Abstract
This report describes a study carried out to gain baseline information on the molecular characteristics of extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli and Klebsiella spp. in Canada. A total of 29,323 E. coli and 5,156 Klebsiella sp. isolates were screened at 12 participating sites. Of these, 505 clinically significant, nonrepeat isolates displaying reduced susceptibility to the NCCLS-recommended beta-lactams were submitted to a central laboratory over a 1-year period ending on 30 September 2000. A total of 116 isolates were confirmed to be ESBL producers. PCR and sequence analysis revealed the presence of TEM-11 (n = 1), TEM-12 (n = 1), TEM-29 (n = 1), TEM-52 (n = 4), CTX-M-13 (n = 1), CTX-M-14 (n = 15), CTX-M-15 (n = 11), SHV-2 (n = 2), SHV-2a (n = 12), SHV-5 (n = 6), SHV-12 (n = 45), and SHV-30 (n = 2). Five novel beta-lactamases were identified and designated TEM-115 (n = 2), TEM-120 (n = 1), SHV-40 (n = 2), SHV-41 (n = 4), and SHV-42 (n = 1). In addition, no molecular mechanism was identified for five isolates displaying an ESBL phenotype. Macrorestriction analysis of all ESBL isolates was conducted, as was restriction fragment length polymorphism analysis of plasmids harboring ESBLs. Although a “clonal” distribution of isolates was observed at some individual sites, there was very little evidence suggesting intrahospital spread. In addition, examples of identical or closely related plasmids that were identified at geographically distinct sites across Canada are given. However, there was considerable diversity with respect to plasmid types observed.
Infections caused by aerobic gram-negative bacilli are common in hospitalized patients and result in serious infections, such as bacteremia and the majority of cases of nosocomial pneumonia (8, 32). These infections also are associated with high rates of mortality; for example, sepsis is one of the most common causes of death in intensive-care-unit patients (32, 35). The emergence of gram-negative bacilli that contain extended-spectrum beta-lactamases (ESBLs) and AmpC cephalosporinases has compounded this problem and has become a worldwide concern (7).
The first ESBL was identified in Germany in 1983; since then, over 200 variants of the clavulanic acid-inhibited form of the enzyme have been described worldwide. The most common extended-spectrum phenotypes arise from point mutations in the blaTEM, blaSHV, or blaCTX gene resulting in alterations of the primary amino acid sequence of the enzyme (7). Since these genes are generally found on plasmids, many of the organisms that harbor ESBLs also are resistant to other classes of antibiotics, such as aminoglycosides, fluoroquinolones, tetracyclines, chloramphenicol, and sulfonamides (24).
In this study, we examined the molecular characteristics of ESBLs isolated over a 1-year period in 12 Canadian hospitals.
MATERIALS AND METHODS
Surveillance network.
Established in 1995, The Canadian Nosocomial Infection Surveillance Program (CNISP) is a collaborative effort involving members of The Canadian Hospital Epidemiology Committee (a subcommittee of the Canadian Infectious Diseases Society), the National Microbiology Laboratory (NML), and the Centre for Infectious Disease Prevention and Control, Health Canada. Currently, CNISP is comprised of 35 hospital sites. The data presented in this 1-year study represent results obtained from 12 participating CNISP tertiary-care hospitals which volunteered to be study sites. To maintain site confidentiality, data were tabulated into two geographical regions: the west, which included British Columbia (two sites), Alberta (one site), and Saskatchewan (one site), and the east, which included Ontario (five sites), Quebec (two sites), and Nova Scotia (one site).
Study design.
This study was a prospective laboratory-based surveillance study (1 October 1999 to 30 September 2000) in which all nonrepeat strains of Escherichia coli and Klebsiella spp. determined to be clinically significant by the participating hospital laboratories were tested against a panel of beta-lactam antibiotics (from the Centre for Infectious Disease Prevention and Control, Health Canada) with ESBL screening criteria as described by NCCLS (26, 27). Strains identified as possible ESBL producers were submitted to NML for further characterization. The total number of strains of E. coli or Klebsiella spp. was used as the denominator in the calculations to determine incidence rates for ESBLs. For patients with a suspected ESBL-producing organism, specific information was collected from charts; this information included the service the patient was on when the specimen was identified, date of birth, gender, date of specimen collection, and type of specimen.
Bacterial strains.
All strains were identified at participating sites by routine methods performed at each laboratory. Strains meeting the study criteria were submitted to NML where, upon receipt, they were stored at −70°C in Microbank vials (Pro-Lab Diagnostics, Richmond Hill, Ontario, Canada). The identification of all submitted strains was confirmed by using Vitek GNI cards (BioMerieux). Control strains used in this study included Klebsiella pneumoniae ATCC 700603, Pseudomonas aeruginosa ATCC 27853, and E. coli ATCC 25922.
Antimicrobial susceptibility testing.
Potential ESBL-producing strains were confirmed by the disk diffusion method as described by NCCLS (26) with disks containing ceftazidime (CTZ), ceftazidime-clavulanic acid (CTZL), cefotaxime (CTX), and cefotaxime-clavulanic acid (CTXL) (Mast Diagnostics). The MICs of cephalosporins and aztreonam with and without clavulanic acid and of meropenem were determined for all novel ESBLs detected in this study (27). Susceptibilities to other classes of antimicrobial agents were determined by using Vitek GNS-121 panels (BioMerieux).
Molecular characterization of Ambler class A resistance determinants.
An attempt was made to identify the gene responsible for the ESBL phenotype in all strains by using a combination of PCR and sequence analysis. It should be noted that not all genes were sequenced in strains where multiple beta-lactamase genes existed. The isoelectric points (pIs) of the beta-lactamases were used to predict the most likely ESBL candidate, and this gene was sequenced. For example, if a strain contained blaTEM and isoelectric focusing (IEF) results showed a beta-lactamase with a pI of 5.4, an assumption was made that this beta-lactamase most likely represented the non-ESBL TEM-1 and the other gene was sequenced. In addition, for strains with the same macrorestriction pattern, only a single representative strain was characterized further.
To determine the number and pIs of the beta-lactamase(s) present in the confirmed ESBL-positive strains, IEF and visualization of the beta-lactamase were conducted as previously described (23).
PCRs with universal primer sets to detect blaTEM (40) and blaSHV (28) were conducted for all confirmed ESBL-positive strains. CTX-M-type genes were detected by using in-house-designed universal primers CTX-M-U1 (5′-ATGTGCAGYACCAGTAARGTKATGGC) and CTX-M-U2 (5′-TGGGTRAARTARGTSACCAGAAYCAGCGG) (where R is purine, Y is pyrimidine, and S is G or C) under conditions similar to those described for blaTEM, except that the annealing temperature was changed to 58°C. Entire CTX-M-type genes were amplified for sequencing purposes by using either CTX-M-A (5′-TGGTTAAAAAATCACTGCG) and CTX-M-B (5′-ATTACAAACCGTCGGTGAC) or TOHO2-1 (5′-ATGGTGACAAAGAGAGTGCAACG) and TOHO2-2 (5′-ACTGCCCTTCGGCGATGATTC) as described above. Amplicons were purified (Amicon), and sequence identification was conducted with an ABI 3100 sequencer at the DNA Core Facility, NML.
Molecular subtyping by PFGE.
ESBL producers were subtyped by pulsed-field gel electrophoresis (PFGE) in accordance with the standardized E. coli (O157:H7) protocol (41) after analysis with the BioNumerics software program, version 2.5 (Applied Maths, St. Martens-Latem, Belgium). Gels were normalized by using the molecular-weight-standard strain Salmonella enterica serovar Braenderup universal marker (kindly provided by B. Swaminathan, Centers for Disease Control and Prevention). A 1.0% tolerance and a 1.5% optimization were used during cluster analysis with the unweighted pair-group method, and DNA relatedness was calculated based on the Dice coefficient. Isolates were considered to be genetically related if their macrorestriction DNA patterns differed by fewer than three bands and the Dice coefficient of correlation was 85% or greater.
Bacterial transformation and plasmid mapping.
