Abstract
Pituitary adenylyl cyclase activating peptide (PACAP) is expressed in central, sensory, autonomic, and enteric neurons. Although it classically acts as a neurotransmitter/neuromodulator, recent studies indicate that PACAP can also regulate immune function. To this effect, PACAP has been shown to reduce clinical symptoms and inflammation in mouse models of human immune-based diseases such as rheumatoid arthritis, Crohn’s Disease, septic shock and multiple sclerosis. Despite these findings, the role of the endogenous peptide in regulating immune function is unknown. To determine if endogenous PACAP plays a protective role in inflammatory bowel disease (IBD) and IBD-associated colorectal cancer in mice, PACAP-deficient (KO) mice were subjected to 3 cycles of dextran sulfate sodium (DSS) in drinking water over 2 months, an established mouse model for colitis. Compared to wild type (WT) controls, PACAP KO mice exhibited more severe clinical symptoms of colitis and had significantly higher colonic inflammation on pathological examination. Moreover, 60% of the PACAP KO mice developed colorectal tumors with an aggressive-appearing pathology. Consistent with published data, DSS-treated WT mice did not develop such tumors. The results demonstrate a new mouse model which rapidly develops inflammation-associated colorectal cancer in the absence of a carcinogen.
Keywords: pituitary adenylyl cyclase activating peptide (PACAP), inflammation, colorectal cancer, colitis, cancer
Chronic inflammatory diseases are known to be linked to the development of cancer.1 In one of the more prominent examples, colorectal cancer (CRC) occurs at a significantly higher rate in patients with inflammatory bowel disease (IBD) compared to the normal population. The increased incidence of CRC in IBD patients appears to be most pronounced in individuals with extensive and long-term ulcerative colitis.2 The rate of CRC begins to increase significantly after 10 years of colitis, with a cumulative probability of 18% after 30-year duration. Like its more common counterpart, sporadic CRC, the prognosis for IBD-associated CRC is poor: the 5-year survival rate is about 50%. Preventative treatment includes colectomy and the use of drugs. Retrospective studies suggest that chronic treatment with 5-aminosalicylic acid or related drugs can reduce the incidence of CRC in patients with IBD, although the studies are not conclusive, and the mechanism of drug action is uncertain. COX-2 inhibitors may also provide protection and appear to have less potential to aggravate IBD than other nonsteroidal antiinflammatory drugs. Despite potential preventative measures in these patients, the incidence of CRC remains high, and new therapeutic modalities must be explored. Moreover, improved animal models are needed to better understand the disease, to test new treatments, and to understand the mechanisms of action of various treatments. In this regard, several rodent models have been developed to study the pathogenesis of chronic ulcerative colitis and how it relates directly to the development of CRC.3,4 These include natural mutants or genetically-engineered mice that develop the disease spontaneously or after chronic intermittent treatment with agents which induce chronic destruction and repair of the intestinal mucosa such as dextran sulfate sodium (DSS) over a treatment period of 6–12 months. Another popular model utilizes DSS in wild type mice but involves the administration of a procarcinogen such as azoxymethane (AOM) to shorten the time frame for mutations and tumors to occur. While these and other models have had great utility, each has some shortcomings.
Pituitary adenylyl cyclase activating peptide (PACAP) is a 38 amino acid antiinflammatory neuropeptide expressed in the brain and in neurons innervating the intestinal tract and lymphoid organs, and is also produced in immune cells (reviewed in Refs. 5 and 6). Although PACAP is classically viewed as a neurotransmitter or neuromodulator, PACAP can exert powerful antiinflammatory actions when administered exogenously. These actions are mediated by high affinity receptors that are expressed on several immune cell types (reviewed in Ref. 5). In this regard PACAP and the closely related peptide vasoactive intestinal peptide (VIP) have recently been shown to have therapeutic activity in several experimental models of human immune disease. For example, PACAP and/or VIP have been shown to have substantial beneficial actions in the treatment of murine models of rheumatoid arthritis,7,8 Crohn’s disease,9 septic shock, and multiple sclerosis.10,11 The therapeutic effects were associated with down-regulation of proinflammatory responses involving cytokines, chemokines, and receptors. Based on theses studies, PACAP and VIP have emerged as candidates for the treatment of autoimmune and other inflammatory disease. We thus hypothesized that endogenous PACAP might normally provide protection against inflammatory disease and subsequent development of neoplasia. To test for these, we subjected PACAP-deficient mice to a 2-month protocol of 3 cycles of DSS administered in drinking water, without addition of a procarcinogen, a protocol which merely produces mild intermittent colitis in WT mice.
