Abstract
Rationale
In drug self-administration procedures, extended-access test sessions allow researchers to model maladaptive patterns of excessive and escalating drug intake that are characteristic of human substance-abusing populations.
Objectives
The purpose of the present study was to examine the ability of aerobic exercise to decrease excessive and escalating patterns of drug intake in male and female rats responding under extended-access conditions.
Methods
Male and female Long-Evans rats were obtained at weaning and divided into sedentary (no running wheel) and exercising (running wheel) groups immediately upon arrival. After six weeks, rats were surgically implanted with intravenous catheters and allowed to self-administer cocaine under positive reinforcement contingencies. In Experiment 1, cocaine self-administration was examined during 23-hour test sessions that occurred every four days. In Experiment 2, the escalation of cocaine intake was examined during daily 6-hour test sessions over 14 consecutive days.
Results
In Experiment 1, sedentary rats self-administered significantly more cocaine than exercising rats during uninterrupted 23-hour test sessions, and this effect was apparent in both males and females. In Experiment 2, sedentary rats escalated their cocaine intake to a significantly greater degree than exercising rats over the 14 days of testing. Although females escalated their cocaine intake to a greater extent than males, exercise effectively attenuated the escalation of cocaine intake in both sexes.
Conclusions
These data indicate that aerobic exercise decreases maladaptive patterns of excessive and escalating cocaine intake under extended-access conditions.
A growing number of preclinical studies report that exercise reduces cocaine-seeking behavior in a variety of experimental procedures. For instance, a history of physical activity reduces cocaine-maintained breakpoints in rats responding on a progressive-ratio schedule of reinforcement (Smith et al. 2008), and concurrent access to a running wheel reduces cocaine self-administration in rats responding on a fixed-ratio schedule of reinforcement (Cosgrove et al. 2002). Aerobic exercise also reduces drug-primed and cue-induced reinstatement in rats with a history of cocaine self-administration (Lynch et al. 2010; Zlebnik et al. 2010), suggesting that exercise may attenuate some of the underlying behavioral processes that are responsible for maladaptive patterns of drug intake.
Traditional laboratory procedures measuring drug self-administration are normally limited to 1 or 2 hours in duration and generate very stable levels of drug intake over time. This pattern of drug intake contrasts with that typically observed in substance-abusing individuals, who exhibit “binges” of excessive drug use (Burkett et al. 1994) and who progressively escalate their drug use over time (Gawin 1991). Such patterns of maladaptive drug intake are a cardinal feature of drug addiction and comprise a critical component of the diagnostic criteria for substance use disorders (American Psychiatric Association 1994). These patterns of excessive and escalating drug intake can be modeled in the laboratory by giving subjects prolonged access to a drug during test sessions lasting 6 hours or longer (i.e., extended-access). For instance, animals given 24-hour access to cocaine exhibit high levels of drug intake that are associated with a loss of circadian patterns of activity, a dysregulation of homeostatic autonomic functions, and acute withdrawal symptoms upon termination (Mutschler and Miczek 1998; Tornatzky and Miczek 2000; Fowler et al. 2007). Similar disruptions in behavioral and physiological processes are observed in human laboratory studies (Foltin and Fischman 1997; Pace-Schott et al. 2005; Reed et al. 2009), suggesting a high degree of homogeny between human and animal models of excessive drug intake. Furthermore, animals given 6-hour access to cocaine in daily self-administration sessions escalate their drug intake over a period of several days, often doubling their daily cocaine intake in as little as 30 days (Ahmed and Koob 1998; Ahmed et al. 2005). Similar increases in drug intake are seen in human populations and may mark a transition into pathological states of substance use (Gawin and Kleber 1988). Thus, extended-access procedures model critical aspects of the addictive process and provide a platform from which to examine potential interventions for these maladaptive patterns of drug use.
The primary aim of the present study was to examine the effects of aerobic exercise on maladaptive patterns of drug intake during extended-access test sessions. In one experiment, excessive drug intake was examined in sedentary and exercising rats during 23-hour “binges” of cocaine self-administration. Although the use of the term “binge” has varied across studies using animal models of repeated drug administration (cf. Maisonneuve and Kreek 1994; Morgan et al. 2005; Fowler et al. 2007), a binge was operationally defined in the present study as a loss of circadian control of drug intake, with high levels of responding extending into the inactive phase of the light/dark cycle (see Roberts et al. 2002 for example and discussion). In a second experiment, the escalation of cocaine intake was examined in sedentary and exercising rats during daily 6-hour test sessions. In both experiments, no limit was placed on the number of cocaine infusions that could be earned, other than those set by the session length and post-infusion timeout. Previous studies report that females maintain higher levels of exercise output than males (Eikelboom and Mills 1988) and show greater dysregulation of responding under extended-access conditions than males (Lynch and Taylor 2004). Consequently, both male and female rats were examined in the present study.
Methods
General Methods
Animals
Male and female Long-Evans rats were obtained at weaning from Charles River Laboratories (Raleigh, NC) and divided into sedentary and exercising groups upon arrival. Sedentary rats were housed in polycarbonate cages (50 × 28 × 20 cm) that permitted no exercise beyond normal cage ambulation; exercising rats were housed in cages of equal dimensions with attached running wheels (Harvard Apparatus, Holliston, MA, USA). Running wheels were stainless steel (35 cm diameter) and connected to mechanical switches and electronic counters that recorded each revolution. All rats were housed individually in a temperature- and humidity-controlled colony room maintained on a 12-hour light/dark cycle (lights on 0700). Except during the brief period of lever-press training (see below), food and water were continuously available in the home cages. For the female rats, estrous phase was allowed to cycle normally and was not monitored. All subjects were treated in accordance with the guidelines of the Animal Care and Use Committee of Davidson College and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animals Resources 1996).
Apparatus
All training and test sessions took place in aluminum and polycarbonate operant conditioning chambers from Med Associates, Inc. (St. Albans, VT). Each chamber contained a food receptacle, a house light for general illumination, two retractable response levers, and two white stimulus lights located above the response levers. All experimental events were programmed with software and interfacing from Med Associates, Inc. The left lever was designated the active lever for all rats.
Lever-press training
Five weeks after arrival, all rats were lightly food restricted and trained to respond on a fixed-ratio 1 (FR1) schedule of food reinforcement. On this schedule, each lever press produced a 45 mg Noyes food pellet delivered from the pellet dispenser to the food receptacle. Sessions terminated once 40 reinforcers had been earned or after 2 hours elapsed, whichever occurred first.
Catheter implantation
Six weeks after arrival and at least 48 hours after termination of lever-press training, all rats were anesthetized with a combination of ketamine (100 mg/kg, ip) and xylazine (8.0 mg/kg, ip) and surgically implanted with intravenous catheters (CamCaths, Cambridge, UK) according to methods described previously (Smith et al. 2008; 2009). A solution of heparinized saline (200 units/ml, iv) and ticarcillin (20 mg/kg, iv) was infused through the catheter daily to prevent infection and maintain patency. After seven days, ticarcillin was discontinued and only heparinized saline was used to maintain catheter patency.
