Abstract
Background
Evidence is accumulating that mitochondrial dysfunction is involved in the pathophysiology of bipolar disorder and schizophrenia. Cerebrospinal fluid (CSF) concentration of lactate, a product of extra-mitochondrial glucose metabolism, is commonly elevated in individuals with mitochondrial disorders, especially those with neuropsychiatric symptoms. We tested the hypothesis that patients with bipolar disorder and schizophrenia would, on average, have elevated CSF lactate concentrations compared with healthy control subjects.
Methods
The CSF lactate and CSF and plasma glucose concentrations were measured with a YSI (YSI, Yellow Springs, Ohio) 2300 STAT Plus Glucose & Lactate Analyzer in 15 samples from each of three groups of subjects: bipolar I disorder patients, schizophrenic patients, and healthy control subjects.
Results
Mean CSF lactate concentrations were significantly higher in bipolar (1.76 ±.38) and schizophrenic subjects (1.61 ±.31) compared with control subjects (1.31 ±.21 mmol/L). These differences persisted after adjusting means for CSF glucose concentration, which correlated positively with CSF lactate concentration.
Conclusions
This is the first report of increased CSF lactate concentrations in patients with bipolar disorder and schizophrenia. Elevated CSF lactate indicates increased extra-mitochondrial and anaerobic glucose metabolism and is consistent with impaired mitochondrial metabolism. Measuring CSF lactate concentration might help identify bipolar and schizophrenic patients with mitochondrial dysfunction who might benefit from research to elucidate and ultimately rectify possible mitochondrial pathology underlying these disorders.
Keywords: Bipolar, cerebrospinal fluid, lactate, metabolism, mitochondrial, schizophrenia
Evidence is accumulating that mitochondrial dysfunction is involved in the pathophysiology of bipolar disorder and schizophrenia (for reviews see 1– 4). The evidence consists of: histological studies showing abnormalities in mitochondrial structure (5), molecular studies identifying abnormal expression of mitochondrial proteins and mitochondrially related genes (6 –11), and metabolic studies revealing abnormalities in postmortem and live brain consistent with impaired mitochondrial metabolism (11–13).
The evidence in bipolar disorder is more robust. Mitochondrial disorders seem to be more frequently comorbid with mood disorders (14) than with psychotic disorders (15). Furthermore, evidence for mitochondrial dysfunction in schizophrenia should be treated with greater caution, because antipsychotic medication—particularly typical antipsychotic drugs— have some degree of mitochondrial toxicity (16,17). Postmortem evidence in both disorders is controversial, because there is considerable debate over whether differences reflect lower sample pH or actual mitochondrial pathology (16,18).
Pathophysiologic mechanisms that underlie mitochondrial dysfunction and relate it to psychiatric symptoms are unclear. Identification and characterization of bipolar and schizophrenic patients with impaired mitochondrial metabolism is an important step toward clarifying disease mechanisms and testing novel therapies aimed at ameliorating mitochondrial dysfunction. This study attempted to identify patients who might have mitochondrial dysfunction by measuring cerebrospinal fluid (CSF) concentration of lactate, a metabolite of anaerobic, extra-mitochondrial glucose metabolism that accumulates with mitochondrial dysfunction.
We hypothesized that patients with bipolar disorder and schizophrenia would, on average, have elevated CSF lactate concentrations compared with healthy control subjects. Elevated blood, CSF, and brain lactate concentrations are routinely used as biomarkers of mitochondrial dysfunction in patients suspected of having mitochondrial disorders (19 –21). Under normal homeostatic conditions, brain lactate is produced through anaerobic glycolysis by neurons to meet acutely increased energy demands during activation and by astrocytes to be shuttled to neurons as a substrate for mitochondrial oxidative metabolism (22). With intact mitochondrial metabolism, lactate will not accumulate in brain or CSF. In mitochondrial disorders, however, lactate accumulates because metabolism shifts to extra-mitochondrial glycolysis and mitochondrial metabolism is insufficient to metabolize the lactate that is produced (4).
Cerebrospinal fluid lactate concentration has been shown to correlate positively with neurologic and neuropsychologic impairment in individuals with mitochondrial disorder (23). Importantly, some individuals with mitochondrial disorders have persistent central nervous system (CNS) symptoms and elevated CSF lactate despite no peripheral symptoms of mitochondrial disorder or elevated blood levels of lactate (20,24). Therefore, it is plausible that bipolar and schizophrenic patients could show only CNS-related symptoms or evidence of mitochondrial dysfunction. It is now thought that early mitochondrial dysfunction might be reversible and thus might be a valuable new target for treatment (18).
