Abstract
Although case-control studies suggest that African Americans with common coding variants in the APOL1 gene are 5–29 times more likely than those individuals without such variants to have focal segmental glomerulosclerosis, HIV-associated nephropathy, or ESRD, prospective studies have not yet evaluated the impact of these variants on CKD in a community-based sample of African Americans. Here, we studied whether the APOL1 G1 and G2 risk alleles associate with the development of CKD and progression to ESRD by analyzing data from 3067 African Americans in the Atherosclerosis Risk in Communities Study who did not have CKD at baseline. Carrying two risk alleles associated with a 1.49-fold increased risk of CKD (95% CI=1.02 to 2.17) and a 1.88-fold increased risk of ESRD (95% CI=1.20 to 2.93) compared with zero or one risk allele; associations persisted after adjusting for European ancestry. Among participants who developed CKD, those participants with two risk alleles were more likely to progress to ESRD than their counterparts with zero or one risk allele (HR=2.22, 95% CI=1.01 to 4.84). In conclusion, APOL1 risk variants are risk factors for the development of CKD and progression from CKD to ESRD among African Americans in the general population.
African Americans suffer disproportionally from the most severe forms of CKD, including ESRD, and progress faster from CKD to ESRD, even after accounting for differences in socioeconomic factors.1–5 Recent studies show that genetic variants in the MYH9-APOL1 region on chromosome 22 that are common among individuals with African ancestry but rare in Caucasian populations are associated with prevalent ESRD,6–12 accounting for the excessive risk of kidney disease among African Americans compared with their Caucasian counterparts. In case-control studies, two risk alleles (termed G1 and G2) in the last exon of APOL1, a gene that encodes apolipoprotein-L1, are associated with 5–29 times higher odds of severe kidney disease, such as nondiabetic ESRD, hypertension-attributed ESRD, focal segmental glomerulosclerosis, and HIV-related nephropathy.8,10,11 However, it is not known whether these variants predict the development of incident CKD or ESRD events and whether the risk of progression from CKD to ESRD in a prospective community-based sample of middle-aged African Americans differs from prior work investigating associations with prevalent kidney disease in case-control studies and cross-sectional analyses of population-based or high-risk cohorts.8–14 If APOL1 G1 and G2 variants confer higher risk of incident CKD and progression to ESRD in the general population and an effective intervention is identified, then genetic screening could be used to identify high-risk individuals who can be targeted for intervention. Therefore, we sought to determine whether the APOL1 risk alleles are associated with the development of incident CKD and progression to ESRD events in over 3000 African Americans from the Atherosclerosis Risk in Communities (ARIC) Study with a baseline examination in 1987–1989 (visit 1), follow-up examinations in 1990–1992 (visit 2) and 1996–1998 (visit 4), and follow-up for ESRD hospitalizations through 2008. We hypothesized that African Americans carrying two APOL1 risk alleles would have an increased risk of CKD and ESRD progression compared with those individuals carrying zero or one risk allele.
Results
Study Sample Characteristics
Characteristics of 3067 African-American participants free of CKD at baseline are presented in Table 1. Participants were, on average, 53 years old, and 62.9% were women. The prevalence of hypertension and diabetes at baseline was 53.6% and 17.5%, respectively. The prevalence of hypertension at baseline did not differ significantly by the number of APOL1 risk alleles. Allele frequencies of the G1 and G2 alleles were 22% and 13%, respectively (Table 1). Overall, 13.2% of the study sample carried two APOL1 risk alleles (G1/G1, G1/G2, or G2/G2), whereas 45.4% and 41.4% carried one (wild type [WT]/G1 or WT/G2) or zero (WT/WT) risk alleles, respectively.
Table 1.
