Abstract
Purpose.
The purpose of this investigation was to better understand lipid composition in human meibum.
Methods.
Intact lipids in meibum samples were detected by direct infusion electrospray ionization mass spectrometry (ESI-MS) analysis in positive detection mode using sodium iodide (NaI) as an additive. The peak intensities of all major types of lipid species, that is, wax esters (WEs), cholesteryl esters (CEs), and diesters (DEs) were corrected for peak overlapping and isotopic distribution; an additional ionization efficiency correction was performed for WEs and CEs, which was simplified by the observation that the corresponding ionization efficiency was primarily dependent on the specific lipid class and saturation degree of the lipids while independent of the carbon chain length. A set of WE and CE standards was spiked in meibum samples for ionization efficiency determination and absolute quantitation.
Results.
The absolute amount (μmol/mg) for each of 51 WEs and 31 CEs in meibum samples was determined. The summed masses for 51 WEs and 31 CEs accounted for 48 ± 4% and 40 ± 2%, respectively, of the total meibum lipids. The mass percentages of saturated and unsaturated species were determined to be 75 ± 2% and 25 ± 1% for CEs and 14 ± 1% and 86 ± 1% for WEs. The profiles for two types of DEs were also obtained, which include 42 α,ω Type II DEs, and 21 ω Type I-St DEs.
Conclusions.
Major neutral lipid classes in meibum samples were quantitatively profiled by ESI-MS analysis with NaI additive.
Keywords: mass spectrometry, neutral lipids, profiling, quantitation, sodium iodide
In this study, the molecular species within the major neutral lipid classes in meibum were quantitatively profiled using optimized methods for mass spectrometry detection and data analysis.
Introduction
The lipids in meibomian gland secretions (meibum) have been reported to be mainly composed of neutral lipids including wax esters (WEs),1–8 cholesteryl esters (CEs),1–8 diesters (DEs),1,2,4,6 and triacylglycerols (TGs),1–6,8 along with a small portion of polar lipids including free fatty acids1–6,9 and O-acyl-ω-hydroxyl-fatty acids.6,8,10 These lipids form the outmost layer of tear film and help slow evaporation of aqueous tear film components.11 A change in the quality of meibum is considered a hallmark of meibomian gland dysfunction, and eventually results in dry eye disease.11 Detection of differences in the lipid profiles between normal and diseased eyes may help delineate the etiology of evaporative dry eye disease and ultimately lead to improved diagnosis of dry eye disease and the development of novel therapeutic treatments.
With the development of ionization methods, intact nonvolatile lipids can now be directly analyzed by mass spectrometry (MS) and tandem mass spectrometry (MS/MS) without hydrolysis and/or derivation.12 Among the various types of ionization methods including atmospheric pressure chemical ionization (APCI), electrospray ionization (ESI) is the most suitable for comprehensive analysis of intact lipids since it is the least energetic.13,14 Due to the lack of acidic and basic groups, neutral lipids including TGs, WEs, CEs, and DEs are typically detected by ESI-MS using ammonium salts of acetate or fluoride15–27 as additives and, less frequently, lithium salts of hydroxide or chloride28–31 or sodium salts of acetate or iodide.6,17,19 With the aid of these additives, the detection of TGs,6,15–17,19,21,22,25–29 WEs,6,16,20,30 CEs,6,16,18,21,23,24,27 and DEs6 has been reported.
However, there are still issues related to the use of ammonium and lithium salts as additives for ESI-MS analysis of neutral lipids. When ammonium salts are used, neutral lipids often form multiadducts, including ammonium adducts; sodium adducts (which are often abundant); and proton adducts (due to loss of ammonia from ammonium adducts). The formation of multiadduct lipid species, which has been previously observed in ESI-MS analysis either with direct infusion6,15 or when coupled with high performance liquid chromatography (HPLC) separation16,27 as well as APCI-MS analysis,32 decreases detection sensitivity and complicates the resulting spectra. This multiadduct problem also occurs when lithium salts are utilized as the additive. For instance, ESI-MS analysis of CEs still showed peaks apparently corresponding to sodium adducts in addition to lithium adducts31 after multiple extractions in the presence of 1 mg/mL (i.e., 24 mM) lithium chloride (LiCl) and the addition of 10 mM LiCl to the final solution.
Compared with ammonium and lithium salts, sodium salts appear to be the ideal additive for ESI-MS analysis of these neutral lipids,6 as the sodium ion is the most abundant cation in the natural environment (glassware, solvent, and biological systems) and has a high affinity for neutral species during ESI.33 In contrast to ammonium and lithium salts, the sodium salt additive leads to sodium adducts only. Additionally, since sodium has only one dominant isotope (23Na: 100%), it will not complicate the mass spectra to the level of lithium which has two isotopes (6Li: 7.59%; 7Li: 92.41%). Despite these advantages, there are several concerns about using sodium salts as the ESI-MS additive, including: interference of analyte peaks by sodium salt cluster ion peaks,34 the potential ion-suppression effect,35 and salt deposits blocking the transmission of ions.36 However, when the concentration of sodium ions is low enough, these concerns may be negligible.
