SUMMARY
Primary progressive aphasia (PPA) is a neurodegenerative syndrome characterized by insidious and progressive loss of language. Current diagnostic criteria require symptoms to be largely restricted to language dysfunction for at least the first 2 years of the syndrome. However, as the disorder progresses – and sometimes even in the early stages – patients with PPA may exhibit neuropsychiatric symptoms. In this article, we review the phenomenology and frequency of neuropsychiatric symptoms in PPA. Among the few studies of this topic that have been performed, there is consistent agreement that neuropsychiatric symptoms are not uncommon among PPA patients. In some cases, particularly the semantic variant of PPA, symptoms are similar to those found in the behavioral variant of frontotemporal dementia. We further review the approach to assessment of behavioral symptoms in PPA and their possible management strategies, and speculate regarding their potential neurobiological substrates.
Primary progressive aphasia (PPA) is a neurodegenerative syndrome characterized by the insidiously progressive loss of language abilities [1]. According to contemporary diagnostic criteria, the diagnosis of PPA requires that aphasia must be the most salient symptom and the major cause of impaired daily living activities for approximately the first 2 years of the clinical syndrome [1–3]. PPA is usually viewed as one major clinical form of the frontotemporal lobar degeneration (FTLD) spectrum of neurodegenerative diseases [4]. Depending on the type of language problem, PPA is further sub-categorized into a nonfluent/agrammatic variant (PPA-G, known previously as progressive nonfluent aphasia [PNFA]) involving effortful and agrammatic speech, a semantic variant (PPA-S, also known as semantic dementia [SD]) involving impaired single-word comprehension and a logopenic variant (PPA-L, also known as logopenic progressive aphasia) involving impaired word retrieval and repetition [2].
The presence of prominent early neuropsychiatric or behavioral symptoms is generally considered exclusionary for PPA. Patients with a neurodegenerative syndrome in which these symptoms are the earliest feature may fit criteria for the behavioral variant of frontotemporal dementia (bvFTD) [5]. In spite of this distinction, which is particularly important for clinical research on these disorders, some patients whose diagnosis fits the criteria for PPA have prominent early neuropsychiatric or behavioral symptoms (a point discussed briefly in the new diagnostic criteria) [2]. Many others have relatively mild but notable symptoms in these domains, particularly as PPA progresses to involve abilities beyond language.
In this article, we review the existing medical literature that has investigated the neuropsychiatric symptoms in patients with PPA. We discuss the phenomenology and clinical features of these symptoms and speculate regarding their neurobiological substrates, highlighting relevant studies when considering the approach to treatment.
Review of the literature on neuropsychiatric symptoms in PPA
A systematic search for papers reporting studies of neuropsychiatric symptoms in PPA was conducted. The following MeSH search terms `primary progressive aphasia', `neuropsychiatric', `psychiatric', `behavioral', `neuroanatomy' and `neuroimaging' were used. Subsequently, each subcategory of PPA (`semantic dementia', `progressive non-fluent' or `agrammatic aphasia' and `lopogenic' PPA) was combined with the other search terms and were searched for separately. The studies were identified from the following databases: Pubmed (Medline); Embase; PsychInfo; and Cochrane. The references of all the identified articles were reviewed and relevant references were added to the review list. Finally, we entered each of the review list articles into the Science Citation Index of the Institute for Scientific Information Web of Science, and articles citing those in the review list were reviewed and relevant primary research articles were added to the list. Research and review articles were restricted to the English language from 1949 to present were included. We identified multiple studies reporting on the spectrum of neuropsychiatric symptoms in PPA, eight studies reporting investigations of specific neuropychiatric symptoms and two case reports.
Studies of the spectrum of neuropsychiatric symptoms in PPA
The studies varied in patient selection and comparison groups. In some studies, patients with PPA were analyzed as one group, whereas other studies employed subtype categories. The majority of studies used the Neuropsychiatric Inventory (NPI) to measure the frequency or severity of neuropsychiatric symptoms. A few studies used the Frontal Behavioral Inventory (FBI) or the behavioral domain of the Clinician Dementia Rating Scale (CDR). Except for two longitudinal studies, the rest were either cross-sectional studies or retrospective chart reviews. Table 1 summarizes the studies that investigated the spectrum of neuropsychiatric symptoms in PPA using the NPI, FBI or CDR.
Table 1.
Summary of studies of the spectrum of neuropsychiatric symptoms in primary progressive aphasia.
| Study (year) | Type of study | Patients (n) | Scale | Outcome measure | Major results | Additional notes | Ref. |
|---|---|---|---|---|---|---|---|
| Rosen et al. (2006) | Cross-sectional | PPA-S (33), PPA-G (17), PPA-L (17), bvFTD (50) and AD (119) | NPI | Total NPI score, FTD-specific NPI score | bvFTD > PPA-S > PPA-L ≈ PPA-G ≈ AD | – | [18] |
| Marra et al. (2007) | Cross-sectional | PPA-G (10), bvFTD (22) and AD (20) | NPI | Mean severity score | bvFTD > PPA-G ≈ AD | No PPA-G with delusions, hallucinations or aberrant motor behaviors | [19] |
| Banks and Weintraub (2008) | Cross-sectional | PPA (42) (not subtyped) and bvFTD (28) | NPI | NPI severity score, number of symptoms | bvFTD > PPA Number of symptoms in long-duration PPA (>5 years) ≈ bvFTD |
Mood, agitation, nightmare symptoms, apathy and appetite symptoms common in PPA | [22] |
| Fatemi et al. (2011) | Cross-sectional | PPA (55) (not subtyped), CTL (111) | NPI | Frequency of symptoms | PPA > CTL (apathy, depression, appetite, motor symptoms, anxiety and irritability) PPA ≈ CTL (sleep-related/night-time symptoms) |
Delusions, euphoria and hallucinations were rare or absent in PPA | [20] |
| Liu et al. (2004) | Cross-sectional | SD (27), bvFTD (24) and AD or mild cognitive impairment (22) | NPI | Frequency of symptoms | Elation/euphoria, disinhibition, aberrant motor behaviors: bvFTD ≈ SD > AD Sleep disorders: SD > bvFTD and AD Apathy: bvFTD > SD |
– | [8] |
| Rohrer and Warren (2010) | Cross-sectional | PPA-S (9), PPA-G (14), PPA-L (7) and PPA associated with mutations in the GRN-PPA (3) | NPI | Frequency of symptoms | Frequency >50% in all PPA subtypes: agitation/aggression, depression, anxiety, apathy, disinhibition, irritability/lability and abnormal appetite/eating disorders Frequency >50% in PPA-S: depression, anxiety, irritability, disinhibition and abnormal appetite/eating symptoms Frequency >50% in PPA-G: agitation, depression and apathy Frequency >50% in PPA-L: agitation, anxiety, irritability and apathy |
No relationship between total score and either duration of disease or MMSE score | [9] |
| Xiong et al. (2011) | Retrospective chart review | PPA with AD spectrum pathology (13) and PPA with FTLD spectrum pathology (20) | NPI | Frequency of symptoms | Eating disorders, disinhibition, apathy had highest specificity for FTLD spectrum pathology | The absence of depression not useful in predicting FTLD pathology | [23] |
| Knopman et al. (2008) | Longitudinal (1 year) | PPA-S (26), PPA-G (25), PPA-L (9) and bvFTD (47) | NPI and FBI | Frequency of symptoms | bvFTD and PPA-S > PPA-G > PPA-L After 12 months, on the NPI and FBI, the PPA-S patients showed greater worsening than the other three subtypes |