Material and methods
Animals
All husbandry and experimental procedures were performed in compliance with the Animal Welfare Act, Institutional Policies and Guidelines, and adhered to all principles stated in the Guide for the Care and Use of Laboratory Animals.12 The experimental protocol was approved by the UCLA Animal Resource Committee. Mice were obtained from breeding colonies established and maintained at UCLA (Goldschmied Neuroscience and Genetics Research Center). PACAP-deficient mice were generated and characterized previously.13 Because a high percentage (up to 80%) of C57BL/6-backcrossed PACAP KO mice die between the first and second postnatal week due to a thermoregulatory defect,14 PACAP KO and WT control mice used in these experiments were of a 50/50% mixed C57BL/6 × 129 background. Mice were also age- and sex-matched, and were bred, raised and studied in the same specific pathogen-free colony, which excluded the following pathogens: Sendai virus, pneumonia virus of mice, mouse hepatitis virus, minute virus of mice, Theiler’s murine encephalomyelitis GDVII, reovirus, Mycoplasma pulmonis, lymphocytic choriomeningitis virus, ectromelia, epizootic diarrhea of infant mice, mouse parvovirus (additionally screened for with Parv NS1 ELISA), K virus, polyoma virus, mouse adenovirus FL/K87, murine cytomegalovirus, hantaan virus, Encephalitozoon cuniculi, cilia-associated respiratory bacillus, mouse thymic virus, Syphacia sp, Aspicularis tetraptera, fleas, mice, and fur mites. Mice were housed in 11.5 ×7.5 ×5-in. polycarbonate boxes with filter covers (Micro-Isolator™, Lab Products) and hardwood chip bedding (Harlan Sani-Chips, Harlan Teklad). A commercial rodent diet (NIH-31 Modified Sterilizable Diet, Harlan Teklad) and acidified water were provided ad libitum. All animal handling and husbandry procedures were performed under a laminar flow hood. The general health status of all mice was evaluated daily by a trained laboratory animal veterinarian for general signs of pain, discomfort, or nonspecific illness including dehydration, kyphosis (hunched posture), lethargy, changes in activity, skin lesions, anorexia, paresis, or unkempt appearance.
Chemical agents
DSS was purchased from ICN Chemicals (MW 36–44 kDA).
Experimental design
Mice of each genotype (n = 10), 3–4 months of age, were randomly assigned to control and DSS groups. On day 1, mice were treated with 2.5% DSS in their drinking water for 5 days. All mice were then provided with 16 days of nontreated water. This cycle was repeated one time. Mice then received a final treatment of 2.0% DSS in water for 4 days. After 10 days of nontreated water, all mice were euthanized. To provide a greater assessment of tumor development in DSS-treated mice, additional sets of WT and PACAP KO mice were subjected to the DSS treatment protocol so that a total of 21 WT and 24 PACAP KO mice were assessed for tumor development. All mice were weighed at least once weekly throughout the duration of the experiment. Clinical health observations were recorded daily by a laboratory animal veterinarian, for the duration of the experiment. For cytokine gene expression, an additional set of mice were euthanized at the end of the first DSS cycle and analyzed by real time RT-PCR as described below.
Clinical colitis assessment
Each day mice were given a clinical colitis “score” of 0–4 based on severity of clinical signs (Table I). Mice scored as “4” were euthanized. Only 3 of 24 PACAP KO mice, and 0 of 21 WT mice reached this stage during the entire course of the studies. At the end of the study, the colitis product for each mouse was calculated as the total number of days that each mouse exhibited colitis times the average colitis score over the period of time when colitis symptoms were observed.
TABLE I.
CLINICAL COLITIS SCORING CRITERIA
|
Euthanasia and tissue collection
Mice were euthanized in 100% CO2. The entire colon from the cecum to the anal verge was carefully dissected out, flushed until clean with PBS, injected with 10% formalin, and prepared in a standard “Swiss Roll” technique and fixed in 10% neutral buffered formalin. Colon samples were flushed clean with PBS after formalin fixation, and then placed in 70% ethanol for 24 hr. The samples were routinely dehydrated and paraffin embedded, sectioned, and stained with hematoxylin and eosin for light microscopy.
Histological analysis and scoring methods and tumor assessment
Mucosal inflammation, with or without crypt dysplasia or tumor formation, was analyzed on H & E stained sections. A standard scoring system based on that described by Cooper et al.13 was utilized to score inflammatory lesions (Table II). Tumors were characterized as described in the text and in Table III.
TABLE II.
SCORING/ASSESSMENT OF HISTOLOGICAL LESIONS
|
TABLE III.