The typical methods for analysis of lipids can be divided into two categories: liquid chromatography (LC)–mass spectrometry (MS)–based methods37 and shotgun lipidomics.38 LC-MS–based methods are usually used for analyzing lipids in complex samples, where retention time can be used to confirm the identity of lipids. This method typically requires a long time to perform the experiment and analyze the data, and is therefore less suitable for high-throughput studies. Additionally, the ionization efficiency of lipids may vary with elution order (where mobile phase compositions are different over time), creating challenges for quantitation studies.39 Although LC-MS-based methods have been used to analyze several classes of meibum lipids, the analysis is time-consuming (typically performed for one class at a time) and limited quantitative information was obtained.10,32 In contrast, shotgun lipidomics, which is performed by direct infusion, is faster and may be more suitable when analyzing a large number of samples. In addition, it is easier to compare the relative abundance of different species of the same class or subclass of lipids within samples when using the shotgun lipidomic approach as the solution composition is constant during the analysis.38 However, there are also several issues related to shotgun lipidomics that require optimization. Traditional shotgun lipidomic analyses are based on precursor-ion or neutral-loss scans for one or a few classes of lipids. Therefore, it is difficult to obtain a complete lipid profile of a complex biological sample. In addition, few transitions are utilized for detection, resulting in potentially false discoveries due to interference from species having the same or similar transitions as the species of interest.40 This is particularly the case for spectra obtained on low-resolution mass spectrometers. Furthermore, the potential presence of ion-suppression in traditional shotgun lipidomic techniques is also a concern for quantitation.41
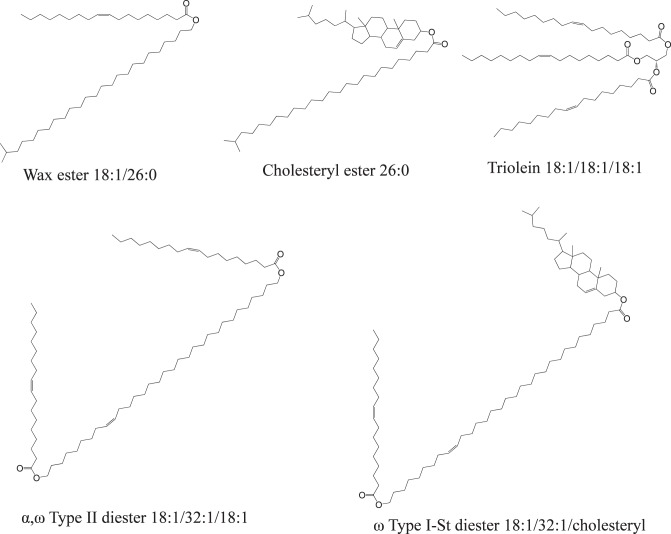
Recently, we reported a modified shotgun lipidomic approach for the analysis of intact lipids in meibomian gland secretions (meibum) using direct infusion electrospray ionization mass spectrometry (ESI-MS) and ammonium acetate (NH4Ac) or sodium iodide (NaI) additive.6 This modified approach is similar to the traditional shotgun analysis as no front-end chromatography separation is needed; however, there is also one important difference: lipids are not detected by precursor-ion or neutral-loss scans but by high resolution MS analysis combined with the identification of major species by MS/MS. The major species are identified by matching their MS/MS spectra pattern with those of lipid standards, which minimizes potential false discoveries; while the minor species are identified by extrapolation from high mass accuracy MS analysis of the same classes of major species (within ±5 ppm and ±12 ppm after internal calibration for meibum lipids detected in positive mode and negative mode, respectively).6 Once the identities of the species are known, they can then be directly detected based on the corresponding high mass accuracy m/z values. Using this modified approach, all the species in meibum samples can be detected simultaneously; as such, meibum was found to be mainly composed of neutral lipids, including WEs, CEs, DEs, and TGs (see Figure 1 for representative structures, based on references4,6,42–44), along with a small portion of free fatty acids and O-acyl-ω-hydroxyl-fatty acids.6 This approach requires minimizing impurity peaks and spectra complexity caused by factors such as formation of multiadducts of lipids.
Figure 1.
Molecular structures of the most abundant meibum lipid species representing five major lipid classes, based on references.4,6,42–44
Herein, we report a method for rapid profiling of neutral lipids in meibum samples by direct infusion ESI-MS. The experimental condition for high sensitivity detection of meibum lipids as well as acquisition of simplified spectra were optimized and combined with an improved data analysis approach to obtain absolute and/or relative quantitation of meibum lipids.
Materials and Methods
Chemicals
Chloroform (HPLC grade, >99.9%, with amylene as the stabilizer, Burdick & Jackson, Muskegon, MI; or Sigma-Aldrich, St. Louis, MO); methanol (HPLC grade, >99.9%, Riedel-de Haen, Seelze, Germany); NH4Ac (>98%), LiCl (>99.99%), and NaI (99.999%) were purchased from Sigma-Aldrich. WE standards, namely, oleyl palmitate (16:0/18:1), oleyl oleate (18:1/18:1), oleyl stearate (18:0/18:1), palmitoyl behenate (22:0/16:1), behenyl palmitate (16:0/22:0), behenyl oleate (18:1/22:0), behenyl stearate (18:0/22:0); and CE standards—namely, cholesteryl linoleate (18:2), cholesteryl oleate (18:1), cholesteryl stearate (18:0), cholesteryl behenate (22:0), cholesteryl nervonate (24:1)—were purchased from Sigma-Aldrich and Nu-Chek Prep, Inc. (Elysian, MN).
Lipid Standard Mixture Preparation
Equimolar lipid standard mixture stock solution was prepared by dissolving 11 WE and CE lipid standards (listed above, except oleyl palmitate) in chloroform/methanol solvent mixture (2:1, vol/vol) with the concentration of each lipid being 1 μM. Working solutions were prepared by diluting the standard stock solution using 2:1 chloroform/methanol solvent mixture or methanol only, with the addition of an appropriate volume of 1 mM additive stock solution (NaI, NH4Ac, or LiCl dissolved in methanol). For the linearity test experiments, the solutions were 1-200 nM equimolar lipid standard mixture dissolved in a mixture of chloroform and methanol, spiked with 10 nM oleyl palmitate; the percentage of chloroform to the total volume ranged from 0.7% to 14%, depending on the concentration of the lipid standard mixture.
Meibum Sample Preparation
The study protocol was approved by The Ohio State University IRB in accordance with the tenets of the Declaration of Helsinki. Meibum samples were collected from both eyes on several occasions from three clinical groups: postmenopausal females with dry eye (n = 5); postmenopausal females without dry eye (normal; n = 4); and nondry eye contact lens wearing subjects (n = 5). The right eye samples were used in this study. A battery of clinical tests, including ocular surface assessment, tear production, and symptom questionnaires, were used to categorize patients according to the study protocol. In short, participants eligible for entry into the study were current contact lens wearers with dry eye symptoms or were postmenopausal women (menses ceased more than 12 months prior to the start of the study). Menopause via hysterectomy was allowable. Additionally, in order to be classified as dry eye, subjects had to have corneal staining ≥ 3 on the NEI scale, while normal women could have a sum score for staining ≤ 1. In addition, at least one of the following abnormal dry eye tests criteria needed to be met for inclusion: OSDI > 12, Schirmer 1 < 10 mm/5 min, tear break-up time < 6 seconds, use of artificial tears regularly, or a previous dry eye diagnosis. Following clinical examination of the five dry eye subjects, three were classified as aqueous deficient (Schirmer 1 < 5 mm/5 min). All of these samples had tear break-up times less than 4 seconds and had mild to moderate meibomian gland dysfunction (quality and expressibility of secretions), indicating that both aqueous deficient and evaporative dry eye components coexisted. In addition to these samples for clinical studies, three normal meibum samples were collected for general study.