Baseline characteristics of participants free of CKD at ARIC visit 1 (1987–1989) overall and by number of APOL1 risk alleles
| Characteristic | Overall Sample (n=3067) | Zero or One APOL1 Risk Allele (n=2663) | Two APOL1 Risk Alleles (n=404) | P Value Comparing Two with Zero or One Allele |
|---|---|---|---|---|
| Age (yr) | 53.2±5.8 | 53.3±5.8 | 52.7±5.6 | 0.04 |
| Women, n (%) | 1927 (62.8) | 1669 (62.7) | 258 (63.9) | 0.65 |
| Study center, n (%) | 0.41 | |||
| Forsyth County, NC | 328 (10.7) | 280 (10.5) | 48 (11.9) | |
| Jackson, MS | 2739 (89.3) | 2383 (89.5) | 356 (88.1) | |
| Hypertension, n (%) | 1644 (53.6) | 1415 (53.1) | 229 (56.7) | 0.18 |
| Systolic BP (mmHg) | 127±20 | 127±20 | 128±20 | 0.43 |
| Diastolic BP (mmHg) | 80±12 | 79±12 | 80±12 | 0.08 |
| Hypertension medication use, n (%) | 1189 (38.8) | 1025 (38.5) | 164 (40.6) | 0.30 |
| Fasting blood glucose (mg/dl)a | 111±42 | 111±42 | 110±38 | 0.52 |
| Diabetes, n (%) | 536 (17.5) | 458 (17.2) | 78 (19.3) | |
| Body mass index (kg/m2)b | 29.6±6.1 | 29.5±6.1 | 30.0±6.1 | 0.20 |
| Total cholesterol (mg/dl) | 215±45 | 215±44 | 212±49 | 0.29 |
| HDL cholesterol (mg/l)c | 55±18 | 55±18 | 56±19 | 0.43 |
| eGFR (ml/min per 1.73 m2) | 104.6±16.7 | 104.7±16.6 | 104.5±17.2 | 0.89 |
| Global ancestry (% European ancestry)d | 18±10 | 18±10 | 15±8 | <0.001 |
| APOL1 risk allele frequencies | ||||
| G1 | 0.22 | — | — | — |
| G2 | 0.13 | — | — | — |
| APOL1 genotype frequencies, n (%) | ||||
| WT/WT | 1270 (41.4) | — | — | — |
| WT/G1 | 859 (28.0) | — | — | — |
| WT/G2 | 534 (17.4) | — | — | — |
| G1/G2 | 201 (6.6) | — | — | — |
| G1/G1 | 153 (5.0) | — | — | — |
| G2/G2 | 50 (1.6) | — | — | — |
Aa±.5±e F Glomerular Filtration
Fasting blood glucose was available in 2877 participants overall (2504 participants carrying zero or one risk allele and 373 participants carrying two risk alleles).
Body mass index was available in 3064 participants (2661 participants with zero or one allele and 403 participants with two alleles).
HDL and total cholesterol were available in 3006 participants (2611 participants with zero or one allele and 395 participants with two alleles).
Global ancestry was available in 2740 participants (2373 participants with zero or one allele and 367 participants with two alleles).
APOL1 Risk Alleles and Incident CKD and ESRD
Among 3067 African-American participants free of CKD at baseline, 190 (6.2%) participants developed CKD. The overall incidence rate of CKD was 7.94 events/1000 person-years among participants carrying zero APOL1 risk alleles, 8.60 events/1000 person-years among participants carrying one risk allele, and 11.64 events/1000 person-years among participants carrying two risk alleles (Table 2); the similar incidence rates among those participants with zero or one allele suggest that the recessive inheritance model is appropriate to use in our analyses. After adjusting for age, sex, study center, and ancestry, participants with two copies of the APOL1 risk alleles had a 51% increased risk of developing CKD compared with those participants with zero or one APOL1 risk allele (hazard ratio [HR]=1.51, P value=0.04) (Table 2). Similar results were observed when models were additionally adjusted for baseline HDL cholesterol level and HDL cholesterol subparticle concentrations (data not shown); APOL1 risk alleles were not associated with HDL cholesterol (β=0.70 mg/dl, P value=0.45). Effect estimates were of similar magnitude among diabetic and nondiabetic individuals (P value for interaction=0.82) (Table 3). The interaction for the APOL1 risk alleles and baseline hypertension status was not statistically significant (P value for interaction=0.08) (Table 3).
Table 2.
Incidence rates and HRs for incident CKD and incident ESRD by the number of APOL1 risk alleles
| Model | Number of APOL1 Risk Alleles | ||
|---|---|---|---|
| Zero | One | Two | |
| Incident CKD | |||
| Events/number at risk | 72/1270 | 85/1393 | 33/404 |
| Incidence rate (per 1000 person-yr) | 7.94 | 8.60 | 11.64 |
| Model 1a HR (95% CI) | Reference | 1.49 (1.02 to 2.17); P=0.04 | |
| Model 2b HR (95% CI) | Reference | 1.51 (1.01 to 2.24); P=0.04 | |
| Incident ESRD | |||
| Events/number at risk | 39/1268 | 50/1393 | 25/404 |
| Incidence rate (per 1000 person-yr) | 1.70 | 1.99 | 3.38 |
| Model 1a HR (95% CI) | Reference | 1.88 (1.20 to 2.93); P=0.005 | |
| Model 2b HR (95% CI) | Reference | 1.92 (1.19 to 3.10); P=0.008 | |
Model 1 adjusted for age, sex, and study center.
Model 2 adjusted for age, sex, study center, and percent European ancestry.
Table 3.