CYTOLOGICAL CHANGES OBSERVED IN COLONIC EPITHELIA OF TUMOR-BEARING WT AND PACAP-DEFICIENT MICE GIVEN DSS1
| Mouse ID | Genotype | Compression-obliteration of surrounding structures | Mitotic figures | Nuclear hyperchromasia and/or polymorphic nuclei | Nuclear stratification and/or loss of polarity | Tubular crypt proliferation | Loss of goblet cells | Basophilic columnar cells | Distorted glandular and crypt structure |
|---|---|---|---|---|---|---|---|---|---|
| 8117 | PACAP KO | − | ++ | +++ | + | ++ | +++ | + | + |
| 8195 | PACAP KO | +/− | ++ | + | +/− | ++ | +++ | + | + |
| 81962 | PACAP KO | + | +++ | ++ | + | ++ | ++ | + | + |
| 8980 | PACAP KO | ++ | ++++ | +++ | +/− | ++ | ++ | ++ | ++ |
| 8982 | PACAP KO | − | ++ | ++ | − | ++ | ++ | ++ | +/− |
| 9028 | PACAP KO | ++++ | ++++ | ++++ | ++++ | ++++ | +++ | ++++ | ++++ |
| 10220 | PACAP KO | − | + | + | − | + | ++ | + | + |
| 10223 | PACAP KO | ++ | ++++ | +++ | +++ | +++ | ++++ | ++ | ++++ |
| 10252 | PACAP KO | − | +/− | + | + | + | + | +/− | +/− |
| 10253 | PACAP KO | ++ | +++ | +++ | +++ | ++ | ++++ | + | ++++ |
| 10254 | PACAP KO | + | + | ++ | ++ | +++ | + | +++ | ++ |
| 102572 | PACAP KO | ++ | +++ | +++ | +++ | ++ | ++++ | + | +++ |
| 102592 | PACAP KO | ++ | ++ | ++ | +++ | +++ | ++++ | ++++ | ++++ |
| 10262 | PACAP KO | +/− | +/− | +/− | +/− | ++ | ++ | + | ++ |
| 8160 | WT | − | + | + | − | ++ | + | − | + |
| 8161 | WT | − | +/− | +/− | − | + | − | − | − |
| 10188 | WT | − | − | − | − | + | + | + | +/− |
| 10243 | WT | − | + | ++ | + | ++ | + | + | + |
21 WT and 24 PACAP KO mice were included in this study. Only those with tumors are shown.
Because of severe colitis symptoms these mice were euthanized after the second DSS cycle.
Real-time RT-PCR
Total RNA was extracted from the entire colon using Guanidine Thiocyanate-Chloroform method. Briefly, tissue was homogenized in 4 M guanidine thiocyanate containing 0.8% ß-mercaptoethanol. RNA was then isolated in aqueous phase in serial additions of sodium acetate (pH 4.0), phenol, and chloroform-isoamyl alcohol. RNA was then precipitated in isopropanol, and treated with DNAse (Ambion, Austin, TX) to remove residual contaminating DNA. About 240 ng of RNA was used to synthesize cDNA in a total reaction of 25 μl, following the protocol provided by iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA). Realtime RT-PCR was performed using SYBR Green detection method (Bio-Rad Laboratories, Hercules, CA), using the following conditions: 2 min at 50°C, 4 min at 95°C, 50 cycles of amplification (96°C for 20 sec, 60°C for 30 sec, and 72°C for 20 sec). Oligonucleotide primer sequences used here were: HPRT (housekeeping gene) (s) TGGTGAAAAGGACCTCTCGAA (as) TCAAGGGCATATCCAACAACA; IL1-ß (s) GTGAATGCCACCTTTTGACA (as) CAAAGGTTTGGAAGCAGCC; IL6 (s) TTCCATCCAGTTGCCTTCTTG (as) TTGGGAGTGGTATCCTCTGTGA.
Statistical analysis
Data analysis and interpretation was performed using 2-way ANOVA and Bonferroni posttests. p < 0.05 were considered significant.
Results
Increased inflammation in DSS-treated PACAP −/− mice
Cohorts of 10 wild type (WT) and PACAP KO mice (3–4 months of age) were given either normal drinking water or 3 separate rounds of DSS treatment (MW 36–44 kD, ICN) over a period of 2 months. The scoring criteria for clinical severity of colitis and for histological assessment of inflammation are described in Tables I and II, respectively. Wild type (WT) and PACAP KO mice receiving normal drinking water showed no appreciable clinical signs of colitis (Fig. 1a) and showed no histological signs of inflammation (Figs. 1b and 2a). Thus PACAP KO mice did not exhibit the type of spontaneous intestinal inflammation observed in certain immunocompromised IBD models such as IL-1015 and Gαi216 deficient mice. In contrast and as expected, DSS produced in WT mice mild to moderate signs of clinical colitis (Fig. 1a) and moderate histological inflammation scores (Fig. 1b). PACAP KO mice had more severe clinical symptoms of colitis (Fig. 1a) and significantly higher histological inflammation scores than WT mice (Fig. 1b). In fact, 8 of 10 PACAP KO mice had inflammation scores or 3 or higher, whereas only 1 mouse in the WT group reached a score of 3. Inflammation was in all cases restricted to the distal part of the colon, typical of ulcerative colitis in humans. Macrophages and lymphocytes, and lymphoid aggregates were typically present in both the mucosa and submucosa, suggestive of chronic inflammation (Fig. 2). However, neutrophils were also present, suggesting some degree of acute inflammation.