Sample preparation was as described previously.6 Briefly, meibum was collected in 0.5-μL × 32-mm glass microcapillary tubes and stored under air without degradation at −80°C for up to several months. All tested samples were essentially homogeneous and inclusion-free. At the time of analysis, the meibum stock solutions were prepared in chloroform-methanol solvent mixture (2:1, vol/vol) at an approximate concentration of 134 μg/mL. This concentration is slightly different from the previously estimated 160 μg/mL due to more accurate calculations of the collected meibum volume and more reliable assumption of meibum density as 0.86 g/mL (based on the reported density of average WEs45). All samples were prepared in glass vials with glass syringes (Hamilton, Reno, NV) or microdispensers (Drummond, Broomall, PA). Contact of sample solutions with any plastics except PTFE (syringe plunger tip) was avoided. Stainless steel tubing was used to connect the syringe to the mass spectrometer (Q-TOF II; Waters Corp., Milford, MA) to minimize adsorption of impurities.
The meibum working solution was prepared by diluting the stock solutions 10- to 100-fold with either a chloroform/methanol mixture (2:1, vol/vol) or methanol only. Assuming the average molecular weight of the lipids to be 800 Da, the lipid concentrations in the working solutions were estimated to be 1.7 to 17 μM. NH4Ac or NaI additive was added to the meibum solutions to a final concentration of 10 μM to 5 mM for NH4Ac or 10 μM to 1 mM for NaI. For the experiment using the lipid standard mixture as the internal standard, meibum solutions of normal samples containing approximately 1.7 μM of total lipids (100-fold dilution of the stock solution) and 10 μM NaI in a mixture of 6.67:93.33 (vol/vol) chloroform/methanol were prepared with or without spiking of the lipid mixture (10 nM for each of the 11 lipid standards).
Mass Spectrometry Analysis
A quadrupole-time-of-flight mass spectrometer (Q-TOF II; Waters Corp.) was used for the MS analysis with no isolation applied. The quadrupole worked as an ion guide and was operated in RF only mode, where the ion transmission was controlled by ramping the mass 1 of m/z 2 to mass 2 of m/z 700 with no dwelling on either of the two masses. According to the manufacturer, “for a given quad mass, there is no transmission below 80% of the mass. The transmission decays much more slowly on the opposite side of the mass scale, with transmission still good at three times the set mass.” This MS profile for ion transmission worked well for sensitive detection and reliable quantitation of meibum lipids.
The samples were directly infused into the mass spectrometer at a flow rate of 10 to 40 μL/min. The spectra were acquired in the m/z range of 100 to 2000. Cone voltage was set at 15 or 50 V unless otherwise indicated. The source temperature was set at 100°C and the desolvation temperature was typically set at 150°C or 250°C unless otherwise indicated. Capillary voltage was 3.2 kV; desolvation gas flow rate was 500 L/h. The instrument was externally calibrated with cluster ions of NaI as recommended by the manufacturer. The spectra were smoothed twice with Savitsky Golay method and centered based on the peak area or peak height. No background subtraction was performed as it affected quantitation reliability. The deposition of involatile NaI ions on the sample cone was minimized due to the Z-spray design of the Q-TOF II instrument (Waters Corp.); the low concentration applied (typically ≤100 μM); and moving the probe away from the sample cone. To minimize impurity peaks, the syringes and ESI capillary were thoroughly rinsed or flushed multiple times, using a clean solvent or solvent mixture (methanol, chloroform, or methanol/chloroform mixture) in a vial or test tube containing no leftover from last washes for each subsequent wash. It was often necessary to rinse the syringe and flush the ESI capillary with alternative solvents. It generally took some time at the beginning when the instrument had been used for other projects; however, after the system had been cleaned, the washing step was much easier and generally took only a couple of minutes. The cleanliness of the setup was verified by direct infusion MS analysis of the solvent mixture before the analysis of the samples. No unwanted adducts of lithium or ammonium were observed after the cleaning.
Peak Intensity Corrections and Quantitative Study
Ideally, the peak intensities of lipids are proportional to their concentrations when their ionization efficiency is the same and can be used for quantitative study. However, for quantitative studies of lipids, correction must be made based on three factors including: (1) overlapping between the third isotopic peak (intensity IM1+2) of the first species (M1) and the monoisotopic peak (intensity IM2) of the second species (M2) of the same class, where the M1 contains one more double bond than M2; (2) the isotopic distribution; and (3) ionization efficiency. Factors 1 and 2 result from the fact that each lipid peak is composed of a series of isotopic peaks, which is mainly due to the abundance of 13C in nature (the abundance of other elements such as 18O is negligible in this study). All three of these corrections have been reported by Han and Gross46 and Yang and Han.47
The first correction performed for peak overlapping was based on the same principle described in previous reports (the second correction in reference).46,47 The actual intensity IM2 of the monoisotopic peak of species 2 was calculated from the apparent intensity IM2' by subtracting the intensity of the third isotopic peak of species 1 (IM1+2), whose intensity was calculated from the intensity of the monoisotopic peak of species 1 (IM1)—that is, IM2 = IM2′ − IM1 × (0.01092× n × [n – 1]/2)—where n is the number of carbon atoms in the molecular lipid species.46,47 For the lipids studied in this work, consideration of the effect of 13C on the isotopic distribution is sufficient, although a full list of the isotopic peaks including the contribution of 18O can be calculated.48 For the molecular lipid species in this study with the total carbon numbers ranging from 32 to 79, only the third (M1+2) and fifth (M1+4) isotopic peaks of species 1 significantly overlapped with the monoisotopic peak (intensity IM2) of species 2, where the intensities of (M1+2) and (M1+4) isotopic peaks (IM1+2 and IM1+4) were 6.99 to 41.6% and 0.91 to 2.97% of its monoisotopic peak (IM1), respectively; thus the correction for overlapping with only the third isotopic peak of species 1 is sufficient for this study. For spectra of meibum samples containing sequential overlapping, the corrections were made one after another. For instance, in addition to overlapping of M1+2 with M2 as mentioned above, M2+2 peak (i.e., the third isotopic peak of species 2), could also overlap with the monoisotopic peak of species 3 (M3). In this case, IM2 was first calculated and then used for the subsequent calculation of IM3 using the formula IM3 = IM3′ − IM2 × (0.01092 × n × [n – 1]/2).