Self-administration training
Three days after surgery, rats were placed into the operant conditioning chambers and connected to infusion pumps via Tygon tubing. Each self-administration session began with illumination of the house light, extension of the response lever into the chamber, and illumination of the white stimulus light above the response lever. During the initial training sessions, lever presses were reinforced on an FR1 schedule of reinforcement. On this schedule, each lever press activated the infusion pump for 2.0 to 3.5 seconds (based on body weight) and delivered 0.5 mg/kg cocaine (Research Triangle Institute, Research Triangle Park, NC, USA). Coincident with each infusion, the response lever retracted and the stimulus light turned off to signal a timeout period during which cocaine was not available. After 20 seconds, the stimulus light turned back on and the lever extended back into the chamber. In the past, we observed seizure activity in rats that were previously trained with food reinforcement on the first day of cocaine training due to exceptionally high rates of responding. Consequently, the number of available infusions was capped at 21 during the first two training sessions. Beginning in the third session, no artificial limit was placed on the number of infusions that could be earned, other than those set by the post-infusion timeout.
Experiment 1
Self-administration training
A total of 32 rats (16 female, 16 male) started the experiment and were implanted with intravenous catheters; rats that lost catheter patency were removed from the study and not replaced (see figure captions for final number of subjects in each group). Self-administration training sessions were conducted as described above and continued for 10 days, with the FR value increasing from FR1 to FR5 according to the following schedule: Days 1–4 (FR1), Day 5 (FR2), Day 6 (FR3), Day 7 (FR4), Days 8–10 (FR5). All 10 sessions were conducted during the light phase of the light/dark cycle so as not to interfere with nocturnal running. All sessions terminated automatically after 2 hours.
Self-administration testing (23-hour sessions)
Two days after the conclusion of self-administration training, rats were placed in the operant conditioning chambers for a 23-hour test session. During this session, lever presses produced an infusion of cocaine on an FR5 schedule of reinforcement. Each session began 30 minutes after the start of the dark phase of the light/dark cycle (start time: 1930) and ended the following day, 30 minutes before the start of the next dark phase (end time: 1830). A small amount of food and water was placed in each chamber at the beginning of the session; otherwise, conditions were identical to those used during the 10 training sessions (see above). Three doses of cocaine (0.5, 1.0, and 2.0 mg/kg/infusion) were tested in an irregular order 73 hours apart. No limit was placed on the number of infusions that could be obtained, other than those set by the post-infusion timeout.
Self-administration testing (2-hour sessions)
Three days after the final 23-hour session, the cocaine dose-effect curve was determined in five daily 2-hour test sessions. Each test session was conducted during the light phase of the light/dark cycle. During each session, cocaine (0.03, 0.1, 0.3, or 1.0 mg/kg/infusion) or saline was available on an FR5 schedule of reinforcement. Doses of cocaine were tested in an irregular order.
Data analysis
All self-administration data were analyzed via mixed-factor ANOVA, with sex and condition serving as between-subjects factors and session (or dose) serving as a within-subjects factor. For exercising rats, a Pearson product-moment correlation was used to determine the correlation between exercise output and responding at each dose of cocaine. Data from the 23-hour sessions were also analyzed by examining the cumulative record of each rat to determine the relative rate of responding and duration of responding over the 23-hour test period. The relative rate of responding was calculated for each individual rat by determining the slope of a regression line fitted to the data for the period of active lever pressing. The period of active lever pressing (i.e., the duration of responding) was operationally defined as the elapsed time between the beginning of the session and the occurrence of the lever press representing the 99th percentile of total lever presses emitted during that session. Due to a programming error, the cumulative record was truncated at 1000 responses for female rats responding at the two lower doses of cocaine (but the session otherwise continued normally and data for total number of infusions were unaffected). Consequently, cumulative record data from males and females were analyzed separately. For males, relative rate of responding and duration of responding were analyzed via mixed-factor ANOVA, using condition as a between-subjects factor and dose as a within-subjects factor. Because of differences in variance in the duration of responding between groups, these data were also rank ordered and analyzed via a nonparametric Mann-Whitney U test. For females, relative rate of responding was analyzed via mixed-factor ANOVA, with the caveat that only data from the first 1000 responses were used in the analysis. For duration of responding, data obtained at the high dose were analyzed via independent-samples t-test using condition as the factor.
Experiment 2
Self-administration training
A total of 40 rats (16 female, 22 male) started the experiment and were implanted with intravenous catheters; rats that lost catheter patency were removed from the study and not replaced (see figure captions for final number of subjects in each group). Self-administration training sessions were conducted as described above (see General Methods) for 7 consecutive days in which responding was always reinforced on an FR1 schedule of reinforcement. All sessions terminated automatically after 1 hour.
Self-administration testing (6-hour sessions)
After 7 days of training, self-administration sessions were lengthened to 6 hours with no limit on the number of infusions that could be earned, other than those set by the post-infusion timeout. All other experimental events were identical to those used during the training sessions. These conditions remained in effect for 14 consecutive days. All self-administration training and testing sessions began at the onset of the light phase of the light/dark cycle (start time: 0700) so as not to interfere with nocturnal running. The dose of cocaine was maintained at 0.5 mg/kg/infusion in all sessions.
Data analysis
Drug self-administration data were expressed as number of infusions obtained and analyzed via three-way ANOVA, with sex and condition serving as between-subjects factors and session serving as a within-subjects factor. In exercising rats, a Pearson product-moment correlation was used to determine the correlation between exercise output and cocaine self-administration. Relative rate of responding and duration of responding were calculated for the first (Day 1) and last (Day 14) day of testing according to the methods described above, with the exception that duration of responding was operationally defined as the elapsed time between the beginning of the session and the occurrence of the final lever press of the session. These data were then analyzed via three-way ANOVA, with sex and condition serving as between-subjects factors and session serving as a within-subjects factor.