Methods and Materials
Subjects and CSF Samples
Patients were recruited from the inpatient and outpatient psychiatric services of the University of Maryland Medical Center, and all provided written informed consent to participate in this Institutional Review Board (IRB)-approved study. Bipolar subjects (n = 15) had bipolar I disorder confirmed by a board-certified psychiatrist (WTR) on the basis of Structured Clinical Interview for the DSM-IV (SCID) criteria (25). Specific diagnoses were: manic, severe with catatonic features (n = 3); manic, severe with psychotic features (n = 3); manic, severe without psychotic features (n = 1); most recent episode, manic, in partial remission (n = 3); most recent episode, manic, in full remission (n = 1); depressed, severe, without psychotic features (n = 3); and most recent episode, depressed, in partial remission (n = 1). Three bipolar subjects were recruited after having lumbar punctures during evaluation for altered mental status by clinicians unaffiliated with this study. In all cases, there was no infectious, toxic, or neurologic cause found, and altered mental status was determined to be a catatonic manifestation of bipolar disorder. Mood state was assessed within 24 hours of lumbar puncture by the 21-item Hamilton Depression Scale (HAM-D-21) (26) and the Young Mania Rating Scale (YMRS) (27). The sum of these scores was an index of acute mood symptom severity. With life chart data (28), we obtained an index of treatment resistance, which was the average number of relapses requiring inpatient admission/year over the previous 5 years, despite compliance with a standard treatment regime and without the emergence or exacerbation of a comorbid disorder. A standard regime was one that included therapeutic range dosing of either lithium or valproate (29).
Schizophrenic subjects (n = 15) had chronic, undifferentiated schizophrenia confirmed by WTR, on the basis of SCID criteria. We assessed symptom severity with the 30-item Positive and Negative Syndrome Scale (30) and the 18-item Scale for the Assessment of Negative Symptoms (31). The sum of scores was an index of acute symptom severity. Schizophrenic treatment resistance index was exactly like the bipolar index except that a standard treatment regime included a typical or atypical antipsychotic medication at a dose in or exceeding the recommended range.
All subjects had to be between the ages of 18 and 59 years. Healthy control subjects (n = 15) had no personal or primary family history of psychiatric disorder. Exclusion criteria for all subjects included: diabetes mellitus; glucocorticoid use within 6 months; acquired immunodeficiency syndrome; ischemic heart disease; stroke; brain tumor; meningitis; head injury with loss of consciousness; neurodevelopmental disorder; hypoxia; seizures (other than electroconvulsive seizures); substance abuse within the past week; history of substance dependence; and cognitive disorder. Additional exclusion criteria for psychiatric subjects included: an axis II disorder or any additional axis I disorder.
Healthy control CSF and plasma samples were obtained from the archives of the National Institute of Mental Health (NIMH) Clinical Neuroendocrinology Branch, Bethesda, Maryland. These samples had anonymous identifiers, and therefore their use in this study was exempt from full IRB review and informed consent. The CSF samples from patients and from healthy control subjects were obtained and handled identically with the same standardized method. The CSF samples were obtained by lumbar puncture in the lateral decubitus position, immediately placed on ice, kept on ice until storage at −80°C, and, if necessary, transported on dry ice. All CSF samples were clear with normal cell counts.
Biochemical Measurements
We measured CSF lactate, CSF glucose, and plasma glucose concentrations, in duplicate and blind to sample identity, with a YSI 2300 STAT Plus Glucose & Lactate Analyzer (YSI, Yellow Springs, Ohio) according to manufacturer directions. Average intra- and inter-assay coefficients of variation were 2.4% and 3.2% for glucose, respectively, and 2.6 % and 3.7% for lactate, respectively. According to the method of mitochondrial disorders investigators, the upper limit of the normal range for CSF lactate concentration was set at 2 SDs above our healthy control mean (20,32).
Statistical Methods
Data distributions were determined to approximate a normal distribution by the Kolmogorov-Smirnov test. We used correlations (Pearson’s r) and dichotomous group comparisons (Student t test) to assess potentially confounding relationships between demographic or clinical variables and CSF lactate concentrations. Group means were compared by one-way analysis of variance or analysis of covariance (ANCOVA). Demographic or metabolic variables found to have significant (p ≤ .05) or trend-level (p ≤ .1) relationships with CSF lactate were included as covariates in ANCOVA of group mean lactate concentrations. Covariates that did not have significant between-subjects effects in the ANCOVA were dropped from the analysis. Categorical variables were analyzed with Pearson’s χ2 test. Degrees of freedom are noted after each test statistic in parentheses. All tests were two-tailed, and α was set at p = .05 unless specified. We used SPSS version 12.0 (SPSS, Chicago, Illinois) for all analyses.
Results
Groups differed significantly by racial composition, CSF lactate, CSF glucose, and plasma glucose concentrations but not by age, gender, treatment resistance, illness duration, or family history of psychiatric illness (Table 1). Bipolar and schizophrenic groups had significantly higher mean CSF lactate concentrations compared with healthy control subjects—34% and 23% higher, respectively; however, group data distributions overlapped considerably (Figure 1). Bipolar subjects also had significantly higher mean plasma glucose concentration compared with control subjects and significantly higher mean CSF glucose concentration compared with both schizophrenic subjects and control subjects (Figure 2).
Table 1.