Once the intensities of the monoisotopic peaks were determined, the second correction for isotopic distribution was calculated using the formula Itotal(Mm) = IMm(1 + 0.0109n + 0.01092 × n × n[n – 1]/2 + … ), where IMm is the intensity of the monoisotopic peak of species m, and n is the number of carbon atoms in the molecular lipid species.46,47 For convenience, in this study only the intensities of the first three isotopic peaks for each species were summed as the total ion intensity; the maximum deviation of this approximation is 2%, 3%, 5%, and 7% for WEs, CEs, type II DEs and type I-St DEs, respectively, compared with the actual total ion intensity taking into consideration all of the isotopic peaks including the contribution of 18O.48 The deviation is less than 1.6% when the relative abundance of lipids is compared within each class.
The third correction, the correction for ionization efficiency of different species, has also been previously proposed by Han et al. for quantitation of TGs.46,47 In that report, the correction was based on the sensitivity correction factors (or response factors), which was determined experimentally from a series of external lipid standards and fitted to a curve for the calculation of the factors for any TGs. In contrast, our method for ionization efficiency correction was based on our observation that under our experimental conditions, the ionization efficiency of WEs and CEs was primarily dependent on the specific lipid class (WEs or CEs) and saturation degree of the lipids while independent of the carbon chain length. As a result, the ionization efficiency correction for all the WEs and CEs can be based on only a few representative species that contain different numbers of double bonds regardless of carbon chain lengths. The ionization efficiency varied between different classes of lipids even when using similar experimental conditions; however, the relative ionization efficiency was essentially constant between lipids of different degrees of saturation within the same class. Compared with saturated species, the increased ionization efficiency of unsaturated lipids was approximately proportional to the number of double bonds. This information was utilized to extrapolate the ionization efficiency of several meibum lipids containing more double bonds than those of the internal standards. Importantly, to achieve a more reliable correction for ionization efficiency, in our study the response factors were determined from a set of internal standards (six WEs and five CEs) of appropriate equimolar concentration spiked in the samples of interest. Due to the lack of commercial standards for DEs, this ionization efficiency correction was applied only to WEs and CEs.
With the three corrections discussed above, lipids can be quantified based on one internal standard for each class47 and even for several classes.49 In our study, the calibration was based on the average normalized response of the 11 internal lipid standards, which is expected to be more accurate than external calibration or the use of one internal standard.
Absolute and Relative Quantitation of Lipid Species in Meibum Samples
For the absolute quantitative studies, two normal meibum samples with relatively large and equivalent amounts (i.e., 3.5 mm long, collected in the 0.5 μL capillary), were utilized. Each meibum sample solution was prepared as mentioned above, split into two samples, and one of them was spiked with an equimolar amount of lipid standards. The final solution composition was a chloroform/methanol mixture (0.67:99.33, vol/vol) containing 1.7 μM (1.8 μM with the internal standards) total lipids and 10 μM NaI. The peak intensities of the spectra were first corrected for isotopic peak overlapping and isotopic distribution as described above. For the peaks of the spiked lipid standards that overlapped with the endogenous meibum lipids, their peak intensities were determined by subtracting the intensities of the corresponding peaks in the spectra of nonspiked samples and in turn were utilized to determine the factors for the third correction—the ionization efficiency.
The intensities of the endogenous lipid peaks and spiked 10 nM lipid standard peaks were normalized by the three corrections; the concentrations of these endogenous lipids were determined from the ratio of their normalized peak intensities to the average for the lipid standards. For endogenous meibum lipid peaks that overlapped with the lipid standards, concentrations were determined by one of the following methods: subtracting the concentration of the standards or using the spectra of the nonspiked sample based on the observation that the normalized intensities of the lipid peaks were proportional for spiked and nonspiked samples. For instance, the peaks of endogenous WE 44:0 (C44H88O2Na1, m/z 671.6682) and the spiked CE 18:2 (C45H76O2Na1, 671.5743) significantly overlapped and they were processed by the mass spectrometry software (MassLynx; Waters Corp., Milford, MA) as one single peak; the corrections for peak overlapping and isotopic distribution could be processed as described above. However, as the ionization efficiencies for the two species were different, the concentrations of WE 44:0 could not be determined from the peak intensities in the spectra of spiked meibum sample by subtracting the concentration of the spiked CE 18:2; instead, their concentrations were determined using the peak intensities in the spectra of nonspiked meibum samples. Generally, the peak intensities based on peak area were more accurate for the overlapping correction, particularly for the peaks with greater differences in their m/z values such as WE 44:0 overlapped with CE 18:2. The absolute amount of WEs and CEs were determined after consideration of meibum sample mass and the sample dilution factor. The compositions of WEs and CEs were calculated as mole% (or mass%, after consideration of the species' molecular weights). The absolute quantitation of DEs was not attempted due to the lack of commercially available lipid standards.
For clinical meibum samples, the collections were 0.5 to 1.5 mm long (in the capillary tubes). The final solution composition was a chloroform/methanol mixture (1.3:98.7, vol/vol) containing ∼3.4 μM total lipids and 5 μg/mL NaI (33 μM). Relative quantitative studies were performed. Lipid standards were not spiked in the meibum samples, which simplified data analysis because the peaks of meibum lipids normally overlap with those of the lipid standards. Additionally, when samples of 0.5 to 1.5 mm length were collected, potential variation in the length measurement was increased, which would likely decrease the accuracy of absolute quantitation; therefore, absolute quantitation was not attempted. The normalized intensities (after the two or three corrections) of each molecular species within the same class were summed and the percentage of each species to the sum total was calculated as intensity% (with the first two corrections) or mole% (or mass%, with all three corrections). Peaks with low signal-to-noise ratios were not included in the profiles, which did not affect the overall distribution of the major molecular species within the same class of lipids. The distribution of molecular species within the TG class was not compiled as almost all the molecular species of TGs except TG 54:3 were of low intensities.