FIGURE 1.
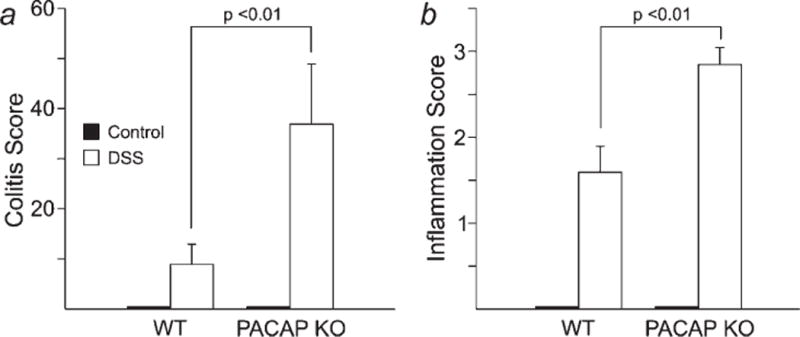
Mean clinical colitis symptoms and (a) and histological inflammatory scores (b) in WT and PACAP mice given normal drinking water (control) or 3 cycles of DSS in drinking water. Scoring criteria for clinical symptoms and histology are indicated in Tables I and II, respectively.
FIGURE 2.

Colon architecture in PACAP KO mice given normal drinking water (a) or 3 cycles of DSS (b and c). Note in panel b the extensive inflammatory infiltration in the mucosa and submucosa, and the complete loss of recognizable crypt structures and surface epithelium, and the presence of a lymphoid aggregate in the mucosa. Panel c is a high power magnification of the boxed area in panel b showing prominent infiltration of macrophages and lymphocytes. Neutrophils are also present. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Proinflammatory cytokines gene expression is hyperinduced in PACAP KO mice
In separate experiments, we examined gene expression of IL-1b, IL-6, and TNF-α, 3 representative proinflammatory cytokines, in WT and PACAP-deficient mice at the end of the first cycle of DSS. In agreement with the higher inflammation scores, induction of IL-1β and IL-6 mRNAs were significantly enhanced in PACAP KO mice (Fig. 3). TNF-α mRNA levels were higher in PACAP KO than WT mice, but the levels were highly variable between animals, and the difference was not significant (data not shown).
FIGURE 3.
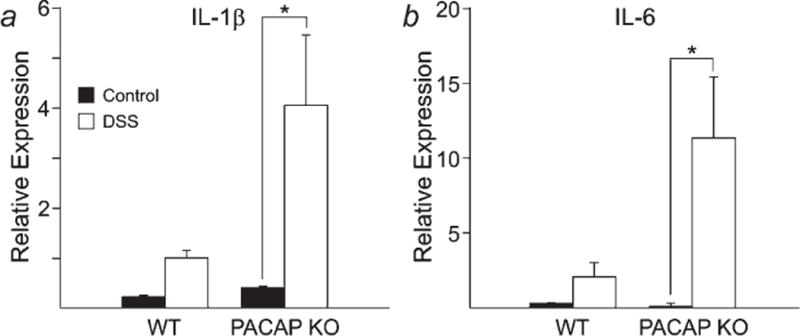
Induction of IL-1β (a) and IL-6 (b) gene expression in DSS-treated WT and PACAP KO mice at the end of the first DSS cycle. *p < 0.05 ANOVA.
