Abstract
Objective
To describe cartilage matrix and morphology changes, assessed using quantitative MRI, after acute anterior cruciate ligament (ACL) injury relative to controls and longitudinally during 2 years following reconstruction.
Method
Fifteen patients with acute ACL injuries and sixteen healthy volunteers with a similar demographic profile but no history of osteoarthritis or knee injury were studied. The injured knee of each participant was imaged with a 3.0 T MR scanner at baseline (prior to ACL reconstruction); patients’ knees were re-imaged 1- and 2-years after ACL reconstruction. Cartilage T1ρ and T2 values in full thickness, superficial layers, and deep layers, and cartilage thickness of the full layer were quantified within subcompartments of the knee joint.
Results
In the posterolateraltibial cartilage, T1ρ values were significantly higher in ACL-injured knees than control knees at baseline and were not fully recovered at the 2-year after ACL reconstruction. T1ρ values of medial tibiofemoral cartilage in ACL-injured knees increased over the 2-year study and were significantly elevated compared to that of the control knees. T2 values in cartilage of the central aspect of the medial femoral condyle at the 2-year follow-up were significantly elevated compared with control knees. Cartilage in the posterior regions of the lateral tibia was significantly thinner, while cartilage in the central aspect of the medial femur was significantly thicker than that of controls. Patients with lesions in the posterior horn of the medial meniscus exhibited significantly higher T1ρ values in weight-bearing regions of the tibiofemoral cartilage than that of control subjects over the two year period, whereas patients without medial meniscal tears did not.
Conclusion
Quantitative MRI provides powerful in vivo tools to quantitatively evaluate early changes of cartilage matrix and morphology after acute ACL injury and reconstruction, which may possibly relate to the development of post-traumatic osteoarthritis in such joints.
Keywords: Anterior cruciate ligament, Post-traumatic osteoarthritis, Magnetic resonance imaging, T1ρ, T2, Cartilage
Introduction
Anterior cruciate ligament (ACL) rupture is a common and serious knee injury. ACL-injured knees are currently treated by reconstructing the ligament with biological tissue grafts, and this surgical procedure has been shown to improve the stability and function of the knee in most patients1. However at 5 to 15 years after surgery, radiographic studies document that approximately 50% of patients who have undergone ACL reconstruction are susceptible to post-traumatic osteoarthritis (OA)2–6. In many young individuals, this injury leads to the development of OA with knee-related symptoms that severely affects their quality of life7,8.
Standard magnetic resonance imaging (MRI) techniques, which include fat-saturated T2-weighted, proton density-weighted fast spin echo (FSE) and T1-weighted spoiled gradient echo (SPGR) sequences, have been found to be useful in detecting morphological changes associated with cartilage breakdown noninvasively9. These sequences, however, are limited from detecting early degenerative changes of the cartilage matrix10,11. Recent developments in MRI techniques, such as T1ρ, T2, and delayed gadolinium-enhanced MRI of cartilage (dGEMRIC), can be used to quantify the biochemical changes in cartilage matrix and detect early cartilage degeneration12–19. A few previous studies applied T1ρ, T2, and dGEMRIC imaging to detect cartilage matrix composition changes after ACL injury and reconstruction20–24.
Our group previously reported that T1ρ quantification was able to detect persistent damage in the lateral tibial cartilage and early degeneration in the medial tibiofemoral cartilage of ACL-injured knees 1 year after reconstruction21. Consistent with previous clinical studies, our study also reported that patients with medial meniscal injury had a higher T1ρ increase than those without, which suggests that meniscal injury is a potential risk factor for post-traumatic OA development3–5.
Despite promising results, the 1-year study warranted a longer follow-up to better understand changes that were observed. Thus, the objectives of the present study are to 1) quantify longitudinal changes in cartilage morphology and matrix in ACL-injured knees two years after ACL reconstruction using quantitative MRI (thickness, T1ρ, and T2 quantification); and 2) identify baseline MR measures that predict cartilage morphology and matrix T1ρ and T2 progression at 2-year. We hypothesize that 1) early degeneration of the lateral and medial tibiofemoral cartilage of ACL-injured knees will persist two years after reconstruction and that 2) baseline meniscal injury and bone marrow edema-like lesions (BMEL) may predict cartilage degeneration progression two years after reconstruction.
Materials and Methods
Subjects
The study was approved by the Committee for Human Research at our institution and all subjects gave informed consent. Sixteen healthy controls and fifteen patients with clinically diagnosed acute ACL rupture were studied. The exclusion criteria included knee radiograph Kellgren-Lawrence (KL) score > 0 for controls and KL score > 2 for patients, prior diagnosed inflammatory arthritis, or previous injury on either knee. Patients who required surgical intervention for other injuries, including collateral ligament and posterior cruciate ligament tears, were excluded from the study. All patients underwent ACL reconstruction (all but one were performed by (C.B.M.), an experienced orthopedic surgeon). One patient underwent concomitant lateral meniscal repair, two patients underwent medial meniscectomy, and one underwent debridement of the posterior lateral horn.
Imaging Protocols
Knee radiography was performed after injury but prior to ACL reconstruction (baseline). The standard knee radiographic protocol included bilateral semiflexed weight-bearing view, 30° flexion lateral view, and bilateral patellofemoral sunrise view. All MR examinations were acquired using a 3 T GE Signa MR scanner (HDx, General Electric Healthcare, Milwaukee, WI) with a transmit/receive quadrature knee coil (Clinical MR Solutions, Brookfield, WI). MR images were taken at baseline (n = 15) and at 1 (n = 15) and 2 (n = 12) years after surgery. Controls were imaged at baseline only. Table 1 summarizes the clinical, T1ρ, and T2 quantification sequences previously developed by our lab21.
Table 1.
Sagittal Imaging Protocol at 3.0 T
| Imaging Parameters | |||||||
|---|---|---|---|---|---|---|---|
| Sequence | TR/TE* (ms) |
FOV (cm) |
Matrix size | Slice Thickness |
Flip Angle |
VPS | Other Parameters |
| T2-weighted fat saturated FSE |
4300/51 | 14 | 512 × 256 | 2.5 mm | - | - | - |
| 3D fat-suppressed High-resolution SPGR |
15/6.7 | 14 | 512 × 512 | 1 cm | 12° | - | - |
| 3D T1ρ | 9.3/3.7 | 14 | 256 × 192 | 4 mm | - | 64 | TSL (ms): 0, 10, 40 80 FSL (Hz): 500 |
| 3D T2 | 9.3/3.7 | 14 | 256 × 192 | 4 mm | - | 64 | Preparation TE (ms): 2.9, 13.6, 24.3, 45.6 |
TR/TE: repetition time/echo time; FOV: field of view; VPS: view per segment; TSL: time of spin lock; FSL: frequency of spin lock.
Conventional Radiographic and Clinical Diagnostic MR Assessment
All radiographs and clinical MR images were reviewed by two experienced musculoskeletal radiologists (L.N. and T.M.L.). The radiographic findings were scored according to the Kellgren-Lawrence scale25. The MR images were analyzed for meniscal lesion, effusion, and cartilage lesion by using modified subscores of the Whole-Organ Magnetic Resonance Imaging Score system, Table 226.
Table 2.
Modified Subscores of the Whole-Organ Magnetic Resonance Imaging Score System
| Articular Features | Grade |
|---|---|
| Meniscal lesion | 0 = normal meniscus, 1 = signal abnormality, 2 = vertical tear, 3 = horizontal tear, 4 = complex tear with both horizontal and vertical components, 5 = maceration of the meniscus |
| Effusion | 0 = normal, 1 = mild, 2 = moderate, 3 = severe |
| Cartilage Lesion | Unmodified 8-point scale26 |
Quantification of Bone Marrow Edema-like Lesions
In all subjects, BMELs were defined as focal subchondral high signal intensity lesions on T2-weighted fat-saturated FSE images. BMELs were segmented semi-automatically using a threshold developed previously by our lab27. The final regions based on the threshold were verified by a radiologist (L.N.).
