Abstract
Isolates of Aspergillus fumigatus that demonstrate resistance to itraconazole (ITZ) have been described previously; however, the prevalence and clinical significance of ITZ resistance are not completely understood. In this study we assessed the ITZ susceptibilities of 128 A. fumigatus isolates that caused invasive infection in 82 stem cell transplant patients before and after the use of ITZ in our institution (study period, 1991 to 2000). The MICs for 10 isolates obtained from seven patients were high, ≥1 μg/ml. The average ITZ MIC increased after institutional use of the drug began in 1995. The majority of the isolates for which MICs were high (6 of 10) and one isolate for which the MIC was low (0.06 μg/ml) demonstrated an unusual phenotype, appearing as predominantly white colonies. For all seven atypical isolates, voriconazole MICs were high (≥2 μg/ml), and minimal effective concentrations of caspofungin were high (≥4 μg/ml). For two of the seven atypical isolates, amphotericin B MICs were high (≥2 μg/ml). The isolates appeared white due to slow sporulation; however, after prolonged incubations, the isolates sporulated with no difference in conidial color or conidiophore morphology compared with typical isolates. Randomly amplified polymorphic DNA-PCR patterns of these isolates were distinct compared with those of other A. fumigatus isolates. Sequencing of 18S rRNA genes confirmed that all were A. fumigatus; however, the mitochondrial cytochrome b gene sequences of all the atypical isolates were unique. These data suggest the potential presence of a genetically unique, poorly sporulating variant of A. fumigatus that demonstrates decreased susceptibilities to several antifungals.
Morbidity and mortality caused by Aspergillus species have increased dramatically in recent years, particularly in patients who receive cytotoxic chemotherapy or hematopoietic stem cell transplantation (SCT). In vitro resistance to itraconazole (ITZ) and amphotericin B (AMB), antifungals used to treat or prevent invasive aspergillosis (IA), has been reported; however, the clinical significance of antifungal drug resistance among Aspergillus species is not presently understood (13).
Most Aspergillus infections in humans appear to be caused by typical A. fumigatus isolates that have green, echinulate conidia (A. fumigatus Fresenius) (24, 25). However, infections with atypical variants that differ based on minor morphological features have been reported; for instance, A. fumigatus var. ellipticus has smooth, ellipsoid conidia, and A. fumigatus var. albus has white conidia (17, 23, 29). While variants have identical 18S rRNA gene sequences, they differ based on unique mitochondrial cytochrome b gene sequences (9, 12, 29). To date, there has been no indication that the variants have different antifungal susceptibility patterns or that antifungal-resistant A. fumigatus isolates are unique variants.
This study was performed to assess the ITZ susceptibilities of a large number of A. fumigatus isolates obtained from SCT patients at the Fred Hutchinson Cancer Research Center (FHCRC) during the 1990s. The results suggest that ITZ resistance is infrequent, but MICs increased in years subsequent to the start of institutional use of the drug. Most of the A. fumigatus isolates for which ITZ MICs were high had a variant phenotype and a unique genotype. Several also displayed decreased susceptibilities to other antifungals (AMB, ITZ, voriconazole [VRZ], and caspofungin [CAS]).
MATERIALS AND METHODS
Patients and Aspergillus isolates.
Patients who developed proven or probable IA at the FHCRC had been previously identified by a review of microbiology, histopathology, and infection control records (15). In this study, the patients' charts were reviewed to determine the basic features of infection (e.g., organ involvement) and prior antifungal therapy. Aspergillus isolates obtained from patients between 1991 and 2000 had been stored frozen on potato dextrose agar (PDA) (Becton Dickinson, Sparks, Md.) slants at −20°C. One hundred twenty-eight A. fumigatus isolates, obtained between 1991 and 2000 from 82 patients with proven or probable IA, were recovered for analysis of ITZ susceptibilities. This study was approved by the FHCRC Institutional Review Board.
All isolates obtained from primary cultures were subcultured at least twice on PDA (at 35°C for 5 to 7 days) to ensure optimal growth. Microscopic evaluation of conidiophore morphology and thermotolerance studies were performed to verify that isolates were A. fumigatus. Growth at an elevated temperature (48°C) was interpreted as confirmatory identification of A. fumigatus (28).
Media, reagents, and antifungals.
The media used in this study included RPMI 1640 (buffered with 0.165 M morpholinepropanesulfonic acid [MOPS] to pH 7.0; Sigma Chemical Co., St. Louis, Mo.) with l-glutamine but without bicarbonate, PDA (Becton Dickinson), Czapek Dox (CZD; Becton Dickinson), malt extract agar (Becton Dickinson), and Sabouraud dextrose agar (Becton Dickinson). 2,3-Bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) and coenzyme Q (CoQ; 2,3-dimethoxy-5-methyl-1,4-benzoquinone) were purchased from Sigma.