HRs (95% CIs) for incident CKD and incident ESRD associated with two copies of APOL1 risk alleles (compared with zero or one risk allele) stratified by baseline diabetes and hypertension status
| Model | HR (95% CI) | P Value | HR (95% CI) | P Value |
|---|---|---|---|---|
| Incident CKD | ||||
| Stratified by diabetes (P for interaction=0.82) | No diabetes (n=2531) | Diabetes (n=536) | ||
| Model 1a | 1.54 (0.95 to 2.49) | 0.08 | 1.41 (0.76 to 2.60) | 0.27 |
| Model 2b | 1.57 (0.95 to 2.61) | 0.08 | 1.43 (0.72 to 2.81) | 0.30 |
| Stratified by hypertension (P for interaction=0.08) | No hypertension (n=1423) | Hypertension (n=1644) | ||
| Model 1a | 2.43 (1.24 to 4.78) | 0.01 | 1.13 (0.71 to 1.79) | 0.60 |
| Model 2b | 2.53 (1.25 to 5.14) | 0.01 | 1.12 (0.69 to 1.83) | 0.64 |
| Incident ESRD | ||||
| Stratified by diabetes (P for interaction=0.66) | No diabetes (n=2529) | Diabetes (n=536) | ||
| Model 1a | 2.00 (0.99 to 4.04) | 0.06 | 1.64 (0.92 to 2.92) | 0.09 |
| Model 2b | 2.04 (0.96 to 4.35) | 0.06 | 1.71 (0.91 to 3.21) | 0.09 |
| Stratified by hypertension (P for interaction=0.14) | No hypertension (n=1423) | Hypertension (n=1642) | ||
| Model 1a | 3.09 (1.42 to 6.73) | 0.004 | 1.45 (0.84 to 2.50) | 0.19 |
| Model 2b | 2.87 (1.20 to 6.87) | 0.02 | 1.53 (0.86 to 2.72) | 0.15 |
Model 1 adjusted for age, sex, and study center.
Model 2 adjusted for age, sex, study center, and percent European ancestry.
Among participants with follow-up for ESRD events through 2008, 114 (3.7%) participants experienced an ESRD event over a median follow-up of 19.7 years. After adjusting for age, sex, and study center, participants with two copies of the APOL1 risk alleles had an 88% increased risk of developing incident ESRD compared with participants with zero or one APOL1 risk allele (HR=1.88, P value=0.005), which persisted after additional adjustment for global ancestry (HR=1.92, P value=0.008) (Table 2). Effect estimates were of similar magnitude after stratifying by baseline diabetes status (P value for interaction=0.66). Similar to CKD, models stratified by hypertension status suggested potential heterogeneity (Table 3), but this interaction was not statistically significant (P value for interaction=0.14).
APOL1 Risk Alleles and Progression to ESRD among Participants with CKD
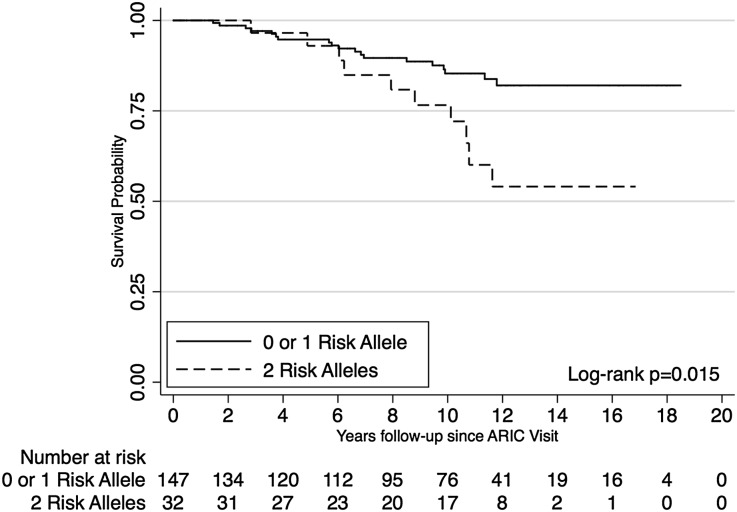
One hundred sevety-nine participants who developed incident CKD stages 3–4 during follow-up were followed for ESRD events for a median of 10.2 years (interquartile range=6.0–12.2 years). Compared with participants with zero or one APOL1 risk allele, those participants with two APOL1 risk alleles progressed faster to ESRD (log-rank P value=0.02) (Figure 1). After accounting for age, sex, study center, and ancestry, participants with two APOL1 risk alleles had about an overall twofold increased risk of progression to ESRD compared with participants with zero or one risk allele (HR=2.43, P value=0.05) (Table 4). In extended Cox models allowing for an interaction with follow-up time, carrying two APOL1 risk alleles was associated with a more than threefold risk of ESRD progression after ≥6 years follow-up (adjusted HR=3.89, P value=0.02) (Table 4).