Results
Experiment 1
Exercise output
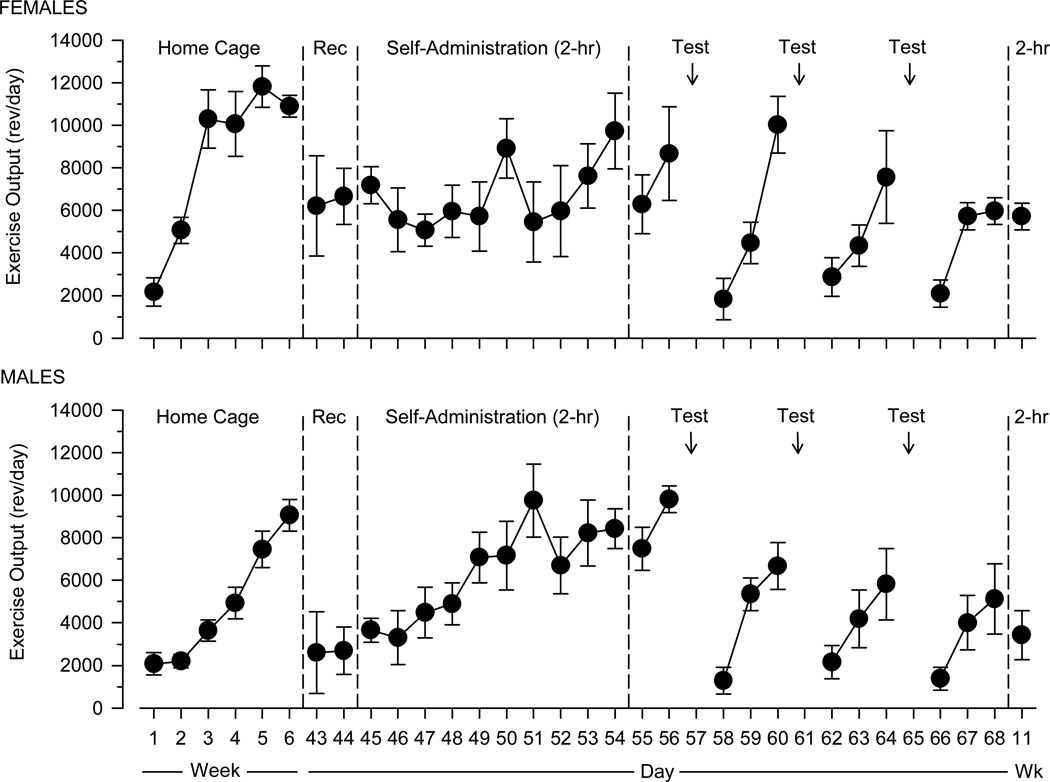
Females increased their running at a faster rate than males and achieved greater maximal levels of exercise output than males over the first six weeks of the study (Fig. 1). Wheel running decreased sharply after each 23-hour session, and this effect was apparent in both males and females. Running recovered during the three days following each session, but did not return to pre-surgery levels in most instances. There was a trend for exercise output to recover more slowly following each session, such that exercise output was only 50% of pre-surgery levels by the end of this phase of the study. Running remained suppressed in both groups in the final week of the study during which cocaine was self-administered in daily 2-hour test sessions.
Fig. 1.
Exercise output over the course of Experiment 1 in female (n = 7) and male (n = 7) rats. Vertical axes depict exercise output in revolutions per day (rev/day); horizontal axes depict time expressed in weeks (Weeks 1–6, 11) and days (Days 43–68). Vertical reference lines indicate transitions between different experimental events: Weeks 1–6, Home Cage, Lever Press Training, and Surgery; Days 43–44, Recovery from Surgery (Rec); Days 45–54, Self-Administration Training (2-hr); Days 55–68, Self-Administration Testing (23-hr); and Week 11, Self-Administration Testing (2-hr)
Self-administration training
All rats responded on the first day of self-administration training and showed stable response rates with regular post-reinforcement pauses by the third day. Over the last 8 days of training (Sessions 3–10), responding varied from day to day [main effect of session: F (7, 182) = 4.030, p < .001], but no increasing or decreasing trends were observed in any group (Online Resource 1). Females responded more than males throughout the training period [main effect of sex: F (1, 26) = 6.942, p = .014], but no significant differences in responding were observed between sedentary and exercising rats of either sex.
Self-administration testing (23-hour sessions)
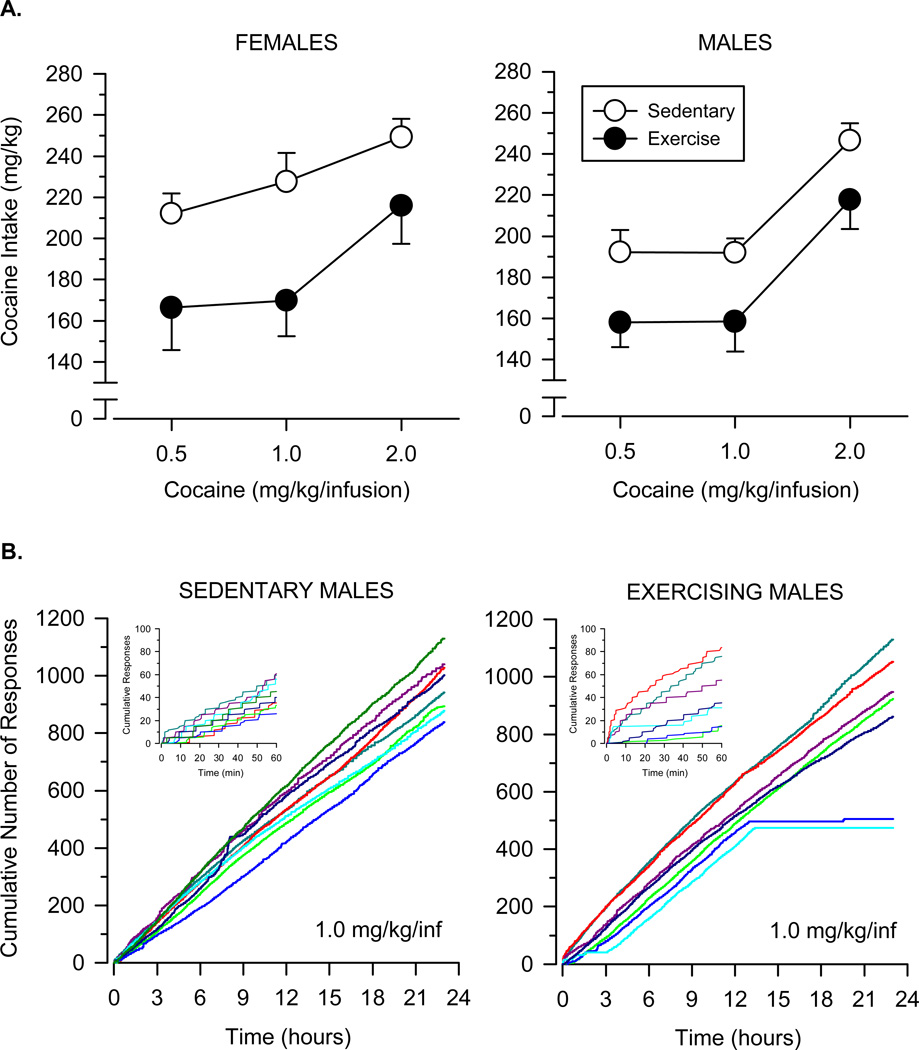
Responding varied across dose and condition during the 23-hour sessions (Fig. 2). Cocaine intake increased significantly across the doses tested [main effect of dose: F (2, 52) = 36.032, p < .001]. At all three doses, cocaine intake was significantly greater in sedentary rats than exercising rats [main effect of condition: F (1, 26) = 12.570, p = .002], and this effect was apparent in both males and females. No significant sex or order effects were observed.
Fig. 2.