Hallucinations and delusions were infrequent, but all other symptoms reported in PPA | [41] |
| Knopman et al. (2011) | Cross-sectional | Probable AD (2550), DLB (281), VaD(88), bvFTD (234) andPPA(137) | CDR supplemental 'behavioral' and'language' domains | Frequency of symptoms | PPA-S > PPA-G likely to have abnormal rating in behavioral domain Most PPAs had nonzero rating on behavioral domain |
– | [62] |
| Kertesz et al. (2000) | Cross-sectional | PPA(11),bvFTD (26), AD (38), VaD (16)and depressive disorders (17) | FBI | Mean FBI score | bvFTD > PPA ≈ AD Vascular dementia > PPA The main symptoms exhibited by PPA were apathy, irritability and, to a lesser extent, inappropriate behavior |
– | [40] |
| Marczinski et al. (2004) | Longitudinal (3 years) | bvFTD (12) and PPA (14) | FBI | Mean FBI score | FBI scores in PPA rose in the third year to >27 FBI scores in bvFTD rose in the second year and reached a plateau in the third year |
Apathy, aspontaneity, personal neglect, disorganization, inattention, logopenia, poor judgment, inappropriateness, restlessness, aggression and hyperorality items increased over time in PPA patients | [21] |
| Bozeat et al. (2000) | Cross-sectional | bvFTD(13), SD (20) and probable AD (37) | CBI | Frequency of symptoms | Mood symptoms: bvFTD ≈ SD ≈ AD Mental rigidity: SD > bvFTD > AD Stereotypic behaviors: SD > bvFTD >AD Social withdrawal: bvFTD > SD > AD Apathy: bvFTD > SD > AD |
Psychotic symptoms were less frequent in the temporal variant. Stereotypic and eating behaviors were the strongest factors differentiating FTD from AD | [7] |
AD: Alzheimer's disease; bvFTD: Behavioral variant of frontotemporal dementia; CBI: Cambridge Behavioral Inventory; CDR: Clinician Dementia Rating Scale; CTL: Control; DLB: Lewy body dementia; FBI: Frontal Behavioral Inventory; FTD: Frontotemporal dementia; FTLD: Frontotemporal lobar degeneration; MMSE: Mini Mental State Examination; NPI: Neuropsychiatry Inventory; PPA: Primary progressive aphasia; PPA-G: Primary progressive aphasia nonfluent/agrammatic variant; PPA-L: Primary progressive aphasia logopenic variant; PPA-S: Primary progressive aphasia semantic variant; SD: Semantic dementia; VaD: Vascular dementia.
In addition to the studies that used behavioral scales to measure neuropsychiatric symptoms, Snowden et al. evaluated the behavior of 30 bvFTD and 11 SD patients using a newly created semi-structured interview conducted with caregivers [6]. With regard to basic emotions, all patients were impaired in their capacity to show basic emotions, but SD patients were less impaired than bvFTD patients in their capacity to express anger, sadness and disgust. They did, however, commonly show impairments in the expression of fear. In the bvFTD group, changes in primary emotions were largely characterized by a reduction in expression of emotion, whereas some SD patients exhibited exaggerated emotional displays, while others showed diminished emotional responses. With regard to social emotions, both groups showed reduced demon strations of empathy or embarrassment, and increased selfishness. As for interest in social interactions, patients with SD and bvFTD showed an opposite pattern: bvFTD patients were more likely to avoid social contact, whereas SD patients were more likely to seek social contact (in contrast to Bozeat et al.'s findings [7]). Eating behaviors were altered in both groups, but with different patterns. bvFTD patients, especially those with prominent dis inhibited behavior, showed increased eating and were indiscriminate in the type of food they ingested, whereas SD patients had food fads and were more selective in their food consumption. Complex repetitive behaviors, including hoarding, verbal stereotypies and obsessive-compulsive symptoms, were prevalent in SD, similar to the findings of Bozeat et al. [7].
Two studies have investigated the neuroanatomic correlates of neuropsychiatric symptoms in PPA. In the first, NPI scores were compared between 27 SD patients, 24 bvFTD patients and 22 patients with either Alzheimer's disease (AD) or mild cognitive impairment [8]. Principal component analysis in all patients showed that the presence of disinhibition was associated with decreased volume in the right anterior temporal, right ventromedial prefrontal cortex and right amygdala. Depression was associated with decreased volume in the right amygdala and right anterior temporal cortex and eating disorders were associated with decreased volume in the right frontal cortex and right ventromedial prefrontal cortex.
Rohrer and Warren recently investigated the neuroanatomic correlates of neuropsychiatric symptoms in PPA subtypes using the NPI and voxel-based morphometry [9]. Anxiety, apathy, irritability/lability and abnormal appetite/eating disorders correlated with reduced gray matter density in the right lateral orbitofrontal cortex, while disinhibition correlated with reduced gray matter density in the left lateral orbitofrontal cortex. Other correlations were found between apathy and atrophy in the right dorsolateral prefrontal cortex, irritability/lability and atrophy in the right anterior cingulate cortex, and disinhibition and atrophy in the left anterior superior temporal gyrus and entorhinal cortex.
Studies of a single neuropsychiatric symptom in PPA
A number of investigations have been performed on a single neuropsychiatric symptom in PPA. With the idea that a relatively isolated language difficulty in a person who is otherwise healthy could make them vulnerable to depression and social withdrawal, Medina and Weintraub examined Geriatric Depression Scale (GDS) scores and found that a group of 61 PPA patients had higher GDS scores than the controls, although on average not in the `depressed' range [10]. Interestingly, the depressed group was much more likely (43%) than the nondepressed group (15%) to have had a premorbid history of depression.
In two studies, Banks and Weintraub investigated the lack of insight in patients with PPA, bvFTD and AD [11,12]. They asked both the patients and caregivers to rate the FBI and the reponses were compared. PPA patients tended to underestimate their own behavioral changes relative to caregiver estimates, although not as prominently as bvFTD or AD patients.
In another study of insight, Eslinger et al. asked patients with bvFTD, PNFA, SD and AD to estimate their performance on tasks and also compared their own ratings on symptom scales to caregivers' ratings [13]. They found that fronto-temporal dementia (FTD) patients significantly underestimated their apathy, meaning that they viewed themselves as much more motivated than their caregivers did. This was also true within the PPA subgroups. Empathic concern was overestimated in both PNFA and SD, as was self-monitoring in the SD subtype.
One study investigated the prevalence of misidentification syndrome among people with different types of dementia [14]. AD and Lewy body dementia groups had the highest prevalence of this syndrome (~15%). Approximately 8% of patients with SD (n = 24) also endorsed some kind of misidentification symptoms (either by themselves or reported by their caregivers). These symptoms were not present in other PPA subtypes (n = 101) or bvFTD (n = 119).
Sollberger et al. longitudinally studied interpersonal traits in a group of patients with AD, bvFTD and SD by having patients' caregivers fill out the Interpersonal Adjective Scales (IAS) [15]. They found that SD was associated with prominent changes in personality trait characteristics. Dominance and warmth scores became abnormally low at the moderate-to-severe disease stage, although both showed an early drop from premorbid levels. The extraversion score already showed a significant drop to an abnormally low level at the very mild disease stage.