Figure 1.
Participants with CKD and two APOL1 risk alleles progressed faster to ESRD than participants with CKD and zero or one APOL1 risk allele. Kaplan–Meier survival curves for ESRD-free survival by number of APOL1 risk alleles among participants with prevalent CKD (n=179).
Table 4.
Incidence rates and HRs for progression to ESRD among participants who developed CKD stages 3–4 during follow-up (n=179) by the number of APOL1 risk alleles
| Model | Number of APOL1 Risk Alleles | |||||
|---|---|---|---|---|---|---|
| Zero | One | Two | ||||
| Events/number at risk | 9/67 | 10/80 | 10/32 | |||
| Incidence rate (per 1000 person-yr) | 15.22 | 12.69 | 35.22 | |||
| HRa (95% CI) | P value | |||||
| Model 1b | Reference | 2.22 (1.01 to 4.84) | 0.05 | |||
| Model 2c | Reference | 2.43 (1.00 to 5.95) | 0.05 | |||
| Extended Cox model allowing for interaction with time | Before year 6 | Year 6 and later | ||||
| HRa (95% CI) | P value | HRa (95% CI) | P value | |||
| Model 1b | Reference | 0.87 (0.19 to 4.07) | 0.86 | 3.51 (1.37 to 9.03) | 0.009 | |
| Model 2c | Reference | 1.12 (0.23 to 5.39) | 0.89 | 3.89 (1.30 to 11.62) | 0.02 | |
HR comparing two APOL1 risk alleles with zero or one APOL1 risk allele.
Model 1 adjusted for age, sex, and study center.
Model 2 adjusted for age, sex, study center, and percent European ancestry.
APOL1 Risk Alleles and Urinary Albumin-to-Creatinine Ratio
Among 1979 participants at visit 4, carrying two APOL1 risk alleles was associated with a higher urinary albumin-to-creatinine ratio (UACR; 74% higher geometric mean UACR, P<0.001) and a higher odds of prevalent microalbuminuria (odds ratio=2.14, 95% confidence interval [CI]=1.55 to 2.97, P<0.001) after adjustment for age, sex, study center, and percent European ancestry. Similar results were observed when the analysis was restricted to participants at visit 4 without CKD (n=1834).
Discussion
The APOL1 G1 and G2 risk alleles are common genetic variants with strong effects in a large group of community-based African Americans in the ARIC Study, with about 13% of participants carrying two copies of these risk alleles. We found that participants carrying two risk alleles were at an increased risk of developing incident CKD as well as ESRD events compared with those participants carrying zero or one risk allele. Furthermore, we found that, among African Americans who developed CKD, over 31% of participants carrying two APOL1 risk alleles progressed to ESRD compared with 13% of participants with zero or one risk allele, with a marked elevation of progression to ESRD after CKD duration exceeded 6 years. The G1 and G2 risk alleles are relatively common among African Americans compared with Americans with European ancestry (minor allele frequencies of 0.028%–0.057% were previously reported in the ARIC Study and the Framingham Heart Study15) and could explain, in part, the increased kidney disease burden in African Americans compared with individuals with European ancestry.
The strong association of the G1 and G2 APOL1 risk alleles with several types of prevalent kidney disease in African Americans has been established in multiple case-control studies.8–11 Genovese et al.8 reported that carrying two APOL1 risk alleles was associated with over a 10- and 7-fold increased odds of focal segmental glomerulosclerosis and hypertension-attributed ESRD, respectively. More recent findings have been consistent with these results, with effect estimates of similar or stronger magnitudes observed for focal segmental glomerulosclerosis and HIV-related nephropathy compared with healthy controls in other African-American samples.10–12 It is important to note that earlier research has focused on advanced kidney disease in case-control settings, which may overestimate the increased risk for kidney disease associated with the APOL1 risk alleles in the general population. More modest cross-sectional associations have recently been observed for milder, prevalent kidney disease in African Americans from cohort studies.13,14 Among 1776 African Americans in the Dallas Heart Study, participants carrying two APOL1 risk alleles compared with participants with zero or one risk allele had about a three- to fourfold increased odds of prevalent microalbuminuria and reduced estimated GFR (eGFR; eGFR<60 ml/min per 1.73 m2); these associations differed by diabetes status, with no significant associations observed in participants without diabetes.13 Among 786 first-degree relatives of patients with nondiabetic ESRD, APOL1 risk alleles were significantly associated with proteinuria (UACR≥300 mg/g) overall and in participants without diabetes; associations with lower eGFR were of borderline significance, potentially because of limited power given the sample size.14
In the present analysis, we have extended the current literature to report absolute risks of CKD and ESRD within a prospective, community-based sample of African Americans and show that the APOL1 risk alleles are also associated with increased risk of incident CKD and ESRD events. Consistent with the cross-sectional studies investigating APOL1 and milder forms of kidney disease, our effect estimates are more modest than observed in prior case-control studies of ESRD. In addition, our study sample is, on average, older at baseline than observed in prior studies,6,7,10,13,14 extending the relevance of these variants for the risk of kidney disease to an older general population-based group of African Americans. Of note, given the strong associations observed in younger samples in earlier work, our findings may represent an underestimation of the overall risk association in the general African-American population, because individuals who developed APOL1-associated kidney disease earlier in life would have been censored in this middle-aged study sample. Consistent with prior work in 92 African Americans with biopsy-proven focal segmental glomerulosclerosis10 and adults with CKD in the African American Study of Kidney Disease and Hypertension Trial,12 our findings show that carrying two APOL1 alleles is also associated with faster progression to ESRD in African Americans with moderately reduced eGFR.