A. Cocaine self-administration in female and male rats during 23-hour test sessions. Vertical axes depict total cocaine intake in mg/kg; horizontal axes depict dose of cocaine in mg/kg/infusion. Open symbols depict data collected in sedentary rats (n = 8 female; n = 8 male); filled symbols depict data collected in exercising rats (n = 7 female; n = 7 male). B. Cumulative records from sedentary and exercising male rats during 23-hour sessions in which responding was maintained by 1.0 mg/kg/infusion cocaine. Vertical axes depict cumulative number of responses; horizontal axes depict time expressed in hours. Inset graphs depict a magnified view of the cumulative record during the first hour of the session to show the step-like response pattern that is typical of an FR5 schedule of reinforcement. Colored lines represent data from individual rats
An analysis of individual cumulative records revealed that all subjects displayed patterns of responding typical of an FR5 schedule of cocaine reinforcement (i.e., a rapid run of five responses followed by a post-reinforcement pause, the length of which was related to the dose of cocaine maintaining the behavior). To compare relative response rates across conditions, regression lines were calculated for the period of active lever pressing and the slopes of these regression lines were determined for each rat (Table 1; Fig. 2). In males, slopes varied as a function of dose, with steeper slopes (i.e., faster response rates) observed at the lower doses of cocaine [main effect of dose: F (2, 26) = 193.547, p < .001]. Response rates were similar between sedentary and exercising male rats at each dose of cocaine, and no significant differences were observed between the two groups. In females, relative response rates varied as a function of dose [F (2, 26) = 164.017, p < .001], but not condition. Omitting data from the first hour of the session (i.e., from the initial “load-up” phase) revealed a similar pattern of effects in both sexes. An analysis of post-reinforcement pause data, which were highly correlated with relative response rates (r = −0.88; p < .001), also produced a similar pattern of results.
Table 1.
Relative response rate and duration of responding across conditions in Experiment 1a
| Sex/Condition | Relative Response Rateb |
Duration of Respondingc |
||||
|---|---|---|---|---|---|---|
| 0.5 mg/kg | 1.0 mg/kg | 2.0 mg/kg | 0.5 mg/kg | 1.0 mg/kg | 2.0 mg/kg | |
| Female | ||||||
| Sedentary | 1.65 (0.11) d | 0.74 (0.04) d | 0.53 (0.04) | ---e | ---e | 1189.43 (60.72) |
| Exercise | 1.49 (0.13) d | 0.67 (0.03) d | 0.50 (0.04) | ---e | ---e | 1089.14 (110.89) |
| Male | ||||||
| Sedentary | 1.39 (0.08) | 0.70 (0.02) | 0.45 (0.02) | 1375.44 (0.45) | 1373.01 (0.76) | 1370.20 (1.10) |
| Exercise | 1.32 (0.08) | 0.70 (0.02) | 0.47 (0.05) | 1244.24 (74.19) | 1204.91 (108.05) | 1224.95 (118.93) |
data reflect the mean (SEM)
relative response rate was calculated as the slope of the regression line fitted to the portion of the cumulative record covering the period of active responding
duration of responding was defined as the time in minutes between the beginning of the session and the occurrence of the lever press representing the 99th percentile of total lever presses emitted
values derived from first 1000 responses only
could not be determined
In order to compare the duration of responding across conditions, the period of active lever pressing (i.e., the period of time before responding ceased) was determined for each rat (Table 1; Fig. 2). In males, a mixed-factor ANOVA revealed that the duration of active lever pressing did not vary across dose, but sedentary rats responded for significantly longer periods of time than exercising rats [main effect of condition: F (1, 13) = 5.035, p = .043]; the latter finding was confirmed by a nonparametric analysis of the data (p = .002). For females, the duration of active lever pressing was longer in sedentary rats than exercising rats at the high dose of cocaine (2.0 mg/kg/infusion), but this effect was not statistically significant. Collapsing across sex and dose, sedentary rats responded, on average, 2.3 hours longer than exercising rats. An examination of the cumulative record revealed that sedentary rats responded in the last 15 minutes of the session in 27 out of 32 instances for which data were available, whereas exercising rats responded in the last 15 minutes of the session in only 14 out of 28 instances.
Self-administration testing (2-hour sessions)
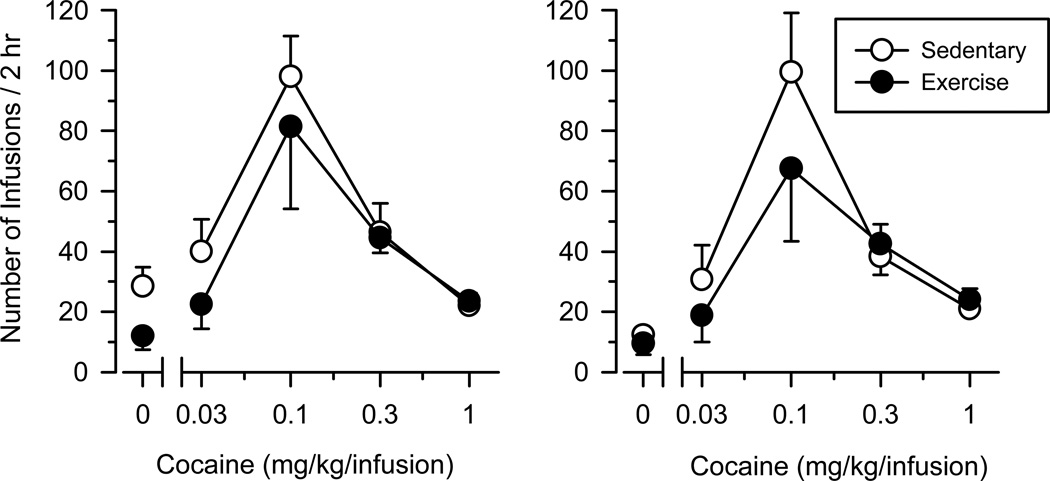
Three days after the final 23-hour session, the full dose-effect curve of cocaine (i.e., both the ascending and descending limb) was determined in all groups during 2-hour test sessions conducted over five consecutive days (Fig. 3). Responding was characterized by an inverted U-shaped dose-effect curve in all groups [main effect of dose: F (3, 48) = 120.483, p < .001]. Exercise did not impact responding maintained by cocaine under these conditions (no significant main effect of condition or significant dose × condition interaction), and no significant sex differences were observed.
Fig. 3.
Dose-effect curve of cocaine in female and male rats as determined during 2-hour test sessions. Vertical axes depict number of infusions obtained; horizontal axes depict dose of cocaine in mg/kg/infusion. Open symbols depict data collected in sedentary rats (n = 6 female; n = 6 male); filled symbols depict data collected in exercising rats (n = 4 female; n = 4 male)
Correlation between exercise output and cocaine self-administration
Responding during the 23-hour sessions was not strongly associated with exercise output during the various phases of the study (e.g., before surgery, before a session, after a session). Only two significant correlations (out of a possible 18) were obtained. For females, there was a significant negative correlation between the number of infusions maintained by the low dose of cocaine and exercise output during the three days immediately preceding the session (r = −.817; p = .025) and the three days immediately following the session (r = −.807; p = .028).