Results
MS Analysis of Lipid Standards With NH4Ac, LiCl or NaI as Additive
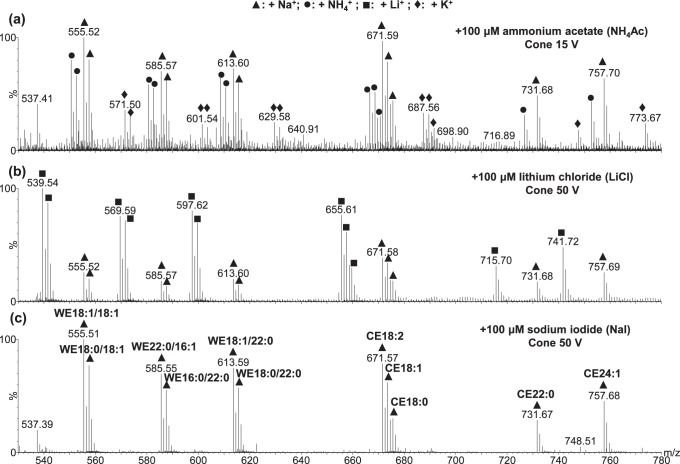
The MS spectra of WE and CE standards acquired with different additives were compared (Fig. 2). NaI showed only one type of adduct and the spectrum was strikingly “clean.” In contrast, NH4Ac showed three types of adducts: ammoniated, sodiated, and potassiated adducts. Formation of ammoniated ions was optimum when the cone voltage was 15V; increasing the cone voltage to 50V resulted in predominantly sodiated peaks. When LiCl was used as the additive, there were two types of adducts (i.e., lithiated and sodiated peaks). Interestingly, potassiated adducts were only observed when NH4Ac was used as the additive. The potassium may be derived from impurities in NH4Ac, extracted from the glassware by NH4Ac or introduced by the standards. The absence of potassiated species peaks in the spectra of meibum samples suggested that potassium was most likely introduced by the lipid standards. The signal-to-noise ratios for the spectra with additives of LiCl and NH4Ac were approximately 3/4 and less than 1/8, respectively, of the ratio observed when using NaI as the additive. As mentioned previously, the high abundance of the different isotopes of lithium further complicates the resulting MS spectra when LiCl is used as the additive. This may interfere with the analysis of complex biological samples such as meibum. Therefore, only NH4Ac and NaI additives were compared in our study.
Figure 2.
Electrospray ionization mass spectra of 11 equimolar WE and CE standards (100 nM each, 1.1 μM total) using 100 μM of the following additives: (a) ammonium acetate, (b) lithium chloride, and (c) sodium iodide. The sample solution was in a mixture of chloroform and methanol (1:14, vol/vol). The flow rate was 40 μL/min, the desolvation temperature was 250°C, and the acquisition time was 1 minute. For clarity, only the peaks in (c) were labeled.
Two primary observations were made when conducting these experiments. First, the number of double bonds in a lipid molecule positively correlates with its ionization efficiency. This has also been observed for TGs and phospholipids at a relatively high concentration.50,51 Secondly, the intensity of lipid peaks in the MS spectra was essentially irrelevant to the carbon chain length within the same class of lipids. Under similar experimental conditions, changes in relative peak intensity have been observed for different classes of lipids when spectra were acquired on different days or when total concentrations or solvent composition differed. However, the ionization efficiency for lipid species within the same class was essentially independent of carbon chain length yet dependent on the saturation level of the chain; the relative ionization efficiency between lipids of different saturation levels within the same class of lipids was essentially constant.
MS Analysis of Meibum Lipids With NH4Ac as Additive
Meibum samples containing lipids of ∼1.3 μg/mL (∼1.7 μM) or ∼13 μg/mL (∼17 μM) were analyzed by direct infusion ESI-MS with 10 μM to 5 mM NH4Ac additive. The observed lipid peaks were of one or more of the three types of cation adducts (i.e., sodiated, ammoniated, and protonated). The relative intensities of these different types of adducts depended on the cone voltage, the concentration of meibum lipids, and the concentration of NH4Ac.
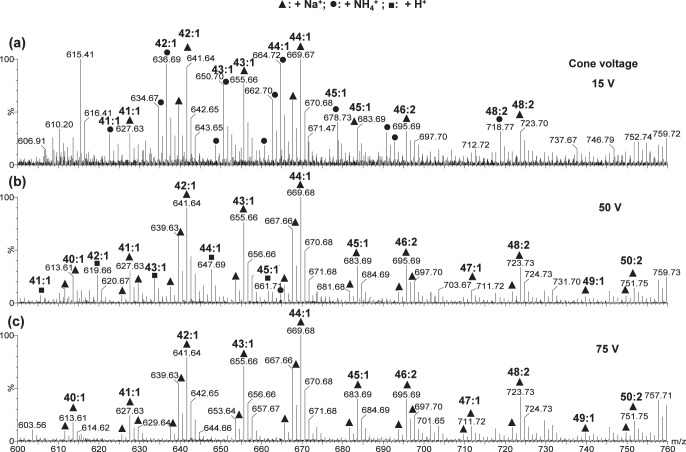
For meibum samples containing lipids of ∼13 μg/mL (∼17 μM), at cone voltage 15V, peaks corresponding to both sodium and ammonium adducts were observed for WEs, CEs, or DEs (Figs. 3a, 4). When 10 μM NH4Ac additive was used, lipid peaks were predominantly sodiated (data not shown). The relative intensities of sodiated and ammoniated peaks varied for different samples, possibly depending on the level of total sodium ions in the meibum samples as well as the relative concentrations between sodium ions, additive cations, and lipids. The use of higher concentrations of NH4Ac slightly increased the relative intensity of ammoniated peaks while decreasing the signal-to-noise ratios of the lipid peaks due to the ion suppression effect.35 The concentration of 100 μM appeared to be optimum for detection sensitivity (Fig. 4). When the cone voltage was increased to 50 V for the analysis of the same meibum sample solutions, the lipid peaks were mainly sodiated regardless of the concentration of NH4Ac additive (Fig. 3b). When the cone voltage was further increased to 75 V, sodiation was often the only type of cationization observed for the lipid peaks (Fig. 3c). No potassiated species were detected, suggesting that the potassiated species for the lipid standards (Fig. 2a) likely resulted from potassium impurity in the standards. The signal-to-noise ratios for the lipid peaks in the spectra acquired at cone voltage of 50 or 75 V were generally higher at a lower concentration of NH4Ac additive, resulting from a decreased ion-suppression effect and an increased sodiation due to the high relative concentration of sodium ions.
Figure 3.