Plasmid DNA was isolated by using a commercial kit (Qiagen). Approximately 1.0 to 10 ng of plasmid DNA was used to transform electrocompetent DH10B (Invitrogen) by using a Gene Pulser (Bio-Rad) set at 200 Ω/1.25 kV with a time constant of 4 to 5 in 1-mm electroporation cuvettes (EquiBio). Transformants were selected on Luria-Bertani agar containing either CTX or CTZ (5 mg/liter). PCR was used to confirm the presence of the beta-lactamase gene. Approximately 2 μg of plasmid DNA was digested with HpaI, and the restriction fragments were separated on 0.7% agarose gels in 0.5× Tris-borate-EDTA for 18 h at 2.8 V/cm with circulating buffer. Fragments were sized, and gels were normalized by using BioNumerics, version 2.5 (Applied Maths), with a 1-kb extension ladder (Gibco) as a standard.
Nomenclature for classifying the various plasmid profiles was devised in the present study as follows: the first letter denotes the gene class (C represents CTX, S represents SHV, and T represents TEM) and is followed by a number that denotes the ESBL variant subtype. This number is followed by a number that represents the major plasmid class harboring the ESBL and then by a letter that denotes the plasmid subtype. For example, C14-1a describes a plasmid carrying the CTX-M-14 class of ESBL with major plasmid backbone 1 and a fragment of subtype a.
Nucleotide sequence accession numbers.
The complete nucleotide sequences of novel ESBL resistance genes were deposited in the GenBank database under the following accession numbers: TEM-115, AF535127; TEM-120, AY243512; SHV-40, AF535128; SHV-41, AF535129; and SHV-42, AF535130.
RESULTS
Epidemiologic characteristics of study strains.
A total of 29,323 E. coli and 5,156 Klebsiella sp. strains were screened at the 12 participating sites for potential ESBL producers. A total of 505 strains of E. coli (n = 425; 84%) and Klebsiella spp. (n = 80; 16%) (1.5% of the total number of strains) met NCCLS screening criteria (26, 27) as potential ESBL producers. These strains were submitted to the NML for confirmatory testing and advanced characterization. Confirmatory testing by the NCCLS disk diffusion method (26) yielded 116 ESBL-positive strains (E. coli, n = 74; K. pneumoniae, n = 37; and Klebsiella oxytoca, n = 5), representing 20% of all strains submitted for testing. The incidence rates for E. coli and Klebsiella spp. were 0.26/100 (range, 0 to 1.79%) and 0.81/100 (range, 0 to 3.25%), respectively. Forty-three ESBL-producing strains were from the west (E. coli, n = 37; K. pneumoniae, n = 5; and K. oxytoca, n = 1), and 73 were from the east (E. coli, n = 37; K. pneumoniae, n = 32; and K. oxytoca, n = 4). The sex distribution of patients harboring ESBL-positive strains was as follows: 68 female patients (E. coli, n = 41; K. pneumoniae, n = 25; and K. oxytoca, n = 2) and 48 male patients (E. coli, n = 33; K. pneumoniae, n = 12; and K. oxytoca, n = 3). The age distributions of the 58 female patients for whom dates of birth were available were as follows: 0 to 21 years, n = 3; 22 to 65 years, n = 27; and over 65 years, n = 28. The age distributions of the 46 male patients for whom ages were reported were as follows: 0 to 21 years, n = 4; 22 to 65 years, n = 15; and over 65 years, n = 27.
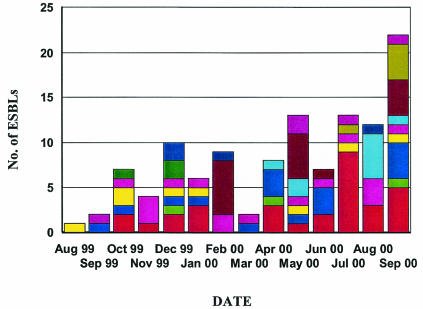
The site of the specimen was reported for 109 strains (Table 1); the highest number of strains was isolated from the urinary tract (n = 68). Ward locations were specified for 75 strains: 18 from surgical wards, 16 from intensive-care units, 16 from acute-medical-care units, 8 from emergency wards, 3 from outpatient wards, 3 from oncology wards, 2 from pediatric wards, and 1 each from dialysis, medical-surgical, pediatric intensive-care, and coronary-care units. A steady increase in ESBL-producing strains was reported in Canada over the 1-year study, from a single strain being reported in the first month of the study to a maximum of 22 strains being reported from nine sites in the last month of the study (Fig. 1).
TABLE 1.
Sites of isolation of ESBL-positive strains
| Site of isolation (n)a | No. of strains of the following organism by patient sex:
|
|||
|---|---|---|---|---|
|
E. coli (n = 69)
|
Klebsiella spp. (n = 40)
|
|||
| Male | Female | Male | Female | |
| Blood (13) | 5 | 4 | 0 | 4 |
| Wound (6) | 3 | 0 | 0 | 3 |
| Abscess (3) | 1 | 1 | 0 | 1 |
| Urine or urinary tract (68) | 17 | 28 | 8 | 15 |
| Upper respiratory tract (8) | 2 | 1 | 3 | 2 |
| Lower respiratory tract (5) | 2 | 0 | 2 | 1 |
| Other (6) | 2 | 3 | 0 | 1 |
| Total | 32 | 37 | 13 | 27 |
Total of 109 of 117 reported sites of isolation.
FIG. 1.
Graph depicting the number of ESBLs isolated by month and by site over the length of the study. Colors represent the total number of ESBL strains at a specific site.
Antimicrobial susceptibilities of study strains.
Of the 505 potential ESBL-producing strains submitted by the participating sites, 195 showed reduced susceptibility to CTZ (disk zone diameter, ≤22 mm) and 260 displayed reduced susceptibility to CTX (disk zone diameter, ≤27 mm); for cefpodoxime (CPD), 347 strains displayed a disk zone diameter of ≤22 mm (CPD22) (26) and 253 strains displayed a disk zone diameter of ≤17 mm (CPD17) on the basis of revised NCCLS criteria (25). On the basis of the revised criteria, the percentage of ESBL-producing strains that would not be detected with CTX, CTZ, CPD, or combinations thereof as a screen are as follows: CTX, 12.8% (≥28 mm; n = 15); CTZ, 23.1% (≥23 mm, n = 27); CPD22, 6.0% (≥23 mm, n = 7); CPD17, 29.1% (≥18 mm, n = 34); CTX-CTZ, 3.4% (n = 4); CTX-CPD17, 12.8% (n = 15); CTX-CPD22, 6.0% (n = 7); CTZ-CPD17, 9.5% (n = 11); CTZ-CPD22, 1.7% (n = 2); CTX-CTZ-CPD22, 1.7% (n = 2); and CTX-CTZ-CPD17, 3.5% (n = 4). A comparison of the antimicrobial resistance patterns for all of the strains submitted as potential ESBL-producing strains for various classes of antimicrobial agents is shown in Table 2.
TABLE 2.
Susceptibilities of study strainsa
| Antimicrobial agent | % (no.) of strains
|
|||
|---|---|---|---|---|
| ESBL producers
|
Non-ESBL producers
|
|||
| E. coli (n = 74) | Klebsiella spp. (n = 42) | E. coli (n = 351) | Klebsiella spp. (n = 38) | |
| Amoxicillin-clavulanate | 19 (14) | 31 (13) | 64 (224) | 26 (10) |
| Ampicillin | 99 (73) | 100 (42) | 87 (305) | 97 (37) |
| Cefazolin | 76 (56) | 38 (16) | 41 (142) | 16 (6) |
| Ceftazidime | 7 (5) | 60 (25) | 0 (0) | 0 (0) |
| Ceftriaxone | 35 (26) | 2 (1) | 0 (0) | 0 (0) |
| Piperacillin-tazobactam | 11 (8) | 17 (7) | 2 (7) | 8 (3) |
| Cefotetan | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Imipenem | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Ciprofloxacin | 51 (38) | 5 (2) | 30 (104) | 11 (4) |
| Levofloxacin | 50 (37) | 5 (2) | 29 (101) | 11 (4) |
| Nitrofurantoin | 7 (5) | 14 (6) | 5 (16) | 18 (7) |
| Gentamicin | 51 (38) | 69 (29) | 21 (74) | 11 (4) |
| Amikacin | 8 (6) | 0 (0) | 0.5 (2) | 0 (0) |
| Tobramycin | 44 (33) | 45 (19) | 18 (64) | 8 (3) |
| Trimethoprim-sulfamethoxazole | 69 (52) | 57 (24) | 37 (130) | 13 (5) |
Determined by using Vitek GNS-121 panels.
Detection and distribution of Ambler class A genes in Canada.