Experiment 2
Exercise output
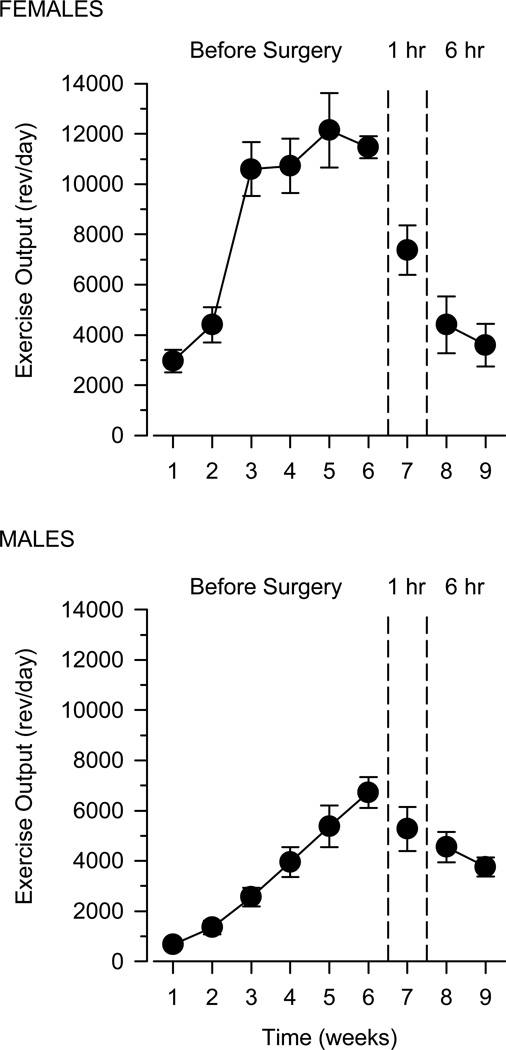
Exercise output differed markedly between males and females during the first 6 weeks of the study (Fig. 4). Females ran more during the first week of wheel exposure than males, increased their running at a faster rate than males, and reached a greater maximal level of exercise output than males. Following catheter implantation, running decreased markedly in females to approximately 50% of that observed prior to surgery. In males, running declined slightly after surgery and was comparable to that of females for the remainder of the study.
Fig. 4.
Exercise output over the course of Experiment 2 in female (n = 6) and male (n = 8) rats. Vertical axes depict exercise output in revolutions per day (rev/day). Horizontal axes depict time expressed in weeks. Vertical reference lines after weeks 6 and 7 indicate transitions between different experimental events: home cage and running wheel acclimation (weeks 1–6); self-administration training (week 7); extended-access cocaine self-administration (weeks 8–9)
Self-administration training
All rats responded on the first day of self-administration training and stable response rates were observed by the third day of training. Over the next five days of training, females responded more than males, but no differences were observed between sedentary and exercising rats (Online Resource 2). Consistent with these observations, a repeated-measures ANOVA revealed a significant main effect of sex [F (1, 24) = 31.435; p < .001], but no other significant effects were obtained.
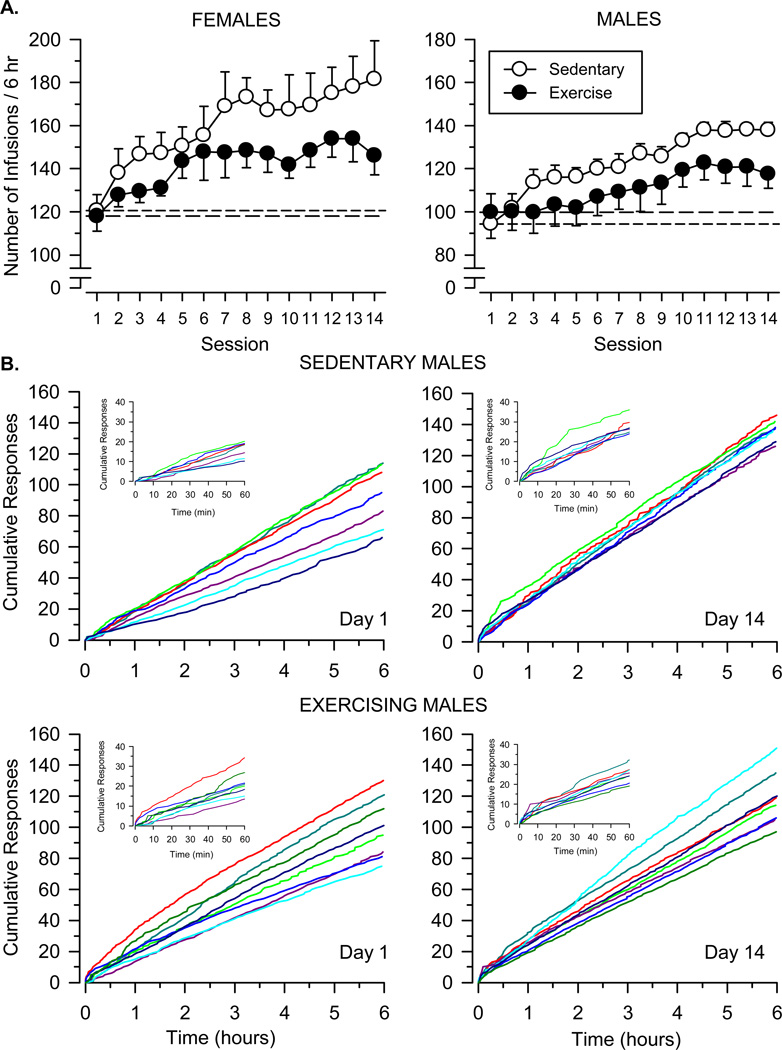
Self-administration testing (6-hour sessions)
Significant differences in responding were observed between males and females, between sedentary and exercising rats, and across the 14 test sessions (Fig. 5). Progressive increases in responding were observed in all groups across the 14-day period [main effect of session: F (13, 312) = 11.235; p < .001]. Females responded more than males throughout the testing period [main effect of sex: F (1, 24) = 35.201; p < .001], and this effect was apparent in both sedentary and exercising rats. Sedentary rats of both sexes responded to a significantly greater degree than exercising rats [main effect of condition: F (1, 24) = 6.742; p = .016]. Importantly, for both females and males, sedentary and exercising rats did not differ on the first day of testing, but differences between groups emerged rapidly over subsequent test sessions.
Fig. 5.
A. Escalation of cocaine intake over 14 daily 6-hour test sessions in female and male rats. Open symbols depict data collected in sedentary rats (n = 7 female; n = 7 male); filled symbols depict data collected in exercising rats (n = 6 female; n = 8 male). Vertical axes depict number of infusions obtained during 6-hour test sessions (note different axes for males and females). Horizontal axes indicate session number relative to the beginning of extended-access conditions. Short-dashed lines indicate control responding for sedentary rats (data from Day 1); long-dashed lines indicate control responding for exercising rats (data from Day 1). The dose of cocaine was 0.5 mg/kg/infusion in each session. B. Cumulative records from sedentary and exercising male rats during the first (Day 1) and last (Day 14) test session. Vertical axes depict cumulative number of responses; horizontal axes depict time expressed in hours. Inset graphs depict a magnified view of the cumulative record during the first hour of the session. Colored lines represent data from individual rats; colors representing individual rats are consistent within a group.
The relative rate of responding and duration of responding were determined for all rats using data from individual cumulative records (Table 2, Fig. 5). The relative rate of responding was greater in females than males [main effect of sex: F (1, 24) = 22.927; p < .001], and greater on the last day of testing than on the first day of testing [main effect of session: F (1, 24) = 32.250; p < .001]. Importantly, response rates did not differ between sedentary and exercising rats on the first day, but were significantly greater in sedentary rats on the last day [condition × session interaction: F (1, 24) = 4.795; p = .039]. In contrast, no significant main effects or interactions were observed for duration of responding. All rats responded until the end of the session (i.e., obtained at least one infusion during the final 10 minutes of the session) on both the first and last day of testing.
Table 2.
