Abstract
AKI affects both quality of life and health care costs and is an independent risk factor for mortality. At present, there are few effective treatment options for AKI. Here, we describe a nonpharmacologic, noninvasive, ultrasound-based method to prevent renal ischemia-reperfusion injury in mice, which is a model for human AKI. We exposed anesthetized mice to an ultrasound protocol 24 hours before renal ischemia. After 24 hours of reperfusion, ultrasound-treated mice exhibited preserved kidney morphology and function compared with sham-treated mice. Ultrasound exposure before renal ischemia reduced the accumulation of CD11b+Ly6Ghigh neutrophils and CD11b+F4/80high myeloid cells in kidney tissue. Furthermore, splenectomy and adoptive transfer studies revealed that the spleen and CD4+ T cells mediated the protective effects of ultrasound. Last, blockade or genetic deficiency of the α7 nicotinic acetylcholine receptor abrogated the protective effect of ultrasound, suggesting the involvement of the cholinergic anti-inflammatory pathway. Taken together, these results suggest that an ultrasound-based treatment could have therapeutic potential for the prevention of AKI, possibly by stimulating a splenic anti-inflammatory pathway.
The immune response after ischemia-reperfusion injury (IRI) contributes to tissue damage and reduced GFR. CD45+ leukocyte infiltration begins as early as 30 minutes after reperfusion, with the appearance of CD4+ and CD8+ T cells, B220+ B cells, and the myeloid/monocyte populations (including Ly6G+ neutrophils, Ly6C+CCR2+ monocytes, and F4/80+ macrophages).1,2 Attenuating this ensuing inflammatory response markedly reduces the development of IRI3–9 by preventing tubular epithelial cell apoptosis, rarefaction, and scarring.10 The severity of tissue injury depends on the duration of ischemia and results in acute loss of kidney function, progressive kidney fibrosis,11 and, in some cases, CKD or ESRD.11,12
Given the role of the innate immune system in the development of AKI,1,13,14 treatments targeting inflammation could be valuable therapeutic tools. However, current immunosuppressive agents elicit adverse effects and increase the onset of various comorbid conditions.15,16 An inherent splenic anti-inflammatory pathway has recently been described, and this pathway can be stimulated pharmacologically with nicotinic agonists or by electrical stimulation of the vagus nerve. Referred to as the cholinergic anti-inflammatory pathway, this cascade depends on the spleen, CD4+ T cells, and the α-7 nicotinic acetylcholine receptor (α7nAChR).17 This pathway modulates inflammation and benefits animals in models of myocardial ischemia,18 hepatic injury,19 sepsis and endotoxemia,17,20,21 IRI,22–24 and the response of humans injected with lipopolysaccharide.25 Because of its efficacy in humans and the preclinical data from human tissues, the cholinergic anti-inflammatory pathway is a promising therapeutic target. However, improved methods to stimulate this anti-inflammatory pathway are needed.
Using a modification of contrast-enhanced ultrasound (CEU), our original intent was to develop a method to precondition the renal vasculature before IRI.26 This concept stems from observations that a modified CEU protocol improves blood flow in ischemic skeletal muscle.27–29 Serendipitously, results from our initial studies revealed that prior ultrasound (US) exposure alone, in the absence of a contrast agent, prevented kidney IRI. Further studies indicated that the cholinergic anti-inflammatory pathway may be involved because of the dependence of the US treatment on an intact spleen and the α7nAChR. These studies provide evidence for a simple, portable, noninvasive, and nonpharmacologic approach to prevent AKI.
Results
Prior Exposure to US Reduces IRI in Mice
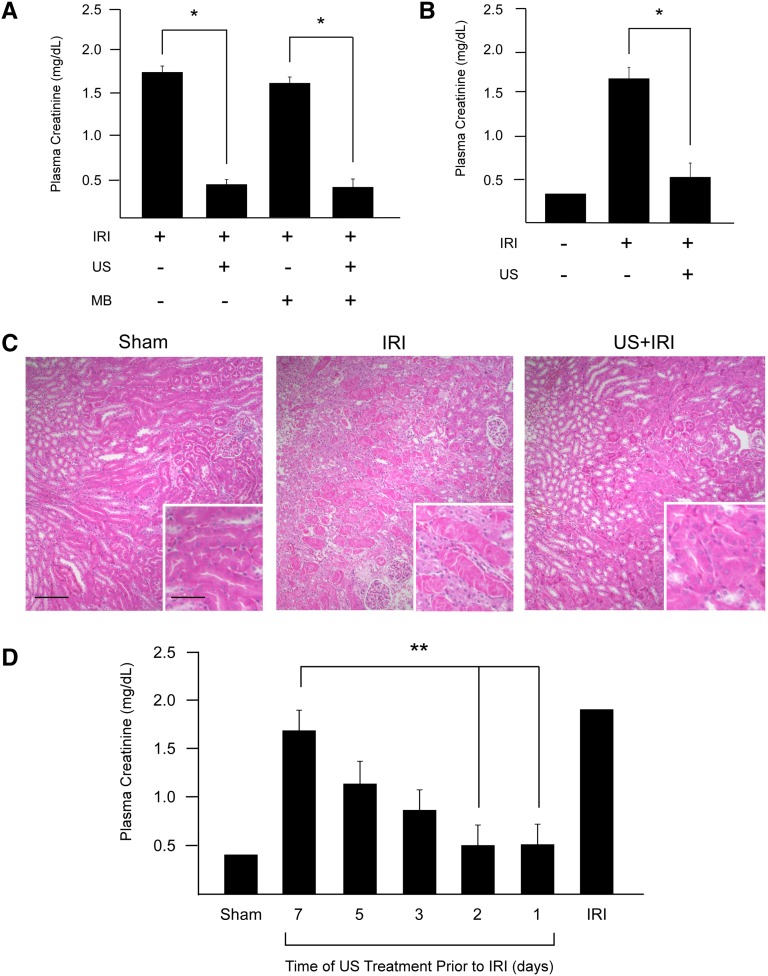
Our initial interest was to investigate the potential use of CEU to prevent IRI. Twenty-four hours before IRI, mice were infused intravenously with a gas-microbubble contrast agent that was destroyed in the kidney using US. Interestingly, we found marked attenuation of AKI (determined by plasma creatinine) in all mice receiving US, regardless of infusion media (contrast agent or saline) (Figure 1A). The study was repeated with the same US settings but without intravenous infusion, and again US exposure alone 24 hours before IRI preserved renal function (Figure 1B). Quantitative stereologic analysis of hematoxylin and eosin (H&E)–stained kidney sections revealed that 18.8%±0.8% of the tissue section surface area consisted of necrotic tubules in mice subjected to IRI alone. This was reduced in mice exposed to US before IRI to 6.0%±1.4% (P<0.001 versus IRI alone), a value similar to that in sham-operated mice (1.7%±0.8%, P=0.33) (Figure 1C). To determine the duration of US-mediated protection, mice were exposed to US up to a week before IRI. Kidney injury was significantly attenuated when mice were exposed to a single US treatment 1 or 2 days before IRI. The efficacy waned in a time-dependent manner when US was applied 3–7 days before IRI (Figure 1D).
Figure 1.
Prior exposure to US alone prevents IRI in naive mice. (A) Mice were infused (intravenously) with the microbubble (MB) contrast agent (or saline) and exposed to US 24 hours before 26 minutes of kidney ischemia, followed by 24 hours of reperfusion (IRI). The preservation of kidney function (as assessed by plasma creatinine) depends on the US application alone. (B) Preservation was replicated in animals without intravenous infusion. (C) Renal morphology (as assessed in H&E-stained tissue section) confirms prevention of IRI in animals exposed to US 24 hours before IRI. n=5–16. (D) Mice were exposed to US 1, 2, 3, 5, or 7 days before IRI. IRI was reduced in animals exposed to US 2 days before injury. n=5. *P<0.001 compared with groups without US; **P<0.005 compared with animals exposed to US 7 days before IRI. Scale bars, 100 μm in low-power image, 50 μm in the inset. Data in bar graphs are shown as mean ± SEM.