Universal primers were used in PCR to detect the blaTEM, blaSHV, or blaCTX gene in all phenotypically confirmed ESBL-producing strains. Multiple beta-lactamase genes were identified in 71% of strains (n = 82), with 69% of strains containing two genes (TEM and SHV, n = 55, or TEM and CTX, n = 24) and 2.6% (n = 3) harboring all three genes. Thirty percent of strains (n = 34) contained a single beta-lactamase gene. Seventy-seven percent of strains contained blaTEM (n = 90), 67.5% contained blaSHV (n = 79), and 28% contained blaCTX (n = 32).
Macrorestriction analysis of phenotypically confirmed ESBL-producing strains.
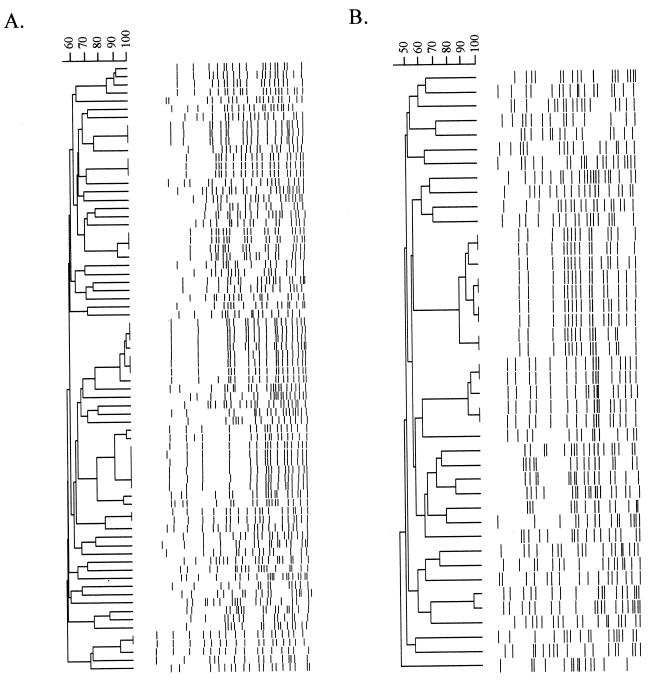
A dendrogram showing the different molecular subtypes is shown in Fig. 2 for E. coli or Klebsiella spp. Sixty-one unique subtypes were identified from the 74 E. coli ESBL-producing strains, whereas 36 unique subtypes were identified from the 42 Klebsiella sp. ESBL-producing strains. On the basis of macrorestriction analysis, clonal spread of E. coli or Klebsiella sp. strains was suggested to have occurred at 4 of the 12 sites participating in the study. All of the outbreaks were small, with the largest being comprised of five clonal strains. In addition, all four sites identified as having outbreaks had more than one outbreak. There were only two examples of an indistinguishable or similar pattern (a three-band difference) being observed at more than one site, that is, a multicenter clonal outbreak. In both cases, only a single E. coli strain was observed at the second site, suggesting that the strain did not spread at the other hospital site.
FIG. 2.
Dendrograms of DNA macrorestriction patterns generated with XbaI. (A) E. coli ESBL-positive isolates (n = 74). (B) Klebsiella sp. ESBL-positive isolates (n = 42).
blaTEM ESBLs.
Only 10 strains (8.7%) carried a blaTEM ESBL gene; the majority of these (7 [70%]) were E. coli. The distribution of TEM ESBLs, as determined by sequence analysis, was TEM-11 (n = 1), TEM-12 (n = 1), TEM-29 (n = 1), TEM-52 (n = 4), TEM-115 (n = 2), and TEM-120 (n = 1). The most prevalent was TEM-52, which was observed in four nonclonal E. coli strains identified at four sites, two in the west and two in the east. Plasmids were transferred to DH10B by electroporation, and the presence of the TEM gene in the transformants was confirmed by PCR. Plasmid restriction fragment length polymorphism (pRFLP) profiling demonstrated that three of the strains had identical plasmids (pT52-1a), and one plasmid generated a unique fingerprint (pT52-2a) (Table 3). Gentamicin resistance was cotransferred with TEM-52 in both plasmids (pT52-1a and pT52-2a), and tobramycin resistance also was cotransferred with pT52-2a.
TABLE 3.
Characteristics of various plasmids harboring ESBLs
| Plasmid | ESBL gene | Clinical strain(s) | Estimated size (kb) | Additional non-beta-lactam resistance transferred to DH10Ba | Region submitting the strainb |
|---|---|---|---|---|---|
| pT52-1a | TEM-52 | ESBL 424 | 146 | GM | W (site 1) |
| pT52-2a | TEM-52 | ESBL 140 | 81 | GM/TB | E (site 2) |
| pT115-1a | TEM-115 | ESBL 187, ESBL 218 | 70 | GM/TB | E (site 3, both strains) |
| pT120-1a | TEM-120 | ESBL 511 | 72 | GM | E (site 3) |
| pC14-1a | CTX-M-14 | ESBL 1 | 68 | None | E (site 4) |
| pC14-1b | CTX-M-14 | ESBL 144 | 75 | None | E (site 2) |
| pC14-1c | CTX-M-14 | ESBL 154 | 59 | None | E (site 2) |
| pC14-2a | CTX-M-14 | ESBL 138 | 73 | None | E (site 2) |
| pC14-3a | CTX-M-14 | ESBL 326 | 147 | AK/GM/TB/TS | W (site 5) |
| pC14-4a | CTX-M-14 | ESBL 339 | 84 | TS | W (site 5) |
| pC14-5a | CTX-M-14 | ESBL 351 | 80 | None | W (site 5) |
| pC14-6a | CTX-M-14 | ESBL 512 | 64 | None | E (site 3) |
| pC15-1a | CTX-M-15 | ESBL 304 | 72 | GM/TB | W (site 5) |
| pC15-1b | CTX-M-15 | ESBL 35 | 77 | GM/TB | W (site 5) |
| pC15-2a | CTX-M-15 | ESBL 123 | 111 | TS | W (site 5) |
| pC15-2b | CTX-M-15 | ESBL 480 | 131 | GM/TB | E (site 4) |
| pC15-3a | CTX-M-15 | ESBL 3 | 70 | None | E (site 4) |
| pC15-3b | CTX-M-15 | ESBL 122 | 81 | None | W (site 5) |
| pS2a-1a | SHV-2a | ESBL 32 | 96 | TS | W (site 5) |
| pS2a-2a | SHV-2a | ESBL 435 | 67 | TS | W (site 1) |
| pS2a-2b | SHV-2a | ESBL 338 | 71 | TS | W (site 5) |
| pS2a-2c | SHV-2a | ESBL 344 | 68 | TS | W (site 5) |
| pS2a-3a | SHV-2a | ESBL 333 | 65 | None | W (site 5) |
| pS2a-4a | SHV-2a | ESBL 85 | 129 | TS | W (site 5) |
| pS2a-5a | SHV-2a | ESBL 97 | 109 | None | W (site 5) |
| pS2a-6a | SHV-2a | ESBL 53 | 131 | TS | W (site 5) |
| pS5-1a | SHV-5 | ESBL 261 | 80 | TB/TS | W (site 6) |
| pS5-1b | SHV-5 | ESBL 12 | 85 | TB/TS | E (site 4) |
| pS5-1c | SHV-5 | ESBL 10 | 84 | TB/TS | E (site 4) |
| pS12-1a | SHV-12 | ESBL 131 | 115 | TB/TS | W (site 5) |
| pS12-2a | SHV-12 | ESBL 229 | 174 | AK/TB/TS | W (site 7) |
| pS12-3a | SHV-12 | ESBL 292 | 159 | GM/TB/TS | E (site 8) |
| pS12-4a | SHV-12 | ESBL 444 | 101 | GM/TB | W (site 7) |
| pS12-5a | SHV-12 | ESBL 182 | 173 | None | E (site 3) |
| pS12-6a | SHV-12 | ESBL 364 | 107 | AK/GM/TB | W (site 5) |
| pS12-6b | SHV-12 | ESBL 513 | 109 | AK/GM/TB | E (site 3) |
| pS12-7a | SHV-12 | ESBL 320 | 115 | TS | W (site 5) |
| pS12-8a | SHV-12 | ESBL 5 | 79 | TB | E (site 4) |
| pS12-9a | SHV-12 | ESBL 184 | 79 | GM | E (site 3) |
| pS12-9b | SHV-12 | ESBL 200 | 74 | None | E (site 3) |
| pS12-10a | SHV-12 | ESBL 18 | 115 | None | E (site 8) |
| pS12-11a | SHV-12 | ESBL 1 | 83 | None | E (site 4) |
| pS12-12a | SHV-12 | ESBL 264 | 133 | None | W (site 6) |
| pS12-13a | SHV-12 | ESBL 8 | 157 | GM | E (site 4) |
| pS12-14a | SHV-12 | ESBL 152 | 60 | GM/TB | E (site 2) |
| pS12-15a | SHV-12 | ESBL 93 | 79 | TB | W (site 5) |
| pS12-16a | SHV-12 | ESBL 457, ESBL 460 | 208 | GM/TS | E (site 9, both strains) |
| pS12H3-1a | SHV-12 | ESBL 17, ESBL 272, ESBL 277 | 141 | GM/TS | E (site 8, all three strains) |
| pS12H3-1b | SHV-12 | ESBL 271 | 135 | GM/TS | E (site 8) |
| pS12H3-1c | SHV-12 | ESBL 69 | 131 | TS | W (site 5) |
| pS12H3-1d | SHV-12 | ESBL 275 | 128 | GM/TS | E (site 8) |
| pS12H3-1e | SHV-12 | ESBL 273 | 139 | TS | E (site 8) |
| pS30-1a | SHV-30 | ESBL 290, ESBL 295 | 64 | GM/TB | E (site 8, both strains) |
Determined by using Vitek GNS-121 panels. AK, amikacin; GM, gentamicin; TB, tobramycin; TS, trimethoprim-sulfamethoxazole; CP, ciprofloxacin; LV, levofloxacin; NF, nitrofurantoin.