Demographic, Clinical, and Metabolic Information by Subject Group
| Variablea | Subject Group
|
Analysisb | ||
|---|---|---|---|---|
| Healthy Control | Bipolar Disorder | Schizophrenia | ||
| n | 15 | 15 | 15 | |
| Age (yrs) | 39.0 ± 7.2 | 47.5 ± 10.3 | 41.1 ± 11.9 | F(2,42) = 2.92, p = .065 |
| Race (n) (Caucasian/Afro-American) | 15/0 | 5/10 | 6/9 | χ2(2) = 16.6, p < .001 |
| Gender (n) (Male/Female) | 9/6 | 8/7 | 10/5 | χ2(2) = .486, p = .784 |
| Acute Symptom severityc | N/A | 28.5 ± 15.8 | 83.9 ± 18.0 | N/A |
| Treatment Resistanced | N/A | .76 ± .32 | .59 ± .24 | t(28) = 1.64, p = .112 |
| Illness Duration (yrs) | N/A | 21.6 ± 8.8 | 22.4 ± 9.6 | t(28) = .24, p = .813 |
| Family Psychiatric History, positive (n) | N/A | 5 | 11 | χ2(1) = 3.35, p = .067e |
| CSF Lactate (mmol/L) | 1.31 ± .21 | 1.76 ± .38 | 1.61 ± .31 | F(2,42) = 8.24, p = .001 BD>HC, p = .001 SCHZ>HC, p = .035 |
| Plasma Glucose (mg/dL) | 83.9 ± 8.6 | 107.3 ± 16.2 | 95.6 ± 23.8 | F(2,42) = 6.83, p = .003 BD>HC, p = .002 |
| CSF Glucose (mg/dL) | 59.2 ± 6.3 | 69.1 ± 12.8 | 59.9 ± 9.6 | F(2,42) = 4.66, p = .015 BD>HC, p = .027 BD>SCHZ, p = .046 |
CSF, cerebrospinal fluid; BD, bipolar disorder; HC, healthy control; SCHZ, schizophrenia.
Values for continuous variables are means ± SDs.
F tests are one-way analyses of variance with post hoc Bonferroni t tests.
Combined 21-item Hamilton Depression Scale and the Young Mania Rating Scale scores for bipolar and combined 30-item Positive and Negative Syndrome Scale and the 18-item Scale for the Assessment of Negative Symptoms scores for schizophrenic subjects.
Yearly average number of hospitalizations over previous 5 years.
Continuity corrected.
Figure 1.
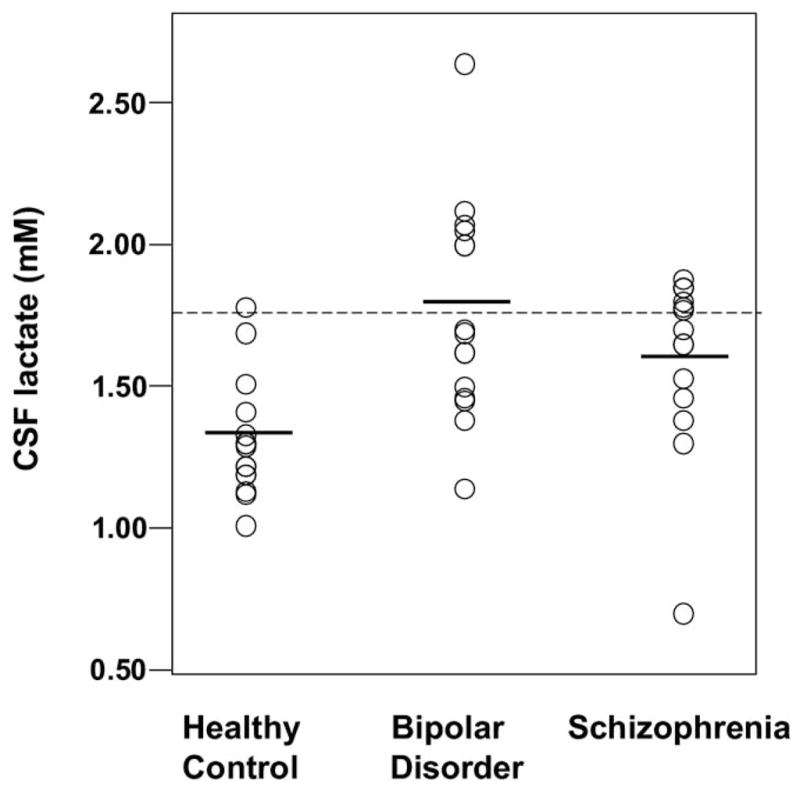
Scatter plots of cerebrospinal fluid (CSF) lactate concentration data distributions for healthy control subjects (n =15), patients with bipolar disorder (n = 15), and patients with schizophrenia (n = 15). Horizontal bars represent group means. Dotted line represents upper cut-off of normal range for this study (1.73 mmol/L), 2 SDs above the healthy control mean.
Figure 2.