Our findings suggest that associations of APOL1 risk alleles and kidney disease do not differ substantially by baseline diabetes status, and although no significant interaction was observed for APOL1 and baseline hypertension status, our stratified models suggest that the APOL1 and kidney disease association may be slightly stronger among those individuals without baseline hypertension. This suggestion is of interest, because prior work has observed significant associations specifically with nondiabetic and hypertension-attributed ESRD,8,9 and findings from the Dallas Heart Study suggest that the associations with prevalent microalbuminuria and reduced eGFR are driven by the increased odds ratio observed in nondiabetic participants.13 A potential explanation of our findings is that the presence or absence of either diabetes or hypertension at baseline does not necessarily reflect the underlying cause of kidney disease in participants who develop CKD or ESRD in this population, which is, on average, at least 10 years older than the population of the Dallas Heart Study.
The APOL1 gene encodes the protein apolipoprotein-1, a trypanosome lytic factor found in human serum16 and a component of HDL particles.17 A recent biopsy study found that apolipoprotein-1 localized within smooth muscle cells in the walls of medium-sized arteries and arterioles in patients with focal segmental glomerulosclerosis or HIV-related nephropathy but not within samples from a normal kidney, suggesting that the APOL1 variants may contribute to kidney disease and disease progression through arterial wall damage and vascular dysfunction.18 This mechanism is also supported by our observed associations with UACR and albuminuria and is consistent with a greater role of APOL1 variants later in disease, because albuminuria is one of the first signs of vascular damage.19 Other possible mechanisms include effects of APOL1 variants on lipid metabolism or autophagy.20 However, lipid-related mechanisms are not supported by our findings given that we did not observe significant associations with the APOL1 risk alleles and HDL cholesterol and that additional adjustment for HDL cholesterol or HDL cholesterol subparticle concentrations did not materially change our findings. Finally, it may be possible that the association of APOL1 variants with kidney disease is not causal and because of these variants being in linkage disequilibrium with the true causal variants.
This study is the first study to characterize common APOL1 variants with moderately strong genetic associations for kidney disease within a large community-based cohort of African Americans with >25 years of follow-up data. We were able to establish population estimates for these variants and prospectively evaluate the association of the APOL1 risk alleles with incident CKD, CKD progression, and ESRD in African-American adults not selected for kidney disease, which would be informative to potential policy or practice guidelines related to APOL1 genotyping in African Americans as a high-risk population. Nonetheless, there are a few limitations to our study that should be considered. In contrast to current guidelines,21 we defined CKD with eGFR based on single serum creatinine measurements from the ARIC Study visits and did not include assessment of proteinuria, leading to potential outcome misclassification. However, this misclassification is likely nondifferential with respect to exposure status and would bias our results to the null, suggesting that our findings represent conservative estimates for the increased risk associated with the APOL1 risk variants. The hospitalization surveillance used to identify ESRD events would miss individuals who developed ESRD but were never hospitalized with such an International Classification of Diseases (ICD) code. Finally, we do not have more detailed information on the specific diagnoses for each CKD or ESRD event, which limits our ability to evaluate the association of APOL1 with kidney disease events with different underlying causes, such as diabetic nephropathy or hypertensive-attributed ESRD.