W, west; E, east.
Single E. coli strains harboring TEM-11, TEM-12, and TEM-29 were also identified in this study. Only TEM-12 and TEM-29 were transferable to DH10B under our electroporation conditions, and no additional resistance phenotypes were cotransferred with the plasmids (Table 3). All of these TEMs were readily detected with CTZ-CTZL disk combinations, with the exception of TEM-120 (described below); however, TEM-12, TEM-29, and the novel TEMs described below were not readily confirmed with CTX-CTXL disk combinations. Two novel TEM variants were identified, and both were found in Klebsiella spp.
Two different strains, found at the same site in the east, were found to harbor a novel TEM variant which contained two mutations (L21F and R164H). The first was isolated from K. oxytoca on 11 December 1999, and the second was identified in K pneumoniae on 3 May 2000. The sequence has been deposited in GenBank and has been named TEM-115 (Table 4). The pRFLP patterns were the same for both TEM-115 genes (data not shown). The MICs of most of the beta-lactams tested for TEM-115-producing clinical strains remained low, with the exception of the CTZ MIC (≥16 mg/liter) (Table 4). Resistance to gentamicin and tobramycin was cotransferred with TEM-115 on the 70-kb plasmid (Table 3). The same site yielded an additional novel TEM variant that was isolated on 25 August 2000 from K. oxytoca and that contained the same L21F substitution as TEM-115 but also included a G238S substitution. This novel ESBL has been designated TEM-120. The susceptibilities to various beta-lactams for this novel enzyme are shown in Table 4. CPD was the only drug that had an MIC of ≥2 mg/liter for this strain. This strain was detected as a potential ESBL-producing strain only with CPD (zone diameter, 22 mm) and, upon confirmatory testing, displayed a zone diameter of ≥5 mm only with CTX.
TABLE 4.
Characteristics of novel ESBL genesa
| Novel bla gene (strain) | Organismb | DNA fingerprint pattern of strain | Amino acid change(s) | pI | GenBank accession no. | MIC (mg/liter)c
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CPD | CPD/C | CTR | CTR/C | CTX | CTX/C | CTZ | CTZ/C | AZT | AZT/C | CPM | FOX | MER | ||||||
| TEM-115 (ESBL 218) | KP | EKX.0036 | L21F, R164H | 5.4 | AF535127 | 4 | ≤0.25 | ≤0.25 | ≤0.25 | ≤0.25 | ≤0.25 | 16 | 0.5 | 1 | ≤0.25 | 1 | 4 | ≤0.25 |
| TEM-115 (ESBL 187) | KO | EKX.0035 | L21F, R164H | 5.4 | AF535127 | 4 | ≤0.25 | 0.5 | ≤0.25 | 0.5 | ≤0.25 | >16 | 1 | 2 | ≤0.25 | 2 | 8 | ≤0.25 |
| TEM-120 (ESBL 511) | KO | EKX.0014 | L21F, G238S | 5.4 | AY243512 | 4 | ≤0.25 | 0.5 | ≤0.25 | 0.5 | ≤0.25 | 0.5 | ≤0.25 | ≤0.25 | ≤0.25 | <0.5 | 2 | ≤0.25 |
| SHV-40 (ESBL 214) | KP | EKX.0034 | L35Q, A234G | 7.6 | AF535128 | 1 | 0.5 | ≤0.25 | ≤0.25 | ≤0.25 | ≤0.25 | 16 | 1 | 1 | ≤0.25 | 1 | 8 | ≤0.25 |
| SHV-40 (ESBL 263) | KP | EKX.0032 | L35Q, A234G | 7.6 | AF535128 | 4 | 0.5 | 0.5 | ≤0.25 | ≤0.25 | ≤0.25 | 16 | 1 | 1 | ≤0.25 | 2 | 16 | ≤0.25 |
| SHV-41 (ESBL 180) | KP | EKX.0001 | V142F | 7.6 | AF535129 | 1 | 0.5 | 2 | 0.5 | 1 | ≤0.25 | 16 | 16 | >16 | 16 | <0.5 | 32 | 4 |
| SHV-41 (ESBL 494) | KP | EKX.0003 | V142F | 7.6 | AF535129 | 4 | 4 | 0.5 | ≤0.25 | 0.5 | 0.25 | >16 | 0.5 | 4 | ≤0.25 | 2 | 16 | ≤0.25 |
| SHV-42 (ESBL 168) | KP | EKX.0018 | A25S, M129V | 7.6 | AF535130 | 8 | 8 | 2 | 2 | 8 | 8 | 16 | 16 | 16 | ≤0.25 | 2 | 8 | ≤0.25 |
KP, K. pneumoniae; KO, K. oxytoca.
Determined by broth microdilution. CTR, ceftriaxone; AZT, aztreonam; CPM, cefipime; FOX, cefoxitin; MER, meropenem; /C, with clavulanic acid.
blaCTX ESBLs.
Twenty-seven strains of E. coli were found to harbor CTX-M-type genes, including CTX-M-13 (n = 1), CTX-M-14 (n = 15), and CTX-M-15 (n = 11). The single strain harboring the CTX-M-13 gene was isolated in western Canada in June 2000 from a urine specimen. The macrorestriction pattern of the E. coli strain harboring CTX-M-13 was unique.
CTX-M-14 was identified eight times in the west and seven times in the east. A strain outbreak with closely related PFGE patterns and comprised of six strains was observed at two sites in the west (site 1, n = 4, and site 2, n = 1) and one site in the east (n = 1). The first and most distantly related outbreak strain was first isolated in January 2000 at the site in eastern Canada (PFGE pattern, EEX.0051). Closely related strains (n = 4) appeared at site 1 in the west from July 2000 through September 2000 (PFGE pattern, EEX.0053). A strain with an indistinguishable DNA pattern from site 1 emerged at a second location in the west at the end of the study period in September 2000. The remaining eight strains harboring the CTX-M-14 gene were not found to be closely related by macrorestriction analysis.