Colorectal tumors in PACAP KO mice
In the first cohort of WT and PACAP KO mice, 2 of the 10 mice in the PACAP KO group and none of 10 in the WT group exhibited severe clinical signs of illness that necessitated euthanasia before the third DSS cycle. We subjected these mice to histological examination as well as the remaining mice in this cohort after 3 DSS cycles to systematically examine for the presence of tumors. Additional cohorts of mice were treated with 3 DSS cycles so that a total of 24 PACAP KO and 21 WT mice were investigated. Tumors were observed in 14 of 24 PACAP KO mice and in 4 out of 21 WT mice (Table III). In all cases, tumors were restricted to distal 1/3 to 1/2 of the colon, similar to chronic colitis-associated adenocarcinomas in humans. Grossly, they were focally extensive, very firm, and were tan and irregular in appearance. The neoplastic lesions were mainly flat, although some more advanced lesions were polypoid. Microscopically, it was notable that the neoplastic glands were usually associated with collagenous stroma and inflamed spindle cells. This is typical of inflammatory desmoplasia in human invasive colon carcinoma, and delineated in the analyses of invasive lesions associated with immune colitis in mouse genetic models,17 although no metastatic lesions were found in the animals reported here. In scoring neoplastic changes and other features of these tumors, we considered the fact that classification of colorectal tumors based on histological criteria has been a subject of much debate.18–20 For example, pathologists do not always agree on the distinction between adenoma and adenocarcinoma, with some pathologists considering the characteristic of invasiveness as of greatest importance in making the distinction, whereas others place greater emphasis on cytological changes. Moreover, the term “dysplastic” is controversial because an apparent dysplastic phenotype may be observed in the normal process of regeneration that occurs after destruction of the epithelia19 We thus scored all mice with respect to specific cytological changes in epithelial cells commonly associated with neoplasia,18–20 such as loss of polarity, high rate of mitotic figures, and intense nuclear staining (Table III). Strikingly, more than half of the DSS-treated PACAP KO mice (14 out of 24) had tumors with highly aggressive characteristics. In contrast, epithelial cells in WT mice exhibited only minor neoplastic changes, and these occurred in only 4 of 21 mice. The latter is consistent with published data indicating that WT mice given DSS only rarely develop aggressive colorectal tumors, and then only after a considerably more extensive 6–8 months of intermittent DSS treatment.21,22 Notably, all of the tumors in PACAP KO mice exhibited multiple changes associated with an aggressive pathology, including loss of crypt structure and goblet cells, stacked columnar basophilic cells, loss of polarity, high rate of mitotic figures, intense nuclear staining, prominent nucleoli, and obliteration of crypts and nearby structures. Examples of the cytological changes observed in tumors of PACAP KO mice are shown in Figure 4.
FIGURE 4.

Aggressive-appearing tumors in DSS-treated PACAP KO mice. (a) Lower portion of lumen in a treated PACAP deficient mouse shows cystic and solid areas, loss of crypt structure and goblet cells, stacked, columnar basophilic cells, loss of polarity, high rate of mitotic figures, obliteration of crypts and nearby structures. Upper part of the lumen shows advanced stage 3 inflammation with massive infiltration of lymphocytes and loss of identifiable crypts (right side) and an island of dysplastic crypts surrounded by fibrous tissue (left side). (b and c) High power photomicrographs of tumors in DSS-treated PACAP KO mice showing mitotic figures, loss of polarity, intense nuclear staining, and prominent nucleoli, loss of organizational crypt structure. (d) Tumor in a DSS-treated PACAP KO mouse showing possible invasion into the lamina propria. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Discussion
Well-defined animal models are important research tools to identify early stages of colitis-induced malignancies, to test subsequent interventions to delay or stop the development of tumors in predisposed individuals, and finally to study mechanisms of therapeutic interventions. The data reported here establish PACAP knockout mice as an animal model in which aggressive-appearing colorectal tumors rapidly develop after induction of mild to moderate colitis, without the use of a carcinogen. Several rodent models have been developed to study the pathogenesis of chronic ulcerative colitis and how it relates directly to the development of CRC. While these models have had great utility, each has some shortcomings. For example, while wild type rodents develop colorectal tumors after DSS alone, this generally requires many rounds of DSS, requiring up to 8 months of study, and very few of these have aggressive features.21,22 Thus, investigators have attempted to accelerate the process of tumor generation by administering procarcinogens just prior to administration of DSS.23–27 For example, administration of AOM along with DSS results in the occurrence of multiple lesions in the colon after about 2-month treatment. However, the carcinogen-induced mutations are not random; this mutagen induces mainly G:C to A:T transitions, as documented in K-ras and β-catenin genes in AOM-treated rodents,28 and is presumed to involve O6-methyl-deoxyguanine adducts.