The antifungal agents AMB (Bristol-Myers Squibb Pharmaceutical Research Institute, Wallingford, Conn.), ITZ (Ortho Biotech, Bridgewater, N.J.), and VRZ (Pfizer Pharmaceuticals, New York, N.Y.) were dissolved in dimethyl sulfoxide, and CAS (Merck & Co., Inc., Rathway, N.J.) was dissolved in distilled water. Further dilutions were made in RPMI 1640, as previously outlined in National Committee for Clinical Laboratory Standards (NCCLS) document M38-A (20).
Antifungal drug susceptibility testing.
ITZ susceptibilities for all isolates were first determined by the E-test (6). In brief, A. fumigatus conidia (106) were plated onto RPMI 1640 agar supplemented with 2% glucose (Sigma) and the plates were allowed to dry. E-test strips containing ITZ (AB Biodisk, Solna, Sweden) were applied, and MICs were determined after 48 h of incubation at 35°C. The E-test MIC was considered to be the drug concentration at the point where dense colonial growth intersected the strip; sparse subsurface hyphal growth at the margins was ignored.
Those A. fumigatus isolates for which MICs of ITZ as determined by the E-test were ≥1.0 μg/ml were reevaluated for susceptibilities to ITZ using the NCCLS M-38A broth microdilution method (20). VRZ and AMB MICs and minimal effective concentrations (MECs) of CAS were determined for the seven atypical isolates by the NCCLS method as well. Briefly, conidial inocula were prepared by flooding Aspergillus colonies with sterile RPMI 1640 containing 0.025% Tween 20 and gently probing the colonies with a pipette tip. The resulting suspension was vortexed, heavy particles were allowed to settle for 3 to 5 min, and the upper layer was adjusted to a percentage of transmittance of 80 to 82% with a spectrophotometer (wavelength, 530 nm). The adjusted inoculum was diluted 1:50 in RPMI 1640, 100 μl was dispensed into microtiter wells containing serial twofold dilutions of the respective antifungals, and plates were incubated for 48 h at 35°C. Optical densities (OD) were read at 450 nm with an LP 400 microplate reader (Bio-Rad Laboratories, Richmond, Calif.). Growth in each well was calculated relative to that of a growth control by the following formula: [(OD of drug-containing well − background OD)/(OD of drug-free well − background OD)] × 100. Per the NCCLS recommendation, MICs of ITZ, VRZ, and AMB were defined as the lowest concentration of the drug that resulted in 100% growth reduction compared to the growth of the drug-free control. The minimal fungicidal concentration was determined by culturing 100 μl of medium from each of the four wells exhibiting no growth with concentrations above the MIC onto Sabouraud dextrose agar plates and incubating the plates at 35°C. The minimal fungicidal concentration was defined as the lowest concentration of antifungal compound that resulted in the growth of three or fewer colonies. For CAS, the MEC was defined as the minimum concentration of drug that produced morphological alterations, such as abnormal hyphal growth with highly branched tips, swollen germ tubes, and distended balloon-like hyphae, as viewed under a light microscope (10). Susceptibility was determined by duplicate measures in three different experiments.
Since the accuracy of a MIC determined by spectrophotometric readings may be hampered by the nonhomogenous growth of filamentous fungi, a colorimetric method, the XTT assay, was used to reassess the susceptibilities of the seven atypical isolates to AMB, ITZ, and VRZ according to a previously published method (18). Briefly, microdilution plates were prepared and incubated at 35°C as described above. After 48 h, the plates were centrifuged at 1200 × g, the medium was removed, and 200 μl of an XTT-CoQ suspension (50 μg of XTT/ml and 50 μg of CoQ/ml in phosphate-buffered saline) was added. Plates were incubated at 37°C on a rotating shaker. After 1 h, the supernatant was transferred to a fresh 96-well plate and the absorbance was read at 450 nm using a Bio-Tek Synergy HT microplate reader (Bio-Tek Instruments, Winooski, Vt.). The MIC was determined to be the drug concentration at which the OD was 90% lower than that of the growth control (18)
Growth studies.
Since 7 of 128 isolates sporulated poorly on PDA, the growth patterns were studied in more depth on multiple media (CZD, malt extract agar, PDA, and RPMI 1640 with 2% glucose). A comparison of conidiations on solid media was also performed by previously described methods (27). In brief, 10 μl of conidial suspension (106 conidia/ml) of each isolate was spread on medium plates and incubated at 37°C for 5 days, at the end of which a plug of agar (1 cm2) was removed and suspended in 1 ml of distilled water, and the number of conidia per square millimeter was calculated. To verify whether the hyphal growth rates of these atypical isolates were similar to those of wild-type A. fumigatus isolates, radial growth on solid media was measured for all seven atypical isolates and five randomly selected wild-type A. fumigatus isolates. In brief, 10 μl of the conidial suspension (105 conidia/ml) was placed on the center of the agar plates and incubated at 25 and 37°C, and the diameter of the growing colony was measured every 24 h up to 92 h. To quantify hyphal growth in liquid medium, 10 μl of conidial suspension (106 conidia/ml) was inoculated into 25 ml of RPMI 1640 and incubated for 5 days at 35°C with shaking. After the incubation period, the fungal mat was separated from the media by filtration and dried on Whatman no. 1 filter paper for 30 min at 37°C, and the wet weight was determined. An assessment of growth differences in liquid media was also performed by the XTT assay, which was previously shown to be a reliable indicator of fungal biomass (18). In brief, 200 μl of 105 conidia/ml of RPMI 1640 was incubated at 25 and 35°C for 0, 16, 20, and 40 h. After each of the respective incubation times, the XTT assay was performed as described above and the absorbance at 450 nm was plotted for both the wild-type and atypical isolates. The percentages of atypical and wild-type A. fumigatus conidia that had germinated after 8 to 10 h of incubation in liquid media in 96-well plates were also determined. At least 200 conidia per well were counted, and the experiments were repeated three times.