Electrospray ionization mass spectra of a meibum sample using 1 mM ammonium acetate additive at different cone voltages: (a) 15 V, (b) 50 V, and (c) 75 V. The sample contained ∼13 μg/mL (∼17 μM) total lipids and was dissolved in a mixture of chloroform and methanol (2:1, vol/vol). The flow rate was 40 μL/min, the desolvation temperature was 150°C, and the acquisition time was 1 minute. The range m/z 600 to 760 of the spectra is shown and only the WE peaks were labeled. Some of the unlabeled peaks corresponded to CEs. Since these peaks were previously identified by tandem mass spectrometry and high mass accuracy (mass spectrometry analysis with internal calibration), no internal calibrations for mass accuracy were performed in this work.
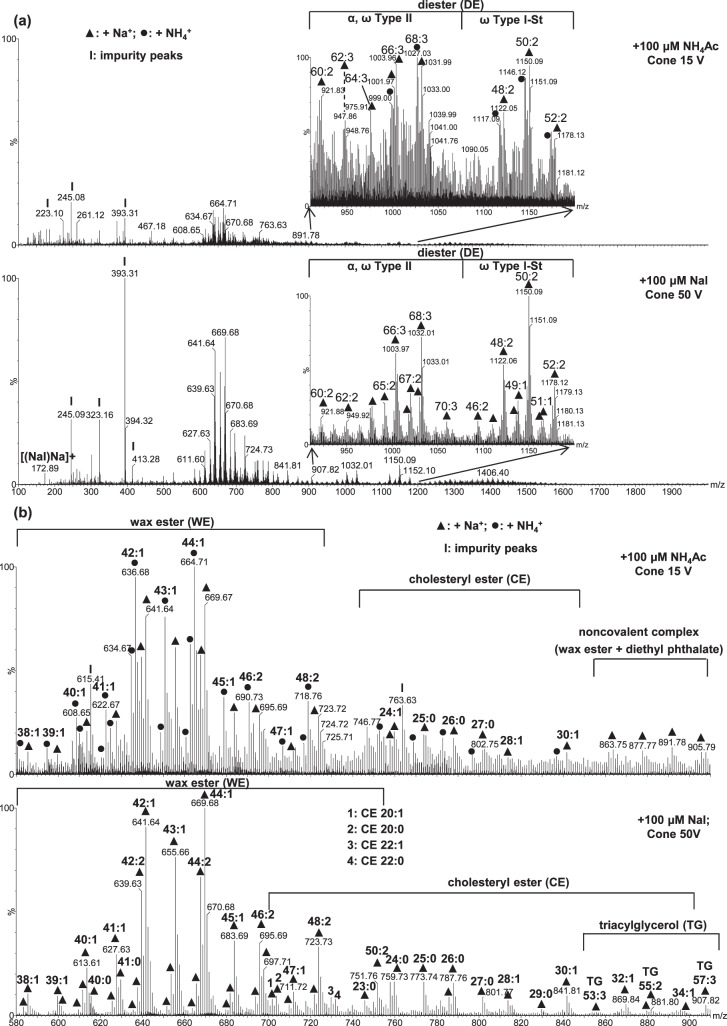
Figure 4.
Electrospray ionization mass spectra of a meibum sample (containing ∼17 μM total lipids) acquired using 100 μM NH4Ac as the additive at cone voltage 15 V, and 100 μM NaI as the additive at cone voltage 50 V: (a) The full range m/z 100 to 2000 of the spectra (almost all the peaks below m/z 495 are impurity peaks) with the insets showing the range m/z 910 to 1200 of the spectra. (b) The range m/z 600 to 910 of the spectra, which mainly contains WEs and CEs. For easier visual comparison of the intensity changes when using the two additives, the intensities of the peaks of two panels in (a) were normalized to the same most intense peak. The experimental conditions utilized were the same as in Figure 3.
For meibum samples containing ∼1.3 μg/mL (∼1.7 μM) of lipids, similar results were obtained. The lipid peaks observed were in mixed adduct forms when using a cone voltage of 15V and were predominantly in sodiated form when a cone voltage of 50 or 75 V was used. Detection sensitivity was higher when the additive concentration was 10 μM and the cone voltage was 50 or 75 V. Increasing cone voltage increased detection sensitivity; however, it also enhanced the dissociation of some lipids including CEs or ω Type I-St DEs, as the relative intensities of the peak around m/z 369.35 were also increased.6 However, the dissociation of sodiated or ammoniated WEs due to increased cone voltage was negligible. Thus, the summed intensity for the different adduct peaks of the most abundant WE could be used to normalize the intensity for the peak m/z 369.35 to estimate the dissociation degree of cholesteryl-containing lipids. The most abundant WE observed here and in our previous work6 was 44:1, which was present in one or more of the sodiated (theoretical m/z 669.65); ammoniated (theoretical m/z 664.70); and protonated (theoretical m/z 647.67) forms. The normalized intensity for the peak m/z 369.35 was calculated using the ratio of the intensity of this peak to the summed intensity of the different adducts of WE 44:1. This normalized intensity was generally higher when a high concentration of NH4Ac additive was combined with a high cone voltage (Table 1), although it varied depending on meibum samples and the instrument conditions.
Table 1.
Dependence of Normalized Intensity for the Peak m/z 369.35 on the Cone Voltage and Additives
|
Cone Voltage, V |
Im/z
369.35/IWE 44:1 |
|||
|
NH4Ac |
NaI |
|||
|
1 mM, % |
100 μM, % |
1 mM, % |
100 μM, % |
|
| 15 | 4.3 | 4.2 | 2.2 | 1.0 |
| 50 | 44.2 | 23.0 | 0.4 | 0.8 |
| 75 | 61.8 | 27.0 | 1.2 | 1.3 |
Mass spectra were acquired at the desolvation temperature of 150°C for meibum sample containing ∼13 μg/mL (∼17 μM). IWE44:1 is the sum of the intensities of sodiated, ammoniated, and protonated peaks of WE 44:1. The normalized intensities for spectra with 10 μM additives are not listed due to the low signal-to-noise ratio, likely resulting from inadequate amount of additive to promote the ionization of lipids in the samples.