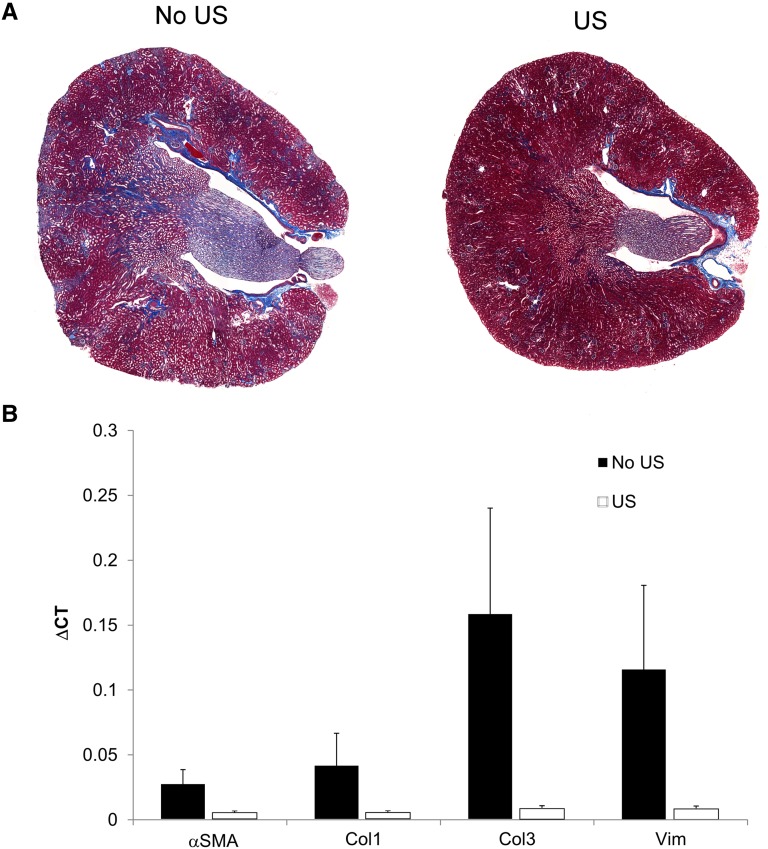
Long-term studies also revealed the preservation of tissue morphology in mice pretreated with US before IRI. Mice were exposed to US 24 hours before bilateral IRI. However, to ensure survival for the duration of the experiment (4 weeks), a reduced period of ischemia (25 minutes) was used. Renal tubulointerstitial fibrosis was assessed 4 weeks later by quantifying Masson trichrome–stained kidney sections. Stereologic analysis revealed that 13%±1% of the tissue section surface area consisted of trichrome-positive tubulointerstitial fibrotic tissue in mice subjected to IRI alone. This value was reduced to 1.5%±0.5% (P<0.001) in mice pretreated with US (Figure 2A), indicating that prior US treatment preserved tissue morphology beyond 24 hours. There was also a >85% reduction in the renal expression of vimentin, α-smooth muscle actin, collagen I, and collagen III mRNA compared with mice exposed to IRI alone (Figure 2B). These results demonstrate that mice exposed to US up to 2 days before IRI have attenuated AKI (24 hours) as well as long-term (4 weeks) preservation of tissue morphology compared with mice challenged with IRI alone.
Figure 2.
The preservation of tissue morphology by prior US exposure is observed 4 weeks after IRI. Mice were exposed to US 24 hours before mild (25 minutes) IRI and were maintained for 4 weeks. Mice receiving US before IRI had (A) less tubulointerstitial fibrosis (quantified using Masson trichrome staining) and (B) >85% reduction in renal mRNA expression of profibrotic genes. n=3. Data in B are the mean ± SEM for ∆CT (change in cycle threshold) values calculated on the basis of tissue glyceraldehyde 3-phosphate dehydrogenase expression. αSMA, α-smooth muscle actin; Col1, collagen 1; Col3, collagen 3; Vim, vimentin.
Prior Exposure to US Reduces Kidney Inflammation in Mice Challenged with IRI
We next examined the effect of US treatment on the infiltration of immune cells that typically occurs in kidneys after IRI.1 FACS and tissue immunofluorescence have shown that infiltrating leukocytes (predominantly neutrophils) accumulate in the interstitium of the cortico-medullary junction after IRI.30 Compared with mice receiving IRI alone, exposure to US 24 hours before IRI reduced infiltration of CD45+ leukocytes in the kidney. The reduced CD45+ leukocyte population consisted primarily of CD11b+Ly6Ghigh and CD11b+F4/80high immune cells (Table 1 and Supplemental Figure 1).
Table 1.
FACS analysis of renal tissue
| FACS Staining (Cell Type) | CD45+ (Total Leukocytes) | CD45+CD11b+F4/80high (Dendritic Cells) | CD45+CD11b+F4/80low (Macrophages) | CD45+CD11b+Ly6Ghigh (Neutrophils) |
|---|---|---|---|---|
| IRI | 1179±208 | 175±32 | 80±36 | 450±137 |
| US + IRI | 158±156 | 39±24 | 42±27 | 53±103 |
| P Value | 0.003 | 0.006 | 0.4 | 0.04 |
Unless otherwise noted, values are cell number (×1000)/g tissue, expressed as mean ± SEM. n=6–10.