In summary, African Americans carrying two APOL1 risk alleles are at an increased risk of developing CKD and ESRD and progress faster to ESRD after they have developed CKD. Given that the G1 and G2 risk alleles are common among individuals with African ancestry, with about 13% of African Americans in our sample carrying two copies of these risk variants, these findings highlight the potential of APOL1 risk alleles for identifying individuals who are more likely to have forms of CKD with poorer prognosis and faster disease progression. Although individuals with CKD who carry two APOL1 risk variants may potentially benefit from earlier or more aggressive treatment options to prevent further renal function decline, additional research in a randomized setting is required to evaluate the impact of such treatment. It will be important to determine whether aggressive application of current intervention improves outcomes among individuals with CKD who carry two APOL1 risk variants as well as to develop and test new interventions that lower risk using methods specific to pathogenesis of ESRD as a result of APOL1.
Concise Methods
Study Sample
Our study sample was drawn from the ARIC Study, a prospective, community-based sample of 15,792 adults ages 45–64 years recruited from four US communities between 1987 and 1989.22 Participants attended a baseline examination (visit 1) and follow-up examinations in 1990–1992 (visit 2), 1993–1995 (visit 3), and 1995–1998 (visit 4), with an additional examination currently underway. A total of 4211 African Americans was enrolled in Jackson, Mississippi, and Forsyth County, North Carolina, of which 3757 individuals gave informed consent for genetic studies and underwent genotyping for three APOL1 single-nucleotide polymorphisms. Participants with an eGFR<60 ml/min per 1.73 m2, missing serum creatinine at visit 1, missing follow-up at visits 2 and 4 (n=666), or missing information on diabetes or hypertension status at visit 1 (n=24) were excluded, resulting in a final sample size of 3067 participants. Similar allele frequencies were observed in those individuals with and without eGFR<60 ml/min per 1.73 m2 at visit 1 (G1: 0.23 versus 0.22; G2: 0.10 versus 0.14, respectively).
Exposure Assessment
Direct genotyping of three APOL1 risk variants within the last exon of APOL1 used to define the G1 and G2 risk alleles was performed using Taqman. The G1 risk allele consists of two missense mutations (rs73885319 [Ser342Gly] and rs60919145 [Ile384Met]) that are in almost total positive linkage disequilibrium, and the G2 risk allele consists of the third variant rs71785313, a 6-bp deletion; in the G1, single-nucleotide polymorphisms are both in total negative disequilibrium with the G2 single-nucleotide polymorphism.8
Outcome Assessment
Serum creatinine was measured at ARIC visits 1, 2, and 4 using a modified kinetic Jaffe method, and values were calibrated to the Cleveland Clinic.23 The Chronic Kidney Disease Epidemiology Collaboration Equation was used to calculate eGFR based on serum creatinine.24 Incident CKD was defined as an eGFR<60 ml/min per 1.73 m2 at ARIC visit 2 or 4 among participants with an eGFR≥60 ml/min per 1.73 m2 at the baseline ARIC visit 1.
Incident ESRD was ascertained through active hospitalization surveillance through December 31, 2008. Using ICD codes, ESRD events included all hospitalizations with ICD codes for kidney transplants, dialysis, or dialysis procedures with the following exceptions: a concomitant code for traumatic anuria (ICD 958.5), a concomitant code for AKI (ICD-9 584–584.9 and 586 and ICD-10 N17.0-N17.9) without a prior history of CKD, and a concomitant code for AKI as an underlying cause of death in participants with a prior history of CKD.25 We examined the association of APOL1 risk alleles with incident ESRD in two ways. First, the association with incident ESRD was evaluated among African-American adults without CKD at baseline visit 1. Second, the association with progression to ESRD events was only examined among 179 participants who developed CKD stages 3–4 during follow-up. In this analysis, nine individuals with eGFR<15 ml/min per 1.72 m2 at the time of incident CKD development (either visit 2 or 4) and two individuals without ESRD surveillance data were excluded.
Covariate Assessment
During clinic visits, participants provided information on demographics and medical history, underwent BP measurements, and provided a blood sample. Systolic and diastolic BPs were defined as the average of two of three seated measurements. Hypertension was defined as a systolic BP≥140 mmHg, a diastolic BP≥90 mmHg, or self-reported use of antihypertensive medication. Total cholesterol, HDL cholesterol, HDL cholesterol subparticle types 2 and 3 concentration, and blood glucose were determined using blood samples collected during clinic visits. The UACR at visit 4 was computed by dividing urinary albumin by urinary creatinine (mg/g) with albuminuria defined as UACR≥30 mg/g. Body mass index (kg/m2) was determined based on weight and height measurements. Diabetes was defined as fasting glucose≥126 mg/dl, nonfasting glucose≥200 mg/dl, self-reported physician diagnosis of diabetes, or self-reported use of oral hypoglycemic medication or insulin. The global percentage of European ancestry for each participant was estimated using ANCESTRYMAP based on approximately 1350 ancestry informative markers.6,26
Statistical Methods
Haplotype frequencies for the G1 and G2 alleles were estimated using SAS, Version 9.2 (SAS Institute Inc.; http://www.sas.com/). Prior work indicated that the reference genotype group consisted of participants with either zero or one APOL1 risk allele (WT/WT, G1/WT, or G2/WT), and the at-risk group consisted of individuals with two APOL1 risk alleles (G1/G1, G1/G2, or G2/G2).8 We examined the incidence of both CKD and ESRD by the number of risk alleles (zero, one, or two) to check if this recessive genetic model assumption was appropriate.