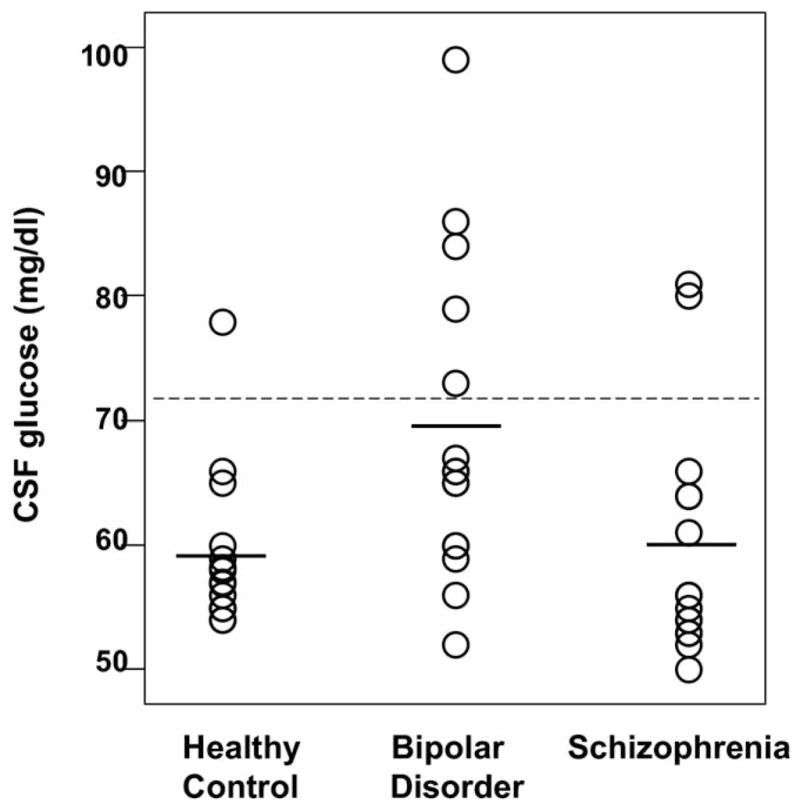
Scatter plots of cerebrospinal fluid (CSF) glucose concentration data distributions for healthy control subjects (n =15), patients with bipolar disorder (n = 15), and patients with schizophrenia (n = 15). Horizontal bars represent group means. Dotted line represents upper cut-off of normal range for this study (71.8 mg/dL), 2 SDs above the healthy control mean.
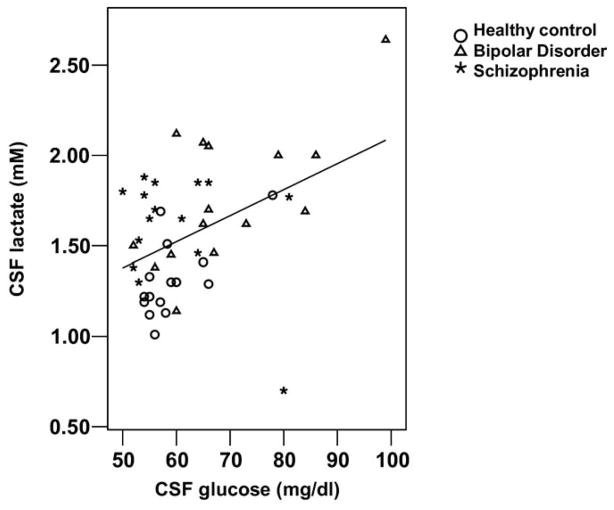
We assessed potentially confounding relationships of age, race, gender, and glucose concentrations with CSF lactate concentration. We found significant relationships with race [t (43) = 2.68, p = .010] and CSF glucose (r = .435, p = .003) (Figure 3), a trend-level relationship with plasma glucose (r = .288, p = .055), and no significant relationship with age (r = −.029, p = .850) and gender [t (43) = 1.24, p = .222] in the 45-subject sample. The ANCOVA comparison of group mean CSF lactate concentrations with race, CSF glucose, and plasma glucose as covariates revealed significant difference among groups (p = .040) with a significant effect of CSF glucose (p = .008) but not of race and plasma glucose (p ≥ .240). The ANCOVA with CSF glucose as the only covariate revealed that CSF lactate concentration differed between groups [F (2,41) = 5.47, p = .008] with an effect of CSF glucose (p = .030). The CSF lactate means adjusted for CSF glucose concentration in healthy control, bipolar, and schizophrenic groups were: 1.35, 1.70, and 1.64 mmol/L, respectively. Post hoc comparisons of adjusted means by Bryant-Paulson simultaneous tests showed that bipolar and schizophrenic mean CSF lactate concentrations differed significantly (p < .05) from the healthy control mean.
Figure 3.
Scatter plot of cerebrospinal fluid (CSF) lactate concentration data plotted against CSF glucose concentration data for all 45 subjects. Pearson’s r = .435; r2 = .189.