Relative response rate and duration of responding across conditions in Experiment 2a
| Sex/Condition | Relative Response Rateb |
Duration of Respondingc |
||
|---|---|---|---|---|
| Day 1 | Day 14 | Day 1 | Day 14 | |
| Female | ||||
| Sedentary | 0.33 (0.02) | 0.50 (0.05) | 357.76 (0.85) | 358.69 (0.38) |
| Exercise | 0.32 (0.03) | 0.41 (0.02) | 357.82 (0.63) | 358.54 (0.61) |
| Male | ||||
| Sedentary | 0.24 (0.02) | 0.37 (0.01) | 358.29 (0.28) | 357.73 (0.51) |
| Exercise | 0.27 (0.02) | 0.32 (0.02) | 358.71 (0.37) | 358.73 (0.26) |
data reflect the mean (SEM)
relative response rate was calculated as the slope of the regression line fitted to the portion of the cumulative record covering the period of active responding
duration of responding was defined as the time in minutes between the beginning of the session and the occurrence of the final lever press
Correlation between exercise output and cocaine self-administration
Pearson product-moment correlations failed to reveal a significant relationship between exercise output during any phase of the study [before surgery (weeks 1–6); during testing (weeks 8–9); throughout the entire study (weeks 1–9)] and responding on Day 1 of testing, responding on Day 14 of testing, and the difference between Day 1 and Day 14 of testing (correlations not shown).
Discussion
The principal finding of this study is that aerobic exercise reduces maladaptive patterns of drug intake in male and female rats self-administering cocaine under extended-access conditions. In Experiment 1, exercise reduced the duration of responding during uninterrupted, 23-hour test sessions. In Experiment 2, exercise reduced the escalation of responding during daily, 6-hour test sessions over 14 consecutive days. These data add to a growing body of preclinical literature reporting that physical activity reduces cocaine self-administration and attenuates maladaptive patterns of drug intake that are characteristic of substance use disorders (Cosgrove et al. 2002; Smith et al. 2008; Zlebnik et al. 2010).
Consistent with previous studies reporting that females run more than males (Eikelboom and Mills 1988; Boakes et al. 1999), females in both experiments increased their running at a faster rate than males and achieved greater maximal levels of exercise output than males. Wheel running was reduced immediately after catheter implantation in both sexes, and was reduced further when the extended-access phase of the experiments began. In Experiment 1, exercise output on the day following each 23-hour test session was only 10–20% of that observed on the day preceding the session. Mutschler and Miczek (1998) reported that termination of a 12- or 48-hour binge results in a withdrawal syndrome as indicated by ultrasonic vocalizations during the first 24 hours after the session. It is likely that the reduction in wheel running after each test session was the result of acute withdrawal from cocaine. Consistent with this possibility, Santucci et al (2008) reported that withdrawal from experimenter-delivered cocaine is associated with a significant reduction in both locomotor activity and wheel running. In the present study, wheel running increased during the three days following each test session, suggesting that physical and/or affective withdrawal symptoms gradually abated over time. Interestingly, wheel running typically did not return to the level observed prior to the session, and there was a tendency for recovery to be progressively less robust following each subsequent test session. Tornatzky and Miczek (2000) reported that while some autonomic processes return to normal within 3 days after a prolonged binge (e.g., heart rate), other autonomic processes remain dysregulated for up to 15 days (e.g., core body temperature). It is likely that autonomic processes related to wheel running and physical activity had not fully recovered before the start of the next test session, and that each subsequent binge further exacerbated the dysregulation of those processes.
In Experiment 1, physical activity markedly reduced cocaine self-administration during the 23-hour test sessions. These effects were observed in both males and females and across all three doses of cocaine. Because decreases in cocaine intake on a fixed-ratio schedule could be due to either a slower rate of responding or to an earlier cessation of responding, we calculated both relative response rate and duration of responding for each rat. The slopes of regression lines fitted to cumulative records did not differ between sedentary and exercising rats, indicating that response rates were similar between the two groups. In contrast, the duration of responding was shorter in exercising rats, indicating that this group terminated the binge earlier than sedentary rats. In fact, exercising rats ended their binge before the conclusion of the 23-hour session in 14 of the 28 instances for which data were available; in sedentary rats, early termination was observed in only 5 of 32 instances. Given that the majority of sedentary rats were still responding at the end of each session, the differences in cocaine intake between the two groups likely reflect a conservative estimate of exercise’s protective effects. All rats that ceased responding before the end of the session did so during their inactive phase (i.e., during the last 12 hours of the session). Given that voluntary exercise entrains circadian rhythms in both rodents (Edgar and Dement 1991) and humans (Buxton et al. 2003), it is possible that exercise’s ability to synchronize behavioral rhythms is responsible for the early termination of responding in exercising rats, and for its protective effects against excessive cocaine intake under extended-access conditions.
Responding under fixed-ratio schedules of drug reinforcement is characterized by an inverted U-shaped dose-effect curve, and all doses tested during the 23-hour sessions fall on the descending limb of the curve. This is potentially significant, because reductions in responding on the descending limb could indicate either a leftward shift in the dose-effect curve or a downward shift in the dose-effect curve. Exercising rats have lower body weights, less adipose tissue, and smaller livers than sedentary rats (Pitts and Bull 1977), all of which could lead to differences in the pharmacokinetics of cocaine between sedentary and exercising groups. A previous study reported that plasma concentrations of an intravenous infusion of cocaine were greater in a group of forced-exercise rats while they were exercising than in a group of rested controls (Han et al. 1996). Although running wheels were never concurrently available during test sessions in the present study, increased plasma concentrations at the time of testing could increase the effects of cocaine in exercising rats. To examine this possibility, the full dose-effect curve (i.e., both ascending and descending limbs) was determined during 2-hour test sessions over five consecutive days. In these tests, no differences in sensitivity to cocaine were apparent between sedentary and exercising rats.
In Experiment 2, female rats escalated their cocaine intake under extended-access conditions to a greater degree than male rats. Although this finding contrasts with the lack of sex differences noted in Experiment 1, it is consistent with a large body of literature reporting that females self-administer more cocaine than males (see reviews by Carroll et al. 2004; Roth et al. 2004; Lynch 2006) and escalate their cocaine intake to a greater extent than males (Roth and Carroll 2004). In females, hormonal fluctuations due to the estrous cycle influence both wheel running (Steiner et al. 1982; Kent et al. 1991) and cocaine self-administration (Roberts et al. 1989; Feltenstein and See 2007). Although females ran more than males in both experiments, no sex differences were observed in the effects of exercise on cocaine self-administration in either experiment.
In Experiment 2, sedentary rats escalated their cocaine intake to a significantly greater degree than exercising rats, even though the two groups did not differ during training or during the first day of responding under extended-access conditions. Thus, any effect of exercise on the escalation of cocaine intake cannot be attributed to differences in pharmacological history or to baseline differences in cocaine sensitivity. Similar to that observed in Experiment 1, differences between sedentary and exercising rats did not emerge until cocaine was available during extended-access test sessions, under conditions specifically designed to model maladaptive patterns of drug intake. Unlike that seen in Experiment 1, the differences observed between sedentary and exercising rats after 14 days of 6-hour test session were due to differences in the rate of responding, rather than to differences in the duration of responding. These data suggest that different mechanisms are likely responsible for the protective effects of exercise in these two procedures.