Studies of neuropsychiatric syndromes in single cases
One case report described a 75-year-old female without any remarkable medical or psychiatric history who developed a full blown panic disorder with agoraphobia at 71 years of age [16]. A year after the panic disorder, the patient exhibited a decline in linguistic fluency, word-finding difficulties, effortful speech and hesitant utterances with frequent pauses, phonemic paraphasias and transpositional errors. Approximately 2 years after the onset of aphasia, she was reported to have developed cognitive decline substantial enough to be consistent with dementia. Neuroimaging highlighted left temporal and inferior frontal abnormalities. Although the neuroimaging abnormalities in this case are consistent with those of PPA-S, the clinical description of language characteristics is inconsistent with this diagnosis, thus leaving the subtype unclear.
The second case was a 57-year-old woman who had received a diagnosis of PPA by her neurologist 6 years earlier [17]. Initially, she developed gradual but progressive difficulties in word finding and object naming with intact comprehension. As PPA advanced, her speech output and understanding of language became compromised. However, she continued to maintain her social activities. Computed tomography was reportedly unremarkable. Her medical history was significant for hypertension. There was no personal or family history of psychiatric illness. Later during the course of PPA, she started to disengage herself from family and social activities, eat poorly and neglect her appearance. Her family interpreted these symptoms as a result of worsening language problems. She then developed restlessness and episodes of crying. She was admitted to the hospital after she attempted suicide by running into traffic. She was treated with venlafaxine, and was engaged in art, group exercise and pet therapy. She showed improvements in self-care, appetite and nonverbal interactions after receiving treatment; thus, her mood appeared to improve. In our opinion, this case description is consistent with the PPA-S and illustrates the types of neuropsychiatric symptoms described quantitatively in the studies above.
General synthesis of literature on specific neuropsychiatric symptoms
The review of the existing literature indicates that, even when measured using a general screening instrument, such as the NPI, neuropsychiatric symptoms are not infrequent in patients with PPA. As this instrument has been used so frequently, we performed a synthesis of the literature reviewed above, focusing on each individual neuropsychiatric symptom construct using the NPI as a framework. In addition, at the end of this section we highlight a few other symptom constructs relevant to PPA that are not captured by the NPI. Table 2 demonstrates the frequency range of each neuropsychiatric symptom reported in PPA.
Table 2.
Frequency ranges of neuropsychiatric symptoms in primary progressive aphasia.
| Symptom | All PPAs (%) | PPA-S (%) | PPA-G (%) | PPA-L (%) | Ref. |
|---|---|---|---|---|---|
| Delusions | 2–9 | 5–19 | 0–7 | 14 | [7–9,19,20,22] |
| Hallucinations | 0–6 | 0–11 | 0 | 14 | [7–9,19,20,22] |
| Agitation/aggression | 20–50 | 30–64 | 50 | 57 | [6,7,9,20,22] |
| Depression/dysphoria | 38–56 | 44–78 | 57 | 29 | [7–9,20,22] |
| Anxiety | 15–50 | 41–56 | 36 | 71 | [8,9,20,22] |
| Elation/euphoria | 8–19 | 22–37 | 14 | 14 | [8,9,20,22] |
| Apathy/indifference | 32–56 | 33–65 | 64 | 57 | [7–9,20,22] |
| Disinhibition | 12–38 | 36–74 | 14 | 43 | [6,8,9,20,22] |
| Irritability | 18–56 | 33–82 | 29 | 71 | [6–9,20,22] |
| Aberrant motor behavior | 4–24 | 22–52 | 0–21 | 29 | [7–9,19,20,22] |
| Night-time behaviors | 22–30 | 44–52 | 21 | 14 | [8,9,20,22] |
| Appetite/eating behaviors | 26–50 | 35–67 | 43 | 43 | [7–9,20,22] |
| Mental rigidity | – | 80 | – | – | [7] |
| Stereotypies/rituals/compulsions | – | 35–91 | – | – | [6,7] |
| Distractibility | – | 90 | – | – | [7] |
| Decrease in self-care | – | 64–65 | – | – | [6,7] |
| Poor judgment | – | 65 | – | – | [7] |
| Social withdrawal | – | 18–60 | – | – | [6,7] |
| Lack of empathy/selfishness | – | 75–91 | – | – | [6,7] |
PPA: Primary progressive aphasia; PPA-G: Primary progressive aphasia nonfluent/agrammatic variant; PPA-L: Primary progressive aphasia logopenic variant; PPA-S: Primary progressive aphasia semantic variant.
Delusions & hallucinations
Psychotic symptoms in PPA are either rare or absent [18–20]. The study by Rohrer and Warren reported a higher rate of psychotic symptoms in PPA-L and PPA-S (14 and 11%, respectively), which could be related to longer duration or more severe illness [9].
Agitation/aggression
Agitation/aggression is more frequent in PPA patients than in controls (11 vs 1%) and increases over time [21]. It is present in all types of PPA [9,18]; however, it is less severe than in bvFTD [19]. In one study, agitation/aggression was reported in up to 50% of patients with PPA [9].
Depression/dysphoria
Depression/dysphoria are consistently reported and appear to be the most prevalent psychiatric symptoms across all types of PPA [9,20,22]. In fact, one of the case reports described a PPA patient who gradually became depressed and attempted suicide, but she eventually responded to treatment [17]. We have observed a similar case in our practice. Nevertheless, only a subset of patients with these symptoms meet formal diagnostic criteria for clinical depression [10].
Anxiety
Anxiety is reported as frequently as depression in PPA [9,22]. It is observed in up to 70% of PPA-L patients [9]. Anxiety can be an isolated symptom or a cluster of symptoms severe enough to form an anxiety disorder. In one case report, a patient with no previous anxiety disorder developed a classic panic disorder with agoraphobia, fulfilling DSM criteria, 1 year before the symptoms of PPA slowly emerged [16].
Elation/euphoria
Mood elation/euphoria is not as common as depression in PPA, although it is similarly prevalent in bvFTD and in PPA [22], its frequency in the studies ranged between 5 and 20% [9,20,22].
Apathy/indifference
Apathy is one of the most common symptoms in PPA patients [9,22] and compared with controls, the frequency of apathy is significantly higher in PPA [20] and increases over time [21]. In pathologically confirmed PPA cases, apathy was one of the clinical features that suggested FTLD rather than AD pathology [23].
Disinhibition
Disinhibition is less frequent and less severe in PPA than in bvFTD [19,22]; however, its frequency increases over time [22]. In one study, up to 70% of PPA-S patients exhibited disinhibition [9]. In PPA, disinhibition is one of the distinguishing features that points toward FTLD pathology rather than AD pathology [23].
Irritability
Banks and Weintraub reported a similar proportion of mood symptoms including irritability in PPA and in bvFTD [22]. Irritability is more frequent in PPA when compared with controls [20], and is one of the most frequent symptoms reported in all types of PPA [9].
Aberrant motor behaviors
Aberrant motor behaviors are present in PPA, although they appear to be less frequent than other neuropsychiatric symptoms [9,20,22] and less severe than similar symptoms in bvFTD [18] (except one study that did not find any difference in the frequency between PPA and bvFTD [22]).
Night-time behaviors
PPA patients seem to have a relatively low frequency of night-time behaviors (in one study the frequency was similar to that of controls) [20], although the frequency increases over time [22].