Cartilage Thickness and MR Relaxation Time Quantification
Cartilage was segmented semi-automatically on sagittal SPGR images by using a in-house program28. The LFC, MFC, LT, and MT were further divided into subcompartments with regard to the meniscus as shown in our 1-year report21. An iterative minimization process was used to calculate the thickness of each subcompartment.
The T1ρ and T2 maps were reconstructed by fitting the images pixel by pixel to the following equations: S(TSL)∝ S0exp(-TSL/T1ρ) for T1ρ and S(TE) ∝ S0exp(-TE/T2) for T2, where TSL is the time of spin lock, TE is the echo time, and S is the signal intensity. The signal-to-noise ratio for each subcompartment in images with TSL = 80 ms or TE = 45.6 ms ranged from 6.8 to 14.8, which is sufficient for robust T1ρ and T2 quantification.
T1ρ and T2 maps were registered to SPGR images, and cartilage contours generated from the SPGR images were overlaid onto the registered T1ρ and T2 maps. To reduce artifacts caused by partial volume effects with synovial fluid, relaxation times greater than 150 ms on T1ρ and relaxation times greater than 100 ms on T2 were automatically removed from quantification. In addition, T1ρ and T2 values were quantified for two equally spaced layers, the deep and the superficial, by using an in-house program29.
Statistical Analysis
Restricted maximum-likelihood mixed-effects regression models were used to analyze outcomes that were measured at multiple times and/or locations within individuals. Subjects are modeled as random effects via variance components correlation matrix to ensure that standard error estimates account for correlated outcomes within subjects. To allow close estimation of effects while avoiding over-fitting, the models include two-way interactions among fixed-effect covariates.
Among all subjects, cartilage thickness was modeled as a function of Bone (femur, tibia, or patella), Side (lateral or medial), Group (controls vs patients), and Year (baseline, 1-year, or 2-year, 2 degrees of freedom (DF)), adjusted for subcompartment and baseline T1ρ. Among patients, linear regression was used to estimate associations of baseline BMEL volume with bone, age, sex, and BMI. BMEL volumes at each time point were compared using rank tests. Also among patients, we modeled the longitudinal pattern in T1ρ and T2 to determine if they vary significantly as a function of clinical (presence of baseline meniscal injury, and baseline BMEL volume) and demographic characteristics (age, sex, BMI) and if any of the latter should be included in the primary analyses.
We separately modeled T1ρ and T2 as functions of Group, Year, Bone, Side, Layer (superficial or deep), and Subcompartment (femur included 4–5 (4 DF) and tibia included 3 (2 DF) per side, whereas patella had no side or subcompartments). We further modeled T1ρ and T2 as functions of Group and Year in models stratified by bone, side, and layer.
In post-hoc analysis, Wilcoxon signed-rank tests were employed to compare the T1ρ and T2 of all subcompartments between the patients and controls. A Spearman rank correlation was performed between mean T1ρ and T2 values of different subcompartments. Data were reported as mean (SD) unless otherwise noted as mean (SE) in the results.
Results
Clinical Profiles
Control and patient groups were similar in age, gender, and BMI (Table 3(a)). The mean time from injury to baseline MRI was 46.1 days and from injury to ACL reconstruction was 83.1 days.
Table 3(a).
Baseline characteristics of study participants by gender and injury status. Mean (Min -Max).
| Women | Men | All | |||||
|---|---|---|---|---|---|---|---|
| Healthy (n=8) |
Injured (n=8) |
Healthy (n=8) |
Injured (n=7) |
Healthy (n=16) |
Injured (n=15) |
2-sided KW test† |
|
| Age (years) | 29.4 (24 – 38) |
33.6 (23 – 49) |
36.3 (23 – 57) |
36.9 (30 – 45) |
32.8 (23 – 57) |
35.1 (23 – 49) |
p = 0.15 |
| BMI (kg/m2) | 22.9 (20 – 29)* |
22.4 (19 – 25) |
25.9 (22 – 29) |
24.3 (21 – 27) |
24.4 (20 – 29) |
23.3 (19 – 27) |
p = 0.44 |
| Injury to Baseline MRI(days) |
- | 37.3 (8 – 56) |
- | 56.3 (15 – 147) |
- | 46.1 (8 – 147) |
- |
| MRI to Surgery (days) |
- | 36.6 (9 – 98) |
- | 37.3 (1 – 92) |
- | 36.9 (1 – 98) |
- |
| Injury to Surgery (days) |
- | 73.9 (41 – 114) |
- | 93.6 (31 – 152) |
- | 83.1 (31 – 152) |
- |
One value missing
Compares healthy and injured participants without adjusting for gender. KW=Kruskal-Wallis.
Based on radiographs, 12 patients had KL score = 0, two had KL = 1 and one had KL = 2. On the basis of MR images, all ACL-injured knees exhibited effusion (grade 1 (n = 5), grade 2 (n = 9), grade 3 (n = 1)).
Ten patients had a mensical lesion involving either the posterior horn of the medial or the lateral meniscus: 3 had isolated medial tears, 3 had isolated lateral tears, and 4 had both medial and lateral meniscal tears, Table 3(b). At baseline, 2 patients had no cartilage lesions, 5 had one, and 8 had more than one. At the 1-year follow-up, all 15 patients had a lesion in at least one compartment with 31 cartilage lesions being observed: 7 in the patella, 6 in MFC, 6 in LFC, 1 in MT and 11 in LT. In the 12 ACL-injured patients who had 2-year scans, 23 cartilage lesions were observed: 6 in the patella, 4 in MFC, 5 in LFC, and 8 in LT.
Table 3(b).
Baseline clinical characteristics of injured participants by gender: counts of categorical variables. Mean (Min - Max).
| Women (n = 8) |
Men (n = 7) |
All (n = 15) |
|
|---|---|---|---|
| KL Scores | |||
| 0 | 6 | 6 | 12 |
| 1 | 1 | 1 | 2 |
| 2 | 1 | 0 | 1 |
| Effusion | |||
| 0 | 0 | 0 | 0 |
| 1 | 3 | 2 | 5 |
| 2 | 4 | 5 | 9 |
| 3 | 1 | 0 | 1 |
| Cartilage Lesion | |||
| Patella | 3 | 3 | 6 |
| MFC | 1 | 3 | 4 |
| LFC | 2 | 0 | 2 |
| MT | 0 | 0 | 0 |
| LT | 5 | 4 | 9 |
| Max | 2.1 (0 – 5) | 2.3(0 – 5) | 2.2 (0 – 5) |
| Total | 3.1 (0 – 7) | 3.4 (0 – 8) | 3.3 (0 – 8) |
| Graft Type | |||
| Hamstring | 4 | 4 | 8 |
| Allograft | 4 | 3 | 7 |
| Medial Meniscal Lesion |
|||
| 0 | 4 | 4 | 8 |
| 1 | 2 | 2 | 4 |
| 2 | 0 | 1 | 1 |
| 3 | 0 | 0 | 0 |
| 4 | 1 | 0 | 1 |
| 5 | 1 | 0 | 1 |
| Lateral Meniscal Lesion* |
|||
| 0 | 6 | 2 | 8 |
| 1 | 0 | 0 | 0 |
| 2 | 0 | 1 | 1 |
| 3 | 1 | 0 | 1 |
| 4 | 0 | 3 | 3 |
| 5 | 1 | 1 | 2 |
One patient showed a root tear in the lateral posterior horn.