Molecular analyses.
To ensure that the atypical isolates were A. fumigatus, 18S rRNA genes of representative wild-type (n = 4) and all atypical (n = 7) A. fumigatus isolates were sequenced and compared with the published A. fumigatus sequence (GenBank accession no. AF109329) and Neosartorya fischeri sequence (GenBank accession no. AF176661). Fungal mats were grown in liquid culture and disrupted with glass beads, and the extracted RNA was purified in an RNeasy mini kit (QIAGEN, Valencia, Calif.). rRNA genes were sequenced after PCR amplification of the 18S region using 5′-CTGGTTGATCCTGCCAGTAGT-3′ as the forward primer and 5′-ACCTACGGAAACCTTGTTACGA-3′ as the reverse primer. Amplification conditions consisted of an initial denaturation step of 2 min at 94°C followed by 30 cycles of 15 s at 94°C, 30 s at 59°C, and 1 min at 72°C. A final extension of 7 min at 72°C followed the last amplification cycle. Products were gel isolated, purified, and sequenced with a Dye Terminator cycle sequencing ready reaction kit on a model 377 DNA sequencer (Applied Biosystems, Foster City, Calif.). Sequences were compared using the Bioedit sequence alignment editor software (North Carolina State University).
Randomly amplified polymorphic DNA-PCR (RAPD-PCR) was performed to determine if the atypical isolates had distinct banding patterns compared to those of other typical A. fumigatus isolates. Genomic DNA was isolated from a 7-day-old mycelial mat cultured in Sabouraud dextrose broth by liquid nitrogen hyphal disruption and was purified with a QIAGEN mini kit. PCR amplification was performed in 25-μl volumes containing 50 mM KCl, 10 mM Tris-HCl (pH 8.0), 1.25 mM MgCl2, a 200 μM concentration of each deoxynucleoside triphosphate, 1.0 μM primer, 2 μg of genomic DNA, and 0.625 U of Taq DNA polymerase (Invitrogen, Carlsbad, Calif.). Primers included p2 (5′-GCTGGTGG), p5 (5′-GCGCACGG), R108 (5′-GTATTGCCCT), and R151 (5′-GCTGTAGTGT) as previously described (1). Reactions were carried out in a model 9700 GeneAmp PCR system (Applied Biosystems), starting with an initial denaturation step of 5 min at 94°C, followed by 45 cycles of 1 min at 94°C, 2 min at 35°C, and 2 min at 72°C. A final extension of 10 min at 72°C followed the last amplification cycle. Ribosomal intergenic spacer regions were also amplified by PCR using primers IGS-L (5′-TAGTACGAGAGGAACCGT) and IGS-R (5′-CATATGACTACTGGCAG) (22). Reactions consisted of an initial denaturation step of 3 min at 94°C, followed by 40 cycles of 30 s at 94°C, 1 min at 45°C, and 2 min at 72°C. A final extension of 8 min at 72°C followed the last amplification cycle. Amplification products were visualized on a 2.0% agarose gel containing 0.5 μg of ethidium bromide/ml.
To determine if the atypical isolates were distinct variants of A. fumigatus, a fragment of mitochondrial cytochrome b DNA was amplified by PCR and sequenced; this method had been shown previously to distinguish variants of A. fumigatus (29). Genomic DNA was isolated as previously described (2). E1m (5′-TGAGGTGCTACAGTTATTAC) was used as the forward primer, and E2 (5′-GGTATAGMTCTTAAWATAGC) was used as the reverse primer. Amplification conditions consisted of an initial denaturation step of 2 min at 94°C followed by 30 cycles of 1 min at 94°C, 1 min at 50°C, and 2 min at 72°C. A final extension of 8 min at 72°C followed the last amplification cycle. Products were gel isolated and purified with a QIAquick gel extraction kit (QIAGEN). Both strands were sequenced as described above and compared with published sequences by using Bioedit sequence alignment editor software.
RESULTS
Susceptibility to antifungals.