Appetite/eating behaviors
Compared with controls, changes in eating behaviors are more evident in PPA patients [20]. Over 3 years of longitudinal follow-up, hyper-orality significantly increased over time in PPA patients [21]. This symptom (sweet food preference) was also highly associated with FTLD pathology rather than AD pathology in PPA [23].
Insight
PPA patients are better than bvFTD and AD patients at estimating their ability to perform tasks such as memory tasks. However, relative to other general or physical characteristics, such as weight or eyesight, their estimation of behavioral symptoms is less accurate [11,12,24]. For example, PPA patients may lack insight into loss of empathic concern and apathy [13].
Obsessive–compulsive spectrum symptoms
Compulsive and repetitive behaviors are quite common in the PPA-S subtype of PPA. In fact, the study by Snowden et al. showed that simple motor stereotypies such as lip smacking, hand rubbing and foot tapping were as common in PPA-S as those in bvFTD, and complex routines, verbal stereotypies and repetitive themes were more frequent in PPA-S than bvFTD patients [6]. In the same study, more than 90% of PPA-S patients exhibited some form of repetitive theme and more than 70% of them were reported to have verbal stereotypies. Furthermore, PPA-S patients exhibited obsessive and compulsive symptoms, such as excessive worrying and performing the same tasks again and again, more frequently than bvFTD patients. Similar observations were reported by Bozeat et al. [7].
Loss of empathy
Loss of empathy, selfishness and no sense of embarrassment were ubiquitously found in PPA-S patients [6].
Personality changes
Personality changes are a classic symptom early in the course of bvFTD. By contrast, to fulfill diagnostic criteria for PPA, a patient must be largely free of these symptoms during the first 2 years of the disease [1]. However, one recent study demonstrated that PPA-S is accompanied by subtle or overt changes in personality (dominance, warmth and extraversion) even during the early stages [15]. Other symptoms common in PPA-S, such as selfishness, lack of empathy and disinhibition, may also be viewed as personality changes [6].
Neuropsychiatric symptoms across PPA subtypes
Taken together, the literature suggests that PPA-S patients commonly exhibit neuropsychiatric symptoms, often relatively early and in a fairly stereotypical fashion. Many of these symptoms are similar to those of bvFTD, including loss of empathy, changes in eating behavior, compulsive behavior and disinhibition. Although these symptoms are highly consistent with FTD, depending on when they begin and how they are reported by informants, it may be difficult for the clinician to be confident in assigning a subtype diagnosis (i.e., bvFTD vs PPA-S vs SD). In fact, in the Neary et al. diagnostic criteria for FTD, features considered supportive of a diagnosis of SD included loss of sympathy or empathy and narrowed preoccupations (mental rigidity) [25]. Aberrant motor behavior is also commonly reported in some studies; in our experience this often includes elaborate movements related to repetitive or compulsive behaviors, although there has been little focused study of this topic. Depression is also reported as common in PPA-S in some studies; in our experience, however, at least some patients say certain phrases repetitively (i.e., catchphrases) that appear to express negative emotion (e.g., “I feel so stupid” or “I used to know that and now I just don't know anything”), but with minimal affective behavior consistent with depression, and a structured interview with some of these patients' caregivers reveals little behavior in daily life that appears consistent with a diagnosis of depression.
In PPA-G, neuropsychiatric symptoms are less frequent initially, but as the illness progresses it becomes increasingly common to see apathy, depression or irritability. In some cases these symptoms are present early in the illness, which may lead to misdiagnosis as a primary psychiatric disorder (commonly depression).
In PPA-L, neuropsychiatric symptoms are relatively infrequent early on, but increase as the illness progresses and include agitation, anxiety, irritability and apathy. In many cases the clinical phenomenology of neuropsychiatric symptoms appears similar to that seen in AD.
Neuroanatomic substrates of neuropsychiatric symptoms in PPA
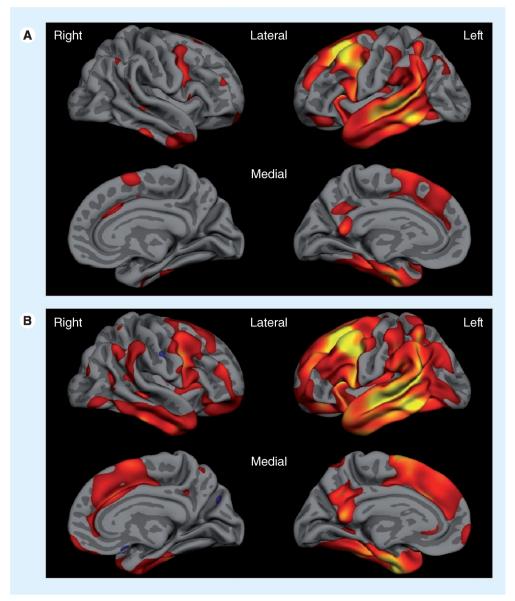
A predominantly right-sided distribution of frontal and temporal areas has been implicated in the pathogenesis of many behavioral symptoms in FTD, such as apathy, disinhibition and abnormal eating behavior [26–29]. Right anterior temporal lobe atrophy is correlated with disinhibition and depression [8]. Right orbitofrontal, insular and striatal atrophy is correlated with eating disorders [30] and right ventromedial prefrontal cortex with aberrant motor behaviors, eating disorders and disinhibition [8,26]. In the study by Rohrer and Warren, anxiety, apathy, irritability and appetite changes were correlated with right hemisphere atrophy in PPA [9]. In PPA-S, atrophy is usually reported to be predominant in the left temporal pole and ventral left temporal lobe, but many patients exhibit subtle atrophy or hypometabolism in orbital and ventromedial prefrontal regions, as well as the ventral anterior insula and striatum; furthermore, a lesser but nontrivial degree of right hemisphere involvement is common [31,32]. Right frontal abnormalities have also been observed in nonfluent aphasia [33–35]. In our experience, there is often at least subtle atrophy in homologous regions of the right hemisphere in PPA at baseline assessment; right hemisphere structures commonly become involved as the condition progresses (Figure 1).
Figure 1. Cortical atrophy in a mixed sample of patients with primary progressive aphasia.
(A) Baseline cortical atrophy and (B) progression of atrophy after 2 years, illustrating not only the spread within the left hemisphere, but also the increasing degree of atrophy within right hemisphere structures. Red–yellow indicate localization of thinner cortex in the group of primary progressive aphasia patients compared with the matched control group.
Management of neuropsychiatric symptoms in PPA
Given the heterogeneity of the underlying pathology in FTD, and the wide range of clinical phenotypes involving behavioral/psychiatric, cognitive and motor symptoms, clinical trials with a homogeneous group of patients are sparse [36]. The selection of outcome measures is also challenging. General behavioral rating scales such as the Comprehensive Psychiatric Rating Scale (CPRS) do not capture all the behavioral abnormalities of FTD. While the NPI [37], the NPI-questionnaire and the Behavioral Pathology in Alzheimer's Disease (BEHAVE-AD) [38] scales are aimed at a general dementia population rather than specifically at the FTD spectrum, they have been employed as outcome measures in some trials in FTD. Symptoms that commonly present in FTD, such as obsessive–compulsive spectrum behaviors, personality changes and lack of empathy, are not captured by these scales. The FBI and Cambridge Behavioral Inventory (CBI) were developed to more specifically capture the behavioral features of FTD [7,39,40], but to date they have only been used in a handful of trials. Table 3 lists some instruments that are used to measure behavioral/psychiatric symptoms in patients with dementia. Knopman et al. studied the validity of different cognitive and behavioral tests as outcome measures in a population of patients with FTD [41]. They found that while the Modified CDR and Clinical Global Impression of Change scale demonstrated decline in the majority of patients, almost a third of patients improved on behavioral scales (NPI and FBI), suggesting that these instruments may not be ideal for use as outcome measures in FTD. Nevertheless, they may be of use in trials of interventions targeting specific types of symptoms, particularly if such trials recruit patients with more prominent neuropsychiatric symptoms at baseline.