Quantification of Bone Marrow Edema-like Lesions
At baseline, all 15 patients had a BMEL in at least one compartment. No significant effect of age, sex, or BMI on baseline BMEL volume was found. The LT was most affected and had the largest mean volume (n = 14; 5.79 (4.4) cm3), followed by the LFC (n = 9; 3.64 (4.1) cm3), MT (n = 9; 1.32 (1.2) cm3), and the MFC (n = 5; 1.61 (1.5) cm3).
BMEL volumes decreased significantly at 1-year and 2-year follow-ups compared to baseline (p < 0.001). At 1-year, 11 patients had a BMEL in at least one compartment (volume: 0.42 (0.5) cm3), with three patients developing new lesions in the LFC (n = 1) or MFC (n = 2). In the second year, four patients had a BMEL in one compartment (0.24 (0.1) cm3), with one patient developing a new lesion in the LFC.
Cartilage Thickness
The estimated mean cartilage thickness did not differ significantly between patients and controls (p = 0.31). The cartilage thickness varied significantly among compartment and subcompartment, being highest in the patella, followed by LT, LFC, MFC, and MT (p < 0.001)(Table 4). After adjustment for baseline T1ρ, the estimated mean cartilage thickness increased significantly during follow-up in patients (p = 0.027).
Table 4.
Adjusted mean cartilage thickness in defined compartments. Mean (SD).
| Bone | Side | Controls | Patients | ||
|---|---|---|---|---|---|
| Year 0 | Year 1 | Year 2 | |||
| Femur | |||||
| Medial | 1.06 (0.21) | 1.13 (0.26) | 1.13 (0.27) | 1.19 (0.30) | |
| Lateral | 1.15 (0.30) | 1.22 (0.30) | 1.22 (0.26) | 1.28 (0.32) | |
| Tibia | |||||
| Medial | 0.82 (0.23) | 0.89 (0.18) | 0.89 (0.21) | 0.95 (0.18) | |
| Lateral | 1.36 (0.49) | 1.44 (0.47) | 1.43 (0.49) | 1.49 (0.45) | |
| Patella | 2.04 (0.36) | 2.11 (0.43) | 2.11 (0.52) | 2.17 (0.63) | |
Post-hoc analysis showed that ACL-injured patients displayed significant cartilage thickening in MFC 3 (p = 0.029), and significant cartilage thinning in LT 3 (p = 0.006) compared to controls at baseline. At the 2-year follow-up, the cartilage in MFC 3 (p = 0.002) and MFC 4 (p = 0.01) of ACL-injured knees were both significantly thicker than the cartilage in controls. In addition, cartilage in LT 3 thickened with respect to the cartilage at baseline, but remained significantly thinner than the cartilage in controls (p = 0.05).
T1ρ and T2 Quantification of Cartilage
The estimated mean relaxation times were significantly higher in patients than controls, whether assessed via T1ρ (p = 0.026) or T2 (p = 0.013). Significant changes during the two-year follow-up were identified by both T1ρ (p = 0.004) and T2 (p = 0.02). Interestingly for T1ρ measurements there was a significant interaction between side and year (p < 0.001), with T1ρ increasing in the medial side and decreasing in the lateral side during follow-up; while no significant interaction between side and year was detected for T2 (p = 0.2), Table 5. Neither T1ρ nor T2 values varied significantly between sides but both varied significantly among bones, among compartments, and between layers (all p < 0.001), Table 5.
Table 5.
T1ρ and T2 tests for fixed effects.
| T1ρ (1816 Obs) | T2 (1720 Obs) | |||||
|---|---|---|---|---|---|---|
| Effect | Num DF | F Value | Pr > F | Num DF | F Value | Pr > F |
| Group | 1 | 4.99 | 0.0256 | 1 | 6.19 | 0.0130 |
| Year | 2 | 5.50 | 0.0042 | 2 | 3.89 | 0.0205 |
| Cpmt | 4 | 23.45 | <.0001 | 4 | 61.76 | <.0001 |
| Group * Cpmt | 5 | 1.36 | 0.2367 | 5 | 0.94 | 0.4520 |
| Year * Cpmt | 8 | 1.66 | 0.1041 | 8 | 3.58 | 0.0004 |
| Side | 1 | 0.08 | 0.7810 | 1 | 1.78 | 0.1821 |
| Side * Year | 2 | 9.97 | <.0001 | 2 | 1.68 | 0.1859 |
| Side * Cpmt | 3 | 21.25 | <.0001 | 3 | 36.01 | <.0001 |
| Layer | 1 | 458.31 | <.0001 | 1 | 175.20 | <.0001 |
| Layer * Year | 2 | 1.12 | 0.3255 | 2 | 1.43 | 0.2394 |
| Layer * Cpmt | 4 | 55.34 | <.0001 | 4 | 60.10 | <.0001 |
| Bone | 1 | 69.75 | <.0001 | 1 | 149.94 | <.0001 |
| Bone * Year | 2 | 1.35 | 0.2601 | 2 | 0.07 | 0.9363 |
| Bone * Cpmt | 2 | 13.05 | <.0001 | 2 | 8.67 | 0.0002 |
| Side * Bone | 1 | 14.06 | 0.0002 | 1 | 19.18 | <.0001 |
| Layer * Bone | 1 | 19.01 | <.0001 | 1 | 8.22 | 0.0042 |
Cmpt: Subcompartment
Models of T1ρ and T2 levels stratified by bone, side, and layer showed the effect of ACL injury on cartilage was more pronounced in some locations than others - a finding which was further explored in post-hoc analyses. According to both T1ρ and T2, injury effects at baseline and over time were very strong in the superficial cartilage of the LT (T1ρ: Group p = 0.05, Year p = 0.06; T2: Group p = 0.008, Year p = 0.015)(Table 6(b) and (e)). At this location, the effect was strongest at baseline and appeared to resolve over time. The T1ρ of deep cartilage of the LFC showed a similar strong initial effect that resolved over time (Table 6(a)), but according to T2 these effects were not as strong and appeared to persist (Table 6(d)). Finally, the relaxation times appeared to increase over time in the superficial cartilage of the MFC; however, this was statistically significant for T1ρ (Year p = 0.022)(Table 6(a)) and not T2.
Table 6(b).
Mean T1ρ (ms) in tibia.
| Medial | Superficial | Deep | ||||||
|---|---|---|---|---|---|---|---|---|
| Controls | Patients | Controls | Patients | |||||
| Subcompartment | Year 0 | Year 1 | Year 2 | Year 0 | Year 1 | Year 2 | ||
| 1 | 30.9 | 32.7 | 31.6 | 31.3 | 32.2 | 31.8 | 33.8 | 33.8 |
| 2 | 37.5 | 39.9 | 44.1 | 47.3 | 26.4 | 24.1 | 26.4 | 28.6 |
| 3 | 37.9 | 35.7 | 37.3 | 37.6 | 31.5 | 32.2 | 32.6 | 35.9 |
| Mean (SE) | 35.6 (1.33) |
36.7 (1.39) |
37.7 (1.36) |
38.8 (1.46) |
30.0 (1.31) |
30.2 (1.38) |
30.9 (1.35) |
32.5 (1.47) |
| Mean Difference (95% CI) |
-- | 1.10 (−2.73, 4.94) |
2.10 (−1.70, 5.89) |
3.26 (−0.68, 7.19) |
-- | 0.18 (−3.61, 3.98) |
0.96 (−2.78, 4.71) |
2.50 (−1.43, 6.42) |
| Group p = 0.57, Year p = 0.27 | Group p = 0.92, Year p = 0.30 | |||||||
| Lateral | Superficial | Deep | ||||||
| Controls | Patients | Controls | Patients | |||||
| Subcompartment | Year 0 | Year 1 | Year 2 | Year 0 | Year 1 | Year 2 | ||
| 1 | 36.6 | 40.6 | 36.7 | 34.7 | 30.5 | 30.1 | 31.2 | 32 |
| 2 | 38.5 | 37.1 | 40.4 | 38.9 | 26.1 | 24.5 | 25.4 | 26.6 |
| 3 | 41.5 | 46.3 | 42.6 | 42.8 | 33.9 | 37.4 | 36.1 | 39.6 |
| Mean (SE) | 38.9 (0.88) |
41.4 (0.93) |
39.9 (0.91) |
39.2 (0.98) |
30.2 (1.05) |
31.1 (1.11) |
30.9 (1.09) |
32.4 (1.18) |
| Mean Difference (95% CI) |
-- | 2.49 (−0.06, 5.04) |
1.02 (−1.50, 3.54) |
0.27 (−2.35, 2.89) |
-- | 0.87 (−2.18, 3.93) |
0.74 (−2.27, 3.76) |
2.23 (−0.92, 5.37) |
| Group p = 0.05, Year p = 0.06 | Group p = 0.57, Year p = 0.42 | |||||||
Table 6(e).