The Spleen Is the Tissue Target for US-Mediated Tissue Protection
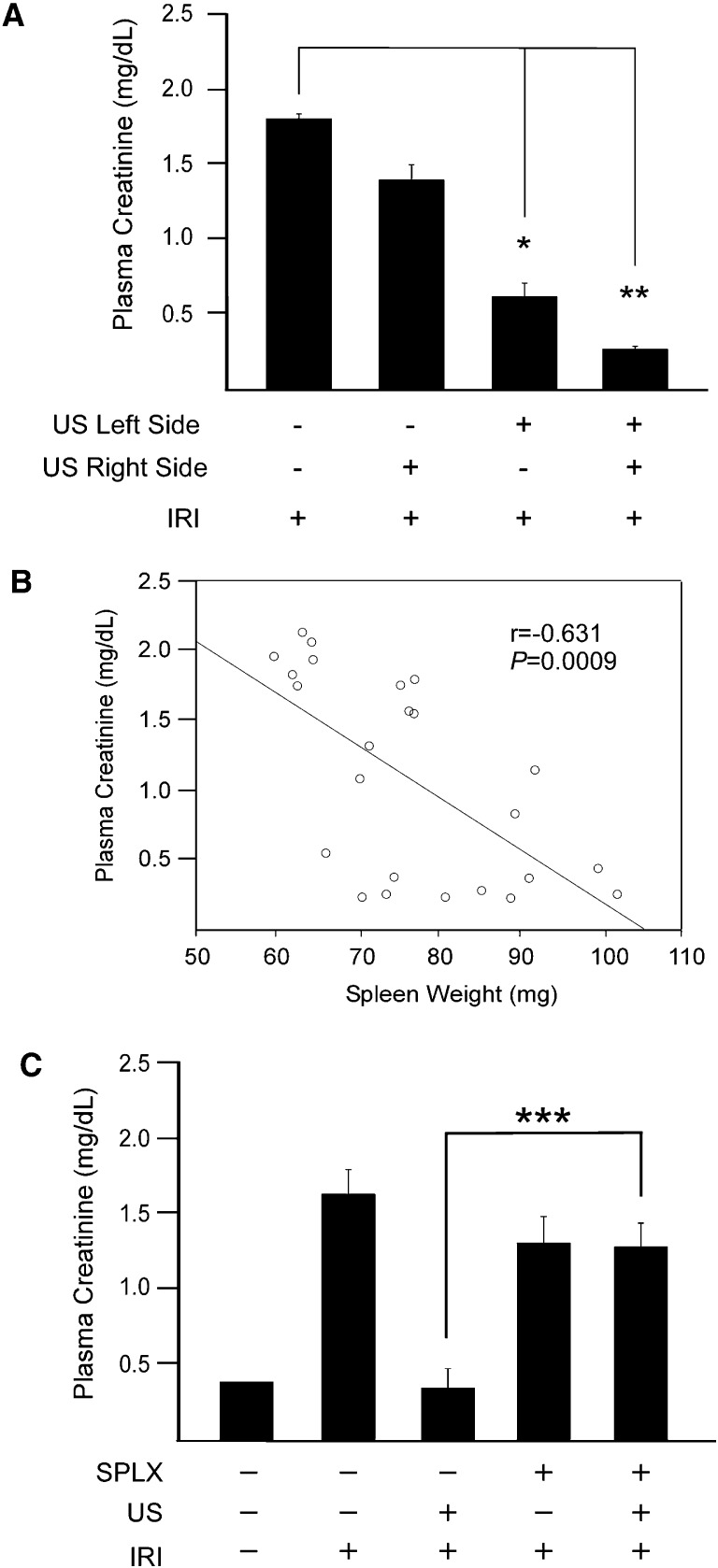
To elucidate the tissue target of US, the US treatment was applied to the right or left side of the animals before bilateral IRI in an attempt to prevent tissue injury in the ipsilateral kidney alone. Interestingly, we observed that US treatment of the left side, but not the right, attenuated the increase in plasma creatinine (60% reduction) (Figure 3A). We also noted visual differences in spleen size; spleen weight was negatively correlated with plasma creatinine after 24 hours of reperfusion (Figure 3B). Combined, these data suggested the spleen was mediating the US-mediated tissue protection. To address this hypothesis, splenectomy was performed 7 days before US treatment. Twenty-four hours after US treatment, mice were subjected to IRI. Splenectomized mice receiving US before IRI had higher plasma creatinine levels than mice receiving the same treatment with intact spleens (Figure 3C). These results suggest that the spleen is the target of US-mediated tissue protection.
Figure 3.
The spleen is required for US-induced protection from IRI. (A) Insonation of both sides of the animal 24 hours before IRI or the left side alone prevented IRI. n=3–8. (B) Splenic weight correlates with kidney function (data are compiled from mice from experiments shown in Figure 1D). (C) US does not prevent IRI in mice splenectomized 7 days before US and IRI. n=5–7. *P=0.03, **P=0.007, ***P=0.003. Data presented as mean ± SEM. SPLX, splenectomized.
Splenic CD4+ T Cells Are Required for US-Mediated Reduction in IRI
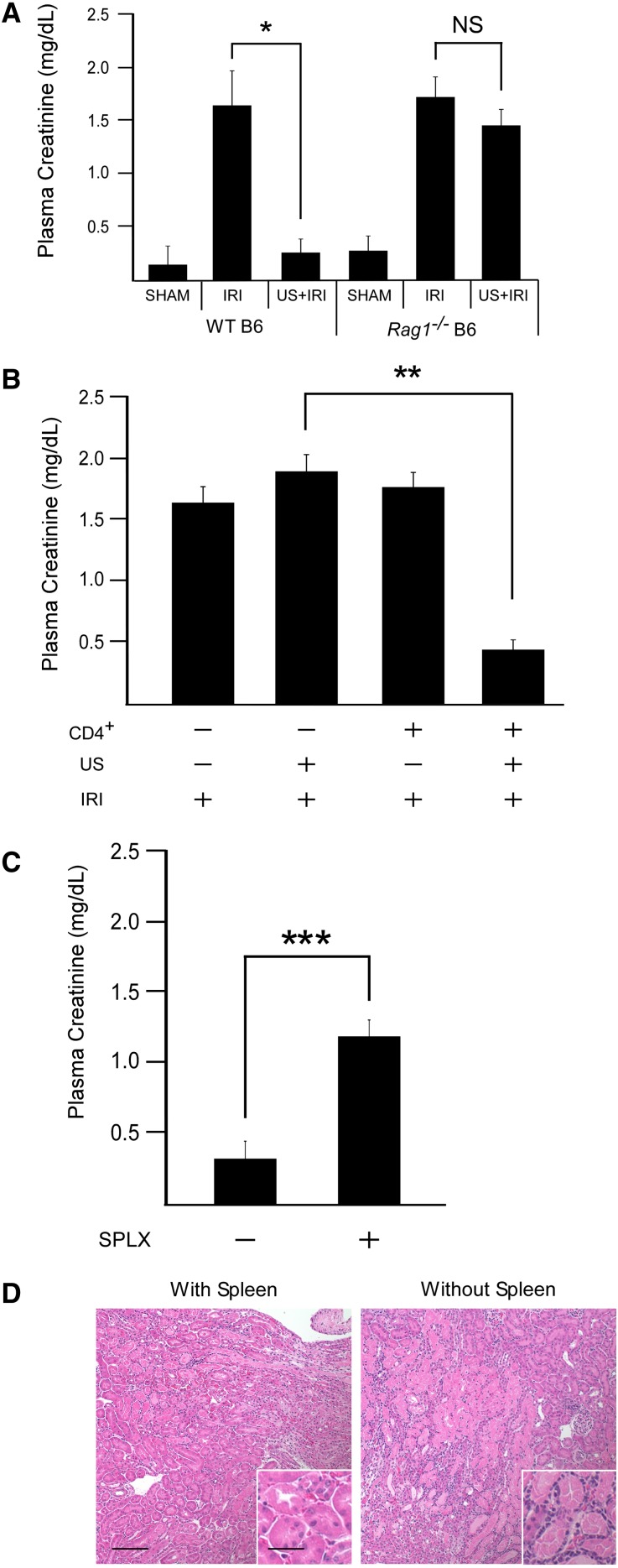
Given the complex cellular composition of the spleen, we sought to determine whether US-mediated kidney tissue protection depended on immune cells. US and IRI treatments were performed in Rag1−/− mice lacking functional T and B lymphocytes. Prior US treatment again markedly reduced IRI in wild-type mice. Rag1−/− mice, however, did not benefit from prior US exposure when subjected to IRI (Figure 4A). Reconstitution of Rag1−/− mice with 0.5–2.0×106 wild-type CD4+ cells 10 days earlier restored US-induced protection from IRI (Figure 4B). To investigate the contribution of the spleen in this process, Rag1−/− mice were splenectomized 7 days before CD4+ T-cell administration and US/IRI treatments. Compared with Rag1−/− mice with intact spleens, splenectomy reduced the CD4+ T cell–specific restoration of US-induced protection from IRI (Figure 4, C and D). These data suggest that both the spleen and CD4+ T cells are required for the US-mediated reduction in IRI.
Figure 4.