Table 3.
Neuropsychiatric and behavioral scales used in dementia.
| Scales | Symptoms measured | Ref. |
|---|---|---|
| NPI and NPI-Q | Delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, elation/euphoria, apathy/indifference, disinhibition, irritability, aberrant motor behavior, night-time behaviors and eating/appetite behaviors (NPI is a caregiver/informant interview; NPI-Q is a self-administered questionnaire with written instructions) | [37,63] |
| FBI | Apathy, aspontaneity, indifference/emotional flatness, inflexibility, disorganization, inattention, personal neglect, loss of insight, preservation, obsession (stereotypy), hoarding, inappropriateness, poor judgment and impulsivity, restlessness/roaming, irritability, aggression, hyperorality/food fads, hypersexuality and utilization behavior | [39] |
| FRS | Lack of interest, lack of normal affection, confusion in unusual surroundings, restlessness, impulsivity and lack of judgment | [12] |
| CBI | Challenging behaviors (uncooperative, threatens to harm self/others), disinhibition, eating habits, sleep, stereotypic and motor behaviors, motivation and insight/awareness | [7] |
| BEHAVE-AD | Paranoid and delusional ideation, hallucinations, activity disturbances, aggressiveness, diurnal rhythm disturbances, affective disturbances, anxieties and phobias | [4] |
| NPI-C | Delusions, hallucinations, agitation, aggression+, dysphoria, anxiety, elation/euphoria, apathy, disinhibition, irritability/lability, motor disorders, sleep disorders, appetite and eating disorders, and aberrant vocalization | [8] |
| FTD Inventory | Behavioral disinhibition, violation of social norms, apathy, hypomania-like behaviors, loss of interpersonal warmth or empathy, loss of insight, decline in personal hygiene and grooming, mental rigidity and inflexibility, distractibility and impersistence, hyperorality and dietary changes, compulsive and stereotyped behavior, and environmental dependency | [64] |
| GDS (short form) | Satisfaction with life, dropping activities or interests, feeling life is empty, getting bored, being in good spirits, being afraid that something bad is going to happen, feeling happy, feeling helpless, preference to stay at home, having problems with memory, thinking it is wonderful to be alive, feeling worthless, feeling full of energy, feeling hopeless and thinking most people are better off than they are | [59] |
Note that aggression has been separated from agitation.
BEHAVE-AD: Behavioral Pathology in Alzheimer's Disease; CBI: Cambridge Behavioral Inventory; FBI: Frontal Behavioral Inventory; FRS: Frontotemporal Dementia Rating Scale; FTD: Frontotemporal dementia; GDS: Geriatric Depression Scale; NPI: Neuropsychiatry Inventory; NPI-C: Neuropsychiatric Inventory-Clinician Rating Scale; NPI-Q: Neuropsychiatry Inventory Questionnaire.
In clinical practice, neuropsychiatric symptoms do not often resolve completely, yet over time, their frequency or severity may intensify or attenuate. It is critical to try to use valid scales to monitor these symptoms over time, particularly if empirical management strategies are attempted. The management of symptoms should be tailored to individual patients and caregivers. Education of caregivers (as well as patients if possible) about the safety issues and the nature of the neuropsychiatric symptoms is an important first step in management. Referral of families to the Association for Frontotemporal Degeneration and the Alzheimer's Association will provide further information and help families connect with the existing support groups. One reasonable strategy for prioritizing the goals of management is to choose one symptom at a time to focus on (usually the most disabling symptom). A multidisciplinary care plan involving a psychiatrist or neurologist, or geriatrician specializing in these symptoms, a social worker, and, if possible, a nurse practitioner or psychologist can assist families in developing behavioral strategies and providing psychosocial support [42]. Most symptoms are best managed by behavioral interventions. However, if the response is inadequate or symptoms are prominent, empirical pharmacological treatments can be tried.
To date, no published studies report on clinical trials attempting to treat neuropsychiatric symptoms specifically in a PPA sample. However, as is apparent from this literature review, these symptoms are not uncommon in PPA and despite the lack of adequate research-based evidence, we need to manage the neuropsychiatric symptoms of these patients in day-to-day clinical practice. Therefore, we must look to studies in bvFTD patients, mixed samples of FTD or other dementia patients for guidance. Below we summarize the results of clinical trials that have measured behavioral or psychiatric symptoms as outcomes in patients with FTD, organized by the type of pharmacological agent tested.
Antidepressants
A randomized double-blind study in 26 subjects with bvFTD using trazodone demonstrated a decrease in NPI scores by improvement of irritability, agitation, depression and eating disorders [43]. Data regarding paroxetine are conflicting; while one study did not find any improvement in the NPI or CBI of patients with bvFTD (n = 10) [44], two others showed an improvement in behavioral symptoms [45,46]. In one randomized study, eight bvFTD patients per group received 20 mg paroxetine or 1200 mg piracetam for 14 months. Patients treated with paroxetine showed an improvement in behavioral symptoms reflected by a reduction of caregivers' distress [45]. In another study, 11 patients with bvFTD were treated with fluoxetine, sertraline or paroxetine for 3 months. After treatment, disinhibition, depressive symptoms, carbohydrate craving and compulsions all showed improvement in at least half of the subjects in whom they had been present [46]. In four PPA-S patients with hostile behavior, sertraline reduced aggressive behaviors and total NPI questionnaire scores [47].
Two case series studied the efficacy of moclobemide and selegiline in managing behavioral symptoms in FTD. Six patients with bvFTD treated with moclobemide for 14 days showed variable improvement in depression, aggressive symptoms, irritability, distractibility, mental rigidity, and stereotypy of speech and perseveration [48]. In three bvFTD patients, selegiline improved NPI scores and demonstrated some benefit on cognition by reducing errors on the Stroop and Paced Auditory Serial Addition Task [49].
Cognitive enhancers
An open-label study of 21 bvFTD, 13 PPA-S and nine PPA patients using memantine for 26 weeks caused a transient improvement in NPI scores predominantly in the bvFTD group [50,51]. In another case series, memantine improved total NPI scores in three bvFTD subjects with specific improvement in scores of apathy, agitation and anxiety [51]. However, in a different study, 16-week treatment of bvFTD patients with memantine did not change the NPI or the FBI scores [52]. Unfortunately, the data from the most recent clinical trial of memantine in FTD do not look encouraging with regard to NPI or other outcome measures [Boxer A, Pers. Comm.]. Rivastigmine reduced caregiver burden as well as NPI, BEHAVE-AD and Cornell Depression Scale scores, when given to bvFTD patients (n = 20) for 12 months [53]. On the other hand, donepezil worsened bvFTD patients' (n = 12) scores on a novel scale called the FTD Inventory and increased disinhibition and compulsivity [54].