Mean T2 (ms) in tibia.
| Medial | Superficial | Deep | ||||||
|---|---|---|---|---|---|---|---|---|
| Controls | Patients | Controls | Patients | |||||
| Subcompartment | Year 0 | Year 1 | Year 2 | Year 0 | Year 1 | Year 2 | ||
| 1 | 21.1 | 23.5 | 23.5 | 23.7 | 24.2 | 25.1 | 25.3 | 25.8 |
| 2 | 31.2 | 32.4 | 32.3 | 32.9 | 22.1 | 20.0 | 20.3 | 22.7 |
| 3 | 27.9 | 27.0 | 27.7 | 27.3 | 24.7 | 25.9 | 25.0 | 26.9 |
| Mean (SE) | 26.9 (0.89) |
28.2 (0.93) |
27.8 (0.88) |
28.0 (0.94) |
23.6 (1.37) |
24.5 (1.40) |
23.5 (1.36) |
24.8 (1.41) |
| Mean Difference (95% CI) |
-- | 1.36 (−1.22, 3.93) |
0.95 (−1.56, 3.46) |
1.14 (−1.44, 3.72) |
-- | 0.89 (−3.01, 4.81) |
0.00 (−3.85, 3.85) |
1.18 (−2.73, 5.10) |
| Group p = 0.30, Year p = 0.86 | Group p = 0.65, Year p = 0.33 | |||||||
| Lateral | Superficial | Deep | ||||||
| Controls | Patients | Controls | Patients | |||||
| Subcompartment | Year 0 | Year 1 | Year 2 | Year 0 | Year 1 | Year 2 | ||
| 1 | 23.7 | 29.0 | 26.2 | 24.3 | 22.9 | 22.8 | 21.7 | 21.5 |
| 2 | 28.2 | 27.1 | 30.3 | 27.4 | 19.5 | 18.1 | 20.6 | 20.8 |
| 3 | 30.8 | 34.9 | 33.2 | 31.4 | 25.6 | 29.9 | 31.5 | 30.4 |
| Mean (SE) | 27.6 (0.81) |
30.8 (0.87) |
30.0 (0.80) |
28.4 (0.87) |
22.6 (1.06) |
23.7 (1.12) |
24.6 (1.07) |
24.6 (1.10) |
| Mean Difference (95% CI) |
-- | 3.24 (0.86, 5.62) |
2.39 (0.09, 4.69) |
0.81 (−1.57, 3.19) |
-- | 1.09 (−2.01, 4.18) |
1.95 (−1.07, 4.96) |
1.96 (−1.13, 5.05) |
| Group p = 0.008, Year p = 0.015 | Group p = 0.49, Year p = 0.55 | |||||||
Table 6(a).
Mean T1ρ (ms) in femur.
| Medial | Superficial | Deep | ||||||
|---|---|---|---|---|---|---|---|---|
| Controls | Patients | Controls | Patients | |||||
| Subcompartment | Year 0 | Year 1 | Year 2 | Year 0 | Year 1 | Year 2 | ||
| 2 | 39.1 | 39.7 | 43.9 | 44.3 | 33.3 | 35.1 | 36.1 | 36.6 |
| 3 | 41.4 | 46.1 | 49.8 | 50.3 | 29.0 | 28.8 | 30.2 | 28.8 |
| 4 | 39.4 | 41.3 | 44.0 | 43.7 | 31.0 | 34.2 | 33.5 | 30.9 |
| 5 | 40.7 | 43.5 | 42.3 | 42.4 | 36.9 | 39.1 | 39.0 | 41.2 |
| Mean (SE) | 40.2 (1.07) |
42.7 (1.13) |
45.0 (1.11) |
45.5 (1.18) |
32.5 (0.86) |
34.5 (0.91) |
34.7 (0.89) |
34.6 (0.97) |
| Mean Difference* (95% CI) |
-- | 2.55 (−0.54, 5.64) |
4.83 (1.77, 7.89) |
5.33 (2.16, 8.49) |
-- | 1.97 (−0.51, 4.46) |
2.13 (−0.33, 4.58) |
2.09 (−0.48, 4.66) |
| Group p = 0.10, Year p = 0.022† | Group p = 0.12, Year p = 0.99 | |||||||
| Lateral | Superficial | Deep | ||||||
| Controls | Patients | Controls | Patients | |||||
| Subcompartment | Year 0 | Year 1 | Year 2 | Year 0 | Year 1 | Year 2 | ||
| 1 | 46.7 | 46.4 | 44.0 | 48.6 | 37.8 | 39.3 | 37.1 | 38.7 |
| 2 | 37.1 | 38.4 | 37.0 | 37.3 | 28.8 | 30.4 | 27.8 | 27.7 |
| 3 | 43.6 | 44.1 | 44.8 | 43.0 | 32.8 | 34.3 | 31.7 | 31.4 |
| 4 | 43.2 | 43.3 | 43.9 | 44.6 | 36.0 | 37.9 | 35.7 | 35.1 |
| 5 | 40.0 | 45.6 | 42.6 | 43.9 | 36.5 | 39.7 | 37.8 | 39.2 |
| Mean (SE) | 42.1 (0.87) |
43.9 (0.91) |
42.4 (0.89) |
43.7 (0.94) |
34.4 (0.73) |
36.5 (0.76) |
34.0 (0.75) |
34.5 (0.79) |
| Mean Difference (95% CI) |
-- | 1.76 (−0.73, 4.26) |
0.31 (−2.17, 2.78) |
1.54 (−1.00, 4.08) |
-- | 2.09 (0.01, 4.18) |
−0.35 (−2.42, 1.71) |
0.083 (−2.05, 2.22) |
| Group p = 0.16, Year p = 0.08 | Group p = 0.049, Year p = 0.001 | |||||||
For all subtables in Table 6:
Differences are relative to healthy controls.
Group effect compares patients with controls at Year 0. Among patients, Year effect compares Years 1 and 2 with Year 0.
The values highlighted with red are those with significant difference compared to controls during post-hoc analysis.
Table 6(d).