Identifying the physiological mechanisms responsible for exercise’s protective effects in extended-access models is difficult, primarily because the underlying processes contributing to excessive and escalating patterns of drug intake are poorly understood. For instance, previous investigations have suggested that tolerance (Oleson and Robert 2009), sensitization (Ferrario et al. 2005), and changes in homeostatic set points (Ahmed and Koob 1998) may all be involved in the escalation of drug intake in extended-access models. Complicating matters further, exercise may be engaging satiety or “stop” mechanisms under some conditions (e.g., 23-hour test sessions) and engaging mechanisms responsible for the incentive value of cocaine under other conditions (e.g., repeated 6-hour test sessions). Nevertheless, several mechanisms that influence cocaine self-administration are known to be modulated by exercise.
Cocaine self-administration is associated with an increase in the number of dendritic spines on striatal medium spiny neurons in the nucleus accumbens and pyramidal neurons in the cortex (Robinson et al. 2001), and these changes may contribute to the development of compulsive patterns of drug intake (Robinson and Kolb 2004). Wheel running promotes neuroplastic changes throughout brain (van Praag 2008) and modulates the neuroplastic changes produced by cocaine (Willuhn et al. 2003). Acute exercise also stimulates the release of dopamine (Meeusen and De Meirleir 1995), and prolonged exercise leads to alterations in dopamine binding proteins (MacRae et al. 1987; Fisher et al. 2004). These alterations could blunt the impact of prolonged exposure to cocaine and attenuate excessive and escalating patterns of cocaine intake. Exercise also stimulates the release of the opioid peptides beta-endorphin (Mehl et al. 2000) and dynorphin (Fontana et al. 1994), which serve as endogenous ligands at mu and kappa receptors, respectively. The opioid receptor system plays an important modulatory role in the reinforcing effects of cocaine (Herz 1998; Mello and Negus 2000), and the kappa opioid receptor system is critically involved in cocaine self-administration under extended-access conditions (Wee and Koob 2010). Exercise also induces neurogenesis in the hippocampus (Rhodes et al. 2003; Uda et al. 2006). Reductions in hippocampal neurogenesis have been implicated in cocaine-seeking behavior (Noonan et al. 2010), and exercise may enhance the ability of this structure to buffer against compulsive patterns of drug intake. Exercise also alters mRNA levels for several catecholaminergic proteins in both reward (Greenwood et al. 2011) and motor (Foley and Fleshner 2008) pathways, any of which may mitigate the maladaptive changes in motivational circuits caused by cocaine. Given the diffuse nature of exercise’s effect on the central nervous system, multiple mechanisms are likely at play and isolating any one mechanism will remain a challenge.
Supplementary Material
Acknowledgements
This study was supported by the National Institutes of Health (NIDA Grants DA14255 and DA027485 to MAS). Additional support was provided by the Howard Hughes Medical Institute (Grant 52006292), the Duke Endowment, and Davidson College. The authors wish to thank the National Institute on Drug Abuse for supplying the study drug and Amy Sullivan for providing expert animal care.
Footnotes
Portions of these data were presented at the annual meeting of the College on Problems of Drug Dependence in Reno, NV on June 24, 2009 and the annual meeting of the Society for Neuroscience in San Diego, CA on November 16, 2010.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Lutjens R, van der Stap LD, Lekic D, Romano-Spica V, Morales M, Koob GF, Repunte-Canonigo V, Sanna PP. Gene expression evidence for remodeling of lateral hypothalamic circuitry in cocaine addiction. Proc Natl Acad Sci U S A. 2005;102:11533–11538. doi: 10.1073/pnas.0504438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- Boakes RA, Mills KJ, Single JP. Sex differences in the relationship between activity and weight loss in the rat. Behav Neurosci. 1999;113:1080–1089. [PubMed] [Google Scholar]
- Brown RA, Abrantes AM, Read JP, Marcus BH, Jakicic J, Strong DR, Oakley JR, Ramsey SE, Kahler CW, Stuart GG, Dubreuil ME, Gordon AA. A pilot study of aerobic exercise as an adjunctive treatment for drug dependence. Ment Health Phys Act. 2010;3:27–34. doi: 10.1016/j.mhpa.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett G, Yasin SY, Palow D, LaVoie L, Martinez M. Patterns of cocaine binging: effect on pregnancy. Am J Obstet Gynecol. 1994;171:372–378. doi: 10.1016/s0002-9378(94)70037-0. [DOI] [PubMed] [Google Scholar]
- Buxton OM, Lee CW, L'Hermite-Baleriaux M, Turek FW, Van Cauter E. Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. Am J Physiol Regul Integr Comp Physiol. 2003;284:R714–R724. doi: 10.1152/ajpregu.00355.2002. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Hunter RG, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharmacol Biochem Behav. 2002;73:663–671. doi: 10.1016/s0091-3057(02)00853-5. [DOI] [PubMed] [Google Scholar]
- Edgar DM, Dement WC. Regularly scheduled voluntary exercise synchronizes the mouse circadian clock. Am J Physiol. 1991;261:R928–R933. doi: 10.1152/ajpregu.1991.261.4.R928. [DOI] [PubMed] [Google Scholar]
- Eikelboom R, Mills R. A microanalysis of wheel running in male and female rats. Physiol Behav. 1988;43:625–630. doi: 10.1016/0031-9384(88)90217-x. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend. 2007;89:183–189. doi: 10.1016/j.drugalcdep.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Fisher BE, Petzinger GM, Nixon K, Hogg E, Bremmer S, Meshul CK, Jakowec MW. Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse basal ganglia. J Neurosci Res. 2004;77:378–390. doi: 10.1002/jnr.20162. [DOI] [PubMed] [Google Scholar]
- Foley TE, Fleshner M. Neuroplasticity of dopamine circuits after exercise: implications for central fatigue. Neuromolecular Med. 2008;10:67–80. doi: 10.1007/s12017-008-8032-3. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. A laboratory model of cocaine withdrawal in humans: intravenous cocaine. Exp Clin Psychopharmacol. 1997;5:404–411. doi: 10.1037//1064-1297.5.4.404. [DOI] [PubMed] [Google Scholar]
- Fontana F, Bernardi P, Merlo Pich E, Boschi S, De Iasio R, Capelli M, Carboni L, Spampinato S. Endogenous opioid system and atrial natriuretic factor in normotensive offspring of hypertensive parents at rest and during exercise test. J Hypertens. 1994;12:1285–1290. [PubMed] [Google Scholar]
- Fowler SC, Covington HE, 3rd, Miczek KA. Stereotyped and complex motor routines expressed during cocaine self-administration: results from a 24-h binge of unlimited cocaine access in rats. Psychopharmacology. 2007;192:465–478. doi: 10.1007/s00213-007-0739-6. [DOI] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Evolving conceptualizations of cocaine dependence. Yale J Biol Med. 1988;61:123–136. [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB, Day HE, Fleshner M. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav Brain Res. 2011;217:354–362. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han DH, Kelly KP, Fellingham GW, Conlee RK. Cocaine and exercise: temporal changes in plasma levels of catecholamines, lactate, glucose, and cocaine. Am J Physiol. 1996;270:E438–E444. doi: 10.1152/ajpendo.1996.270.3.E438. [DOI] [PubMed] [Google Scholar]