Atypical antipsychotics
In a case report, risperidone improved agitation, delusions and hallucinations in bvFTD [55]. Another case study demonstrated that aripeprazole partially restored frontal glucose metabolism in a bvFTD patient, which the authors interpret as suggesting a beneficial role in frontal functions [56]. Czarnecki et al. reported that three patients with bvFTD developed extrapyramidal symptoms and tardive antecollis after treatment with olanzapine, risperidone or quetiapine [57]. It is not clear from this report whether there was any benefit in any of these cases.
Miscellaneous agents
In a single case of bvFTD, quantitative EEG demonstrated profound greater left than right bi-frontotemporal slowing, which partially normalized after methylphenidate administration [58]. In a randomized double-blind trial, methylphenidate reduced risk betting in the Cambridge Gambling Task in bvFTD patients (n = 8) but had no effect on any other measures [59]. In a case report, topiramate reduced alcohol abuse in bvFTD but not other obsessive–compulsive tendencies [60]. A very recent study of oxytocin in bvFTD demonstrated preliminary data showing a beneficial effect on the NPI [61].
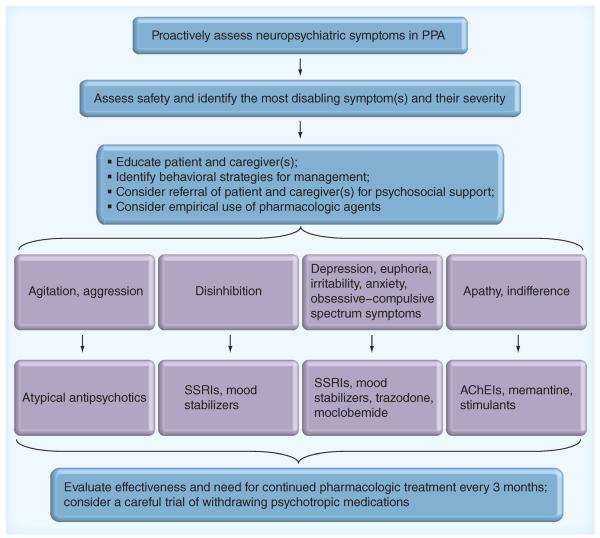
In summary, while it is helpful to have this small amount of literature, the small sample sizes (less than 20 participants), heterogeneity of participants and variability in outcome measures leave many questions about the utility of existing agents in the management of neuropsychiatric symptoms in FTD. At present, the treatment of these symptoms in FTD continues to be challenging and largely reliant on individualized empirical approaches. Figure 2 displays a diagram of our usual approach to the assessment and management of neuropsychiatric symptoms in FTD, with the caveat that `one size does not fit all' in this or any other dementia.
Figure 2. Clinical algorithm for empirical assessment and management of behavioral and neuropsychiatric symptoms in primary progressive aphasia.
AChEIs: Acetylcholinesterase inhibitors; PPA: Primary progressive aphasia; SSRI: Selective serotonin reuptake inhibitor.
Conclusion & future perspective
Although much of the scientific and clinical literature on PPA focuses on the canonical language impairments, as the illness progresses and extends beyond brain language networks neuropsychiatric symptoms become more common. Furthermore, it is not infrequent for neuropsychiatric symptoms to be present during the early stages of PPA. In the PPA-S subtype, in particular, behavioral symptoms are very common and similar to those in bvFTD. On the other hand, PPA-G and PPA-L exhibit fewer behavioral symptoms that some have characterized, at least in some patients, as more reminiscent of those observed in AD [18].
While existing instruments are valuable, it is worth considering the development of new instruments for the measurement of neuropsychiatric symptoms tailored toward PPA. It might be useful for studies of putative disease-modifying therapies to measure neuropsychiatric symptoms, in addition to language, as an indicator that an intervention can slow progression of or otherwise ameliorate PPA. Ultimately, clinical trials of potential treatments aimed at improving the lives of PPA patients and their families need to focus not only on language and general cognitive function but also on neuropsychiatric symptoms.
Practice Points
-
■
Primary progressive aphasia is typically conceptualized as a disorder primarily affecting language, but clinical practice and a review of the literature indicates that neuropsychiatric symptoms are common.
-
■
Very little is known about the biological basis of neuropsychiatric symptoms in primary progressive aphasia, but a few studies suggest that neurodegeneration in particular brain regions or circuits may underlie some of these symptoms.
-
■
There is relatively little systematic research on the clinical characteristics of neuropsychiatric symptoms in primary progressive aphasia, and no evidence-based literature on their management.
Acknowledgements
The authors wish to thank the patients and families who participated in their research. BC Dickerson would also like to thank D Hochberg for her continued partnership in their Primary Progressive Aphasia Program.
This study was supported by NIH grants from the US National Institute on Aging (R01-AG029411 and P50-AG005134), the National Institute of Neurological Disorders and Stroke (R21-NS077059), the Alzheimer's Association and the Sidney R Baer Jr Foundation.
Footnotes
Publisher's Disclaimer: The content of this article is the sole responsibility of the authors and does not necessarily represent the official views of the NIH.
Financial & competing interests disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: ■ of interest ■■ of considerable interest
- 1.Mesulam MM. Primary progressive aphasia: a 25-year retrospective. Alzheimer Dis. Assoc. Disord. 2007;21(4):S8–S11. doi: 10.1097/WAD.0b013e31815bf7e1. [DOI] [PubMed] [Google Scholar]
- 2.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mesulam MM, Wieneke C, Thompson C, Rogalski E, Weintraub S. Quantitative classification of primary progressive aphasia at early and mild impairment stages. Brain. 2012;135(Pt 5):1537–1553. doi: 10.1093/brain/aws080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat. Rev. Neurol. 2010;6(2):88–97. doi: 10.1038/nrneurol.2009.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(Pt 9):2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ Provides the new diagnostic criteria for behavioral variant frontotemporal dementia (FTD) and emphasizes the point that new scales or instruments may be required that are more specific for the neuropsychiatric symptoms present in FTD versus other neurodegenerative syndromes.
- 6.Snowden JS, Bathgate D, Varma A, et al. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J. Neurol. Neurosurg. Psychiatry. 2001;70(3):323–332. doi: 10.1136/jnnp.70.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozeat S, Gregory CA, Ralph MA, Hodges JR. Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer's disease? J. Neurol. Neurosurg. Psychiatry. 2000;69(2):178–186. doi: 10.1136/jnnp.69.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu W, Miller BL, Kramer JH, et al. Behavioral disorders in the frontal and temporal variants of frontotemporal dementia. Neurology. 2004;62(5):742–748. doi: 10.1212/01.wnl.0000113729.77161.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohrer JD, Warren JD. Phenomenology and anatomy of abnormal behaviours in primary progressive aphasia. J. Neurol. Sci. 2010;293(1–2):35–38. doi: 10.1016/j.jns.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ One of only a few studies that have investigated the correlation between neuropsychiatric symptoms in primary progressive aphasia (PPA) and regional brain atrophy.