Mice with mutations in specific genes have also been used as models for CRC. ApcMin+ mice, for example, are an excellent models sporadic CRC. However, these mice develop spontaneous tumors in the absence of observable inflammation. Unfortunately, no specific germ line mutations have been linked to the increased incidence of CRC associated with IBD. However, certain genetically engineered mice spontaneously develop a severe colitis-like condition and subsequently develop colon tumors. These include IL-1015 and Gαi216 KO mice. However, these mice exhibit overt abnormalities of immune function, in contrast to most patients with IBD. Nonetheless, IL-10 and Gαi2 KO mice have had great utility in the study of IBD; they have been used to determine the roles of specific lymphocyte subsets in colitis29,30 and to test various therapeutic strategies. Recently, p53-deficient mice have been shown to develop a high rate of invasive colorectal tumors when treated with DSS.31 This is of interest because p53 mutations occur relatively early in the course of IBD-associated CRC.4
Despite these excellent models, we believe that PACAP −/− mice reported here exhibit a unique combination of features which will make them useful in studies of IBD-associated CRC. Unlike mice with null mutations in IL-10 and Gαi2 genes, PACAP KO mice are relatively normal in the resting state, but can be induced to develop aggressive tumors in a controlled manner over a short period of time. Administration of carcinogens, which might induce biased sets of mutations, is unnecessary. In this regard, it will be of interest to determine in this model if the pattern of specific mutations that occurs in tumors at various stages mirrors that seen in IBD-associated tumors in humans. So far, no animal models have recapitulated the progression of mutations that have been observed in IBD-associated tumors. For example, although mutations in the p53 gene appear to be an early event in IBD-associated tumors (unlike in sporadic CRC).4 This is a very uncommon occurrence in animal models of IBD-associated CRC.
The mechanism by which PACAP protects against DSS-induced tumors remains to be determined. Clinical symptoms, inflammatory changes, and proinflammatory cytokine responses were significantly more severe in PACAP KO vs. WT controls, suggesting that the increase in tumor incidence and severity was due to the heightened inflammatory response to DSS in PACAP-deficient mice. PACAP has multiple actions on immune cells, and like most cytokines, it actions are complex.5 Most, but not all of its immunomodulatory actions are mediate by receptors which interact with high affinity to both VIP and PACAP (VPAC1 and VPAC2 receptors). The best-studied actions of these peptides are on macrophages. Numerous studies indicate that PACAP and VIP inhibit the production and release of proinflammatory cytokines and certain cytokines from macrophages and stimulate the production of ant-inflammatory cytokines such as IL-10 and chemokines.32 Moreover, these peptides inhibit the expression of costimulatory molecules B7.1 and B7.2 in macrophages and dendritic cells.33,34 Evidence also suggest that PACAP and VIP can promote a positive Th2 to Th1 balance by acting directly on Th cells during differentiation, and specifically on Th2-differentiated cells to increase their proliferation, survival, and chemotaxis (reviewed in Ref. 5). Recent studies indicate that the related peptide VIP can induce through tolerogenic dendritic cells and regulatory T cells via the common VIP/PACAP receptor VPAC1.35–37
Although PACAP KO mice showed much higher degrees of inflammation after DSS treatment than WT mice, loss of this peptide could have contributed to induction of aggressive tumors in other ways. PACAP has diverse biological actions, including the capacity to regulate the growth and survival of many cell types6,38 and to potentially regulate angiogenesis.39 For example, the related peptide VIP was found to be an antiproliferative factor on human colonic epithelial cells in an explant assay,40 and both PACAP and VIP inhibited proliferation in 4 different human colo-nic cell lines.41 Thus, loss of PACAP could potentially result in an increase in tumor rate of growth independent of its actions on immune cells.
Acknowledgments
The authors thank Dr. Gardenia Cheung-Lau, Hongmei Dong and Matthew Mossanen for their assistance with these experiments.
Grant sponsor: NIH; Grant numbers: HD06576, HD34475, CA110384.
Footnotes
Lucy Duong was a recipient of a Student Research Fellowship Award from the Crohn’s and Colitis Foundation of America.
References
- 1.Lotze MT, Herberman RB. Cancer as a chronic inflammatory disease: role of immunotherapy in cancer and inflammation. In: Morgan DW, Nakada M, Forssmann UJ, editors. Cancer and inflammationed. Boston: Bir-khauser Verlag; 2004. pp. 21–51. [Google Scholar]