Mean T2 (ms) in femur.
| Medial | Superficial | Deep | ||||||
|---|---|---|---|---|---|---|---|---|
| Controls | Patients | Controls | Patients | |||||
| Subcompartment | Year 0 | Year 1 | Year 2 | Year 0 | Year 1 | Year 2 | ||
| 2 | 30.1 | 30.7 | 30.5 | 32.2 | 26.8 | 31.2 | 28.8 | 28.7 |
| 3 | 33.1 | 37.3 | 37.8 | 37.3 | 22.8 | 24.2 | 26.7 | 25.7 |
| 4 | 31.8 | 31.7 | 33.3 | 32.5 | 26.5 | 29.0 | 28.6 | 28.6 |
| 5 | 31.1 | 32.7 | 32.1 | 33.9 | 32.1 | 33 | 33.2 | 35.2 |
| Mean (SE) | 31.5 (0.73) |
33.2 (0.79) |
33.4 (0.72) |
34.1 (0.78) |
27.1 (0.83) |
29.5 (0.89) |
29.3 (0.83) |
29.6 (0.89) |
| Mean Difference (95% CI) |
-- | 1.72 (−0.41, 3.85) |
1.89 (−0.16, 3.93) |
2.57 (0.44, 4.69) |
-- | 2.42 (0.0, 4.83) |
2.24 (−0.08, 4.57) |
2.50 (0.09, 4.91) |
| Group p = 0.11, Year p = 0.54 | Group p = 0.049, Year p = 0.95 | |||||||
| Lateral | Superficial | Deep | ||||||
| Controls | Patients | Controls | Patients | |||||
| Subcompartment | Year 0 | Year 1 | Year 2 | Year 0 | Year 1 | Year 2 | ||
| 1 | 35.1 | 34.3 | 35.8 | 38.7 | 30.1 | 32.4 | 32.2 | 37.1 |
| 2 | 27.4 | 28.0 | 26.9 | 25.5 | 20.0 | 22.2 | 20.7 | 20.6 |
| 3 | 34.4 | 33.1 | 34.0 | 33.2 | 25.3 | 26.6 | 25.0 | 26.7 |
| 4 | 33.8 | 33.9 | 33.6 | 32.8 | 29.6 | 30.1 | 31.2 | 30.2 |
| 5 | 29.6 | 33.2 | 33.0 | 35.8 | 30.1 | 31.5 | 32.5 | 34.8 |
| Mean (SE) | 32.0 (0.77) |
33.1 (0.81) |
32.6 (0.76) |
33.2 (0.80) |
27.1 (0.79) |
28.8 (0.82) |
28.3 (0.77) |
29.8 (0.81) |
| Mean Difference (95% CI) |
-- | 1.00 (−1.21, 3.21) |
0.59 (−1.56, 2.75) |
1,19 (−1.02, 3.40) |
-- | 1.69 (−0.56, 3.93) |
1.24 (−0.95, 3.42) |
2.67 (0.43, 4.92) |
| Group p = 0.37, Year p = 0.63 | Group p = 0.14, Year p = 0.09 | |||||||
Post-hoc analysis showed that at baseline, the T1ρ values of LT 3 and MFC 4 were significantly elevated compared with that of the control subjects (LT 3: 42.9 (6.2) ms vs 36.9 (2.6) ms, p = 0.001; MFC 4: 39.2 (6.9) ms vs 34.3 (4.7) ms, p = 0.018). Significance was also observed in the T2 of LT 3 (32.5 (4.8) ms vs 28.3 (3.2) ms, p = 0.01) and MFC 2 (31.5 (2.4) ms vs 29.1 (2.7) ms, p = 0.041) between patients and controls.
At 1-year, T1ρ in LT 3 decreased but increased in MFC 4. Both values continued to be significantly higher than knees of control subjects (LT 3: 39.5 (3.6) ms, p = 0.033; MFC 4: 39.1 (4.5) ms, p = 0.004). In addition, T1ρ values in MFC 2 (p = 0.011), MFC 3 (p = 0.006), and MT 2 (p = 0.001) of ACL-injured knees were significantly higher than controls. T2 of LT 3 (p = 0.011) in patients was also significantly greater than that of controls.
At 2-year, T1ρ in LT 3 increased compared with that at 1-year, and stayed significantly higher than controls (41.2 (5.3) ms, p = 0.012). T1ρ values in MFC 2 (p = 0.016), MFC 3 (p = 0.011), and MFC 5 (p = 0.011) of ACL-injured knees were also significantly greater than control values.
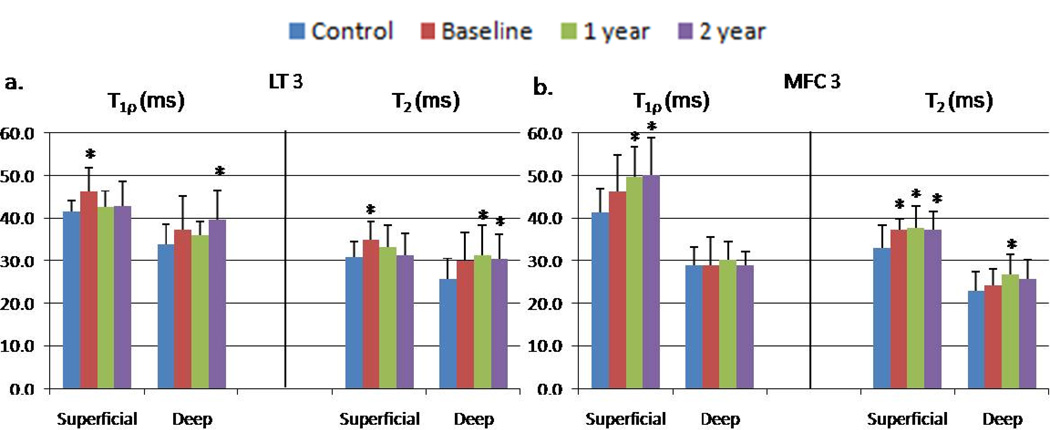
Laminar analysis showed T1ρ values in the LT 3 superficial layer were significantly higher than controls at baseline (p = 0.004), but decreased at 1-year and 2-year. In the deep layer of LT 3, T1ρ values at baseline and 1-year were not significantly different from the control values; but became significantly elevated compared to controls at 2-year (p = 0.014). T2 in LT 3 was very similar to that of T1ρ (Figure 1).
Figure 1.
T1ρ and T2 values in superficial and deep layers of cartilage in (a) LT 3 and (b) MFC 3. *The difference between controls and ACL-injured knees at the given time point was statistically significant (p < 0.05).
At 1-year follow-up, T1ρ values in the superficial layer of MFC 2 (p = 0.009), MFC 3 (p = 0.001), and MT 2 (p = 0.002) were significantly elevated compared to that of control knees. T1ρ values continued to increase in the superficial layer of MFC 2 (p = 0.01), MFC 3 (p = 0.002), and MT 2 (p = 0.01) at 2-year. Only the T2 value of MFC 3 superficial layer increased significantly over the two-year period (p = 0.05). The T2 value of MFC 3 deep layer was also significantly elevated compared to controls at 1-year (p = 0.04).
In both groups, T1ρ and T2 in MFC 3 at baseline were correlated with MFC 4. At 1-year and 2-year, T1ρ in MFC 3 remained highly correlated with MFC 4, and became significantly correlated with MT 2 (p < 0.05).
Relationship between T1ρ Progression and Baseline Meniscal Damage and BMEL
No significant effect of meniscal injury, BMEL volume at baseline, or demographic characteristics (age, sex, BMI) on T1ρ and T2 progression was observed. Post-hoc analysis showed that patients with meniscal lesions at baseline had significantly higher T1ρ values in MFC 3, MFC 4, and MT 2 and significantly higher T2 values in MFC 3 at 2-year follow-up compared to controls (p < 0.05), while no significant difference in T1ρ and T2 values was observed between patients without meniscal lesions and controls (Table 7).
Table 7.
