory tract infections: antimicrobial susceptibility patterns from the SENTRY Antimicrobial Surveillance Program (United States and Canada, 1997). Antimicrob. Agents Chemother. 43:385-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inui, T., T. Endo, and T. Matsushita. 2000. Morphological changes and lysis induced by β-lactams associated with the characteristic profiles of affinities of penicillin-binding proteins in Actinobacillus pleuropneumoniae. Antimicrob. Agents Chemother. 44:1518-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs, M. R., S. Bajaksouzian, A. Zilles, G. Lin, G. A. Pankuch, and P. C. Appelbaum. 1999. Susceptibilities of Streptococcus pneumoniae and Haemophilus influenzae to 10 oral antimicrobial agents based on pharmacodynamic parameters: 1997 U.S. surveillance study. Antimicrob. Agents Chemother. 43:1901-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jorgensen, J. H., G. V. Doern, L. A. Maher, A. W. Howell, and J. A. Redding. 1990. Antimicrobial resistance among respiratory isolates of Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae in the United States. Antimicrob. Agents Chemother. 34:2075-2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jorgensen, J. H., L. A. Maher, and A. W. Howell. 1991. Activity of a new carbapenem antibiotic, meropenem, against Haemophilus influenzae strains with β-lactamase- and non-enzyme-mediated resistance to ampicillin. Antimicrob. Agents Chemother. 35:600-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamiya, H., S. Uehara, T. Kato, K. Shiraki, T. Togashi, T. Morishima, Y. Goto, O. Satoh, and S. M. Standaert. 1998. Childhood bacterial meningitis in Japan. Pediatr. Infect. Dis. J. 17:S183-S185. [DOI] [PubMed] [Google Scholar]
- 13.Malouin, F., and L. E. Bryan. 1988. Haemophilus influenzae penicillin-binding proteins 1a and 3 possess distinct and opposite temperature-modulated penicillin-binding activities. Antimicrob. Agents Chemother. 32:498-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manninen, R., P. Huovinen, A. Nissinen, and the Finnish Study Group for Antimicrobial Resistance. 1997. Increasing antimicrobial resistance in Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis in Finland. J. Antimicrob. Chemother. 40:387-392. [DOI] [PubMed] [Google Scholar]
- 15.Marco, F., J. Garcia-De-Lomas, C. Garcia-Rey, E. Bouza, L. Aguilar, and C. Fernandez-Mazarrasa. 2001. Antimicrobial Susceptibilities of 1, 730 Haemophilus influenzae Respiratory Tract Isolates in Spain in 1998-1999. Antimicrob. Agents Chemother. 45:3226-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuzaki, K., H. Koyama, K. Omika, M. Hasegawa, Y. Sato, I. Kobayashi, and A. Watanabe. 2000. Antibacterial activities of piperacillin for several resistant strains from respiratory infections—in reference to MRSA, PRSP, BLNAR and P. aeruginosa. Jpn. J. Antibiot. 53:566-572. [PubMed] [Google Scholar]
- 17.Mendelman, P. M., D. O. Chaffin, T. L. Stull, C. E. Rubens, K. D. Mack, and A. L. Smith. 1984. Characterization of non-beta-lactamase-mediated ampicillin resistance in Haemophilus influenzae. Antimicrob. Agents Chemother. 26:235-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. 1995. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. M7-A4. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 19.Nissinen, A., E. Herva, M. L. Katila, S. Kontiainen, O. L iimatainen, S. Oinonen, A. K. Takala, and P. Huovinen. 1995. Antimicrobial resistance in Haemophilus influenzae isolated from blood, cerebrospinal fluid, middle ear fluid and throat samples of children. A nationwide study in Finland in 1988-1990. Scand. J. Infect. Dis. 27:57-61. [DOI] [PubMed] [Google Scholar]