MICs of ITZ for 128 A. fumigatus isolates were determined with the E-test. One hundred eighteen of 128 isolates (92%) were susceptible to ITZ (MIC < 1 μg/ml), and MICs were elevated (≥1 μg/ml) for 10 of 128 isolates (7.8%). MICs for these isolates were confirmed to be 1 μg/ml by the NCCLS microdilution method. All of the isolates for which MICs were high were obtained from patients who developed infections after 1995; the mean MIC for A. fumigatus isolates obtained from patients after 1995 was higher than the MICs for isolates obtained between 1991 and 1994 (0.4 versus 0.07 μg/ml; P < 0.0001, Mann-Whitney test).
The majority of the isolates for which ITZ MICs were high (6 of 10) and one isolate that was susceptible to ITZ grew with a slow-sporulation phenotype. These seven atypical isolates were recovered from four different patients who had undergone allogeneic SCTs between 1995 and 2000. All patients had had prior exposure to fluconazole (FLU); none had been previously exposed to ITZ (Table 1). These atypical A. fumigatus isolates were also tested for susceptibilities to AMB, VRZ, and CAS. For all seven isolates, MICs of VRZ were high (≥2 μg/ml) and MECs of CAS were also high (≥4 μg/ml). For four of the seven, AMB MICs were high (≥2 μg/ml) (Table 1). The ITZ, VRZ, and AMB MICs were confirmed by the XTT reduction assay (data not shown). The other three A. fumigatus isolates for which ITZ MICs were high had normal-sporulation phenotypes and were highly susceptible to AMB, VRZ, and CAS (data not shown).
TABLE 1.
Origins and antifungal susceptibilities of atypical isolates
| Patient | Isolate | Yra | Prior therapyb | Site of isolationc | MIC/MFCd (μg/ml)
|
MEC of CAS (μg/ml) | ||
|---|---|---|---|---|---|---|---|---|
| ITZ | VRZ | AMB | ||||||
| 1 | W1 | 1995 | FLU, AMB | Lung | 0.5/1 | 2/4 | 2/4 | 16 |
| W2 | 1995 | FLU, AMB | Kidney | 1/2 | 2/4 | 1/2 | 4 | |
| 2 | W3 | 1998 | FLU | Mouth | 1/2 | 2/4 | 2/2 | 16 |
| W4 | 1998 | FLU | Lung | 1/2 | 2/4 | 1/2 | >32 | |
| 3 | W5 | 1999 | FLU | Lung | 1/2 | 2/2 | 2/4 | 16 |
| 4 | W6 | 2000 | AMB, VRZ | Lung | 1/2 | 2/4 | 1/4 | 32 |
| W7 | 2000 | AMB, VRZ | Mouth | 1/2 | 2/4 | 2/4 | 32 | |
Year during which isolates were recovered from the indicated site.
Antifungal therapy prior to day of infection.
Isolates recovered from either bronchoalveolar lavage or lung biopsy specimens are indicated as “lung.” Kidney isolates were recovered at autopsy.
MICs shown were determined by the NCCLS method, as described in Materials and Methods.
Phenotypic characterization of the atypical isolates.
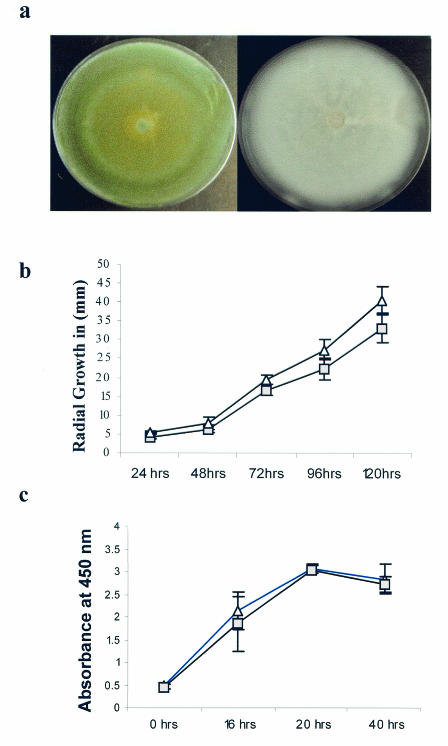
All seven of the atypical isolates were confirmed to be A. fumigatus by microscopic evaluation and thermotolerance studies. The atypical A. fumigatus isolates grew as fluffy white colonies consisting predominantly of vegetative hyphae after 7 days of incubation (Fig. 1a, right panel). After prolonged incubation (10 to 12 days) on PDA, the colonies began sporulating, macroscopically appearing similar to typical isolates that sporulate within 7 days of incubation. The slow-sporulation phenotype was reproducible in all media and temperatures tested. Averages of conidial production per unit area of all seven atypical isolates were 10-fold lower than those of wild-type isolates (data not shown).
FIG. 1.