To assess the effect of psychotropic medication on CSF lactate concentration, we divided patients by medication use into groups of 10 or more according to use of: any psychotropic (n = 23), any antipsychotic (n = 16), typical antipsychotics (n = 12), atypical antipsychotics (n = 14), any mood stabilizer (n = 14), and valproate (n = 10). We found a significant or trend-level effect only for antipsychotic use (typical and atypical combined) [t (28) = 1.97, p = .058] with use associated with lower CSF lactate concentration: 1.57 ± .30 mmol/L versus 1.82 ± .37 mmol/L. When antipsychotic use was added as a covariate to an ANCOVA comparing bipolar and schizophrenic mean lactate concentrations, however, this effect was not significant (p = .204).
Correlation between CSF lactate and the index of treatment resistance in the 30-patient group revealed a trend-level positive correlation with treatment resistance (r = .329, p = .075). Correlation between CSF lactate and indices of acute symptom severity was not significant for both patient groups: bipolar (r = .057, p = .840) and schizophrenic (r = −.153, p = .587). Separate analyses in bipolar subjects also did not reveal significant correlations with CSF lactate for HAM-D-21 (r = .02, p = .942) or YMRS (r = −.056, p = .841) scores.
Although no subject had a known family history of mitochondrial disorder, mitochondrial disorders are often familial (21). Therefore, we compared mean CSF lactate concentrations in subjects with family history of psychiatric illness with those without in all 30 patients and separately for bipolar and schizophrenic patients. We found a significant difference [t (13) = 3.10, p = .008] only in the bipolar group, where mean ± SD CSF lactate for the five subjects with family history of psychiatric illness was 2.10 ± .34 mmol/L compared with 1.59 ± .28 mmol/L for those without.
Discussion
In this study we found evidence to support our hypothesis that CSF concentration of lactate is, on average, elevated in bipolar and schizophrenic patients compared with healthy control subjects. This novel finding indicates increased extra-mitochondrial, anaerobic glucose metabolism and is consistent with impaired mitochondrial metabolism in some patients with these disorders. We discuss this finding and others in the following text in relation to normal CNS glucose and lactate metabolism, literature implicating mitochondrial dysfunction in these disorders, findings in classic mitochondrial disorders, study limitations, and potential relevance to the pathophysiology and treatment of these disorders.
Elevated CSF lactate concentration obtained through lumbar puncture specifically indicates abnormal CNS energy metabolism, because lumbar CSF lactate concentration correlates strongly with brain and ventricular lactate concentrations and is independent of serum lactate concentration (33–35). Mean concentrations we observed in bipolar and schizophrenic patients are clearly abnormal relative to our healthy control mean CSF lactate concentration as well as to healthy control means of 1.21 ± .182 mmol/L (36) and 1.39 ± .106 mmol/L (20) reported by investigators of mitochondrial disorders.
Reviewing the literature (PubMed search 1966 –present) we found no reports of CSF lactate concentration in bipolar subjects and only one report in schizophrenic subjects. Goff et al. (37) reported a mean CSF lactate of 1.6 ± .4 mmol/L in 25 schizophrenic subjects from their study of the relationship between CSF concentrations of brain energy metabolites, tardive dyskinesia, and neuroleptic medications. This concentration is very close to our schizophrenic mean, and like ours, it did not vary significantly with medication use or, in their study, with presence of tardive dyskinesia. They did not, however, report control values for comparison. A recent CSF metabolic profiling study by Holmes et al. (38) found that CSF lactate concentration was reduced by 17.3% in schizophrenic patients compared with matched healthy control subjects; however, individual or group mean concentrations were not reported. Furthermore, CSF glucose concentration was mildly although significantly elevated in this group of patients (62.3 ± 5.5 mg/dL) relative to control subjects (58.5 ± 4.6 mg/dL). However, their sample, consisting of drug-naïve younger (28.1 ± 9.4 years) individuals with first episode schizophrenia or brief psychotic disorder, differed markedly from ours and that of Goff et al.
We observed a significant positive correlation between CSF glucose and lactate concentrations. This is consistent with reports that CSF glucose and lactate concentrations correlated positively in samples from 177 patients with various medical, neurologic, and psychiatric diagnoses (39) and that CSF lactate is elevated in diabetic patients (1.78 mmol/L) compared with control subjects (1.40 mmol/L) (40). Increased CNS glucose can beget increased CNS lactate, because glucose is a precursor of lactate. Also, unlike lactate, CNS glucose is derived from serum glucose. Therefore, there can be an indirect relationship between serum glucose and CSF lactate concentration. The r2 value of .189 for this correlation suggests, however, that only approximately 19% of the variance in CSF lactate concentration can be explained by variance in CSF glucose concentration.
The considerable overlap we found among group distributions of CSF lactate is indirect evidence that mitochondrial dysfunction occurs in patients with bipolar disorder and schizophrenia but is potentially involved in the pathophysiology of only a portion of patients. Forty-three percent (13 of 30) of patients (6 bipolar and 7 schizophrenic) had CSF lactate concentrations exceeding the control mean by 2 SDs. This finding is consistent with genetic studies that suggest some commonality but a great deal of genetic complexity and heterogeneity underlying the pathophysiologies of these 2 disorders (41,42).