The association of APOL1 risk alleles with incident CKD and ESRD was assessed using Cox proportional hazards regression in the overall study sample as well as stratified by baseline diabetes status and hypertension status. Interactions with diabetes and hypertension status were assessed by including an interaction term with APOL1 risk allele status in the overall sample adjusted for age, sex, and study visit center. We evaluated the association of APOL1 risk alleles with progression to ESRD among participants who developed CKD using Kaplan–Meier survival curves with log-rank tests, Cox proportional hazards regression, and extended Cox regression to allow HR to vary with time (<6 years versus after ≥6 years follow-up). We selected 6 years for evaluating the time interaction based on the shape of our Kaplan–Meier curve for ESRD progression. Models were initially adjusted for baseline age, sex, and study center, and in the sensitivity analyses, they were further adjusted for the global percentage of European ancestry (modeled continuously and as ≥15% versus <15% European ancestry) to account for population stratification. Unless otherwise specified, statistical analyses were performed using Stata/IC, Version 11.1 (StataCorp; http://www.stata.com/).
In secondary analyses, we additionally adjusted our models for baseline HDL cholesterol and evaluated the association of APOL1 risk alleles with baseline HDL cholesterol to determine whether any observed associations of APOL1 and CKD or ESRD are independent of HDL cholesterol. UACR was only available at ARIC visit 4; thus, we performed secondary analyses to evaluate the cross-sectional association of APOL1 risk alleles with log-transformed UACR and albuminuria at visit 4.
Disclosures
None.
Acknowledgments
The authors thank the staff and participants of the Atherosclerosis Risk in Communities (ARIC) Study for their important contributions.
This work is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01DK076770 and R21DK073482. Genotyping services were provided by the Johns Hopkins University under Federal Contract Number N01-HV-48195 from the National Heart, Lung, and Blood Institute (NHLBI) and the Center for Inherited Disease Research (CIDR). CIDR is fully funded through a federal contract from the National Institutes of Health (NIH) to the Johns Hopkins University (Contract Number N01-HG-65403). M.C.F. is supported by NIH/NHLBI Cardiovascular Epidemiology Training Grant T32HL007024. The ARIC Study is carried out as a collaborative study supported by NHLBI Contracts HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C.
This work was previously presented as an abstract (poster presentation) at the American Society of Nephrology’s Kidney Week in San Diego, CA, October 30–November 4, 2012.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “APOL1 and Progression of Nondiabetic Nephropathy,” on pages 1344–1346.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2.US Renal Data System : USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2011 [Google Scholar]
- 3.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J: End-stage renal disease in African-American and white men. 16-year MRFIT findings. JAMA 277: 1293–1298, 1997 [PubMed] [Google Scholar]
- 4.Tarver-Carr ME, Powe NR, Eberhardt MS, LaVeist TA, Kington RS, Coresh J, Brancati FL: Excess risk of chronic kidney disease among African-American versus white subjects in the United States: A population-based study of potential explanatory factors. J Am Soc Nephrol 13: 2363–2370, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Perneger TV, Whelton PK, Klag MJ: Race and end-stage renal disease. Socioeconomic status and access to health care as mediating factors. Arch Intern Med 155: 1201–1208, 1995 [PubMed] [Google Scholar]
- 6.Kao WH, Klag MJ, Meoni LA, Reich D, Berthier-Schaad Y, Li M, Coresh J, Patterson N, Tandon A, Powe NR, Fink NE, Sadler JH, Weir MR, Abboud HE, Adler SG, Divers J, Iyengar SK, Freedman BI, Kimmel PL, Knowler WC, Kohn OF, Kramp K, Leehey DJ, Nicholas SB, Pahl MV, Schelling JR, Sedor JR, Thornley-Brown D, Winkler CA, Smith MW, Parekh RS, Family Investigation of Nephropathy and Diabetes Research Group : MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet 40: 1185–1192, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopp JB, Smith MW, Nelson GW, Johnson RC, Freedman BI, Bowden DW, Oleksyk T, McKenzie LM, Kajiyama H, Ahuja TS, Berns JS, Briggs W, Cho ME, Dart RA, Kimmel PL, Korbet SM, Michel DM, Mokrzycki MH, Schelling JR, Simon E, Trachtman H, Vlahov