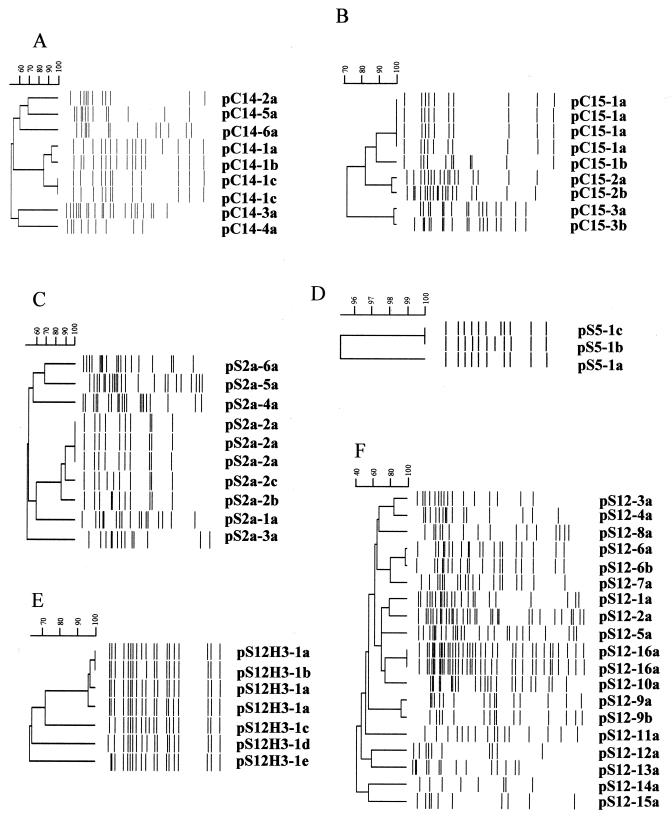
The ESBL phenotype was transferred to E. coli DH10B for all of the strains harboring the CTX-M-14 gene, with the exception of a group of four strains with identical PFGE profiles, described above (EEX.0053). Plasmid restriction enzyme profiles were generated from DH10B transformants, and all recipient strains were shown to contain the CTX gene by PCR (data not shown). A dendrogram representing the plasmid profiles is shown in Fig. 3A. Six major plasmid groups, pC14-1 to pC14-6, are evident. A cluster of four plasmid profiles, two of which are indistinguishable (pC14-1c), is evident in Fig. 3A. Interestingly, the four closely related plasmids were isolated from four sites in the east. The two indistinguishable plasmids were identified from different sites and were isolated within 11 days of each other in January 2000. The other plasmid subtypes (pC14-1a and pC14-1b) were similar, and approximately 70% of their restriction fragments were of similar sizes, suggesting a possible relationship. All of these pC14-1 subtypes confer similar antibiograms to DH10B transformants, i.e., sensitivity to all non-beta-lactams tested (Table 3). Interestingly, a single strain harboring the CTX-M-14 gene (ESBL 1) was found to contain an SHV amplicon. This amplicon was sequenced and identified as SHV-12. However, SHV-12 was identified only in the clinical strain and was not found to be associated with the plasmid harboring the CTX-M-14 gene (pC14-1a). This particular SHV-12 will not be discussed in the SHV section below. The remaining plasmid groups consisted of single strains, all of which harbored CTX-M-14 genes on large (64- to 140-kb) plasmids, only one of which (pC14-3a) carried an additional antimicrobial resistance phenotype along with the ESBL phenotype (Table 3).
FIG. 3.
Dendrograms depicting pRFLPs of the elements containing ESBL genes that were transferred to E. coli DH10B via electroporation. (A) CTX-M-14 HpaI profiles. (B) CTX-M-15 HpaI profiles. (C) SHV-2a HpaI profiles. (D) SHV-5 HpaI profiles. (E) SHV-12 HindIII profiles. (F) SHV-12 HpaI profiles.
The remaining 11 CTX-M PCR-positive strains contained the CTX-M-15 gene, as identified by DNA sequence analysis. All of the strains were isolated at two sites in Canada, one site in the west (n = 9) and one site in the east (n = 2). All of the strains were positive for the TEM gene, which most likely represents TEM-1, because the strains contained a beta-lactamase with an isoelectric point of 5.4. Macrorestriction analysis of bacterial strains revealed nine unique patterns, with only a single observed outbreak occurring at the western site and involving three clonal strains (PFGE pattern, EEX.0038) isolated in the last month of the study.
The ESBL phenotype was transferred to E. coli DH10B for all of the strains with unique representative PFGE profiles. All DH10B recipient strains contained the CTX gene, as demonstrated by PCR (data not shown). A dendrogram depicting the restriction profiles of HpaI-digested plasmids harboring the CTX-M-15 gene from DH10B transformants is shown in Fig. 3B. Three major plasmid profiles, pC15-1 to pC15-3, were observed; plasmid profile pC15-1 was comprised of two plasmid subtypes, pC15-1a, which was identified at two sites (west, n = 5, and east, n = 1), and pC15-1b, which was identified at the site in the west. Resistance to tobramycin and gentamicin was cotransferred with the ESBL phenotype for both pC15-1 subtypes (Table 3). The two pC15-2 subtypes, pC15-2a, which was isolated in the west, and pC15-2b, which was isolated in the east, varied by two bands (Fig. 3B). Trimethoprim-sulfamethoxazole resistance was cotransferred with the ESBL phenotype. Two subtypes of pC15-3 were identified (in the west and east) and varied by three bands (Fig. 3B). No additional resistance phenotypes tested were cotransferred with the ESBL phenotype.
blaSHV ESBLs.
The majority of ESBLs identified in Canada were of the blaSHV type. This enzyme comprises 64% of all ESBLs (n = 74) identified in this study and includes SHV-2 (n = 2), SHV-2a (n = 12), SHV-5 (n = 6), SHV-12 (n = 45), SHV-30 (n = 2), SHV-40 (n = 2), SHV-41 (n = 4), and SHV-42 (n = 1). Fifty-four percent of the SHV ESBLs (n = 40) were found in E. coli. Two K. pneumoniae strains with unique PFGE patterns and isolated at different sites were found to harbor SHV-2 genes. All of the strains harboring SHV-2a genes were identified in the west (n = 12; three sites); the majority of the strains (n = 10) were identified at a single site. All strains were E. coli, with the exception of a single K. pneumoniae strain. A clonal strain outbreak (n = 3; EEX.0027) of E. coli involving a specific plasmid, pSHV2a-3a, occurred with no additional dissemination of the strain or the plasmid (Table 3 and Fig. 3C). Dissemination of a clonal plasmid group, consisting of pS2a-2a to pS2a-2c, occurred at three sites in the west (Fig. 3C). It was first observed at one site in December 1999 (pS2a-2a) and was next identified three times in July 2000 at a different site (pS2a-2a to pS2a-2c), with the final observation occurring at the third site in September 2000 (pS2a-2a). Four additional plasmids harboring unique SHV-2a genes were identified in genetically distinct E. coli strains during the course of this study and were labeled pS2a-1a, pS2a-4a, pS2a-5a, and pS2a-6a. All of the plasmids harboring SHV-2a genes were large (65 to 131 kb) and, with the exception of pS2a-5a, all harbored at least the trimethoprim-sulfamethoxazole resistance determinant in addition to the ESBL (Table 3).
SHV-5 strains were identified at four sites, two in the west (n = 2) and two in the east (n = 3). A single E. coli strain and four K. pneumoniae strains, all with different PFGE profiles, harbored SHV-5 genes. Only three of the SHV-5 genes were transferable to DH10B by electroporation. pRFLP analysis revealed that the three plasmids differed by only a single band shift (Fig. 3D), and all harbored additional tobramycin and trimethoprim-sulfamethoxazole resistance determinants (Table 3). Two of these plasmids (pS5a-1b and pS5a-1c) were isolated within 5 days of each other in April 2000 at a single site in the east, whereas the third plasmid was identified in September 2000 at a site in the west.
Strains harboring SHV-12 genes made up the largest proportion of strains with SHV genes identified in Canada. A disproportionate number of strains harboring SHV-12 genes were identified in the east (n = 66; five sites) compared to the west (n = 9; three sites). A single clonal E. coli outbreak involving eight strains occurred at a site in the east over a 5-month period. The plasmids harboring SHV-12 genes in these strains were unique in that restriction enzyme HpaI yielded too many bands to accurately record in BioNumerics; therefore, HindIII was chosen as an alternative to generate a pRFLP pattern (Fig. 3E). pRFLP analysis with HindIII revealed three additional nonclonal E. coli strains which also harbored closely related plasmids. Two were from the original site, while one was from a site in the west (pS12H3-1c). In all instances, these plasmids carried additional resistance determinants (Table 3). In addition to this plasmid group, 16 other unrelated plasmid types were identified in the strains harboring SHV-12 genes (Table 3 and Fig. 3F).
Undefined ESBLs.
Five strains were identified phenotypically as being ESBL positive; however, all attempts to identify the molecular basis for the ESBL were unsuccessful. All strains described below were not found to be related by PFGE pattern analysis, and all were sensitive to cefoxitin, suggesting that an AmpC-type mechanism (Ambler class C) did not contribute to the ESBL phenotype.