(a) Typical blue-green sporulating colonies of A. fumigatus Fresenius (left panel) are shown compared to fluffy white colonies of the poorly sporulating variant, which are composed of predominantly sterile hyphae (right panel). Both pictures were taken after 7 days of incubation on CZD at 35°C. (b) Comparison of the radial growth of the atypical and wild-type A. fumigatus strains, with both isolates growing on CZD. Each gray square represents the average radius of colony growth of seven atypical A. fumigatus isolates, and the white triangles represent the average radius of colony growth of A. fumigatus wild-type isolates from three individual experiments (± the standard deviation). Radial growth was measured as the radius of the expanding colony in millimeters (y axis) relative to hours of incubation (x axis). Growth rates during a 37°C incubation are shown. Growth rates did not differ when the isolates were incubated at 25°C or when they were grown on multiple media (not shown). (c) Reduction of the XTT dye by the atypical (white triangles) and wild-type (gray squares) isolates over time. The XTT dye was used as an indicator of fungal burden.
We considered the possibility that susceptibility results may have been impacted by decreased quantities of conidia comprising the testing inocula. Susceptibilities were reevaluated and reconfirmed using NCCLS methods after extended incubations and microscopic confirmation of sporulation. We also considered the possibility that the MICs may have been impacted by differential hyphal growth rates. As MICs are calculated by comparing growth (turbidity) in the presence of a drug to growth in a drug-free well, testing of an isolate that does not grow adequately in culture may yield a falsely high MIC that reflects decreased overall turbidities. Also, it is possible that the drugs tested may not have a measurable effect on cells that are not actively replicating. There was no difference in the radii of growth of wild-type and atypical isolates, regardless of the medium tested (Fig. 1b). We also compared the wet weights of the atypical isolates with those of representative wild-type A. fumigatus isolates (in the absence of drug) and found no difference in overall fungal mass (wet weight) of Aspergillus isolates. After 5 days of incubation, the mean wet weight of five wild-type isolates was 54 ± 14 mg, and that of the seven atypical isolates was 60 ± 24 mg. Similarities in growth rates were confirmed by the XTT reduction assay (Fig. 1c). There was also no difference in the percentages of conidia that had germinated after 8 to 10 h of incubation in RPMI 1640 (52% of wild-type versus 56% of atypical isolates). Finally, the absolute ODs of the growth controls were no different among isolates for which the MICs were high or low (data not shown). Thus, the growth rates of the wild-type and atypical isolates on multiple media and at two different temperatures did not differ when they were measured by multiple methods.
Molecular characterization of the variants.
The asexual state of the teleomorph N. fischeri is very similar to that of A. fumigatus (14), and these organisms can be misidentified by microscopic examination. To verify that the seven atypical isolates recovered in this study were A. fumigatus, rRNA genes of the atypical isolates were sequenced and compared with the published A. fumigatus and N. fischeri sequences. All sequences were 100% identical to the published A. fumigatus sequence and different from that for N. fischeri, confirming the atypical isolates to be A. fumigatus (data not shown).
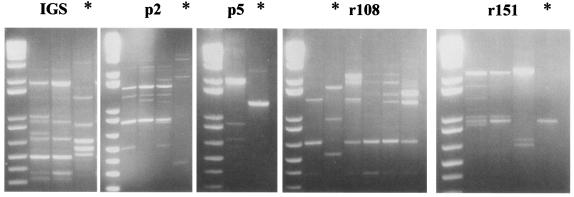
To determine if the atypical isolates were distinct from other A. fumigatus isolates and to compare them to each other, genotypes were examined by RAPD-PCR (8, 22). The profiles of all atypical isolates were similar to each other but differed substantially from the patterns of the representative typical A. fumigatus isolates (Fig. 2).
FIG. 2.
RAPD-PCR profiles. Profiles of several typical A. fumigatus isolates and one atypical variant (indicated by *) are shown. Primers utilized for each reaction are indicated. DNA molecular weight markers are shown in the leftmost lane of each panel.