T1ρ and T2 data for cartilage in ACL-injured patients with (+) and without (−) meniscal tears in the medial posterior horn. Mean (SD).
| Baseline | 1 year | 2 year | |||||
|---|---|---|---|---|---|---|---|
| + | − | + | − | + | − | ||
| T1ρ | MFC 3 | 39.76 (3.4) | 36.24 (7.6) | 42.00 (3.4) | 37.27 (5.2) | 40.40 (4.3) | 38.62 (6.1) |
| MFC 4 | 42.53 (8.3) | 36.74 (4.7) | 41.37 (3.1) | 37.12 (4.8) | 38.18 (3.6) | 36.43 (8.5) | |
| MT 2 | 30.22 (3.9) | 33.51 (6.3) | 36.72 (2.1) | 34.75 (4.1) | 39.05 (3.9) | 35.26 (9.2) | |
| MT 3 | 34.18 (3.5) | 34.90 (4.8) | 35.62 (5.9) | 34.07 (3.5) | 36.26 (2.9) | 32.40 (5.3) | |
| T2 | MFC 3 | 30.96 (1.1) | 29.57 (3.8) | 34.58 (3.2) | 28.84 (2.9) | 34.07 (3.1) | 28.93 (2.9) |
| MFC 4 | 31.35 (3.7) | 29.13 (3.6) | 32.12 (2.7) | 29.21 (3.7) | 32.38 (2.6) | 28.58 (2.8) | |
| MT 2 | 27.22 (5.8) | 26.8 (7.6) | 26.14 (4.8) | 26.87 (4.4) | 27.35 (3.2) | 28.10 (7.2) | |
| MT 3 | 26.59 (4.1) | 27.34 (5.1) | 27.18 (5.0) | 26.21 (4.0) | 29.04 (5.4) | 25.24 (2.4) | |
The values highlighted with red are those with significant difference compared to controls during post-hoc analysis.
Discussion
In this study, quantitative MRI techniques at 3 T were employed to characterize the cartilage matrix and morphology of ACL-injured knees two years after surgical reconstruction. Significantly elevated T1ρ and T2 values were observed at baseline and during follow up in ACL-injured knees compared to controls, and significant changes were also found in cartilage thickness of patients compared to controls.
At baseline, T1ρ and T2 measurements in the posterolateral tibia (LT 3) was significantly elevated compared with values in control knees. In addition, cartilage lesions were identified in LT 3 of 9 ACL-injured patients (60.0%), while BMELs were found in the lateral tibia of 14 ACL-injured patients (93.3%). The high prevalence of BMEL in LT was consistent with previous reports30–32. The elevation of T1ρ values in regions overlying BMELs are consistent with our previous cross-sectional studies10, 27. These results suggest that lateral tibia, especially LT 3, had the most severe damage during acute ACL injury and T1ρ and T2 can detect the early changes within the cartilage matrix initiated at the time of injury.
A significant interaction between the side and year dependency of T1ρ values was evident among ACL-injured patients. T1ρ values increased in the medial compartments and decreased in the lateral compartments over the two-year study, suggesting early degeneration in the medial side and partial recovery in the lateral side. At 1-year follow-up, T1ρ values in LT 3 decreased significantly from the baseline measurement, but remained significantly elevated compared with T1ρ values in control subjects. This result implies that despite the complete resolution of seven of the BMELs (50%) in the lateral tibia, the cartilage overlying these BMELs may not be fully repaired. Interestingly, at the 2-year follow-up, T1ρ values in LT 3 increased from its 1-year measurement, and remained significantly elevated compared with T1ρ values in controls. This may be due to the increased loss of proteoglycans associated with the progressive degenerative processes seen in early stages of OA. This potential partial recovery and early degeneration in the lateral side needs to be confirmed in future studies with a larger cohort and a longer follow up.
Furthermore, laminar analysis of the posterolateral tibia at baseline revealed that T1ρ values in the superficial layer decreased from baseline to two-year follow-up, while T1ρ in the deep layer increased over the two year period. The initial increase in T1ρ in the superficial layer may be due to local loss of proteoglycans caused by the initial injury and is compensated for by recovery mechanisms two years after injury. The observed T1ρ elevation in the deep layer of LT 3 two years after injury indicated potential cartilage degeneration and suggested different biochemical responses and recovery mechanisms in the two layers.
In the medial side, there was a general increase in the T1ρ values of the tibiofemoral cartilage in ACL-injured knees. In particular, T1ρ values of weight-bearing and cartilage-on-cartilage regions of the femoral condyle showed the most significant increase from baseline to the two-year follow-up when compared to values of control knees. Moreover, strong correlations between the T1ρ elevation in the weight-bearing regions of the tibiofemoral cartilage of ACL-injured patients at the 1-year and 2-year follow-up were observed. Previous kinematic studies of ACL-reconstructed knees have reported substantially altered tibiofemoral motion, resulting in a shift of which regions of cartilage are in contact33–35. These results suggest abnormal joint kinematics in the medial side of ACL-injured knees may cause articular cartilage damage and the initiation of the early stages of OA. Laminar analysis of the weight bearing tibiofemoral cartilage showed that T1ρ of the superficial layers were significantly elevated compared with values of control knees at the 1-year and 2-year follow-up. These results are consistent with a previous study that reported surface changes including damage and loss of proteoglycans in load-bearing regions of ACL-injured knees35. As shown in Figure 1, the site of early degeneration in LT 3 and MFC 3 at 2-year is different, with damage originating in the deep layer of LT 3 and the superficial layer of MFC 3. This implies that ACL injury may induce different degenerative mechanisms in these cartilage regions.
In this study, one patient had KL = 2, indicating moderate OA. At baseline, this patient had greater T1ρ in all subcompartments than the other patients except for LT 3. No significant difference was observed for T2. This patient had a higher rate of T1ρ increase from baseline to 2-year in all subcompartments of the LT and MT than the mean T1ρ increase from other patients. Not much difference was observed in the LFC or MFC. This observation suggested that a higher baseline degree of OA may cause a higher rate of cartilage degradation after ACL injury, which warrants further investigations with larger cohorts.
Significant spatial variation of cartilage morphology was also observed among subcompartments. In the posterolateral tibia of ACL-reconstructed knees at baseline, cartilage was significantly thinner than the cartilage of control knees. Previous studies have also reported similar findings that the lateral tibia of reconstructed knees showed cartilage thinning, albeit the difference was not significant30, 36. The increased thickness and decreased T1ρ values in the posterolateral tibia during follow up of the patients suggest partial recovery of cartilage in these regions.
In the medial side, cartilage was significantly thicker in weight-bearing regions of the femoral condyle in ACL-injured knees compared with control knees over the two years. As previously reported, the thickest areas of cartilage occur where cartilage-on-cartilage contact was present, and most likely develop as a response to loading37. Cartilage swelling in the medial tibiofemoral compartment has also been reported in patients with minimal severity of radiographic OA38. In conjunction with increased T1ρ values of the weight-bearing medial tibiofemoral cartilage, these results suggest early degeneration in these regions with increase of water content, decrease of proteoglycan, and cartilage swelling.
Previous studies have demonstrated strong association among meniscal injury, BMEL volume, and cartilage degeneration39–41. The current study was unable to identify meniscal injury and baseline measurement of BMEL volume as risk factors for elevated T1ρ or T2 in cartilage. This may be due to the small sample size and larger cohorts are required to increase the statistical power. However, post-hoc analysis indicated that ACL-injured patients with lesions in the posterior horns of the medial meniscus had significantly higher T1ρ values in weight-bearing regions of the tibiofemoral cartilage than that of controls over the two year period, whereas patients without medial meniscal tears did not. Interestingly, only the cartilage-on-cartilage regions of the medial tibia exhibited an increase in T1ρ values from baseline to two-year follow-up. In addition, five patients (33%) had BMEL in the MFC at baseline. The prevalence of BMEL in the MFC may be due to traumatic chondral shear. All five patients all had cartilage lesions (with scores of 2 or higher) in this region and no lesions in the medial meniscus. Three patients also developed new bone marrow lesions in the lateral or the medial aspect of the femur at 1-year, and one patient developed a new lesion in the lateral femur after two years. Of the new BMELs that developed at 1-year, all became resolved by the 2-year follow-up, indicating transient bone remodeling.