A single K. pneumoniae strain, ESBL 443, was found to harbor both TEM and SHV sequences by PCR; however, only a single beta-lactamase with a pI of 5.4 was observed in this strain. Sequence analysis of both of the amplicons revealed the presence of a TEM-1 gene with a strong Pa/Pb promoter (12) and an SHV-1 gene. The disk zone diameters revealed the phenotypic presence of an ESBL, as follows: CTX, 19 mm; CTXL, 32 mm; CTZ, 13 mm; and CTZL, 27 mm. Strain ESBL 28 was found by the methods used in this study to contain only a TEM-1 gene. IEF analysis revealed that the strain had pIs of 5.4 and 8.2. The disk zone diameter data clearly indicated the presence of an ESBL, with CTX and CTZ zone diameters of 10 and 9 mm, respectively, both of which increased by ≥5 mm in the presence of clavulanic acid. Two K. pneumoniae strains, ESBL 241 and ESBL 262, produced an SHV amplicon; sequence analysis revealed two potential sequences, SHV-1 and LEN-1, within this amplicon.These two potential sequences were previously described to exist in tandem in some strains of K. pneumoniae (11). These strains clearly displayed reduced susceptibility to CTX (20 mm) and CTZ (14 mm). Finally, a K. oxytoca strain, ESBL 465, met the definition of an ESBL-producing strain, with CTX and CTZ zone diameters of 18 and 20 mm, respectively. No TEM, SHV, or CTX gene was detected in this strain with the PCR primer pairs described above.
DISCUSSION
In a recent SENTRY worldwide surveillance program report, ESBL phenotypes were detected in 45% of K. pneumoniae strains from Latin America, 23% from the western Pacific, 23% from Europe, 8% from the United States, and 5% from Canada (45). The lower ESBL prevalence in this study than in the SENTRY study may be attributable to the use of a broth dilution method rather than the disk diffusion method for ESBL determinations or to the examination of different tertiary-care centers. Surveys of U.S. hospitals have found that approximately 5 to 15% of Klebsiella sp. strains and 2 to 10% of E. coli strains harbor ESBLs (16). The prevalence of ESBL-producing Enterobacteriaceae in Europe is highly variable among countries. In The Netherlands, <1% of E. coli or K. pneumoniae strains are ESBL producers; however, in France, up to 40% of K. pneumoniae strains are CTZ resistant (5). One encouraging finding in this study is the lower rate of ESBLs in Canadian tertiary-care facilities than in those of other countries. Although the reasons for this discrepancy are difficult determine, this trend appears to be a general one for multidrug-resistant nosocomial pathogens in Canada. In addition to the low rates of ESBLs, Canada has documented similar findings for methicillin-resistant Staphylococcus aureus (38, 39) and vancomycin-resistant enterococci (14) compared to some other countries.
blaTEM ESBLs.
In the United States, most hospital outbreaks have been due to TEM-12, TEM-10, and TEM-26 (6, 19). However in Canada, TEM is the least prevalent type, comprising only 7.7% of the ESBLs identified. This is the first report of TEM-52 in North America. This variant was first described by Poyart et al. (33); it was found in a strain isolated from a 2-year-old child in France and since then has been identified in Asia (29). Although all of the E. coli strains harboring TEM-52 had different PFGE patterns in the present study, three of the four strains harbored identical HpaI pRFLP patterns, suggesting the dissemination of a plasmid rather than a clonal strain in Canada. A single strain harboring TEM-29 was identified in this study. Globally, this type is rare, having been reported in clinical isolates from Korea and The Netherlands (1, 46). The TEM-11 ESBL identified in this study is the second such report; the first report originally characterized strains in Belgium (13). The Canadian strain harboring TEM-11 also had an enzyme with an identical pI (5.6), although the MIC of CTZ (>16 mg/liter) for this strain was higher, a finding that may be attributable to the presence of a TEM-1 in the clinical strain and/or to alterations in membrane permeability through porin changes in the clinical strain (5, 21, 44).
blaCTX ESBLs.
The CTX-type beta-lactamases constitute the most recent Ambler class A plasmid-encoded enzymes; they were initially isolated from strains in Europe and Argentina in the late 1980s and early 1990s (2, 36). Since then, numerous reports from eastern Europe, Asia, and South America have identified CTX-type ESBLs (reviewed in reference 42). Although only three types of CTX genes have been observed in Canada, plasmid profiling data indicate that multiple plasmid types exist. This report is only the second describing CTX-M-13; the first identified CTX-M-1 on a 35-kb plasmid from K pneumoniae in the People's Republic of China (9).
CTX-M-14 has been described in Taiwan, Korea, China, France, and Spain (reviewed in reference 42). A subset of CTX-M-14 correlated with an E. coli clonal strain (EEX.0053) which has been implicated in a community-acquired urinary tract infection outbreak in western Canada (M. Louie, personal communication). A report from Spain has documented a high percentage of community-acquired urinary tract infections involving E. coli strains harboring CTX-M-14 (3), and one can speculate that there may be genetic similarities between the two geographically disparate sets of strains. Studies are under way to determine the potential source and scope of the problem in Canada.
CTX-M-15 is rare and was first reported in a GenBank entry as UOE-1 (accession no. AY013478). Karim et al. (17) described the first outbreak involving CTX-M-15 in a New Delhi hospital. The third and largest outbreak involving CTX-M-15 to date was reported in Toronto, Canada, and occurred in multiple long-term-care facilities between 2000 and 2002 (22). This outbreak involved the plasmid fingerprint pattern pC15-1a, identified in this study at a single hospital site in the east and three times at a single site in the west (Fig. 3B).
blaSHV ESBLs.
The SHV-type variants are the largest group of ESBLs identified in this study (64%). Many countries, including the United States (15, 31, 34, 43), have reported the SHV subtypes identified in Canada; however, a striking feature of the Canadian experience is the high numbers of strains harboring SHV-2a and SHV-12 relative to the frequency of other SHV types. This similarity seems to be mirrored by the Korean experience (18, 29). Analysis of the Korean strains harboring SHV-2a and SHV-12 suggests the possibility of direct evolution of SHV-12 from SHV-2a or coevolution of both genes (19, 20). PFGE and plasmid analyses clearly demonstrated diverse origins for strains harboring SHV-2a and SHV-12 in Canada. In addition, a comparison of plasmid profiles between SHV-2a and SHV-12 did not reveal any common RFLP types, further suggesting that, at least in Canada, the evolution of SHV-2a into SHV-12 has not taken place.
Novel bla ESBLs.
TEM-115, which contained Leu21Phe and Arg164His changes, was identified in this study. Leu21Phe is thought not to play a role in any expanded enzyme activity in vivo (30). Thus, the mature TEM-115 enzyme is identical to the TEM-29 enzyme, in which the Arg164His mutation has been shown to increase the hydrolysis of CTZ but not of CTX, as in clinical strains harboring TEM-115 (4).
SHV-40 contained the Leu35Gln change that has been found in the non-ESBL SHV-11 (40) as well as an Ala243Gly change that has been found only in the noncharacterized SHV-35 (GenBank accession no. AY070258). The two clinical strains harboring SHV-40 showed decreased susceptibility to CTZ, similar to what has been observed for strains harboring SHV ESBLs with changes at positions 238 and 240, which also showed increased resistance to CTZ (37). These data lead us to speculate that the Ala243Gly change in SHV-40 may be at least partially responsible for the ESBL phenotype observed in ESBL 214 and ESBL 263.
SHV-41 contains only a Val142Phe change compared to SHV-1 and has not been described before. The three strains harboring this variant showed decreased resistance to CTZ but remained susceptible to CTX.
SHV-42 contains an Ala25Ser change and a Met129Val change compared to SHV-1. The first change has not been described before, but the second has been found in SHV-25 (10) and SHV-37 (GenBank accession no. AF467948) in association with Thr18Ala and Leu35Gln changes in both enzymes and with an additional Gln278His change in SHV-37. SHV-25 is not an ESBL; therefore, it is unlikely that the Met129Val change contributes to the decreased susceptibility to CTZ and CTX in ESBL 168. The alanine at position 25 is the cleavage point of the leader peptide; therefore, the Ala25Ser change may affect the transfer of the enzyme precursor into the periplasmic space and/or cause a shift in the cleavage site, thereby changing the sequence of the mature protein. The Ile8Phe change in SHV-7 compared to SHV-1 was shown by site-directed mutagenesis to play a role in the decreased susceptibility to CTZ and CTX, and it was speculated that this enzyme may be more efficiently transferred into the periplasmic space (37).