Splenic CD4+ T cells mediate the protective effect of US. (A) US does not prevent IRI in mice deficient of functional T and B lymphocytes (Rag1−/−). n=4–11. (B) Reconstitution of Rag1−/− mice with 0.5–2×106 wild-type CD4+ T cells (intravenously) 10 days before US restores the protective effect of US in mice challenged with IRI. n=5–7. (C) Splenectomy (7 days before T cell administration) removes the restoration of US protection in Rag1−/− mice receiving CD4+ T cells. (D) Representative H&E image of mice from C. n=4–5. *P=0.003, **P<0.001, ***P=0.002. Scale bars, 100 μm in low-power image, 50 μm in the inset. Data presented as mean ± SEM. SPLX, splenectomized.
US Reduces IRI in an α7nAChR-Dependent Manner
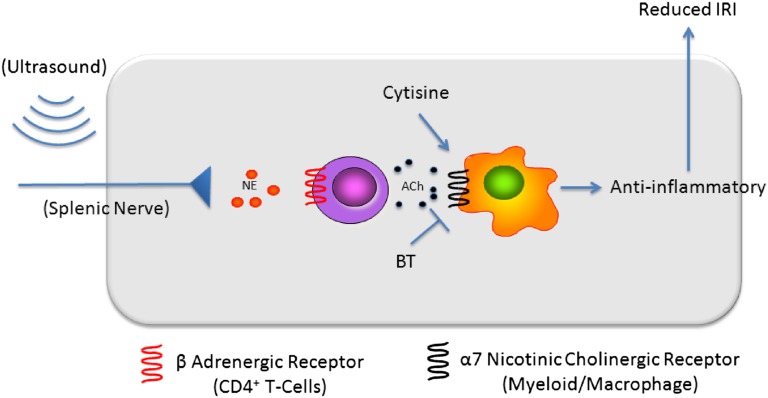
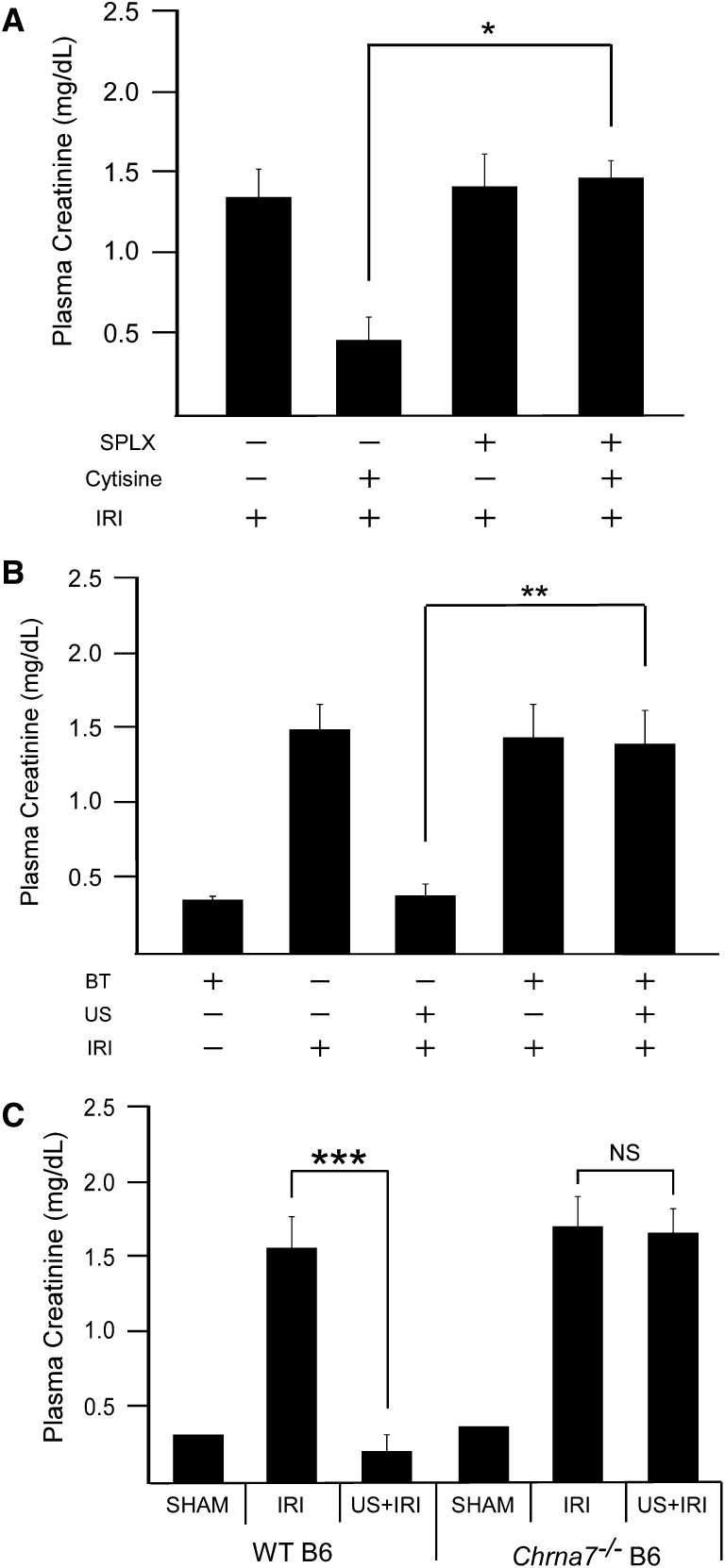
US reportedly modulates nerve activity and can stimulate the motor cortex and elicit limb movement in rodents.31 Nerve stimulation can activate the cholinergic anti-inflammatory pathway, and therefore we hypothesized that US is modulating IRI through activation of this pathway (Figure 5). Previous reports showed that systemic administration of nicotinic agonists reduced renal IRI. However, it was concluded that the kidney (and not the spleen) was the tissue target.22 To verify the involvement of the spleen in preventing IRI with cholinergic agonists, cytisine (an α7nAChR agonist) was administered to splenectomized animals subjected to IRI. Cytisine reduced IRI in animals with intact spleens but was ineffective in splenectomized animals (Figure 6A). These data suggest that prevention of IRI by α7nAChR stimulation, similar to our US treatment, depends on an intact spleen.
Figure 5.
The cholinergic anti-inflammatory pathway provides a potential mechanism for US-mediated protection from IRI. Activation of the adrenergic splenic nerve results in the release of norepinephrine, which binds to adrenergic receptors on nearby CD4+ T cells. This stimulates the production of acetylcholine, which binds to α7nAChRs on splenic myeloid cells (macrophages) and results in reduced inflammation and IRI. Cytisine, an α7nAChR agonist, mimics the effect of acetylcholine, whereas α-bungarotoxin, an α7nAChR antagonist, blocks the effect of endogenous acetylcholine. BT, α-bungarotoxin. Adapted from reference 65.
Figure 6.
US-induced protection depends on the α7nAChR. (A) Administration of the α7nAChR agonist cytisine (100 ng/g, intravenously) 1 hour before IRI prevents kidney injury in a spleen-dependent manner, n=4–8. (B) Prior administration of the α7nAChR antagonist α-bungarotoxin (30 ng/g, intravenously) or (C) use of α7 knockout mice (Chrna7−/−) prevents the US-induced protection from IRI. n=4–7. *P=0.007, **P=0.001, ***P<0.001. Data presented as mean ± SEM. SPLX, splenectomized.
To investigate whether the protective effects of US were mediated through the cholinergic anti-inflammatory pathway and α7nAChR signaling, US was performed in wild-type mice pretreated with the α7nAChR antagonist α-bungarotoxin or in mice lacking a functional α7nAChR (Chrna7−/−). Both the α-bungarotoxin–treated wild-type mice and untreated Chrna7−/− mice developed AKI after IRI. However, prior pharmacologic blockade of the α7nAChR (Figure 6B), or lack of the α7 subunit (Figure 6C), removed the protection elicited by US exposure. Therefore, these data demonstrate that US attenuates IRI in an α7nAChR-dependent manner.