The findings of this study were consistent with our previous 1-year study. The previous study's cohort was small and may have lacked the statistical power to reveal significance in the T2 of several layers. Nevertheless, large scale studies are needed to confirm the findings of the current study and to correlate different types of meniscal tears to cartilage injury at baseline and longitudinal follow-up. Another limitation of the present report was that data from uninjured contralateral knees in patients with ACL injuries were not available. The current study also lacks longitudinal data in controls. However in unpublished data from our lab, a control group with a similar age and BMI range (38.8 ± 11.1 years, 24.0 ± 3.4 kg/m2) did not show significant differences between baseline T1ρ (p > 0.27) , T2, (p > 0.33) and thickness (p > 0.24) values in compartments and those at 2-year.
In conclusion, T1ρ and T2 quantifications revealed persistent damage in the posterolateral tibial cartilage and progressive degeneration in the central regions of the medial tibiofemoral cartilage in ACL-injured knees two years after reconstruction. This study also found that cartilage thinning occurs in the posterolateral tibia after an acute ACL injury, while cartilage thickening occurs in the central medial aspect of the femur. Quantitative MRI provides powerful in vivo tools to quantitatively evaluate early changes of cartilage matrix and morphology after acute ACL injury and reconstruction. Such quantitative tools will help stratify injury, monitor and potentially predict post-traumatic OA development in acutely injured joints.
Table 6(c).
Mean T1ρ (ms) in patella.
| Superficial | Deep | |||||||
|---|---|---|---|---|---|---|---|---|
| Controls | Patients | Controls | Patients | |||||
| Year 0 | Year 1 | Year 2 | Year 0 | Year 1 | Year 2 | |||
| Mean (SE) | 43.9 (1.53) |
46.5 (1.62) |
43.5 (1.58) |
45.6 (1.73) |
33.0 (1.44) |
36.0 (1.52) |
32.0 (1.49) |
34.3 (1.61) |
| Mean Difference (95% CI) |
-- | 2.58 (−2.02, 7.18) |
−0.39 (−4.93, 4.14) |
1.71 (−3.05, 6.47) |
-- | 2.99 (−1.33, 7.32) |
−1.03 (−5.29, 3.24) |
1.31 (−3.15, 5.77) |
| Group p = 0.26, Year p = 0.25 | Group p = 0.17, Year p = 0.055 | |||||||
Table 6(f).
Mean T2 (ms) in patella.
| Superficial | Deep | |||||||
|---|---|---|---|---|---|---|---|---|
| Controls | Patients | Controls | Patients | |||||
| Year 0 | Year 1 | Year 2 | Year 0 | Year 1 | Year 2 | |||
| Mean (SE) | 33.2 (0.99) |
35.7 (1.09) |
35.3 (1.00) |
36.1 (1.09) |
26.5 (0.89) |
26.9 (0.97) |
28.7 (0.88) |
29.4 (0.97) |
| Mean Difference (95% CI) |
-- | 2.55 (−0.51, 5.62) |
2.15 (−0.78, 5.07) |
2.86 (−0.21, 5.93) |
-- | 0.42 (−2.31, 3.15) |
2.24 (−0.35, 4.84) |
2.85 (0.12, 5.58) |
| Group p = 0.10, Year p = 0.83 | Group p = 0.75, Year p = 0.11 | |||||||
Acknowledgements
This study was supported by K25-AR053633, R01-AR46905, P50-AR060752.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflict of interest to disclose with regard to the subject matter of this present manuscript.
Authors' Contributions
All authors approved the final version of the manuscript.
Favian Su: data collection, analysis, and interpretation; drafting and critically revised the article.
Joan F. Hilton: statistical data analysis; critically revised the article for intellectual content.
Lorenzo Nardo: data analysis and interpretation; critically revised the article for intellectual content.
Samuel Wu: data collection and processing; provided technical support.
Fei Liang: data collection and processing; provided technical support.
Thomas M. Link: data analysis and interpretation; critically revised the article for intellectual content.
C. Benjamin Ma: conception and design of the study, patient recruitment, and management; provided clinical input.
Xiaojuan Li: conception and design of the study, data analysis and interpretation; drafting and critically revised the article for intellectual content.
Contributor Information
Favian Su, Email: faviansu@gmail.com.
Joan F. Hilton, Email: joan@biostat.ucsf.edu.
Lorenzo Nardo, Email: Lorenzo.Nardo@ucsf.edu.
Samuel Wu, Email: Samuel.Wu@ucsf.edu.
Fei Liang, Email: fy.liang1@gmail.com.
Thomas M. Link, Email: thomas.link@ucsf.edu.
C. Benjamin Ma, Email: MaBen@orthosurg.ucsf.edu.
References
- 1.Fu FH, Bennett CH, Ma CB, Menetrey J, Lattermann C. Current trends in anterior cruciate ligament reconstruction. Part II. Operative procedures and clinical correlations. Am J Sports Med. 2000;28:124–130. doi: 10.1177/03635465000280010801. [DOI] [PubMed] [Google Scholar]
- 2.Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994;22:632–644. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 3.Hanypsiak BT, Spindler KP, Rothrock CR, Calabrese GJ, Richmond B, Herrenbruck TM, et al. Twelve-year follow-up on anterior cruciate ligament reconstruction: long-term outcomes of prospectively studied osseous and articular injuries. Am J Sports Med. 2008;36:671–677. doi: 10.1177/0363546508315468. [DOI] [PubMed] [Google Scholar]
- 4.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50:3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 5.von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63:269–273. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Hart CP, van den Bekerom MP, Patt TW. The occurrence of osteoarthritis at a minimum of ten years after reconstruction of the anterior cruciate ligament. J Orthop Surg. 2008;3:24. doi: 10.1186/1749-799X-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 8.Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis Cartilage. 1995;3:261–267. doi: 10.1016/s1063-4584(05)80017-2. [DOI] [PubMed] [Google Scholar]
- 9.Eckstein F, Burstein D, Link TM. Quantitative MRI of cartilage and bone: degenerative changes in osteoarthritis. NMR Biomed. 2006;19:822–854. doi: 10.1002/nbm.1063. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Ma CB, Link TM, Castillo DD, Blumenkrantz G, Lozano J, et al. In Vivo T1rho and T2 Mapping of Articular Cartilage in Osteoarthritis of the Knee Using 3 Tesla Mri. Osteoarthritis Cartilage. 2007;15:789–797. doi: 10.1016/j.joca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dijkgraaf LC, de Bont LG, Boering G, Liem RS. The structure, biochemistry, and metabolism of osteoarthritic cartilage: a review of the literature. J Oral Maxillofac Surg. 1995;53:1182–1192. doi: 10.1016/0278-2391(95)90632-0. [DOI] [PubMed] [Google Scholar]
- 12.Duvvuri U, Reddy R, Patel SD, Kaufman JH, Kneeland JB, Leigh JS. T1rho-relaxation in articular cartilage: effects of enzymatic degradation. Magn Reson Med. 1997;38:863–867. doi: 10.1002/mrm.1910380602. [DOI] [PubMed] [Google Scholar]
- 13.Regatte RR, Akella SV, Borthakur A, Kneeland JB, Reddy R. Proteoglycan depletion-induced changes in transverse relaxation maps of cartilage: comparison of T2 and T1rho. Acad Radiol. 2002;9:1388–1394. doi: 10.1016/s1076-6332(03)80666-9. [DOI] [PubMed] [Google Scholar]
- 14.Lozano J, Li X, Link TM, Safran M, Majumdar S, Ma CB. Detection of posttraumatic cartilage injury using quantitative T1rho magnetic resonance imaging. A report of two cases with arthroscopic findings. J Bone Joint Surg Am. 2006;88:1349–1352. doi: 10.2106/JBJS.E.01051. [DOI] [PubMed] [Google Scholar]