NCCLS recently revised the criteria for screening potential ESBLs by changing the breakpoints for CPD from a disk zone diameter of ≤22 mm to one of ≤17 mm (25, 26). With the data presented in this report, 23% additional ESBLs would have been missed if this new criterion had been used as the sole screening method compared to the old guidelines. As suggested by NCCLS, the use of more than one antimicrobial agent for screening improves the sensitivity of detection (25, 26). The highest sensitivity was determined with either the CTX-CTZ-CPD22 combination or the CTZ-CPD22 combination, each of which predicted all but two ESBLs in this study. The two potential ESBLs not identified by these combinations were two novel SHVs (SHV-40 and SHV-41). Further studies are under way to determine whether these novel beta-lactamases are indeed ESBLs. Therefore, on the basis of the findings of this study, we suggest that laboratories use a minimum combination of CTZ and CPD22 to identify potential ESBLs in Canada.
This study documents the emergence of ESBLs in Canada, with only a single isolate being identified in the first month but 22 isolates being identified in the last month. In addition, the emergence was multicenter in nature and not due to a widespread geographical distribution of an epidemic strain or plasmid, as has been observed with another common Canadian nosocomial pathogen, methicillin-resistant S. aureus (38). All three of the most common Ambler class A genes (TEM, SHV, and CTX) have been identified in Canada. Laboratories across North America should be aware of these findings and evaluate their current screening protocols to ensure that all ESBLs will be identified.
Acknowledgments
We thank Romeo Hizon for contributions related to strain identification and antimicrobial susceptibility testing and Jennifer Campbell for molecular typing. We also acknowledge the contributions of students Rebekah Heibert and Darryl Johnstone for work involving plasmid profiling and Shaun Tyler and the staff of the DNA Core Facility at NML for generating the sequence information and synthesizing oligonucleotides.
This project was partially funded by Astra Zeneca Canada.
The members of The Canadian Nosocomial Infection Surveillance Program, Health Canada, are E. Bryce, Vancouver General Hospital, Vancouver, British Columbia, Canada; J. Conly, University Health Network, Toronto, Ontario, Canada; J. Embil, Health Sciences Centre, Winnipeg, Manitoba, Canada; J. Embree, Health Sciences Centre, Winnipeg, Manitoba, Canada; M. Gourdeau, Hôpital de l'Enfant-Jésus, Quebec City, Quebec, Canada; K. Green, Community and Hospital Infection Control Association—Canada; D. Gregson, St. Joseph's Health Centre, London, Ontario, Canada; B. A. Henderson, Peter Lougheed Centre, Calgary, Alberta, Canada.; J. Hutchinson, Health Sciences Centre, St. John's, Newfoundland, Canada; M. Ishak, Centre Hospitalier Angrignon, Verdun, Quebec, Canada; P. Jessamine, The Ottawa Hospital, Ottawa, Ontario, Canada; L. Johnston, Queen Elizabeth II Health Sciences Centre, Halifax, Nova Scotia, Canada; J. Langley, I. W. K. Grace Health Science Centre, Halifax, Nova Scotia, Canada; M. Loeb, Hamilton Health Sciences Corp., Hamilton, Ontario, Canada; A. Matlow, Hospital for Sick Children, Toronto, Ontario, Canada; A. McGeer, Mount Sinai Hospital, Toronto, Ontario, Canada; M. Miller, Jewish General Hospital, Montreal, Quebec, Canada; D. Moore, Montreal Children's Hospital, Montreal, Quebec, Canada; M. Mulvey, Canadian Science Centre for Human and Animal Health, Health Canada; M. Ofner-Agostini, Centre for Infectious Disease Prevention and Control, Health Canada; S. Paton, Centre for Infectious Disease Prevention and Control, Health Canada; A. Simor, Sunnybrook and Women's College Health Sciences Centre, Toronto, Ontario, Canada; G. Taylor, University of Alberta, Edmonton, Alberta, Canada; W. Thompson, The Moncton Hospital, Moncton, New Brunswick, Canada; M. Vearncombe, Sunnybrook and Women's College Health Sciences Centre, Toronto, Ontario, Canada; K. Weiss, Hopital Maisonneuve-Rosemont, Montreal, Quebec, Canada; A. Wong, Royal University Hospital, Saskatoon, Saskatchewan, Canada; and D. Zoutman, Kingston General Hospital, Kingston, Ontario, Canada.
REFERENCES
- 1.Arlet, G., G. Brami, D. Decre, A. Flippo, O. Gaillot, P. H. Lagrange, and A. Philippon. 1995. Molecular characterisation by PCR-restriction fragment length polymorphism of TEM beta-lactamases. FEMS Microbiol. Lett. 134:203-208. [DOI] [PubMed] [Google Scholar]
- 2.Bauernfeind, A., H. Grimm, and S. Schweighart. 1990. A new plasmidic cefotaximase in a clinical isolate of Escherichia coli. Infection 18:294-298. [DOI] [PubMed] [Google Scholar]
- 3.Bou, G., M. Cartelle, M. Tomas, D. Canle, F. Molina, R. Moure, J. M. Eiros, and A. Guerrero. 2002. Identification and broad dissemination of the CTX-M-14 beta-lactamase in different Escherichia coli strains in the northwest area of Spain. J. Clin. Microbiol. 40:4030-4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bou, G., J. Martínez-Beltrán, G. Cerveró, and J. C. Pérez-Díaz. 1999. Biochemical and genetic characteristics of TEM-29B, a novel extended spectrum β-lactamase. FEMS Microbiol. Lett. 174:185-190. [DOI] [PubMed] [Google Scholar]
- 5.Bradford, P. 2001. Extended-spectrum beta-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 14:933-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradford, P. A., C. E. Cherubin, V. Idemyor, B. A. Rasmussen, and K. Bush. 1994. Multiply resistant Klebsiella pneumoniae strains from two Chicago hospitals: identification of the extended-spectrum TEM-12 and TEM-10 ceftazidime-hydrolyzing beta-lactamases in a single isolate. Antimicrob. Agents Chemother. 38:761-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bush, K. 2001. New β-lactamases in Gram-negative bacteria: diversity and impact on the selection of antimicrobial therapy. Clin. Infect. Dis. 32:1085-1089. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 1997. Guidelines for the prevention of nosocomial pneumonia. Morb. Mortal. Wkly. Rep. 47:1-70. [Google Scholar]
- 9.Chanawong, A., F. H. M'Zali, J. Heritage, J. H. Xiong, and P. M. Hawkey. 2002. Three cefotaximases, CTX-M-9, CTX-M-13, and CTX-M-14, among Enterobacteriaceae in the People's Republic of China. Antimicrob. Agents Chemother. 46:630-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, F. Y., L. K. Siu, C. P. Fung, M. H. Huang, and M. Ho. 2001. Diversity of SHV and TEM beta-lactamases in Klebsiella pneumoniae: gene evolution in northern Taiwan and two novel beta-lactamases, SHV-25 and SHV-26. Antimicrob. Agents Chemother. 45:2407-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaves, J., M. G. Ladona, C. Segura, A. Coira, R. Reig, and C. Ampurdanes. 2001. SHV-1 beta-lactamase is mainly a chromosomally encoded species-specific enzyme in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 45:2856-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, C. 1984. Two improved promoter sequences for the β-lactamase expression arising from a single base pair substitution. Nucleic Acids Res. 12:3219-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Claeys, G., T. De Baere, M. Vaneechoutte, and G. Verschraegen. 1998. Caz-hi, an extended-spectrum TEM beta-lactamase (TEM-61), is derived from Caz-lo (TEM-11) by in vivo selection. Antimicrob. Agents Chemother. 42:3328-3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conly, J., M. Ofner-Agostini, S. Paton, L. Johnston, M. Mulvey, A. Kureishi, L. Nicolle, and A. Matlow. 2001. The Canadian Epidemiology Committee, and The Canadian Nosocomial Infection Surveillance Program. The emerging epidemiology of vancomycin-resistant enterococci in Canada: results of the Canadian Nosocomial Infection Surveillance Program Passive Reporting Network 1994-1998. Can. J. Infect. Dis. 12:364-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Agata, E., L. Venkataraman, P. DeGirolami, L. Weigel, M. Samore, and F. Tenover. 1998. The molecular and clinical epidemiology of enterobacteriaceae-producing extended-spectrum beta-lactamase in a tertiary care hospital. J. Infect. 36:279-285. [DOI] [PubMed] [Google Scholar]