Discussion
The results of our studies demonstrate that mice exposed to a US regimen before IRI had preserved kidney structure and function accompanied by a reduction in tissue inflammation. The protective effect of a single US exposure lasted for 2 days and depended on the spleen and CD4+ T cells. Cytisine, an α7nAChR agonist, mimicked US-mediated protection of kidneys from IRI, and α-bungarotoxin, an α7nAChR antagonist, blocked the effect of US-mediated tissue protection. Finally, the protective effect of US treatment was absent in α7nAChR deficient (Chrna7−/−) mice. We conclude that US markedly attenuates IRI by activating the splenic cholinergic anti-inflammatory pathway. These results suggest that this US regimen (with US settings within diagnostic imaging guidelines) is simple, noninvasive, portable, nonpharmacologic, and readily translatable.
We and others have shown the involvement of the immune system in IRI and that blocking inflammatory pathways prevents tissue damage and loss of kidney function in animal models of AKI (reviewed elsewhere10,32). Here we report that an US regimen prevents inflammation and subsequent tissue damage in a murine model of AKI. This supports previous reports that prior splenic US exposure induced an anti-inflammatory effect.33,34 The authors reported reduced hemolysis and hemagglutination titers and varied immunoglobulin response in mice immunized with sheep red blood cells. Although controversial,35,36 this early study sparked a series of publications throughout the 1980s on the interaction of US and the immune system.33,37–40 However, the mechanism underlying US modulation of the immune system was never determined, and this topic was seemingly disregarded.
In vitro41–43 and in vivo studies have shown that US interacts with44 and could be used to activate31,45 neurons. The cholinergic anti-inflammatory pathway provides a physiologic means by which US, possibly through interacting with the splenic nerve, may interfere with the pathogenesis of IRI. Initially described in human macrophages in the context of sepsis,46 the cholinergic anti-inflammatory pathway mediates the neural control of systemic inflammation. Recognition of inflammatory mediators results in the stimulation of the efferent vagus nerve and impulse transmission to the spleen via the splenic nerve.21,47 Innervating regions of the spleen rich in splenic CD4+ T cells, splenic nerve terminals release norepinephrine, which binds to β-adrenergic receptors expressed on a subset of splenic CD4+ T cells. This results in the local release of acetylcholine (predominantly from T cells), which binds to α7nAChR on nearby splenic macrophages, reducing their response to noxious stimuli associated with sepsis/endotoxemia.17,48 Macrophages are a major source of circulating cytokines during inflammation,20,49 and controlling the behavior of these cells is believed to substantially modulate the systemic immune response. IRI is a sterile form of inflammation that is initiated by the injury/apoptosis of renal cells. This damage, whether detected locally by resident myeloid cells or systemically after release of damage-associated molecular patterns,50 results in kidney inflammation with the proliferation of resident and the infiltration of circulating immune cells. Given the involvement of inflammation in the pathophysiology of IRI, prior activation of the cholinergic anti-inflammatory pathway could ameliorate its development. Prior nicotine administration prevented IRI in rats22 and in wild-type, but not α7nAChR−/−, mice24 but it was concluded that protection was mediated by renal α7nAChRs.22 However, this conclusion contradicts previous studies showing that the cholinergic anti-inflammatory pathway depends on an intact spleen.20,21 The use of vagotomy in the aforementioned paper as a way to deactivate the cholinergic anti-inflammatory pathway probably explains the discrepancy. Whereas vagotomy could prevent the inherent activation of the cholinergic anti-inflammatory pathway, systemic administration of a cholinergic agonist would bypass the efferent vagus nerve (Figure 5).
Combining the previous concepts, we hypothesized that US is preventing IRI by activating the cholinergic anti-inflammatory pathway. Similar to the cholinergic anti-inflammatory pathway, the US method depends on an intact spleen and CD4+ T cells. Vagal nerve stimulation induces production of acetylcholine, the endogenous α7nAChR ligand, by a splenic CD4+ T cell subset.17 This explains the dependence of an intact spleen for the restoration of US-induced protection by CD4+ T cells in Rag1−/− mice. To further support the involvement of the cholinergic anti-inflammatory in our US treatment, we show that US exposure prevents IRI in an α7nAChR-dependent manner. Using both pharmacologic blockade of the α7nAChR in wild-type mice and genetically modified mice lacking a functional α7nAChR, we demonstrated a critical role of α7nAChR in mediating the protective effect of US. Aside from cholinergic agonists, other biomolecules are capable of modulating hypoxic diseases.14,51 In animal models of IRI, the anti-inflammatory actions of T regulatory and tolerized dendritic cells heavily depend on adenosine signaling.9,52 Both of these cell types are found in the spleen, and therefore future studies are needed to determine the role of adenosine, and other signaling molecules, in the protective effect of US. It is also important to highlight that US appears to influence the innate immune system, as shown in this study, as well as components of adaptive immunity as reported previously.33,34 These data suggest that US could be beneficial to prevent reperfusion injury to other organs such as liver, lung, and heart.
Our data also suggest that the spleen is influenced or involved in the pathophysiology of IRI. The spleen is a reservoir of monocytes that localize to infarcted tissue.53 Other data suggest that splenectomy prevents IRI in rats54 and the spleen plays a deleterious role in animal models of stroke.55,56 These studies portray the spleen as a source of proinflammatory cells that propagate ischemic tissue injury. By contrast, results from our study (showing that prior splenectomy did not reduce the development of IRI) and others57 reveal a beneficial, anti-inflammatory role of the spleen. This discrepancy could be the result of differences in the timing of splenectomy (immediately versus a week earlier) or the severity of IRI induced in these studies (plasma creatinine of approximately 1.6 versus 0.8 mg/dl). However, the identification of the cholinergic anti-inflammatory pathway and other immune modulatory functions of the spleen58 suggests that the spleen is more than a source of proinflammatory cells. Our current study supports this notion, with cytisine administration having no beneficial effect on IRI in animals previously splenectomized. The requirement of an intact spleen also suggests that the beneficial effects of cholinergic agonists are not due to direct drug-related effects on renal parenchyma or renal hemodynamics. Aside from nicotinic agonists, other therapies for AKI may also depend on an intact spleen. Protection from AKI in an animal model of sepsis by prior administration of the antimalarial drug chloroquine was lost in splenectomized mice.59 Therefore, further studies are needed to determine the involvement of the spleen in the pathogenesis of AKI and other ischemic diseases.
In conclusion, we describe an US-based method that prevents renal IRI in mice. This US-based treatment appears to stimulate an inherent anti-inflammatory pathway in the spleen that depends on the α7nAChR. Data presented here also implicate the spleen in the pathophysiology of AKI and as a potential therapeutic target. The US method described here is simple and provided by a routine clinical imaging system. Therefore, rapid translation to human studies in the prevention of AKI and other inflammatory diseases should be feasible.
Concise Methods
Mice and Reagents
All experiments were performed in accordance with the National Institutes of Health and Institutional Animal Care and Use Guidelines. The Animal Care and Use Committee of the University of Virginia approved all procedures and protocols. Male mice (8–12 weeks of age) were used for all experiments. Wild-type C57/Bl6 mice were purchased from the National Cancer Institute (Frederick, MD). Both the Rag1−/− (B6.129S7-Rag1tm1Mom/J) and Chrna7−/− (B6.129S7-Chrna7tm1Bay/J) mice were obtained from Jackson Laboratories (Bar Harbor, ME).