MS Analysis of Meibum Lipids With NaI as Additive
Since sodiation seems to be inevitable with the amount of sodium ions in the environment that cannot be controlled, we developed an alternative approach in which an appropriate concentration of sodium ions was added to the samples so that only one type of adduct was formed. Our initial trial with the addition of 1 mM NaI to meibum solutions resulted in high intensity cluster ions, which significantly suppressed the intensity of the lipids, especially at a low cone voltage (Supplementary Fig. S1). NaI is well known to be able to form cluster ions34; the series of NaI cluster ions has been recommended by the manufacturer of the Q-TOF II mass spectrometer (Waters Corp.) to calibrate the instruments with a concentration of 2 μg/μL NaI (∼13 mM) dissolved in a solvent mixture of isopropanol: water (50:50, vol/vol). However, reducing concentrations of NaI added to meibum solutions minimized the interference from cluster ions. For the analysis of meibum samples containing ∼13 μg/mL lipids, 100 μM NaI was optimum as the cluster ions that interfered with the lipid peaks disappeared when the cone voltage was 50 V or 75 V. The cone voltage of 50 V appeared to be optimum for simultaneous detection of all lipid peaks, which increased the peak intensities to approximately 3.5 times of those observed when using NH4Ac as the additive (Fig. 4), while a cone voltage of 75 V appeared to be more sensitive for detecting lipid species in high m/z regions (data not shown). Decreasing the concentration of NaI to 10 μM resulted in lipid peaks with lower intensities (data not shown), possibly because the concentration of NaI was not high enough to form adducts with all the lipid species. In contrast, in meibum samples containing lipids of a lower total concentration (∼1.3 μg/mL or ∼1.7 μM), 10 μM NaI additive with a cone voltage of 50 V was optimum for detection sensitivity.
When NaI was used as the additive, no significant dissociation of CEs and/or ω Type I-St DEs was observed. The normalized intensity for the peak m/z 369.35 was never more than 1.3% even when the cone voltage was high (75 V). The low intensity peak m/z 369.35 was most likely due to free cholesterol. In contrast, when using NH4Ac as the additive under similar conditions, the normalized intensity for the same peak was as high as 62% (Table 1). The insignificant dissociation of lipids in the presence of NaI additive suggests that sodiated lipids are highly stable.
Desolvation Temperature Effect on MS Analysis of Meibum
The desolvation temperature for the experiments discussed above was set at 150°C. Other desolvation temperatures were also used to investigate their effect on meibum spectra.
In the analysis of meibum using NH4Ac as the additive, high desolvation temperature promotes the conversion of ammoniated peaks to sodiated peaks, yet enhances partial dissociation of ammoniated peaks. For example, in experiments using a cone voltage of 15 V and NH4Ac as the additive, the normalized intensity for the peak m/z 369.35 increased with temperature. Desolvation temperatures of 100°C, 150°C, and 200°C yielded normalized intensities of 2.2%, 7.0%, and 41.6%, respectively, demonstrating increased lipid dissociation with temperature.
In contrast, when NaI was used as the additive, no significant dissociation of CE and ω Type I-St DEs were observed in spectra acquired with a cone voltage of 50 V and varying the desolvation temperature from 100°C to 300°C. The normalized intensity for the peak m/z 369.35 was observed to be less than 3.0%. Under these varying desolvation conditions, the signal-to-noise ratio appeared to be similar for most of the lipids except TGs, which appeared to have a higher signal-to-noise ratio when a desolvation temperature of 200 to 250°C was used. Increasing the temperature further to 300°C appeared to cause partial dissociation of lipid species, including CEs with longer chains (especially unsaturated chains) and TGs, resulting in decreased peak intensities for these species. The much higher stability of sodiated lipid ions suggested that NaI was the best additive to use in experiments performed for quantitative analysis.
Flow Rate Effect on MS Analysis of Meibum
The flow rate did not significantly affect the relative intensities of sodiated or ammoniated peaks. However, the flow rate influenced the spray stability and detection sensitivity of the mass spectrometer; a higher temperature typically requires a higher flow rate to obtain a stable spray, while the detection sensitivity typically first increases with temperature, then levels off and eventually decreases.
Application of NaI Additive to Quantitative Analysis of Lipids in Meibum
Following optimizing the conditions for lipid analysis by ESI-MS, we attempted to quantitate lipids in meibum samples. As mentioned in the Materials and Methods section, when performing quantitative analysis, corrections for isotope overlapping, isotope distribution, and ionization efficiency were made. The first two corrections were relatively simple, while the third one was more complicated. Ideally, to most accurately correct the ionization efficiency, a series of standards corresponding to all the endogenous lipids should be used. However, for biological samples like meibum that contain hundreds of lipids (some of which are of unknown identity), this is not feasible. Therefore, a method combining representative lipid standards with curve fitting is typically applied for lipid quantitation. However, if the ionization efficiency is affected by many factors, such as the carbon chain length, there will be uncertainty in how well the curve fits the actual ionization efficiency of the predicted species.