- Herz A. Opioid reward mechanisms: a key role in drug abuse? Can J Physiol Pharmacol. 1998;76:252–258. doi: 10.1139/cjpp-76-3-252. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources. Guide for the care and use of laboratory animals. Washington DC: National Academy Press; 1996. [Google Scholar]
- Kent S, Hurd M, Satinoff E. Interactions between body temperature and wheel running over the estrous cycle in rats. Physiol. Behav. 1991;49:1079–1084. doi: 10.1016/0031-9384(91)90334-k. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Sex differences in vulnerability to drug self-administration. Exp Clin Psychopharmacol. 2006;14:34–41. doi: 10.1037/1064-1297.14.1.34. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Piehl KB, Acosta G, Peterson AB, Hemby SE. Aerobic exercise attenuates reinstatement of cocaine-seeking behavior and associated neuroadaptations in the prefrontal cortex. Biol Psychiatry. 2010;68:774–777. doi: 10.1016/j.biopsych.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR. Sex differences in the behavioral effects of 24-h/day access to cocaine under a discrete trial procedure. Neuropsychopharmacology. 2004;29:943–951. doi: 10.1038/sj.npp.1300389. [DOI] [PubMed] [Google Scholar]
- MacRae PG, Spirduso WW, Walters TJ, Farrar RP, Wilcox RE. Endurance training effects on striatal D2 dopamine receptor binding and striatal dopamine metabolites in presenescent older rats. Psychopharmacology. 1987;92:236–240. doi: 10.1007/BF00177922. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Kreek MJ. Acute tolerance to the dopamine response induced by a binge pattern of cocaine administration in male rats: an in vivo microdialysis study. J Pharmacol Exp Ther. 1994;268:916–921. [PubMed] [Google Scholar]
- Meeusen R, De Meirleir K. Exercise and brain neurotransmission. Sports Med. 1995;20:160–188. doi: 10.2165/00007256-199520030-00004. [DOI] [PubMed] [Google Scholar]
- Mehl ML, Schott HC, 2nd, Sarkar DK, Bayly WM. Effects of exercise intensity and duration on plasma beta-endorphin concentrations in horses. Am J Vet Res. 2000;61:969–973. doi: 10.2460/ajvr.2000.61.969. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Interactions between kappa opioid agonists and cocaine. Preclinical studies. Ann N Y Acad Sci. 2000;909:104–132. doi: 10.1111/j.1749-6632.2000.tb06678.x. [DOI] [PubMed] [Google Scholar]
- Morgan D, Smith MA, Roberts DC. Binge self-administration and deprivation produces sensitization to the reinforcing effects of cocaine in rats. Psychopharmacology. 2005;178:309–316. doi: 10.1007/s00213-004-1992-6. [DOI] [PubMed] [Google Scholar]
- Mutschler NH, Miczek KA. Withdrawal from a self-administered or non-contingent cocaine binge: differences in ultrasonic distress vocalizations in rats. Psychopharmacology. 1998;136:402–408. doi: 10.1007/s002130050584. [DOI] [PubMed] [Google Scholar]
- Noonan MA, Bulin SE, Fuller DC, Eisch AJ. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J Neurosci. 2010;30:304–315. doi: 10.1523/JNEUROSCI.4256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Roberts DC. Behavioral economic assessment of price and cocaine consumption following self-administration histories that produce escalation of either final ratios or intake. Neuropsychopharmacology. 2009;34:796–804. doi: 10.1038/npp.2008.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Stickgold R, Muzur A, Wigren PE, Ward AS, Hart CL, Clarke D, Morgan A, Hobson JA. Sleep quality deteriorates over a binge--abstinence cycle in chronic smoked cocaine users. Psychopharmacology. 2005;179:873–883. doi: 10.1007/s00213-004-2088-z. [DOI] [PubMed] [Google Scholar]
- Pitts GC, Bull LS. Exercise, dietary obesity, and growth in the rat. Am J Physiol. 1977;232:R38–R44. doi: 10.1152/ajpregu.1977.232.1.R38. [DOI] [PubMed] [Google Scholar]
- Reed SC, Haney M, Evans SM, Vadhan NP, Rubin E, Foltin RW. Cardiovascular and subjective effects of repeated smoked cocaine administration in experienced cocaine users. Drug Alcohol Depend. 2009;102:102–107. doi: 10.1016/j.drugalcdep.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, van Praag H, Jeffrey S, Girard I, Mitchell GS, Garland T, Jr, Gage FH. Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behav Neurosci. 2003;117:1006–1016. doi: 10.1037/0735-7044.117.5.1006. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology. 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Brebner K, Vincler M, Lynch WJ. Patterns of cocaine self-administration in rats produced by various access conditions under a discrete trials procedure. Drug Alcohol Depend. 2002;67:291–299. doi: 10.1016/s0376-8716(02)00083-2. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39:257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 47 Suppl. 2004;1:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav. 2004;78:199–207. doi: 10.1016/j.pbb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev. 2004;28:533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Santucci AC, Hernandez L, Caba J. Wheel-running behavior is altered following withdrawal from repeated cocaine in adult rats. Behav Neurosci. 2008;122:466–470. doi: 10.1037/0735-7044.122.2.466. [DOI] [PubMed] [Google Scholar]
- Smith MA, Iordanou JC, Cohen MB, Cole KT, Gergans SR, Lyle MA, Schmidt KT. Effects of environmental enrichment on sensitivity to cocaine in female rats: importance of control rates of behavior. Behav Pharmacol. 2009;20:312–321. doi: 10.1097/FBP.0b013e32832ec568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Schmidt KT, Iordanou JC, Mustroph ML. Aerobic exercise decreases the positive-reinforcing effects of cocaine. Drug Alcohol Depend. 2008;98:129–135. doi: 10.1016/j.drugalcdep.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner M, Katz RJ, Carroll BJ. Detailed analysis of estrous-related changes in wheel running and self-stimulation. Physiol Behav. 1982;28:201–204. doi: 10.1016/0031-9384(82)90127-5. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Cocaine self-administration “binges”: transition from behavioral and autonomic regulation toward homeostatic dysregulation in rats. Psychopharmacology. 2000;148:289–298. doi: 10.1007/s002130050053. [DOI] [PubMed] [Google Scholar]
- Uda M, Ishido M, Kami K, Masuhara M. Effects of chronic treadmill running on neurogenesis in the dentate gyrus of the hippocampus of adult rat. Brain Res. 2006;1104:64–72. doi: 10.1016/j.brainres.2006.05.066. [DOI] [PubMed] [Google Scholar]
- van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular Med. 2008;10:128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology. 2010;210:121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willuhn I, Sun W, Steiner H. Topography of cocaine-induced gene regulation in the rat striatum: relationship to cortical inputs and role of behavioural context. Eur J Neurosci. 2003;17:1053–1566. doi: 10.1046/j.1460-9568.2003.02525.x. [DOI] [PubMed] [Google Scholar]
- Zlebnik NE, Anker JJ, Gliddon LA, Carroll ME. Reduction of extinction and reinstatement of cocaine seeking by wheel running in female rats. Psychopharmacology. 2010;209:113–125. doi: 10.1007/s00213-010-1776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.