- 16.Itokazu, G., J. Quinn, C. Bell-Dison, F. M. Kahan, and R. A. Weinstein. 1996. Antimicrobial resistance rates among aerobic gram-negative bacilli recovered from patients in intensive care units: evaluation of a national postmarketing surveillance program. Clin. Infect. Dis. 23:770-784. [DOI] [PubMed] [Google Scholar]
- 17.Karim, A., L. Poirel, S. Nagarajan, and P. Nordmann. 2001. Plasmid-mediated extended-spectrum beta-lactamase (CTX-M-3 like) from India and gene association with insertion sequence ISEcp1. FEMS Microbiol. Lett. 201:237-241. [DOI] [PubMed] [Google Scholar]
- 18.Kim, J., Y. Kwon, H. Pai, J. W. Kim, and D. T. Cho. 1998. Survey of Klebsiella pneumoniae strains producing extended-spectrum beta-lactamases: prevalence of SHV-12 and SHV-2a in Korea. J. Clin. Microbiol. 36:1446-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim, Y. K., H. Pai, H. J. Lee, S. E. Park, E. H. Choi, J. Kim, J. H. Kim, and E. C. Kim. 2002. Bloodstream infections by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in children: epidemiology and clinical outcome. Antimicrob. Agents Chemother. 46:1481-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, K. Y., J. D. Hopkins, and M. Syvanen. 1990. Direct involvement of IS26 in an antibiotic resistance operon. J. Bacteriol. 172:3229-3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez-Martinez, L., S. Hernandez-Alles, S. Alberti, J. M. Tomas, V. J. Benedi, and G. A. Jacoby. 1996. In vivo selection of porin-deficient mutants of Klebsiella pneumoniae with increased resistance to cefoxitin and expanded-spectrum cephalosporins. Antimicrob. Agents Chemother. 40:342-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller, M., A. McGeer, B. M. Willey, D. Reynolds, R. Malanczyj, M. Silverman, M. A. Green, and M. Culf. 2002. Outbreaks of multi-drug resistant Escherichia coli in long-term care facilities in the Durham, York and Toronto regions of Ontario, 2000-2002. Can. Commun. Dis. Rep. 28:113-118. [PubMed] [Google Scholar]
- 23.Mulvey, M. R., G. Soule, D. Boyd, W. Demczuk, and R. Ahmed. 2003. Characterization of the first extended-spectrum beta-lactamase-producing salmonella isolate identified in Canada. J. Clin. Microbiol. 41:460-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nathisuwan, S., D. S. Burgess, and J. S. Lewis. 2001. Extended-spectrum beta-lactamases: epidemiology, detection, and treatment. Pharmacotherapy 21:920-928. [DOI] [PubMed] [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards. 2003. Performance standards for disk susceptibility tests, 8th ed. Approved standard M2-A8. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 26.National Committee for Clinical Laboratory Standards. 2000. Methods for disk susceptibility tests for bacteria that grow aerobically, 7th ed. Approved standard M2-A7. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 27.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard NCCLS M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 28.Nuesch-Inderbinen, M. T., H. Hachler, and F. H. Kayser. 1996. Detection of genes coding for extended-spectrum SHV beta-lactamases in clinical isolates by a molecular genetic method, and comparison with E test. Eur. J. Clin. Microbiol. Infect. Dis. 15:398-402. [DOI] [PubMed] [Google Scholar]
- 29.Pai, H., S. Lyu, J. H. Lee, J. Kim, Y. Kwon, J. W. Kim, and K. W. Choe. 1999. Survey of extended-spectrum beta-lactamases in clinical isolates of Escherichia coli and Klebsiella pneumoniae: prevalence of TEM-52 in Korea. J. Clin. Microbiol. 37:1758-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Philippon, A., R. Labia, and G. Jacoby. 1989. Extended-spectrum β-lactamases. Antimicrob. Agents Chemother. 33:1131-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pitout, J. D., K. S. Thomson, N. D. Hanson, A. F. Ehrhardt, P. Coudron, and C. C. Sanders. 1998. Plasmid-mediated resistance to expanded-spectrum cephalosporins among Enterobacter aerogenes strains. Antimicrob. Agents Chemother. 42:596-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pittet, D. 1997. Prevention and control of nosocomial infections, p. 712-769. In R. Wenzel (ed.), Nosocomial blood stream infections, 3rd ed. The Williams & Wilkins Co., Baltimore, Md.
- 33.Poyart, C., P. Mugnier, G. Quesne, P. Berche, and P. Trieu-Cuot. 1998. A novel extended-spectrum TEM-type beta-lactamase (TEM-52) associated with decreased susceptibility to moxalactam in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 42:108-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quale, J. M., D. Landman, P. A. Bradford, M. Visalli, J. Ravishankar, C. Flores, D. Mayorga, K. Vangala, and A. Adedeji. 2002. Molecular epidemiology of a citywide outbreak of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae infection. Clin. Infect. Dis. 35:834-841. [DOI] [PubMed] [Google Scholar]
- 35.Quinn, J. 1998. Clinical strategies for serious infection: a North American perspective. Diagn. Microbiol. Infect. Dis. 31:389-395. [DOI] [PubMed] [Google Scholar]
- 36.Radice, M., P. Power, J. Di Conza, and G. Gutkind. 2002. Early dissemination of CTX-M-derived enzymes in South America. Antimicrob. Agents Chemother. 46:602-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Randegger, C. C., A. Keller, M. Ira, A. Wada, and H. Hächler. 2000. Contribution of natural amino acid substitutions in SHV extended-spectrum β-lactamases to resistance against various β-lactams. Antimicrob. Agents Chemother. 44:2759-2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simor, A. E., M. Ofner-Agostini, E. Bryce, A. McGeer, S. Paton, M. R. Mulvey, et al. 2002. Laboratory characterization of methicillin-resistant Staphylococcus aureus in Canadian hospitals: results of 5 years of national surveillance, 1995-1999. J. Infect. Dis. 186:652-660. [DOI] [PubMed] [Google Scholar]
- 39.Simor, A. E., M. Ofner-Agostini, E. Bryce, K. Green, A. McGeer, M. Mulvey, S. Paton, et al. 2001. The evolution of methicillin-resistant Staphylococcus aureus in Canadian hospitals: five years of national surveillance. Can. Med. Assoc. J. 165:21-26. [PMC free article] [PubMed] [Google Scholar]
- 40.Speldooren, V., B. Heym, R. Labia, and M. H. Nicolas-Chanoine. 1998. Discriminatory detection of inhibitor-resistant β-lactamases in Escherichia coli by single-strand conformation polymorphism-PCR. Antimicrob. Agents Chemother. 42:879-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swaminathan, B., T. Barrett, S. B. Hunter, R. Tauxe, and the CDC PulseNet Task Force. 2001. PulseNet: the molecular subtyping network for foodborne bacterial disease surveillance, United States. Emerg. Infect. Dis. 7:382-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tzouvelekis, L. S., E. Tzelepi, P. T. Tassios, and N. J. Legakis. 2000. CTX-M-type beta-lactamases: an emerging group of extended-spectrum enzymes. Int. J. Antimicrob. Agents 14:137-142. [DOI] [PubMed] [Google Scholar]
- 43.Venezia, R. A., F. J. Scarano, K. E. Preston, L. M. Steele, T. P. Root, R. Limberger, W. Archinal, and M. A. Kacica. 1995. Molecular epidemiology of an SHV-5 extended-spectrum beta-lactamase in Enterobacteriaceae isolated from infants in a neonatal intensive care unit. Clin. Infect. Dis. 21:915-923. [DOI] [PubMed] [Google Scholar]
- 44.Weber, D. A., C. C. Sanders, J. S. Bakken, and J. P. Quinn. 1990. A novel chromosomal TEM derivative and alterations in outer membrane proteins together mediate selective ceftazidime resistance in Escherichia coli. J. Infect. Dis. 162:460-465. [DOI] [PubMed] [Google Scholar]
- 45.Winokur, P. L., R. Canton, J. M. Casellas, and N. Legakis. 2001. Variations in the prevalence of strains expressing an extended-spectrum beta-lactamase phenotype and characterization of isolates from Europe, the Americas, and the Western Pacific region. Clin. Infect. Dis. 32(Suppl. 2):S94-S103. [DOI] [PubMed] [Google Scholar]
- 46.Yuan, M., L. M. Hall, J. Hoogkamp-Korstanje, and D. M. Livermore. 2001. SHV-14, a novel beta-lactamase variant in Klebsiella pneumoniae isolates from Nijmegen, The Netherlands. Antimicrob. Agents Chemother. 45:309-311. [DOI] [PMC free article] [PubMed] [Google Scholar]