US Application
For US exposure, mice were anesthetized with an intraperitoneal injection of a mixture of ketamine (90 mg/kg), xylazine (9 mg/kg), and atropine (0.18 mg/kg). Fur was shaved and removed using a depilatory. Mice were then placed on a modified microscope stage, which was positioned under an US transducer held in place with a ring clamp. Prewarmed US gel was then placed on the depilated skin for US application. Mouse body temperature was monitored via rectal probe (Fine Science Tools, Foster City, CA) and maintained at 36±0.5°C with a heating pad and heat lamp.
A Sequoia 512 US machine with a small-animal 15L8w transducer (Acuson, Malvern, PA) was used for US application. Once the animal’s body temperature was stabilized, the left kidney (or left side) was localized in real-time using conventional B-mode imaging with a frequency of 14 MHz and a mechanical index of 0.99. In preliminary studies only (Figure 1A), microbubble contrast agent was diluted 1:5 in sterile saline and infused intravenously (into tail vein) at a rate of 10 μl/min. Sterile saline was used as a control. The US treatment consisted of imaging with a low-mechanical-index (0.16) for contrast agent (cadence mode) with a frequency of 7 MHz. Pulses of US (burst function) with a mechanical index of 1.2 and duration of 1 second were administered to destroy microbubbles within the renal tissue once every 6 seconds for 2 minutes; this protocol has previously been optimized for vascular imaging using contrast-enhanced US in humans60 and mice (Gigliotti, Kalantari, Rosin, and Okusa, unpublished observations). The same imaging sequences were repeated for the right kidney (or right side). With the exception of those performed for Figure 3A, all experiments were performed in a similar manner with both kidneys, or sides, exposed to US. After the initial studies, in Figure 1A, the same US procedure was performed but in the absence of infusion. Control animals underwent the same preparation procedures but were not exposed to US. Total US exposure was approximately 5 minutes, with small variations in the time required to stabilize body temperature and localize the kidneys. After US treatment, animals were allowed to recover from anesthesia in a temperature-controlled incubator.
IRI and Splenectomy Procedures
Aside from the timeline study, mice underwent renal IRI 24 hours after US treatments. Mice were anesthetized with an intraperitoneal injection of ketamine (120 mg/kg), xylazine (12 mg/kg), and atropine (0.324 mg/kg). Mouse body temperature was monitored via rectal probe (Fine Science Tools, Foster City, CA) and maintained at 35°C±0.5°C with a heating pad and heat lamp.
Bilateral renal IRI was performed through flank incisions by clamping the renal pedicle for 26 minutes as previously published by our laboratory.8,61 This length of ischemic time is based on our laboratory’s experience using male (8–12 weeks old) C57Bl/6 mice to ensure reproducible kidney injury with minimal mortality. The clamps were then removed and the wound sutured after restoration of blood flow was visually observed. Sham-operated mice underwent the same procedure except that the renal pedicles were not clamped. Twenty-four hours after reperfusion, blood was collected by retroorbital puncture with light anesthetic, and mice were then euthanized by cervical dislocation. For experiments that involved splenectomy before IRI, mice were anesthetized with intraperitoneal injection of ketamine (120 mg/kg), xylazine (12 mg/kg), and atropine (0.324 mg/kg). The spleen was then removed through a small flank incision. Sham-operated mice underwent the same procedure with the exception of splenic artery ligation and spleen removal. Buprenorphine (0.15 mg/kg) was administered as a postoperative analgesic for both IRI and splenectomy. Sham and splenectomized mice were allowed to recover for 7 days before US treatment. Twenty-four hours after US treatment, mice were subjected to IRI.
Plasma Creatinine and Stereologic Analysis of Tissue Morphology
Plasma creatinine (mg/dl) was determined via colorimetric analysis following the manufacturer’s protocol (Sigma). Kidneys were dissected and the capsule removed. A center transverse section was cut and placed in paraformaldehyde (4%)/lysine/periodate for 24 hours and then stored in 70% ethanol until paraffin embedding. Five-micrometer paraffin sections were cut and stained with H&E or Masson trichrome following standard procedures.
The extent of acute tubular necrosis (by H&E) or kidney fibrosis (by Masson trichrome) was assessed in an unbiased, systematic manner using design-based stereology to achieve statistically accurate random sampling of kidney sections and yielding the percentage of total area of the section occupied by injured tubules or fibrotic tissue. The investigator was blinded to the experimental identity of the sections. Sections were imaged by using a Zeiss Axio Imager Z2/Apotome Microscope fitted with motorized focus drives and motorized XYZ microscope stage and integrated to a workstation running StereoInvestigator software, version 10.51 (MBF Bioscience, Williston, VT). The area fraction fractionator probe (StereoInvestigator) was used for stereologic analysis of the fractional area of the section occupied by tubular necrosis or tubulointerstitial fibrosis. The following variables were defined: counting frame, 250×250 μm for H&E, 200×20 μm for trichrome; sample grid, 600×600 μm; grid spacing, 50 μm for H&E, 50 μm for trichrome. These values were determined empirically such that adequate numbers of sample sites were visited and adequate numbers of markers (indicating injured tubules or extracellular deposition of collagen) were acquired, in keeping with accepted counting rules for stereology. A total of 604±29 (mean ± SEM) grid sites were evaluated per section; the sampling fraction was 17.4% of a total average area of 8.15×106±0.33 μm2 for each kidney section. Acute tubular necrosis was identified according to the presence of cast formation, tubule dilation, and/or tubular epithelial denucleation. Interstitial fibrosis (i.e., extracellular collagen deposition) was identified by using Masson trichrome stain.
Flow Cytometry and Immunofluorescence Microscopy
To quantify infiltrating leukocytes by flow cytometry, kidney single cell suspensions were prepared from mice subjected to IRI or sham operation. Sample leukocyte subset cell number was calculated as described before.6 The following antibodies (clone) were used to identify neutrophils: PE-Cy7–labeled antimouse CD45 (30-F11; Biolegend, San Diego CA), PE-labeled antimouse CD11b (M1/70; eBiosciences, San Diego, CA), and FITC-labeled antimouse Ly6G (RB6–8C5; eBiosciences). 7-Aminoactinomycin D (Invitrogen) was used to exclude dead cells. Macrophage and dendritic cells were identified on the basis of their expression of allophycocyanin-labeled F4/80 (BM8; eBiosciences). Flow cytometry data were acquired using BD FACSCalibur (BD Biosciences, San Jose, CA) with Cytek eight-color flow cytometry upgrade (Cytek Development, Fremont, CA) and analyzed with FlowJo software 9.0 (Tree Star Inc., Ashland, OR).
Quantitative Real-Time PCR Analysis
Total RNA was extracted from kidneys with TriReagent according to the manufacturer’s protocol (Molecular Research Center Inc., Cincinnati, OH), and cDNA was synthesized using a cDNA transcript kit (Invitrogen). Primers were designed using IDT PrimerQuest (Integrated DNA Technologies). Primer sequences used to detect mRNA expression of genes of interest are as follows (5′– 3′): (Col1) forw - ACTCCTGGACTTCCTGGCTTCAAA, rev - TCCTGCTTGACCTGGAGTTCCATT; (Col3) forw - TCCTAACCAAGGCTGCAAGATGGA, rev - ACCAGAATCTGTCCACCAGTGCTT, (αSMA) forw - ATTGTGCTGGACTCTGGAGATGGT, rev - TGATGTCACGGACAATCTCACGCT, (Vim) forw - AGATGGCTCGTCACCTTCGTGAAT, rev - TTGAGTGGGTGTCAACCAGAGGAA, and (GAPDH) forw - ACGGCAAATTCAACGGCACAGTCA, rev - TGGGGGCATCGGCAGAAGG. Real-time PCR was performed using the iScript 1-step RT-PCR kit with SYBR Green (Bio-Rad) and quantified using a single-color iCycler real-time PCR machine (Bio-Rad). Delta Ct values were calculated on the basis of tissue glyceraldehyde 3-phosphate dehydrogenase expression.