- 10.Medina J, Weintraub S. Depression in primary progressive aphasia. J. Geriatr. Psychiatry Neurol. 2007;20(3):153–160. doi: 10.1177/0891988707303603. [DOI] [PubMed] [Google Scholar]
- 11.Banks S, Weintraub S. Self-awareness and self-monitoring of cognitive and behavioral deficits in behavioral variant frontotemporal dementia, primary progressive aphasia and probable Alzheimer's disease. Brain Cogn. 2008;67(1):58–68. doi: 10.1016/j.bandc.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banks SJ, Weintraub S. Generalized and symptom-specific insight in behavioral variant frontotemporal dementia and primary progressive aphasia. J. Neuropsychiatry Clin. Neurosci. 2009;21(3):299–306. doi: 10.1176/appi.neuropsych.21.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eslinger PJ, Dennis K, Moore P, et al. Metacognitive deficits in frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry. 2005;76(12):1630–1635. doi: 10.1136/jnnp.2004.053157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harciarek M, Kertesz A. The prevalence of misidentification syndromes in neurodegenerative diseases. Alzheimer Dis. Assoc. Disord. 2008;22(2):163–169. doi: 10.1097/WAD.0b013e3181641341. [DOI] [PubMed] [Google Scholar]
- 15.Sollberger M, Neuhaus J, Ketelle R, et al. Interpersonal traits change as a function of disease type and severity in degenerative brain diseases. J. Neurol. Neurosurg. Psychiatry. 2011;82(7):732–739. doi: 10.1136/jnnp.2010.205047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caixeta L, Caixeta M. Primary progressive aphasia beginning with a psychiatric disorder. Clinics (Sao Paulo) 2011;66(8):1505–1508. doi: 10.1590/S1807-59322011000800035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahgoub N, Avari J. A case of primary progressive aphasia associated with depression. Int. J. Geriatr. Psychiatry. 2012;27(4):436–437. doi: 10.1002/gps.2748. [DOI] [PubMed] [Google Scholar]
- 18.Rosen HJ, Allison SC, Ogar JM, et al. Behavioral features in semantic dementia vs other forms of progressive aphasias. Neurology. 2006;67(10):1752–1756. doi: 10.1212/01.wnl.0000247630.29222.34. [DOI] [PubMed] [Google Scholar]; ■ Highlights the much higher prevalence of neuropsychiatric symptoms in PPA semantic variant as compared with other subtypes of PPA.
- 19.Marra C, Quaranta D, Zinno M, et al. Clusters of cognitive and behavioral disorders clearly distinguish primary progressive aphasia from frontal lobe dementia, and Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 2007;24(5):317–326. doi: 10.1159/000108115. [DOI] [PubMed] [Google Scholar]
- 20.Fatemi Y, Boeve BF, Duffy J, et al. Neuropsychiatric aspects of primary progressive aphasia. J. Neuropsychiatry Clin. Neurosci. 2011;23(2):168–172. doi: 10.1176/appi.neuropsych.23.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marczinski CA, Davidson W, Kertesz A. A longitudinal study of behavior in frontotemporal dementia and primary progressive aphasia. Cogn. Behav. Neurol. 2004;17(4):185–190. [PubMed] [Google Scholar]; ■■ Significant because of its longitudinal assessment of PPA patients in contrast to the majority of studies that focus on cross-sectional evaluations of neuropsychiatric symptoms in PPA.
- 22.Banks SJ, Weintraub S. Neuropsychiatric symptoms in behavioral variant frontotemporal dementia and primary progressive aphasia. J. Geriatr. Psychiatry Neurol. 2008;21(2):133–141. doi: 10.1177/0891988708316856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong L, Xuereb JH, Spillantini MG, et al. Clinical comparison of progressive aphasia associated with Alzheimer versus FTD-spectrum pathology. J. Neurol. Neurosurg. Psychiatry. 2011;82(3):254–260. doi: 10.1136/jnnp.2010.209916. [DOI] [PubMed] [Google Scholar]; ■ Examines the occurrence of specific neuropsychiatric symptoms in relation to Alzheimer's disease versus FTD spectrum pathology in autopsied cases.
- 24.Banks SJ, Weintraub S. Cognitive deficits and reduced insight in primary progressive aphasia. Am. J. Alzheimers Dis. Other Dement. 2008;23(4):363–371. doi: 10.1177/1533317508320351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 26.Rosen HJ, Allison SC, Schauer GF, et al. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128(Pt 11):2612–2625. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peters F, Perani D, Herholz K, et al. Orbitofrontal dysfunction related to both apathy and disinhibition in frontotemporal dementia. Dement. Geriatr. Cogn. Disord. 2006;21(5–6):373–379. doi: 10.1159/000091898. [DOI] [PubMed] [Google Scholar]
- 28.Whitwell JL, Sampson EL, Loy CT, et al. VBM signatures of abnormal eating behaviours in frontotemporal lobar degeneration. Neuroimage. 2007;35(1):207–213. doi: 10.1016/j.neuroimage.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Zamboni G, Huey ED, Krueger F, Nichelli PF, Grafman J. Apathy and disinhibition in frontotemporal dementia: insights into their neural correlates. Neurology. 2008;71(10):736–742. doi: 10.1212/01.wnl.0000324920.96835.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woolley JD, Gorno-Tempini ML, Seeley WW, et al. Binge eating is associated with right orbitofrontal-insular-striatal atrophy in frontotemporal dementia. Neurology. 2007;69(14):1424–1433. doi: 10.1212/01.wnl.0000277461.06713.23. [DOI] [PubMed] [Google Scholar]
- 31.Tyrrell PJ, Warrington EK, Frackowiak RS, Rossor MN. Heterogeneity in progressive aphasia due to focal cortical atrophy. A clinical and PET study. Brain. 1990;113(Pt 5):1321–1336. doi: 10.1093/brain/113.5.1321. [DOI] [PubMed] [Google Scholar]
- 32.Tyrrell PJ, Kartsounis LD, Frackowiak RS, Findley LJ, Rossor MN. Progressive loss of speech output and orofacial dyspraxia associated with frontal lobe hypometabolism. J. Neurol. Neurosurg. Psychiatry. 1991;54(4):351–357. doi: 10.1136/jnnp.54.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cummings JL. Anatomic and behavioral aspects of frontal-subcortical circuits. Ann. N.Y. Acad. Sci. 1995;769:1–13. doi: 10.1111/j.1749-6632.1995.tb38127.x. [DOI] [PubMed] [Google Scholar]
- 34.Snowden JS, Neary D, Mann DM, Goulding PJ, Testa HJ. Progressive language disorder due to lobar atrophy. Ann. Neurol. 1992;31(2):174–183. doi: 10.1002/ana.410310208. [DOI] [PubMed] [Google Scholar]
- 35.Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115(Pt 6):1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- 36.Jicha GA, Nelson PT. Management of frontotemporal dementia: targeting symptom management in such a heterogeneous disease requires a wide range of therapeutic options. Neurodegener. Dis. Manag. 2011;1(2):141–156. doi: 10.2217/nmt.11.9. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Outlines existing literature on the management of neuropsychiatric symptoms in FTD. As there are currently few studies specifically evaluating the efficacy of medications in treating these symptoms in PPA, this review article could be used as a resource to guide clinicians in managing the neuropsychiatric symptoms in PPA.