- 20.Ohkusu, K., A. Nakamura, and K. Sawada. 2000. Antibiotic resistance among recent clinical isolates of Haemophilus influenzae in Japanese children. Diagn. Microbiol. Infect. Dis. 36:249-254. [DOI] [PubMed] [Google Scholar]
- 21.Powell, M., and J. D. Williams. 1987. In vitro activities of aztreonam, imipenem, and amoxicillin-clavulanate against ampicillin-resistant Haemophilus influenzae. Antimicrob. Agents Chemother. 31:1871-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powell, M., D. McVey, M. H. Kassim, H. Y. Chen, and J. D. Williams. 1991. Antimicrobial susceptibility of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella (Brahamella) catarrhalis isolated in the UK from sputa. J. Antimicrob. Chemother. 28:249-259. [DOI] [PubMed] [Google Scholar]
- 23.Rittenhouse, S. F., L. A. Miller, R. L. Kaplan, G. H. Mosely, and J. A. Poupard. 1995. A survey of beta-lactamase-producing Haemophilus influenzae. An evaluation of 5750 isolates. Diagn. Microbiol. Infect. Dis. 21:223-225. [DOI] [PubMed] [Google Scholar]
- 24.Scriver, S. R., D. J. Hoban, A. McGeer, T. C. Moore, S. L. Walmsley, and D. E. Low. 1994. Surveillance of susceptibility testing methodologies for Haemophilus influenzae in Canada, including evaluation of disk diffusion test. J. Clin. Microbiol. 32:2013-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seki, H., Y. Kasahara, K. Ohta, K. Ohta, Y. Saikawa, R. Sumita, A. Yachie, S. Fujita, and S. Koizumi. 1999. Increasing prevalence of ampicillin-resistant, non-beta-lactamase-producing strains of Haemophilus influenzae in children in Japan. Chemotherapy (Basel) 45:15-21. [DOI] [PubMed] [Google Scholar]
- 26.Shishido, H., and K. Matsumoto. 1979. Meningitis due to Haemophilus influenzae type e, biotype 4. J. Clin. Microbiol. 10:926-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamaki, S., S. Nakajima, and M. Matsuhashi. 1977. Thermosensitive mutation in Escherichia coli simultaneously causing defects in penicillin-binding protein-1Bs and in enzyme activity for peptidoglycan synthesis in vitro. Proc. Natl. Acad. Sci. USA 74:5472-5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thornsberry, C., M. E. Jones, M. L. Hickey, Y. Mauriz, J. Kahn, and D. F. Sahm. 1999. Resistance surveillance of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis isolated in the United States, 1997-1998. J. Antimicrob. Chemother. 44:749-759. [DOI] [PubMed] [Google Scholar]
- 29.Tomasz, A. 1986. Penicillin-binding proteins and the antibacterial effectiveness of β-lactam antibiotics. Rev. Infect. Dis. 8:S260-S278. [DOI] [PubMed] [Google Scholar]
- 30.Ubukata, K., Y. Shibasaki, K. Yamamoto, N. Chiba, K. Hasegawa, Y. Takeuchi, K. Sunakawa, M. Inoue, and M. Konno. 2001. Association of amino acid substitutions in penicillin-binding protein 3 with beta-lactam resistance in β-lactamase-negative ampicillin-resistant Haemophilus influenzae. Antimicrob. Agents Chemother. 45:1693-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yousif, S. Y., J. K. Broome-Smith, and B. G. Spratt. 1985. Lysis of Escherichia coli by β-lactam antibiotics: deletion analysis of the role of penicillin-binding proteins 1A and 1B. J. Gen. Microbiol. 131:2839-2845. [DOI] [PubMed] [Google Scholar]
- 32.Zhao, G., T. I. Meier, S. D. Kahl, K. R. Gee, and L. C. Blaszczak. 1999. Bocillin FL, a sensitive and commercially available reagent for detection of penicillin-binding proteins. Antimicrob. Agents Chemother. 43:1124-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]