Adoptive Transfer Studies
Total CD4+ cells were isolated from splenic single cell suspensions using the Dynal mouse CD4 negative isolation kit (Invitrogen). Eight- to 10-week-old Rag-1−/− mice were then injected with 0.5–2×106 CD4+ cells or an equal volume (200 μl) of PBS as control. Ten days later, mice were subjected to US and IRI. Because of their potential tolerance to mild (26 minutes) ischemia,62 all studies that included Rag1−/− mice used an ischemic time of 28 minutes. Wild-type mice were included as controls for effectiveness of US treatment.
Pharmacologic Modulation of α7 Nicotinic Acetylcholine Receptors
The α7 nicotinic acetylcholine receptor antagonist α-bungarotoxin (30 ng/g) and agonist cytisine (100 ng/g) were administered intravenously (tail vein) 1 hour before IRI. The concentrations of α-bungarotoxin (Invitrogen, Grand Island, NY) and cytisine (Sigma, St. Louis, MO) were developed on the basis of the 50% lethal dose for α-bungarotoxin (130–160 ng/g)63 and values used in previous studies with cholinergic agonists.22,24,64
Statistical Analyses
All animal studies were conducted using a complete randomized design. Data were analyzed using one- or two-way ANOVA, with a significant difference represented by P<0.05. Means were compared by post hoc multiple-comparison test (Tukey), and all values are presented as mean ± SEM. The correlation between splenic weights and plasma creatinine was determined using Pearson product moment correlation. All statistical analyses were performed using SigmaPlot 11.0 software (Systat, Chicago, IL).
Disclosures
M.D.O.: AM Pharma; Nature Publishing Group; Lilly; Daiichi-Sankyo; American Physiological Society; International Society of Nephrology; PGX Health/Adenosine Therapeutics, LLC; UVA Patent Office. A.L.K.: Targeson, Inc; Philips Research.
Acknowledgments
We would like to acknowledge Drs. Gilbert Kinsey, Li Li, and Peter Lobo for their input and Jacqueline Miller for technical support.
This work was supported by National Institutes of Health grants: to M.D.O., R01-DK062324, R01-DK085259, and R21-093841; to K.K., K23-DK074616; to A.B., K01-DK091444. J.G. was supported by T32-DK072922.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Ultrasonic Stimulation of the Cholinergic Anti-Inflammatory Pathway for Renal Protection,” on pages 1340–1342.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013010084/-/DCSupplemental.
References
- 1.Li L, Okusa MD: Macrophages, dendritic cells, and kidney ischemia-reperfusion injury. Semin Nephrol 30: 268–277, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedewald JJ, Rabb H: Inflammatory cells in ischemic acute renal failure. Kidney Int 66: 486–491, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Miura M, Fu X, Zhang QW, Remick DG, Fairchild RL: Neutralization of Gro alpha and macrophage inflammatory protein-2 attenuates renal ischemia/reperfusion injury. Am J Pathol 159: 2137–2145, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li L, Huang L, Sung SS, Vergis AL, Rosin DL, Rose CE, Jr, Lobo PI, Okusa MD: The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int 74: 1526–1537, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day YJ, Huang L, Ye H, Li L, Linden J, Okusa MD: Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: The role of CD4+ T cells and IFN-gamma. J Immunol 176: 3108–3114, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Li L, Huang L, Sung SS, Lobo PI, Brown MG, Gregg RK, Engelhard VH, Okusa MD: NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol 178: 5899–5911, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Deng J, Kohda Y, Chiao H, Wang Y, Hu X, Hewitt SM, Miyaji T, McLeroy P, Nibhanupudy B, Li S, Star RA: Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int 60: 2118–2128, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Kinsey GR, Sharma R, Huang L, Li L, Vergis AL, Ye H, Ju ST, Okusa MD: Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol 20: 1744–1753, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, Huang L, Ye H, Song SP, Bajwa A, Lee SJ, Moser EK, Jaworska K, Kinsey GR, Day YJ, Linden J, Lobo PI, Rosin DL, Okusa MD: Dendritic cells tolerized with adenosine A₂AR agonist attenuate acute kidney injury. J Clin Invest 122: 3931–3942, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonventre JV, Yang L: Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK: Acute kidney injury: A springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol 298: F1078–F1094, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levey AS, Coresh J: Chronic kidney disease. Lancet 379: 165–180, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Kinsey GR, Okusa MD: Pathogenesis of acute kidney injury: Foundation for clinical practice. Am J Kidney Dis 58: 291–301, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eltzschig HK, Carmeliet P: Hypoxia and inflammation. N Engl J Med 364: 656–665, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srinivas TR, Meier-Kriesche HU: Minimizing immunosuppression, an alternative approach to reducing side effects: Objectives and interim result. Clin J Am Soc Nephrol 3[Suppl 2]: S101–S116, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haskó G, Linden J, Cronstein B, Pacher P: Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov 7: 759–770, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosas-Ballina M, Olofsson PS, Ochani M, Valdés-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW, Tracey KJ: Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334: 98–101, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong J, Yuan YJ, Xue FS, Wang Q, Cheng Y, Li RP, Liao X, Liu JH: Postconditioning with α7nAChR agonist attenuates systemic inflammatory response to myocardial ischemia—reperfusion injury in rats. Inflammation 35: 1357–1364, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Hiramoto T, Chida Y, Sonoda J, Yoshihara K, Sudo N, Kubo C: The hepatic vagus nerve attenuates Fas-induced apoptosis in the mouse liver via alpha7 nicotinic acetylcholine receptor. Gastroenterology 134: 2122–2131, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, Chavan S, Tracey KJ: Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci U S A 105: 11008–11013, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L: Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med 203: 1623–1628, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeboah MM, Xue X, Duan B, Ochani M, Tracey KJ, Susin M, Metz CN: Cholinergic agonists attenuate renal ischemia-reperfusion injury in rats. Kidney Int 74: 62–69, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeboah MM, Xue X, Javdan M, Susin M, Metz CN: Nicotinic acetylcholine receptor expression and regulation in the rat kidney after ischemia-reperfusion injury. Am J Physiol Renal Physiol 295: F654–F661, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadis C, Teske G, Stokman G, Kubjak C, Claessen N, Moore F, Loi P, Diallo B, Barvais L, Goldman M, Florquin S, Le Moine A: Nicotine protects kidney from renal ischemia/reperfusion injury through the cholinergic anti-inflammatory pathway. PLoS ONE 2: e469, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wittebole X, Hahm S, Coyle SM, Kumar A, Calvano SE, Lowry SF: Nicotine exposure alters in vivo human responses to endotoxin. Clin Exp Immunol 147: 28–34, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skyba DM, Price RJ, Linka AZ, Skalak TC, Kaul S: Direct in vivo visualization of intravascular destruction of microbubbles by ultrasound and its local effects on tissue. Circulation 98: 290–293, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Song J, Cottler PS, Klibanov AL, Kaul S, Price RJ: Microvascular remodeling and accelerated hyperemia blood flow restoration in arterially occluded skeletal muscle exposed to ultrasonic microbubble destruction. Am J Physiol Heart Circ Physiol 287: H2754–H2761, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Chappell