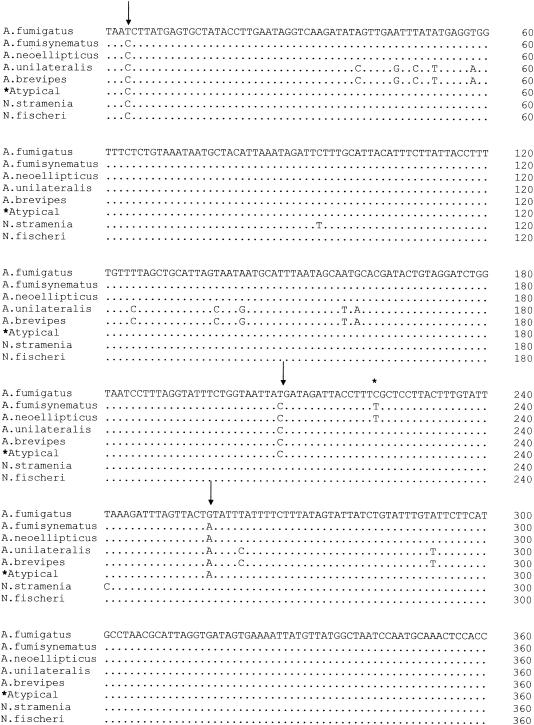
Previous studies have shown that A. fumigatus variants (also referred to as “substrains”) have unique mitochondrial cytochrome b gene sequences (29). We therefore sequenced a region of the mitochondrial cytochrome b gene in atypical isolates (n = 7) and several of the representative typical A. fumigatus isolates (n = 4) and compared these with the published sequences of other A. fumigatus variants and Neosartorya species (Fig. 3). The mitochondrial subunit b gene sequences of the representative typical A. fumigatus isolates (obtained from clinical samples) were 100% homologous with the published sequence of A. fumigatus Fresenius. In contrast, the nucleotide sequence of the atypical isolates was unique and conserved among all seven atypical isolates examined. Positions in which the sequences of the atypical isolates differed from typical A. fumigatus isolates are indicated in Fig. 3. The sequence of the atypical isolates was most similar to that of A. fumigatus var. neoellipticus but differed at position 223 (T→C). In addition, the sequence of the atypical isolates differed from two Neosartorya sequences examined. Collectively, these data suggest that the atypical isolates, which have decreased susceptibilities to several antifungals, are phenotypically and genetically distinct from A. fumigatus Fresnius.
FIG. 3.
Comparison of mitochondrial subunit b gene sequences. Sequences of genes from atypical isolates are compared with A. fumigatus Fresenius, other known Aspergillus variants, and Neosartorya species (29). Only nucleotides that differ from those of A. fumigatus Fresenius (top) are shown. The sequences of the atypical isolates differed from those of A. fumigatus (indicated by arrows), A. fumigatus var. neoellipticus (indicated by *), and Neosartorya species.
DISCUSSION
The resistance of Aspergillus to antifungals has been recognized previously, with most recent studies suggesting that ITZ and AMB resistance among Aspergillus species may, at least in part, contribute to therapeutic failures (5, 11). We have observed a time-dependent increase in ITZ MICs for a large number of invasive Aspergillus isolates recovered from patients with infection in our center. The relative increase in MICs appears to be associated with infections caused by phenotypically and genotypically unique A. fumigatus isolates for which MICs of ITZ and other antifungals with different modes of action are frequently high.
A. fumigatus is identified in the laboratory predominantly by the characteristic green echinulate conidia, produced basipetally in chains from greenish uniseriate phialides. The conidial state of A. fumigatus is morphologically very similar to that of N. fischeri, an ascomycete that has been considered by some taxonomists to be the putative sexual stage of, or at least to be identical to, an ancestor of A. fumigatus (7). In the laboratory, N. fischeri appears at first as white, velvety colonies, which later become granular with formation of the teleomorphic state (ascomata). Although all of our typical isolates initially grew as white colonies (sterile hyphae), they never produced any cleistothecia and all were genotypically distinct from Neosartorya species, differing both in rRNA gene and mitochondrial cytochrome b gene sequence.
A. fumigatus as a species is morphologically heterogeneous, which has led to the description of several varieties of the species, including A. fumigatus var. albus, var. acolumnaris, var. brevipes, var. phialiseptus, var. ellipticus, and var. sclerotiorum. In most of these fungi the distinctions are based on only slight morphological differences, like color of the conidia and septation of the phialides. However, the species distinction for these variants of A. fumigatus is controversial, largely because of similar secondary-metabolite profiles and DNA complementarity (19, 29). The pathogenic potentials and antifungal susceptibilities of these fungi have not been compared. The results of our study suggest that the slowly sporulating isolates of A. fumigatus (all clinical samples), which had unique RAPD-PCR patterns and a distinct mitochondrial cytochrome b gene sequence, have the potential of being less susceptible to several antifungals. A new variant designation for these isolates is being presented, and a taxonomic description will follow in a future publication. Correct identification and characterization of these atypical isolates may have clinical and epidemiological significance.
MICs of multiple antifungal agents are higher for atypical isolates than for the wild-type A. fumigatus isolates evaluated in our laboratory (data not shown) and the average wild-type isolates evaluated in prior studies (3, 5). Growth rates alone are not likely to explain the high MICs measured for the atypical isolates. Whether the high MICs for the atypical A. fumigatus isolates signify true resistance to antifungal drugs is not clear, as breakpoints have not been generated from large studies and in vivo responses have not yet been assessed. Although cross-resistance of A. fumigatus to different azoles has been reported (30), there have been no reports of high MICs of multiple classes of drugs for A. fumigatus. Recently, an isolate of Candida albicans with resistance to both ITZ and CAS was described; overexpression of an ABC transporter was implicated as a potential mechanism explaining resistance to both drugs (26). Transporters or differences in the compositions of cell walls and/or membranes may explain the high MICs for our isolates. Alternatively, the high MICs may be associated with other factors that impact the relative fitness of these isolates upon ex vivo exposure to antifungals. The mechanism of resistance, stability of the phenotype, and significance of the decreased susceptibilities in vivo are currently being determined.