- 37.Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 38.Mendez MF, Perryman KM, Miller BL, Cummings JL. Behavioral differences between frontotemporal dementia and Alzheimer's disease: a comparison on the BEHAVE-AD rating scale. Int. Psychogeriat. 1998;10(2):155–162. doi: 10.1017/s1041610298005262. [DOI] [PubMed] [Google Scholar]
- 39.Kertesz A, Davidson W, Fox H. Frontal behavioral inventory: diagnostic criteria for frontal lobe dementia. Can. J. Neurol. Sci. 1997;24(1):29–36. doi: 10.1017/s0317167100021053. [DOI] [PubMed] [Google Scholar]
- 40.Kertesz A, Nadkarni N, Davidson W, Thomas AW. The frontal behavioral inventory in the differential diagnosis of frontotemporal dementia. J. Int. Neuropsychol. Soc. 2000;6(4):460–468. doi: 10.1017/s1355617700644041. [DOI] [PubMed] [Google Scholar]
- 41.Knopman DS, Kramer JH, Boeve BF, et al. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain. 2008;131(Pt 11):2957–2968. doi: 10.1093/brain/awn234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merrilees J. A model for management of behavioral symptoms in frontotemporal lobar degeneration. Alzheimer Dis. Assoc. Disord. 2007;21(4):S64–S69. doi: 10.1097/WAD.0b013e31815bf774. [DOI] [PubMed] [Google Scholar]
- 43.Lebert F, Stekke W, Hasenbroekx C, Pasquier F. Frontotemporal dementia: a randomised, controlled trial with trazodone. Dement. Geriatr. Cogn. Disord. 2004;17(4):355–359. doi: 10.1159/000077171. [DOI] [PubMed] [Google Scholar]
- 44.Deakin JB, Rahman S, Nestor PJ, Hodges JR, Sahakian BJ. Paroxetine does not improve symptoms and impairs cognition in frontotemporal dementia: a double-blind randomized controlled trial. Psychopharmacology (Berl.) 2004;172(4):400–408. doi: 10.1007/s00213-003-1686-5. [DOI] [PubMed] [Google Scholar]
- 45.Moretti R, Torre P, Antonello RM, Cazzato G, Bava A. Frontotemporal dementia: paroxetine as a possible treatment of behavior symptoms. A randomized, controlled, open 14-month study. Eur. Neurol. 2003;49(1):13–19. doi: 10.1159/000067021. [DOI] [PubMed] [Google Scholar]
- 46.Swartz JR, Miller BL, Lesser IM, Darby AL. Frontotemporal dementia: treatment response to serotonin selective reuptake inhibitors. J. Clin. Psychiatry. 1997;58(5):212–216. [PubMed] [Google Scholar]
- 47.Prodan CI, Monnot M, Ross ED. Behavioural abnormalities associated with rapid deterioration of language functions in semantic dementia respond to sertraline. J. Neurol. Neurosurg. Psychiatry. 2009;80(12):1416–1417. doi: 10.1136/jnnp.2009.173260. [DOI] [PubMed] [Google Scholar]
- 48.Adler G, Teufel M, Drach LM. Pharmacological treatment of frontotemporal dementia: treatment response to the MAO-A inhibitor moclobemide. Int. J. Geriatr. Psychiatry. 2003;18(7):653–655. doi: 10.1002/gps.894. [DOI] [PubMed] [Google Scholar]
- 49.Moretti R, Torre P, Antonello RM, Cazzato G, Bava A. Effects of selegiline on frontotemporal dementia: a neuropsychological evaluation. Int. J. Geriatr. Psychiatry. 2002;17(4):391–392. doi: 10.1002/gps.602. [DOI] [PubMed] [Google Scholar]
- 50.Boxer AL, Lipton AM, Womack K, et al. An open-label study of memantine treatment in 3 subtypes of frontotemporal lobar degeneration. Alzheimer Dis. Assoc. Disord. 2009;23(3):211–217. doi: 10.1097/WAD.0b013e318197852f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swanberg MM. Memantine for behavioral disturbances in frontotemporal dementia: a case series. Alzheimer Dis. Assoc. Disord. 2007;21(2):164–166. doi: 10.1097/WAD.0b013e318047df5d. [DOI] [PubMed] [Google Scholar]
- 52.Diehl-Schmid J, Forstl H, Perneczky R, Pohl C, Kurz A. A 6-month, open-label study of memantine in patients with frontotemporal dementia. Int. J. Geriatr. Psychiatry. 2008;23(7):754–759. doi: 10.1002/gps.1973. [DOI] [PubMed] [Google Scholar]
- 53.Moretti R, Torre P, Antonello RM, et al. Rivastigmine in frontotemporal dementia: an open-label study. Drugs Aging. 2004;21(14):931–937. doi: 10.2165/00002512-200421140-00003. [DOI] [PubMed] [Google Scholar]
- 54.Mendez MF, Shapira JS, McMurtray A, Licht E. Preliminary findings: behavioral worsening on donepezil in patients with frontotemporal dementia. Am. J. Geriatr. Psychiatry. 2007;15(1):84–87. doi: 10.1097/01.JGP.0000231744.69631.33. [DOI] [PubMed] [Google Scholar]
- 55.Curtis RC, Resch DS. Case of pick's central lobar atrophy with apparent stabilization of cognitive decline after treatment with risperidone. J. Clin. Psychopharmacol. 2000;20(3):384–385. doi: 10.1097/00004714-200006000-00018. [DOI] [PubMed] [Google Scholar]
- 56.Fellgiebel A, Muller MJ, Hiemke C, Bartenstein P, Schreckenberger M. Clinical improvement in a case of frontotemporal dementia under aripiprazole treatment corresponds to partial recovery of disturbed frontal glucose metabolism. World J. Biol. Psychiatry. 2007;8(2):123–126. doi: 10.1080/15622970601016538. [DOI] [PubMed] [Google Scholar]
- 57.Czarnecki K, Kumar N, Josephs KA. Parkinsonism and tardive antecollis in frontotemporal dementia – increased sensitivity to newer antipsychotics? Eur. J. Neurol. 2008;15(2):199–201. doi: 10.1111/j.1468-1331.2007.02032.x. [DOI] [PubMed] [Google Scholar]
- 58.Goforth HW, Konopka L, Primeau M, et al. Quantitative electroencephalography in frontotemporal dementia with methylphenidate response: a case study. Clin. EEG Neurosci. 2004;35(2):108–111. doi: 10.1177/155005940403500212. [DOI] [PubMed] [Google Scholar]
- 59.Rahman S, Robbins TW, Hodges JR, et al. Methylphenidate (`Ritalin') can ameliorate abnormal risk-taking behavior in the frontal variant of frontotemporal dementia. Neuropsychopharmacology. 2006;31(3):651–658. doi: 10.1038/sj.npp.1300886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cruz M, Marinho V, Fontenelle LF, Engelhardt E, Laks J. Topiramate may modulate alcohol abuse but not other compulsive behaviors in frontotemporal dementia: case report. Cogn. Behav. Neurol. 2008;21(2):104–106. doi: 10.1097/WNN.0b013e31816bdf73. [DOI] [PubMed] [Google Scholar]
- 61.Jesso S, Morlog D, Ross S, et al. The effects of oxytocin on social cognition and behaviour in frontotemporal dementia. Brain. 2011;134(Pt 9):2493–2501. doi: 10.1093/brain/awr171. [DOI] [PubMed] [Google Scholar]
- 62.Knopman DS, Weintraub S, Pankratz VS. Language and behavior domains enhance the value of the clinical dementia rating scale. Alzheimers Dement. 2011;7(3):293–299. doi: 10.1016/j.jalz.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J. Neuropsychiatry Clin. Neurosci. 2000;12(2):233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 64.McMurtray AM, Chen AK, Shapira JS, et al. Variations in regional SPECT hypoperfusion and clinical features in frontotemporal dementia. Neurology. 2006;66(4):517–522. doi: 10.1212/01.wnl.0000197983.39436.e7. [DOI] [PubMed] [Google Scholar]