Our finding of a greater mean CSF lactate concentration in bipolar subjects but not in schizophrenic subjects with family history of psychiatric illness is interesting in light of the often familial nature of mitochondrial disorders. This finding is consistent with the generally more robust evidence for endogenous mitochondrial abnormalities in bipolar disorder. Future genetic studies might be helpful in clarifying whether known inherited genetic polymorphisms are associated with elevated CSF lactate in these subjects.
It is important to mention the following study limitations. First, we cannot completely exclude the possibility that a number of confounding variables contributed to the differences in CSF lactate concentration observed among subject groups. Groups were not exactly matched on age and race, and a relationship between increasing CSF lactate and age (43) but not race has been reported. However, the results of our ANCOVA showing no significant effect of age on group comparisons of metabolite concentrations suggest that group differences cannot be explained on the basis of age differences. Although diabetic subjects were excluded, subjects were not fasting at the time samples were obtained. Acute changes in serum and CSF glucose might therefore have contributed to some of the variance among groups. Most patients were taking psychotropic medications at the time of lumbar puncture; therefore, medication use could also have contributed to group differences. However, it is noteworthy that, although antipsychotic medication (haloperidol and clozapine) has been recently linked to increased brain lactate in rats (16), other investigators have found no increase in CSF lactate concentration in schizophrenic patients taking anti-psychotics compared with those not taking antipsychotics (37). Moreover, we found a trend-level association between antipsychotic use and decreased CSF lactate concentration in our bipolar and schizophrenic patients, and our ANCOVA results suggest that this association was secondary to greater antipsychotic use among schizophrenic patients, who had a lower mean CSF lactate.
Second, findings in our relatively small subject sample of patients with longstanding illness might not generalize to patients with different clinical characteristics, such as patients without longstanding illness, as in the Holmes et al. study (38) and the many patients with more than one Axis I diagnosis. Third, we cannot conclude whether increased CSF lactate concentration signifies a primary metabolic derangement that drives these illnesses in some patients or whether it is secondary to toxic or degenerative changes that follow repeated episodes of illness.
Finally, although all subjects received a full medical assessment, we did not systematically screen for typical somatic problems associated with mitochondrial disorders, such as ptosis, muscle weakness, or signs and symptoms of cardiac myopathy or heart failure. None of these problems were apparent on history or exam; however, a more systematic approach might have revealed somatic problems associated with mitochondrial disorders.
Regardless of whether increased CSF lactate concentration is a primary or secondary manifestation of these disorders or whether it is influenced by medication use, prevention and amelioration of abnormal metabolism might be an important, untapped therapeutic option for some patients. Identifying these patients is therefore imperative. Elevated CSF lactate might be a useful biomarker to identify patients who might benefit from metabolic therapy. Further studies to assess the relationship between CSF lactate and less invasive biomarkers of mitochondrial dysfunction including genetic polymorphisms and proton or phosphorous magnetic resonance spectroscopy measures of brain energy metabolism would be valuable. Because our data suggest a potential role for mitochondrial dysfunction in slightly < one-half of our patient sample, using biomarkers to select candidate responders to metabolic therapies might be critical to having sufficient statistical power for decisive analysis of data from future trials of metabolic therapies in these disorders.
Acknowledgments
This research was partially funded by a National Institute of Mental Health R21 award (MH066028-01A2) to WTR. We thank the individuals who volunteered for this study for their invaluable contribution and Drs. Mahindranauth Deonarine, Padmaja Kodali, Amelia McPeak, Angela Onwuanibe, and Martin Weiler for referring their patients to the study.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Kato T, Kato N. Mitochondrial dysfunction in bipolar disorder. Bipolar Disord. 2000;2:180–190. doi: 10.1034/j.1399-5618.2000.020305.x. [DOI] [PubMed] [Google Scholar]