It is not clear whether the high MICs found in the present study correlate to innate or acquired traits of the isolates, although innate resistance seems less likely given the variable frequency of high MICs. Another explanation for the apparent resistance could be selection pressure that might occur during azole therapy, as has been described with C. albicans (16). Although none of the patients from whom variants were isolated had prior exposure to ITZ or CAS, all had prior exposure to an azole drug (FLU) and AMB, and one patient had received VRZ.
Whether the atypical isolates described are ubiquitous or represent a strain unique to our institution is not known. The finding that the infected patients were not clustered with regard to either time or place suggests that this isolate may be prevalent in the environment and not acquired from a single source of exposure. Also, there is indirect evidence in the literature that similar isolates have caused disease in other patients. Dannaoui and coworkers reported the isolation of four slowly sporulating A. fumigatus isolates that had similar RAPD patterns among themselves but whose patterns differed from those of other clinical isolates of A. fumigatus (4). These strains also demonstrated an acquired resistance to ITZ, but no attempt was made by these investigators to further characterize the organism. Recently, in an outbreak of A. fumigatus infections among renal transplant recipients (21), the majority of the isolates recovered demonstrated an atypical, slow-sporulation phenotype (A. Panackal and M. Brandt, verbal communication). It will be necessary to document the presence of this organism among other culture collections using molecular studies in order to further assess the clinical significance of our observations.
This is the first report of what appears to be a distinct variant of A. fumigatus that causes invasive infection and demonstrates decreased susceptibilities to multiple antifungal drugs. Further studies are necessary to define the clinical significance, prevalence, and mechanisms of resistance of these atypical isolates of A. fumigatus.
Acknowledgments
This work was supported by grants from the American Lung Association and the National Institutes of Health (K08 AI 01571 and R21 AI 55928).
REFERENCES
- 1.Anderson, M. J., K. Gull, and D. W. Denning. 1996. Molecular typing by random amplification of polymorphic DNA and M13 Southern hybridization of related paired isolates of Aspergillus fumigatus. J. Clin. Microbiol. 34:87-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aufauvre-Brown, A., J. Cohen, and D. W. Holden. 1992. Use of randomly amplified polymorphic DNA markers to distinguish isolates of Aspergillus fumigatus. J. Clin. Microbiol. 30:2991-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chandrasekar, P. H., J. L. Cutright, and E. K. Manavathu. 2001. Aspergillus: rising frequency of clinical isolation and continued susceptibility to antifungal agents, 1994-1999. Diagn. Microbiol. Infect. Dis. 41:211-214. [DOI] [PubMed] [Google Scholar]
- 4.Dannaoui, E., E. Borel, M. F. Monier, M. A. Piens, S. Picot, and F. Persat. 2001. Acquired itraconazole resistance in Aspergillus fumigatus. J. Antimicrob. Chemother. 47:333-340. [DOI] [PubMed] [Google Scholar]
- 5.Denning, D. W., S. A. Radford, K. L. Oakley, L. Hall, E. M. Johnson, and D. W. Warnock. 1997. Correlation between in-vitro susceptibility testing to itraconazole and in-vivo outcome of Aspergillus fumigatus infection. J. Antimicrob. Chemother. 40:401-414. [DOI] [PubMed] [Google Scholar]
- 6.Espinel-Ingroff, A., and A. Rezusta. 2002. E-test method for testing susceptibilities of Aspergillus spp. to the new triazoles voriconazole and posaconazole and to established antifungal agents: comparison with NCCLS broth microdilution method. J. Clin. Microbiol. 40:2101-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Girardin, H., M. Monod, and J.-P. Latge. 1995. Molecular characterization of the food-borne fungus Neosartorya fischeri (Malloch and Cain). Appl. Environ. Microbiol. 61:1378-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henry, T., P. C. Iwen, and S. H. Hinrichs. 2000. Identification of Aspergillus species using internal transcribed spacer regions 1 and 2. J. Clin. Microbiol. 38:1510-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz, M. E., M. McLoon, S. Burrows, and B. F. Cheetham. 1998. Extreme DNA sequence variation in isolates of Aspergillus fumigatus. FEMS Immunol. Med. Microbiol. 20:283-288. [DOI] [PubMed] [Google Scholar]