JC, Song J, Klibanov AL, Price RJ: Ultrasonic microbubble destruction stimulates therapeutic arteriogenesis via the CD18-dependent recruitment of bone marrow-derived cells. Arterioscler Thromb Vasc Biol 28: 1117–1122, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Chappell JC, Klibanov AL, Price RJ: Ultrasound-microbubble-induced neovascularization in mouse skeletal muscle. Ultrasound Med Biol 31: 1411–1422, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Awad AS, Rouse M, Huang L, Vergis AL, Reutershan J, Cathro HP, Linden J, Okusa MD: Compartmentalization of neutrophils in the kidney and lung following acute ischemic kidney injury. Kidney Int 75: 689–698, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tufail Y, Yoshihiro A, Pati S, Li MM, Tyler WJ: Ultrasonic neuromodulation by brain stimulation with transcranial ultrasound. Nat Protoc 6: 1453–1470, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Kinsey GR, Li L, Okusa MD: Inflammation in acute kidney injury. Nephron, Exp Nephrol 109: e102–e107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson DW, Barrett JT: Depression of phagocytosis by ultrasound. Ultrasound Med Biol 7: 267–273, 1981 [DOI] [PubMed] [Google Scholar]
- 34.Anderson DW, Barrett JT: Ultrasound: A new immunosuppressant. Clin Immunol Immunopathol 14: 18–29, 1979 [DOI] [PubMed] [Google Scholar]
- 35.Child SZ, Hare JD, Carstensen EL, Vives B, Davis J, Adler A, Davis HT: Test for the effects of diagnostic levels of ultrasound on the immune response of mice. Clin Immunol Immunopathol 18: 299–302, 1981 [DOI] [PubMed] [Google Scholar]
- 36.Berthold F, Berthold R, Matter I, Reither M, Rother U, Skvaril F, Willems WR: Effect of spleen exposure to ultrasound on cellular and antibody-mediated immune reactions in man. Immunobiology 162: 46–55, 1982 [DOI] [PubMed] [Google Scholar]
- 37.Saad AH, Bahakim HM, Helmi A, Bashandi AM, Lim LK: Therapeutic ultrasound and the liver in vivo: action and possible mechanisms. Ultrasound Med Biol 12: 855–863, 1986 [DOI] [PubMed] [Google Scholar]
- 38.Saad AH, Williams AR: Effects of therapeutic ultrasound on the activity of the mononuclear phagocyte system in vivo. Ultrasound Med Biol 12: 145–150, 1986 [DOI] [PubMed] [Google Scholar]
- 39.Saad AH, Williams AR: Possible mechanisms for the effect of therapeutic ultrasound on the clearance rate of blood borne colloidal particles in vivo. Ultrasound Med Biol [Suppl 2]: 55–60, 1983 [PubMed] [Google Scholar]
- 40.Saad AH, Williams AR: Effects of therapeutic ultrasound on clearance rate of blood borne colloidal particles in vivo. Br J Cancer Suppl 5: 202–205, 1982 [PMC free article] [PubMed] [Google Scholar]
- 41.Khraiche ML, Phillips WB, Jackson N, Muthuswamy J: Ultrasound induced increase in excitability of single neurons. Conf Proc IEEE Eng Med Biol Soc 2008: 4246–4249, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Tsui PH, Wang SH, Huang CC: In vitro effects of ultrasound with different energies on the conduction properties of neural tissue. Ultrasonics 43: 560–565, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Tyler WJ, Tufail Y, Finsterwald M, Tauchmann ML, Olson EJ, Majestic C: Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PLoS ONE 3: e3511, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ang ES, Jr, Gluncic V, Duque A, Schafer ME, Rakic P: Prenatal exposure to ultrasound waves impacts neuronal migration in mice. Proc Natl Acad Sci U S A 103: 12903–12910, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tufail Y, Matyushov A, Baldwin N, Tauchmann ML, Georges J, Yoshihiro A, Tillery SI, Tyler WJ: Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron 66: 681–694, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ: Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405: 458–462, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Tracey KJ: Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 117: 289–296, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ: Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421: 384–388, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Deng J, Muthu K, Gamelli R, Shankar R, Jones SB: Adrenergic modulation of splenic macrophage cytokine release in polymicrobial sepsis. Am J Physiol Cell Physiol 287: C730–C736, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Rosin DL, Okusa MD: Dangers within: DAMP responses to damage and cell death in kidney disease. J Am Soc Nephrol 22: 416–425, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eltzschig HK, Sitkovsky MV, Robson SC: Purinergic signaling during inflammation. N Engl J Med 367: 2322–2333, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kinsey GR, Huang L, Jaworska K, Khutsishvili K, Becker DA, Ye H, Lobo PI, Okusa MD: Autocrine adenosine signaling promotes regulatory T cell-mediated renal protection. J Am Soc Nephrol 23: 1528–1537, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ: Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325: 612–616, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hiroyoshi T, Tsuchida M, Uchiyama K, Fujikawa K, Komatsu T, Kanaoka Y, Matsuyama H: Splenectomy protects the kidneys against ischemic reperfusion injury in the rat. Transpl Immunol 27: 8–11, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Li M, Li F, Luo C, Shan Y, Zhang L, Qian Z, Zhu G, Lin J, Feng H: Immediate splenectomy decreases mortality and improves cognitive function of rats after severe traumatic brain injury. J Trauma 71: 141–147, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Bao Y, Kim E, Bhosle S, Mehta H, Cho S: A role for spleen monocytes in post-ischemic brain inflammation and injury. J Neuroinflammation 7: 92, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andrés-Hernando A, Altmann C, Ahuja N, Lanaspa MA, Nemenoff R, He Z, Ishimoto T, Simpson PA, Weiser-Evans MC, Bacalja J, Faubel S: Splenectomy exacerbates lung injury after ischemic acute kidney injury in mice. Am J Physiol Renal Physiol 301: F907–F916, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ugel S, Peranzoni E, Desantis G, Chioda M, Walter S, Weinschenk T, Ochando JC, Cabrelle A, Mandruzzato S, Bronte V: Immune tolerance to tumor antigens occurs in a specialized environment of the spleen. Cell Rep 2: 628–639, 2012 [DOI] [PubMed] [Google Scholar]
- 59.Leelahavanichkul A, Yasuda H, Doi K, Hu X, Zhou H, Yuen PS, Star RA: Methyl-2-acetamidoacrylate, an ethyl pyruvate analog, decreases sepsis-induced acute kidney injury in mice. Am J Physiol Renal Physiol 295: F1825–F1835, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalantarinia K, Belcik JT, Patrie JT, Wei K: Real-time measurement of renal blood flow in healthy subjects using contrast-enhanced ultrasound. Am J Physiol Renal Physiol 297: F1129–F1134, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kinsey GR, Huang L, Vergis AL, Li L, Okusa MD: Regulatory T cells contribute to the protective effect of ischemic preconditioning in the kidney. Kidney Int 77: 771–780, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bajwa A, Jo SK, Ye H, Huang L, Dondeti KR, Rosin DL, Haase VH, Macdonald TL, Lynch KR, Okusa MD: Activation of sphingosine-1-phosphate 1 receptor in the proximal tubule protects against ischemia-reperfusion injury. J Am Soc Nephrol 21: 955–965, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vernet-der Garabedian B, Bach JF, Morel E: Protective effect of myasthenic immunoglobulins against the lethal toxicity of alpha bungarotoxin. Clin Exp Immunol 68: 130–137, 1987 [PMC free article] [PubMed] [Google Scholar]
- 64.Li YF, Lacroix C, Freeling J: Cytisine induces autonomic cardiovascular responses via activations of different nicotinic receptors. Auton Neurosci 154: 14–19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Andersson U, Tracey KJ: Reflex principles of immunological homeostasis. Annu Rev Immunol 30: 313–335, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]