- 2.Quiroz JA, Gray NA, Kato T, Manji HK. Mitochondrially mediated plasticity in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology. 2008;33:2551–2565. doi: 10.1038/sj.npp.1301671. [DOI] [PubMed] [Google Scholar]
- 3.Shao L, Martin MV, Watson SJ, Schatzberg A, Akil H, Myers RM, et al. Mitochondrial involvement in psychiatric disorders. Ann Med. 2008;40:281–295. doi: 10.1080/07853890801923753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stork C, Renshaw PF. Mitochondrial dysfunction in bipolar disorder: Evidence from magnetic resonance spectroscopy research. Mol Psychiatry. 2005;10:900–919. doi: 10.1038/sj.mp.4001711. [DOI] [PubMed] [Google Scholar]
- 5.Kung L, Roberts RC. Mitochondrial pathology in human schizophrenic striatum: A postmortem ultrastructural study. Synapse. 1999;31:67–75. doi: 10.1002/(SICI)1098-2396(199901)31:1<67::AID-SYN9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 6.Kato T, Kunugi H, Nanko S, Kato N. Association of bipolar disorder with the 5178 polymorphism in mitochondrial DNA. Am J Med Genet. 2000;96:182–186. [PubMed] [Google Scholar]
- 7.Konradi C, Eaton M, MacDonald ML, Walsh J, Benes FM, Heckers S. Molecular evidence for mitochondrial dysfunction in bipolar disorder. Arch Gen Psychiatry. 2004;61:300–308. doi: 10.1001/archpsyc.61.3.300. [DOI] [PubMed] [Google Scholar]
- 8.Kvajo M, Dhilla A, Swor DE, Karayiorgou M, Gogos JA. Evidence implicating the candidate schizophrenia/bipolar disorder susceptibility gene G72 in mitochondrial function. Mol Psychiatry. 2008;13:685–696. doi: 10.1038/sj.mp.4002052. [DOI] [PubMed] [Google Scholar]
- 9.Munakata K, Iwamoto K, Bundo M, Kato T. Mitochondrial DNA 3243A>G mutation and increased expression of LARS2 gene in the brains of patients with bipolar disorder and schizophrenia. Biol Psychiatry. 2005;57:525–532. doi: 10.1016/j.biopsych.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 10.Naydenov AV, MacDonald ML, Ongur D, Konradi C. Differences in lymphocyte electron transport gene expression levels between subjects with bipolar disorder and normal controls in response to glucose deprivation stress. Arch Gen Psychiatry. 2007;64:555–564. doi: 10.1001/archpsyc.64.5.555. [DOI] [PubMed] [Google Scholar]
- 11.Prabakaran S, Swatton JE, Ryan MM, Huffaker SJ, Huang JT, Griffin JL, et al. Mitochondrial dysfunction in schizophrenia: Evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry. 2004;9:684–697. 643. doi: 10.1038/sj.mp.4001511. [DOI] [PubMed] [Google Scholar]
- 12.Dager SR, Friedman SD, Parow A, Demopulos C, Stoll AL, Lyoo IK, et al. Brain metabolic alterations in medication-free patients with bipolar disorder. Arch Gen Psychiatry. 2004;61:450–458. doi: 10.1001/archpsyc.61.5.450. [DOI] [PubMed] [Google Scholar]
- 13.Murashita J, Kato T, Shioiri T, Inubushi T, Kato N. Altered brain energy metabolism in lithium-resistant bipolar disorder detected by photic stimulated 31P-MR spectroscopy. Psychol Med. 2000;30:107–115. doi: 10.1017/s0033291799001439. [DOI] [PubMed] [Google Scholar]
- 14.Fattal O, Link J, Quinn K, Cohen BH, Franco K. Psychiatric comorbidity in 36 adults with mitochondrial cytopathies. CNS Spectr. 2007;12:429–438. doi: 10.1017/s1092852900015303. [DOI] [PubMed] [Google Scholar]
- 15.Kazuno AA, Munakata K, Mori K, Tanaka M, Nanko S, Kunugi H, et al. Mitochondrial DNA sequence analysis of patients with ‘atypical psychosis’. Psychiatry Clin Neurosci. 2005;59:497–503. doi: 10.1111/j.1440-1819.2005.01404.x. [DOI] [PubMed] [Google Scholar]
- 16.Halim ND, Lipska BK, Hyde TM, Deep-Soboslay A, Saylor EM, Herman MM, et al. Increased lactate levels and reduced pH in postmortem brains of schizophrenics: Medication confounds. J Neurosci Methods. 2008;169:208–213. doi: 10.1016/j.jneumeth.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inuwa IM, Peet M, Williams MA. QSAR modeling and transmission electron microscopy stereology of altered mitochondrial ultrastructure of white blood cells in patients diagnosed as schizophrenic and treated with antipsychotic drugs. Biotech Histochem. 2005;80:133–137. doi: 10.1080/10520290500303349. [DOI] [PubMed] [Google Scholar]
- 18.Vawter MP, Tomita H, Meng F, Bolstad B, Li J, Evans S, et al. Mitochondrial-related gene expression changes are sensitive to agonal-pH state: Implications for brain disorders. Mol Psychiatry. 2006;11:615, 663–679. doi: 10.1038/sj.mp.4001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bianchi MC, Sgandurra G, Tosetti M, Battini R, Cioni G. Brain magnetic resonance in the diagnostic evaluation of mitochondrial encephalopathies. Biosci Rep. 2007;27:69–85. doi: 10.1007/s10540-007-9046-z. [DOI] [PubMed] [Google Scholar]