- 10.Kurtz, M. B., I. B. Heath, J. Marrinan, S. Dreikorn, J. Onishi, and C. Douglas. 1994. Morphological effects of lipopeptides against Aspergillus fumigatus correlate with activities against (1,3)-β-d-glucan synthase. Antimicrob. Agents Chemother. 38:1480-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lass-Florl, C., G. Kofler, G. Kropshofer, J. Hermans, A. Kreczy, M. P. Dierich, and D. Niederwieser. 1998. In-vitro testing of susceptibility to amphotericin B is a reliable predictor of clinical outcome in invasive aspergillosis. J. Antimicrob. Chemother. 42:497-502. [DOI] [PubMed] [Google Scholar]
- 12.Leslie, C. E., B. Flannigan, and L. J. Milne. 1988. Morphological studies on clinical isolates of Aspergillus fumigatus. J. Med. Vet. Mycol. 26:335-341. [PubMed] [Google Scholar]
- 13.Loeffler, J., and D. A. Stevens. 2003. Antifungal drug resistance. Clin. Infect. Dis. 36:S31-S41. [DOI] [PubMed] [Google Scholar]
- 14.Lonial, S., L. Williams, G. Carrum, M. Ostrowski, and P. McCarthy, Jr. 1997. Neosartorya fischeri: an invasive fungal pathogen in an allogeneic bone marrow transplant patient. Bone Marrow Transplant. 19:753-755. [DOI] [PubMed] [Google Scholar]
- 15.Marr, K. A., R. A. Carter, M. Boeckh, P. Martin, and L. Corey. 2002. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood 100:4358-4366. [DOI] [PubMed] [Google Scholar]
- 16.Marr, K. A., C. N. Lyons, T. R. Rustad, R. A. Bowden, and T. C. White. 1998. Rapid, transient fluconazole resistance in Candida albicans is associated with increased mRNA levels of CDR. Antimicrob. Agents Chemother. 42:2584-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsella, R., R. Mercantini, A. Stefanini, and L. Volterra. 1983. An atypical isolate of Aspergillus fumigatus from a man affected with a pulmonary disease. Mykosen 26:201-206. [PubMed] [Google Scholar]
- 18.Meletiadis, J., J. W. Mouton, J. F. G. M. Meis, B. A. Bouman, J. P. Donnelly, P. E. Verweij, and Eurofung Network. 2001. Colorimetric assay for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 39:3402-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishra, S. K., F. Staib, C. Rajendran, and U. Folkens. 1982. Serodiagnostic value of culture filtrate antigens from aspergilli with septate phialides. Sabouraudia 20:63-74. [DOI] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M38-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 21.Panackal, A. A., A. Dahlman, K. T. Keil, C. L. Peterson, L. Mascola, S. Mirza, M. Phelan, B. A. Lasker, M. E. Brandt, J. Carpenter, M. Bell, D. W. Warnock, R. A. Hajjeh, and J. Morgan. 2003. Outbreak of invasive aspergillosis among renal transplant recipients. Transplantation 75:1050-1053. [DOI] [PubMed] [Google Scholar]
- 22.Radford, S. A., E. M. Johnson, J. P. Leeming, M. R. Millar, J. M. Cornish, A. B. M. Foot, and D. W. Warnock. 1998. Molecular epidemiological study of Aspergillus fumigatus in a bone marrow transplantation unit by PCR amplification of ribosomal intergenic spacer sequences. J. Clin. Microbiol. 36:1294-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rinyu, E., J. Varga, and L. Ferenczy. 1995. Phenotypic and genotypic analysis of variability in Aspergillus fumigatus. J. Clin. Microbiol. 33:2567-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt, A., and D. I. Schmidt. 1999. J. B. Georg W. Fresenius and the description of the species Aspergillus fumigatus in 1863. Contrib. Microbiol. 2:1-4. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt, A., and M. H. Wolff. 1997. Morphological characteristics of Aspergillus fumigatus strains isolated from patient samples. Mycoses 40:347-351. [DOI] [PubMed] [Google Scholar]
- 26.Schuetzer-Muehlbauer, M., B. Willinger, G. Krapf, S. Enzinger, E. Presterl, and K. Kuchler. 2003. The Candida albicans Cdr2p ATP-binding cassette (ABC) transporter confers resistance to caspofungin. Mol. Microbiol. 48:225-235. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu, K., and N. P. Keller. 2001. Genetic involvement of a cAMP-dependent protein kinase in a G protein signaling pathway regulating morphological and chemical transitions in Aspergillus nidulans. Genetics 157:591-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verweij, P. E., M. Mensink, A. J. Rijs, J. P. Donnelly, J. F. Meis, and D. W. Denning. 1998. In-vitro activities of amphotericin B, itraconazole and voriconazole against 150 clinical and environmental Aspergillus fumigatus isolates. J. Antimicrob. Chemother. 42:389-392. [DOI] [PubMed] [Google Scholar]
- 29.Wang, L., K. Yokoyama, M. Miyaji, and K. Nishimura. 2000. Mitochondrial cytochrome b gene analysis of Aspergillus fumigatus and related species. J. Clin. Microbiol. 38:1352-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warris, A., C. M. Weemaes, and P. E. Verweij. 2002. Multidrug resistance in Aspergillus fumigatus. N. Engl. J. Med. 347:2173-2174. [DOI] [PubMed] [Google Scholar]