- 15.Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2--preliminary findings at 3 T. Radiology. 2000;214:259–266. doi: 10.1148/radiology.214.1.r00ja15259. [DOI] [PubMed] [Google Scholar]
- 16.David-Vaudey E, Ghosh S, Ries M, Majumdar S. T2 relaxation time measurements in osteoarthritis. Magn Reson Imaging. 2004;22:673–682. doi: 10.1016/j.mri.2004.01.071. [DOI] [PubMed] [Google Scholar]
- 17.Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004;232:592–598. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bashir A, Gray ML, Burstein D. Gd-DTPA2- as a measure of cartilage degradation. Magn Reson Med. 1996;36:665–673. doi: 10.1002/mrm.1910360504. [DOI] [PubMed] [Google Scholar]
- 19.Kurkijarvi JE, Nissi MJ, Kiviranta I, Jurvelin JS, Nieminen MT. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) and T2 characteristics of human knee articular cartilage: topographical variation and relationships to mechanical properties. Magn Reson Med. 2004;52:41–46. doi: 10.1002/mrm.20104. [DOI] [PubMed] [Google Scholar]
- 20.Bolbos RI, Ma CB, Link TM, Majumdar S, Li X. In vivo T1rho quantitative assessment of knee cartilage after anterior cruciate ligament injury using 3 Tesla magnetic resonance imaging. Invest Radiol. 2008;43:782–788. doi: 10.1097/RLI.0b013e318184a451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Kuo D, Theologis A, Carballido-Gamio J, Stehling C, Link TM, et al. Cartilage in anterior cruciate ligament-reconstructed knees: MR imaging T1{rho} and T2--initial experience with 1-year follow-up. Radiology. 258:505–514. doi: 10.1148/radiol.10101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Potter HG, Jain SK, Ma Y, Black BR, Fung S, Lyman S. Cartilage injury after acute, isolated anterior cruciate ligament tear: immediate and longitudinal effect with clinical/MRI follow-up. Am J Sports Med. 2012;40:276–285. doi: 10.1177/0363546511423380. [DOI] [PubMed] [Google Scholar]
- 23.Neuman P, Tjornstrand J, Svensson J, Ragnarsson C, Roos H, Englund M, et al. Longitudinal assessment of femoral knee cartilage quality using contrast enhanced MRI (dGEMRIC) in patients with anterior cruciate ligament injury--comparison with asymptomatic volunteers. Osteoarthritis Cartilage. 2011;19:977–983. doi: 10.1016/j.joca.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Tiderius CJ, Olsson LE, Nyquist F, Dahlberg L. Cartilage glycosaminoglycan loss in the acute phase after an anterior cruciate ligament injury: delayed gadolinium-enhanced magnetic resonance imaging of cartilage and synovial fluid analysis. Arthritis Rheum. 2005;52:120–127. doi: 10.1002/art.20795. [DOI] [PubMed] [Google Scholar]
- 25.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Ma BC, Bolbos RI, Stahl R, Lozano J, Zuo J, et al. Quantitative assessment of bone marrow edema-like lesion and overlying cartilage in knees with osteoarthritis and anterior cruciate ligament tear using MR imaging and spectroscopic imaging at 3 Tesla. J Magn Reson Imaging. 2008;28:453–461. doi: 10.1002/jmri.21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carballido-Gamio J, Bauer JS, Stahl R, Lee KY, Krause S, Link TM, et al. Inter-subject comparison of MRI knee cartilage thickness. Med Image Anal. 2008;12:120–135. doi: 10.1016/j.media.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carballido-Gamio J, Link TM, Majumdar S. New techniques for cartilage magnetic resonance imaging relaxation time analysis: texture analysis of flattened cartilage and localized intra- and inter-subject comparisons. Magn Reson Med. 2008;59:1472–1477. doi: 10.1002/mrm.21553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frobell RB. Change in cartilage thickness, posttraumatic bone marrow lesions, and joint fluid volumes after acute ACL disruption: a two-year prospective MRI study of sixty-one subjects. J Bone Joint Surg Am. 2011;93:1096–1103. doi: 10.2106/JBJS.J.00929. [DOI] [PubMed] [Google Scholar]
- 31.Graf BK, Cook DA, De Smet AA, Keene JS. “Bone bruises” on magnetic resonance imaging evaluation of anterior cruciate ligament injuries. Am J Sports Med. 1993;21:220–223. doi: 10.1177/036354659302100210. [DOI] [PubMed] [Google Scholar]
- 32.Hayes CW, Brigido MK, Jamadar DA, Propeck T. Mechanism-based pattern approach to classification of complex injuries of the knee depicted at MR imaging. Radiographics. 2000;20 doi: 10.1148/radiographics.20.suppl_1.g00oc21s121. Spec No: S121–134. [DOI] [PubMed] [Google Scholar]
- 33.Scarvell JM, Smith PN, Refshauge KM, Galloway H, Woods K. Comparison of kinematics in the healthy and ACL injured knee using MRI. J Biomech. 2005;38:255–262. doi: 10.1016/j.jbiomech.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Carpenter RD, Majumdar S, Ma CB. Magnetic resonance imaging of 3-dimensional in vivo tibiofemoral kinematics in anterior cruciate ligament-reconstructed knees. Arthroscopy. 2009;25:760–766. doi: 10.1016/j.arthro.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 35.Chaudhari AM, Briant PL, Bevill SL, Koo S, Andriacchi TP. Knee kinematics, cartilage morphology, and osteoarthritis after ACL injury. Med Sci Sports Exerc. 2008;40:215–222. doi: 10.1249/mss.0b013e31815cbb0e. [DOI] [PubMed] [Google Scholar]
- 36.Andreisek G, White LM, Sussman MS, Kunz M, Hurtig M, Weller I, et al. Quantitative MR imaging evaluation of the cartilage thickness and subchondral bone area in patients with ACL-reconstructions 7 years after surgery. Osteoarthritis Cartilage. 2009;17:871–878. doi: 10.1016/j.joca.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 37.Li G, Park SE, DeFrate LE, Schutzer ME, Ji L, Gill TJ, et al. The cartilage thickness distribution in the tibiofemoral joint and its correlation with cartilage-to-cartilage contact. Clin Biomech (Bristol, Avon) 2005;20:736–744. doi: 10.1016/j.clinbiomech.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Hellio Le Graverand MP, Buck RJ, Wyman BT, Vignon E, Mazzuca SA, Brandt KD, et al. Subregional femorotibial cartilage morphology in women--comparison between healthy controls and participants with different grades of radiographic knee osteoarthritis. Osteoarthritis Cartilage. 2009;17:1177–1185. doi: 10.1016/j.joca.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Nikolic DK. Lateral meniscal tears and their evolution in acute injuries of the anterior cruciate ligament of the knee. Arthroscopic analysis. Knee Surg Sports Traumatol Arthrosc. 1998;6:26–30. doi: 10.1007/s001670050068. [DOI] [PubMed] [Google Scholar]
- 40.Hunter DJ, Zhang YQ, Niu JB, Tu X, Amin S, Clancy M, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54:795–801. doi: 10.1002/art.21724. [DOI] [PubMed] [Google Scholar]
- 41.Zhao J, Li X, Bolbos RI, Link TM, Majumdar S. Longitudinal assessment of bone marrow edema-like lesions and cartilage degeneration in osteoarthritis using 3 T MR T1rho quantification. Skeletal Radiol. 2010;39:523–531. doi: 10.1007/s00256-010-0892-6. [DOI] [PMC free article] [PubMed] [Google Scholar]