- 20.Finsterer J. Cerebrospinal-fluid lactate in adult mitochondriopathy with and without encephalopathy. Acta Med Austriaca. 2001;28:152–155. doi: 10.1046/j.1563-2571.2001.01036.x. [DOI] [PubMed] [Google Scholar]
- 21.Schmiedel J, Jackson S, Schafer J, Reichmann H. Mitochondrial cytopathies. J Neurol. 2003;250:267–277. doi: 10.1007/s00415-003-0978-3. [DOI] [PubMed] [Google Scholar]
- 22.Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond B Biol Sci. 1999;354:1155–1163. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufmann P, Shungu DC, Sano MC, Jhung S, Engelstad K, Mitsis E, et al. Cerebral lactic acidosis correlates with neurological impairment in MELAS. Neurology. 2004;62:1297–1302. doi: 10.1212/01.wnl.0000120557.83907.a8. [DOI] [PubMed] [Google Scholar]
- 24.Hutchesson A, Preece MA, Gray G, Green A. Measurement of lactate in cerebrospinal fluid in investigation of inherited metabolic disease. Clin Chem. 1997;43:158–161. [PubMed] [Google Scholar]
- 25.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders-Non-patient Edition (SCID-I/NP, Version 2.0) New York: Biometrics Research Department New York State Psychiatric Institute; 1998. [Google Scholar]
- 26.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 28.Leverich GS, Post RM. The NIMH Life Chart Manual for Recurrent Affective Illness: Clinician Retrospective (LCM-C/R) Bethesda, Maryland: NIMH; 2002. [Google Scholar]
- 29.Sachs GS, Printz DJ, Kahn DA, Carpenter D, Docherty JP. The expert consensus guideline series: Medication treatment of bipolar disorder 2000. Postgrad Med. 2000;(Spec No):1–104. [PubMed] [Google Scholar]
- 30.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 31.Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Arch Gen Psychiatry. 1982;39:784–788. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- 32.Seyama K, Suzuki K, Mizuno Y, Yoshida M, Tanaka M, Ozawa T. Mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes with special reference to the mechanism of cerebral manifestations. Acta Neurol Scand. 1989;80:561–568. doi: 10.1111/j.1600-0404.1989.tb03927.x. [DOI] [PubMed] [Google Scholar]
- 33.Posner JB, Plum F. Independence of blood and cerebrospinal fluid lactate. Arch Neurol. 1967;16:492–496. doi: 10.1001/archneur.1967.00470230044005. [DOI] [PubMed] [Google Scholar]
- 34.Sommer JB, Gaul C, Heckmann J, Neundorfer B, Erbguth FJ. Does lumbar cerebrospinal fluid reflect ventricular cerebrospinal fluid? A prospective study in patients with external ventricular drainage. Eur Neurol. 2002;47:224–232. doi: 10.1159/000057904. [DOI] [PubMed] [Google Scholar]
- 35.Watson MA, Scott MG. Clinical utility of biochemical analysis of cerebrospinal fluid. Clin Chem. 1995;41:343–360. [PubMed] [Google Scholar]
- 36.Yamamoto M, Ujike H, Wada K, Tsuji T. Cerebrospinal fluid lactate and pyruvate concentrations in patients with Parkinson’s disease and mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) J Neurol Neurosurg Psychiatry. 1997;62:290. doi: 10.1136/jnnp.62.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goff DC, Tsai G, Beal MF, Coyle JT. Tardive dyskinesia and substrates of energy metabolism in CSF. Am J Psychiatry. 1995;152:1730–1736. doi: 10.1176/ajp.152.12.1730. [DOI] [PubMed] [Google Scholar]
- 38.Holmes E, Tsang TM, Huang JT, Leweke FM, Koethe D, Gerth CW, et al. Metabolic profiling of CSF: Evidence that early intervention may impact on disease progression and outcome in schizophrenia. PLoS Med. 2006;3:e327. doi: 10.1371/journal.pmed.0030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pryce JD, Gant PW, Sau KJ. Normal concentrations of lactate, glucose, and protein in cerebrospinal fluid, and the diagnotic implications of abnormal concentrations. Clin Chem. 1970;16:562–565. [PubMed] [Google Scholar]
- 40.Yao H, Sadoshima S, Nishimura Y, Fujii K, Oshima M, Ishitsuka T, Fujishima M. Cerebrospinal fluid lactate in patients with diabetes mellitus and hypoglycaemic coma. J Neurol Neurosurg Psychiatry. 1989;52:372–375. doi: 10.1136/jnnp.52.3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kennedy JL, Farrer LA, Andreasen NC, Mayeux R, St George-Hyslop P. The genetics of adult-onset neuropsychiatric disease: Complexities and conundra? Science. 2003;302:822–826. doi: 10.1126/science.1092132. [DOI] [PubMed] [Google Scholar]
- 42.Maier W, Hofgen B, Zobel A, Rietschel M. Genetic models of schizophrenia and bipolar disorder: Overlapping inheritance or discrete genotypes? Eur Arch Psychiatry Clin Neurosci. 2005;255:159–166. doi: 10.1007/s00406-005-0583-9. [DOI] [PubMed] [Google Scholar]
- 43.Yesavage J, Berger PA. Correlation of cerebrospinal fluid lactate with age. Am J Psychiatry. 1980;137:976–977. doi: 10.1176/ajp.137.8.976. [DOI] [PubMed] [Google Scholar]