- 2.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–33. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 3.Wirtz S, Neurath MF. Animal models of intestinal inflammation: new insights into the molecular pathogenesis and immunotherapy of inflammatory bowel disease. Int J Colorectal Dis. 2000;15:144–60. doi: 10.1007/s003840000227. [DOI] [PubMed] [Google Scholar]
- 4.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–G17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 5.Delgado M, Pozo D, Ganea D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev. 2004;56:249–90. doi: 10.1124/pr.56.2.7. [DOI] [PubMed] [Google Scholar]
- 6.Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000;52:269–324. [PubMed] [Google Scholar]
- 7.Delgado M, Abad C, Martinez C, Leceta J, Gomariz RP. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med. 2001;7:563–8. doi: 10.1038/87887. [DOI] [PubMed] [Google Scholar]
- 8.Abad C, Martinez C, Leceta J, Gomariz RP, Delgado M. Pituitary adenylate cyclase-activating polypeptide inhibits collagen-induced arthritis: an experimental immunomodulatory therapy. J Immunol. 2001;167:3182–9. doi: 10.4049/jimmunol.167.6.3182. [DOI] [PubMed] [Google Scholar]
- 9.Abad C, Martinez C, Juarranz MG, Arranz A, Leceta J, Delgado M, Gomariz RP. Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn’s disease. Gastroenterology. 2003;124:961–71. doi: 10.1053/gast.2003.50141. [DOI] [PubMed] [Google Scholar]
- 10.Kato H, Ito A, Kawanokuchi J, Jin S, Mizuno T, Ojika K, Ueda R, Suzumura A. Pituitary adenylate cyclase-activating polypeptide (PACAP) ameliorates experimental autoimmune encephalomyelitis by suppressing the functions of antigen presenting cells. Mult Scler. 2004;10:651–9. doi: 10.1191/1352458504ms1096oa. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Rey E, Fernandez-Martin A, Chorny A, Martin J, Pozo D, Ganea D, Delgado M. Therapeutic effect of vasoactive intestinal peptide on experimental autoimmune encephalomyelitis: down-regulation of inflammatory and autoimmune responses. Am J Pathol. 2006;168:1179–88. doi: 10.2353/ajpath.2006.051081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Institute of Laboratory Animal Resources CoLS, National Research Council. Guide for the Care and Use of Laboratory Animalsed. Washington, DC: National Academy Press; 1996. [Google Scholar]
- 13.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–49. [PubMed] [Google Scholar]
- 14.Gray SL, Yamaguchi N, Vencova P, Sherwood NM. Temperature-sensitive phenotype in mice lacking pituitary adenylate cyclase-activating polypeptide. Endocrinology. 2002;143:3946–54. doi: 10.1210/en.2002-220401. [DOI] [PubMed] [Google Scholar]
- 15.Sturlan S, Oberhuber G, Beinhauer BG, Tichy B, Kappel S, Wang J, Rogy MA. Interleukin-10-deficient mice and inflammatory bowel disease associated cancer development. Carcinogenesis. 2001;22:665–71. doi: 10.1093/carcin/22.4.665. [DOI] [PubMed] [Google Scholar]
- 16.Rudolph U, Finegold MJ, Rich SS, Harriman GR, Srinivasan Y, Brabet P, Boulay G, Bradley A, Birnbaumer L. Ulcerative colitis and adenocarcinoma of the colon in G alpha i2-deficient mice. Nat Genet. 1995;10:143–50. doi: 10.1038/ng0695-143. [DOI] [PubMed] [Google Scholar]
- 17.Huang EH, Park JC, Appelman H, Weinberg AD, Banerjee M, Logsdon CD, Schmidt AM. Induction of inflammatory bowel disease accelerates adenoma formation in Min +/− mice. Surgery. 2006;139:782–8. doi: 10.1016/j.surg.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Flejou JF, Geboes K, Hattori T, Hirota T, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–5. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riddell RH. Premalignant and early malignant lesions in the gastrointestinal tract: definitions, terminology, and problems. Am J Gastroenterol. 1996;91:864–72. [PubMed] [Google Scholar]
- 20.Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, Russell R, Besselsen DG, Godfrey VL, Doetschman T, Dove WF, Pitot HC, Halberg RB, et al. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;124:762–77. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- 21.Cooper HS, Murthy S, Kido K, Yoshitake H, Flanigan A. Dysplasia and cancer in the dextran sulfate sodium mouse colitis model. Relevance to colitis-associated neoplasia in the human: a study of histopathology, B-catenin and p53 expression and the role of inflammation. Carcinogenesis. 2000;21:757–68. doi: 10.1093/carcin/21.4.757. [DOI] [PubMed] [Google Scholar]
- 22.Okayasu I, Yamada M, Mikami T, Yoshida T, Kanno J, Ohkusa T. Dysplasia and carcinoma development in a repeated dextran sulfate sodium-induced colitis model. J Gastroenterol Hepatol. 2002;17:1078–83. doi: 10.1046/j.1440-1746.2002.02853.x. [DOI] [PubMed] [Google Scholar]