D, Winkler CA: MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet 40: 1175–1184, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tzur S, Rosset S, Shemer R, Yudkovsky G, Selig S, Tarekegn A, Bekele E, Bradman N, Wasser WG, Behar DM, Skorecki K: Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet 128: 345–350, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, Friedman D, Briggs W, Dart R, Korbet S, Mokrzycki MH, Kimmel PL, Limou S, Ahuja TS, Berns JS, Fryc J, Simon EE, Smith MC, Trachtman H, Michel DM, Schelling JR, Vlahov D, Pollak M, Winkler CA: APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 22: 2129–2137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papeta N, Kiryluk K, Patel A, Sterken R, Kacak N, Snyder HJ, Imus PH, Mhatre AN, Lawani AK, Julian BA, Wyatt RJ, Novak J, Wyatt CM, Ross MJ, Winston JA, Klotman ME, Cohen DJ, Appel GB, D’Agati VD, Klotman PE, Gharavi AG: APOL1 variants increase risk for FSGS and HIVAN but not IgA nephropathy. J Am Soc Nephrol 22: 1991–1996, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipkowitz MS, Freedman BI, Langefeld CD, Comeau ME, Bowden DW, Kao WH, Astor BC, Bottinger EP, Iyengar SK, Klotman PE, Freedman RG, Zhang W, Parekh RS, Choi MJ, Nelson GW, Winkler CA, Kopp JB, SK Investigators : Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int 83: 114–120, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman DJ, Kozlitina J, Genovese G, Jog P, Pollak MR: Population-based risk assessment of APOL1 on renal disease. J Am Soc Nephrol 22: 2098–2105, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freedman BI, Langefeld CD, Turner J, Núñez M, High KP, Spainhour M, Hicks PJ, Bowden DW, Reeves-Daniel AM, Murea M, Rocco MV, Divers J: Association of APOL1 variants with mild kidney disease in the first-degree relatives of African American patients with non-diabetic end-stage renal disease. Kidney Int 82: 805–811, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Seaghdha CM, Parekh RS, Hwang SJ, Li M, Köttgen A, Coresh J, Yang Q, Fox CS, Kao WH: The MYH9/APOL1 region and chronic kidney disease in European-Americans. Hum Mol Genet 20: 2450–2456, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanhamme L, Paturiaux-Hanocq F, Poelvoorde P, Nolan DP, Lins L, Van Den Abbeele J, Pays A, Tebabi P, Van Xong H, Jacquet A, Moguilevsky N, Dieu M, Kane JP, De Baetselier P, Brasseur R, Pays E: Apolipoprotein L-I is the trypanosome lytic factor of human serum. Nature 422: 83–87, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Duchateau PN, Pullinger CR, Orellana RE, Kunitake ST, Naya-Vigne J, O’Connor PM, Malloy MJ, Kane JP: Apolipoprotein L, a new human high density lipoprotein apolipoprotein expressed by the pancreas. Identification, cloning, characterization, and plasma distribution of apolipoprotein L. J Biol Chem 272: 25576–25582, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Madhavan SM, O’Toole JF, Konieczkowski M, Ganesan S, Bruggeman LA, Sedor JR: APOL1 localization in normal kidney and nondiabetic kidney disease. J Am Soc Nephrol 22: 2119–2128, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Zeeuw D, Parving HH, Henning RH: Microalbuminuria as an early marker for cardiovascular disease. J Am Soc Nephrol 17: 2100–2105, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Rosset S, Tzur S, Behar DM, Wasser WG, Skorecki K: The population genetics of chronic kidney disease: Insights from the MYH9-APOL1 locus. Nat Rev Nephrol 7: 313–326, 2011 [DOI] [PubMed] [Google Scholar]
- 21.National Kidney Foundation : K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 22.The ARIC Investigators : The Atherosclerosis Risk in Communities (ARIC) Study: Design and objectives. The ARIC investigators. Am J Epidemiol 129: 687–702, 1989 [PubMed] [Google Scholar]
- 23.Matsushita K, Selvin E, Bash LD, Franceschini N, Astor BC, Coresh J: Change in estimated GFR associates with coronary heart disease and mortality. J Am Soc Nephrol 20: 2617–2624, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bash LD, Astor BC, Coresh J: Risk of incident ESRD: A comprehensive look at cardiovascular risk factors and 17 years of follow-up in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 55: 31–41, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Patterson N, Hattangadi N, Lane B, Lohmueller KE, Hafler DA, Oksenberg JR, Hauser SL, Smith MW, O’Brien SJ, Altshuler D, Daly MJ, Reich D: Methods for high-density admixture mapping of disease genes. Am J Hum Genet 74: 979–1000, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]