- 23.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Kohno H, Suzuki R, Sugie S, Tanaka T. Beta-Catenin mutations in a mouse model of inflammation-related colon carcinogenesis induced by 1,2-dimethylhydrazine and dextran sodium sulfate. Cancer Sci. 2005;96:69–76. doi: 10.1111/j.1349-7006.2005.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki R, Kohno H, Sugie S, Tanaka T. Sequential observations on the occurrence of preneoplastic and neoplastic lesions in mouse colon treated with azoxymethane and dextran sodium sulfate. Cancer Sci. 2004;95:721–7. doi: 10.1111/j.1349-7006.2004.tb03252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–73. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang JG, Wang DF, Lv BJ, Si JM. A novel mouse model for colitis-associated colon carcinogenesis induced by 1,2-dimethylhydrazine and dextran sulfate sodium. World J Gastroenterol. 2004;10:2958–62. doi: 10.3748/wjg.v10.i20.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi M, Wakabayashi K. Gene mutations and altered gene expression in azoxymethane-induced colon carcinogenesis in rodents. Cancer Sci. 2004;95:475–80. doi: 10.1111/j.1349-7006.2004.tb03235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rennick DM, Fort MM, Davidson NJ. Studies with IL-10−/− mice: an overview. J Leukoc Biol. 1997;61:389–96. doi: 10.1002/jlb.61.4.389. [DOI] [PubMed] [Google Scholar]
- 30.Wu JY, Jin Y, Edwards RA, Zhang Y, Finegold MJ, Wu MX. Impaired TGF-beta responses in peripheral T cells of G alpha i2−/− mice. J Immunol. 2005;174:6122–8. doi: 10.4049/jimmunol.174.10.6122. [DOI] [PubMed] [Google Scholar]
- 31.Fujii S, Fujimori T, Kawamata H, Takeda J, Kitajima K, Omotehara F, Kaihara T, Kusaka T, Ichikawa K, Ohkura Y, Ono Y, Imura J, et al. Development of colonic neoplasia in p53 deficient mice with experimental colitis induced by dextran sulphate sodium. Gut. 2004;53:710–6. doi: 10.1136/gut.2003.028779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delgado M, Ganea D. Inhibition of endotoxin-induced macrophage chemokine production by vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide in vitro and in vivo. J Immunol. 2001;167:966–75. doi: 10.4049/jimmunol.167.2.966. [DOI] [PubMed] [Google Scholar]
- 33.Delgado M, Gomariz RP, Martinez C, Abad C, Leceta J. Anti-inflammatory properties of the type 1 and type 2 vasoactive intestinal peptide receptors: role in lethal endotoxic shock. Eur J Immunol. 2000;30:3236–46. doi: 10.1002/1521-4141(200011)30:11<3236::AID-IMMU3236>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 34.Delgado M, Reduta A, Sharma V, Ganea D. VIP/PACAP oppositely affects immature and mature dendritic cell expression of CD80/CD86 and the stimulatory activity for CD4(+) T cells. J Leukoc Biol. 2004;75:1122–30. doi: 10.1189/jlb.1203626. [DOI] [PubMed] [Google Scholar]
- 35.Delgado M, Gonzalez-Rey E, Ganea D. The neuropeptide vasoactive intestinal peptide generates tolerogenic dendritic cells. J Immunol. 2005;175:7311–24. doi: 10.4049/jimmunol.175.11.7311. [DOI] [PubMed] [Google Scholar]
- 36.Chorny A, Gonzalez-Rey E, Fernandez-Martin A, Pozo D, Ganea D, Delgado M. Vasoactive intestinal peptide induces regulatory dendritic cells with therapeutic effects on autoimmune disorders. Proc Natl Acad Sci USA. 2005;102:13562–7. doi: 10.1073/pnas.0504484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez-Martin A, Gonzalez-Rey E, Chorny A, Ganea D, Delgado M. Vasoactive intestinal peptide induces regulatory T cells during experimental autoimmune encephalomyelitis. Eur J Immunol. 2006;36:318–26. doi: 10.1002/eji.200535430. [DOI] [PubMed] [Google Scholar]
- 38.Waschek JA. Multiple actions of pituitary adenylyl cyclase activating peptide in nervous system development and regeneration. Dev Neurosci. 2002;24:14–23. doi: 10.1159/000064942. [DOI] [PubMed] [Google Scholar]
- 39.Ogasawara M, Murata J, Kamitani Y, Hayashi K, Saiki I. Inhibition by vasoactive intestinal polypeptide (VIP) of angiogenesis induced by murine Colon 26-L5 carcinoma cells metastasized in liver. Clin Exp Metastasis. 1999;17:283–91. doi: 10.1023/a:1006648402164. [DOI] [PubMed] [Google Scholar]
- 40.Toumi F, Neunlist M, Cassagnau E, Parois S, Laboisse CL, Galmiche JP, Jarry A. Human submucosal neurones regulate intestinal epithelial cell proliferation: evidence from a novel coculture model. Neurogastroenterol Motil. 2003;15:239–42. doi: 10.1046/j.1365-2982.2003.00409.x. [DOI] [PubMed] [Google Scholar]
- 41.Lelievre V, Meunier AC, Caigneaux E, Falcon J, Muller JM. Differential expression and function of PACAP and VIP receptors in four human colonic adenocarcinoma cell lines. Cell Signal. 1998;10:13–26. doi: 10.1016/s0898-6568(97)00067-3. [DOI] [PubMed] [Google Scholar]