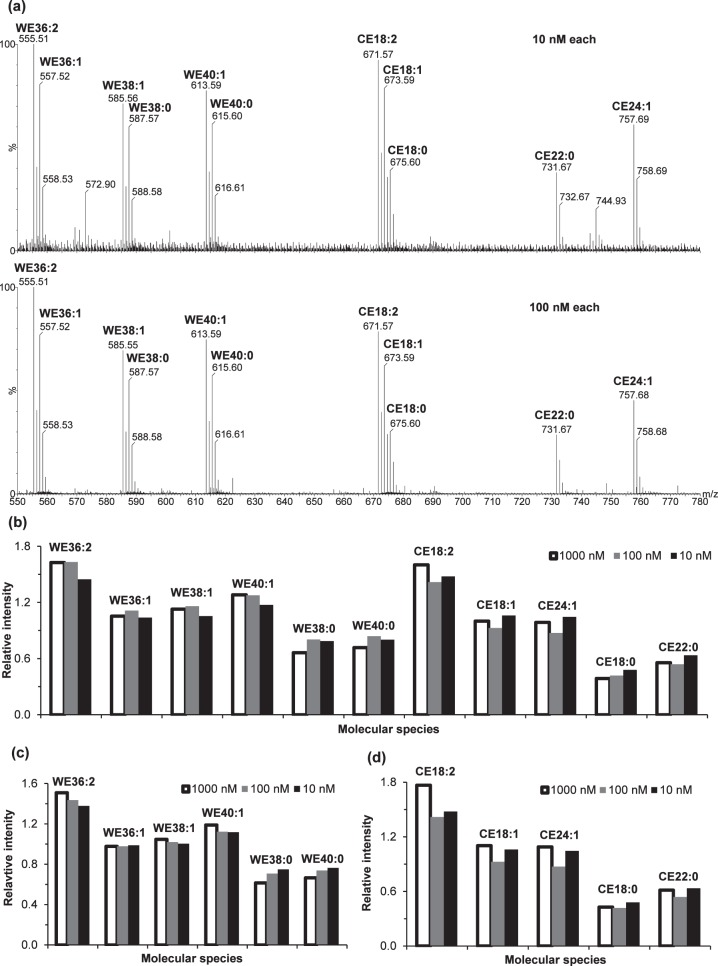
Fortunately, our above analysis of the lipid standards suggested that the ionization efficiency of each species in the solution mixture of WEs and CEs was primarily dependent on the class and saturation degree of the lipids while independent of the carbon chain length (Fig. 2). To confirm this observation, we performed a more detailed ESI analysis using 10-, 100-, and 1000-fold dilution of the 1 μM equimolar lipid standards (Fig. 5). The absolute peak intensities for these lipids versus concentration were not linear over the concentration range of 1 to 1000 nM (data not shown); however, the relative intensities of the esters (versus the average intensity) were essentially constant from 10 to 1000 nM, especially for WEs (Fig. 5c). Some variation was likely due to the slight changes in the sample matrix and experimental condition. When the 1 nM equimolar solution was used, the signal-to-noise ratio of these neutral lipid peaks were not high enough for reliable quantitation; however, at such a low concentration (far below the maximum recommended concentration [i.e., 10 μM, for lipid quantitation38]), lipid-lipid interactions—the major cause for the deviation from linear response—were negligible. Therefore, it was reasonable to assume that the relative peak intensity of the lipids were linear in the range of 1 to 1000 nM. The responses of two lipids in a matrix can be expressed as I1 = k1 × C and I2 = k2 × C, where k1 and k2 are response factors. The absolute intensities of these equimolar lipids were not proportional to their concentration, suggesting that these response factors (k1 and k2) may be matrix-dependent. However, their constant relative intensities indicated the relative response factors (i.e., k1/k2) were constant.
Figure 5.
Electrospray ionization mass spectrometry analysis of 11 WE and CE standards at 10, 100, and 1000 nM equimolar concentrations. (a) The mass spectra for 10 and 100 nM equimolar solution (the spectrum for 1000 or 1 μM equimolar concentration is the same as Fig. 2c). The column graphs of relative peak intensities versus the average intensity for (b) all 11 lipid standards: (c) six WE standards and (d) five CE standards. To maximize detection sensitivity and minimize impurity peaks, slight changes were made to the experimental conditions (solution composition and desolvation temperature) for these different solutions: 10 nM equimolar standards were prepared in 1:140 (vol/vol) chloroform/methanol containing 10 μM sodium iodide; 100 nM equimolar standards were prepared in 1:14 (vol/vol) chloroform/methanol containing 100 μM sodium iodide; and 1000 nM equimolar standards were prepared in 2:1 (vol/vol) chloroform/methanol containing 50 μM sodium iodide. The 10 nM and 100 nM equimolar solutions were analyzed at a desolvation temperature of 250°C, while the 1000 nM equimolar solution were analyzed at a desolvation temperature of 150°C.
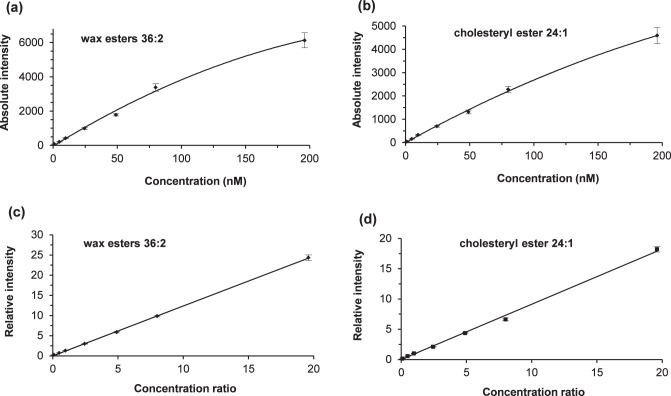
To further characterize the response-concentration relationship, we spiked in a series of equimolar standard mixture solutions with a constant concentration (10 nM) of oleyl palmitate (Fig. 6). The absolute response of equimolar lipid standards was not directly correlated with the concentrations at high concentrations (Figs. 6a, 6b). However, the relationship between relative responses (versus responses for oleyl palmitate) and lipid standard concentrations or the concentration ratios were linear within the range examined (Figs. 6c, 6d). Our observation that the linearity (nonlinear versus linear) of the relationship depending on whether absolute or relative responses are considered is similar to a report on phospholipids.51 Linear relative intensity responses have also been reported for TGs in the range of 1 to 1000 nM,46 phospholipids in the range of 100 nM to 10 μM,51 and CEs in the range of 0.1 to 10 μM.31
Figure 6.
Concentration dependency of electrospray ionization mass spectrometry responses of a representative WE, oleyl oleate (WE 36:2, m/z 555.51), and a representative CE, cholesteryl nervonate (CE 24:1, m/z 757.68). The plots of absolute intensity versus concentration were nonlinear for (a) oleyl oleate and (b) cholesteryl nervonate. However, the plots of relative intensity versus concentration ratio (relative to 10 nM concentration of oleyl palmitate) were linear for (c) oleyl oleate and (d) cholesteryl nervonate. The error bars indicated one standard deviation from 4 to 5 measurements. The equations for the best-fit lines were: (c) y = 1.238x, with a correlation coefficient of 0.9999; and (d) y = 0.913x with a correlation coefficient of 0.9988. The linear relationship between relative intensity and concentration ratio also applies to the other nine lipid standards. The flow rate was 40 μL/min, the desolvation temperature was 250°C, and the concentration of sodium iodide was 100 μM. See text for other experimental details.
Theoretically and practically, one internal standard for each class works for the quantitation based on the intensity ratios and ionization efficiency correction.49,50,52–54 However, to obtain more reliable information, in this study, a set of representative lipid standards was spiked into the meibum samples to achieve absolute quantitation of meibum lipids. The total concentration of the standards was 0.11 μM (11 standards, 10 nM each), a concentration that allows quantitative detection without significantly changing the overall meibum lipid profile.
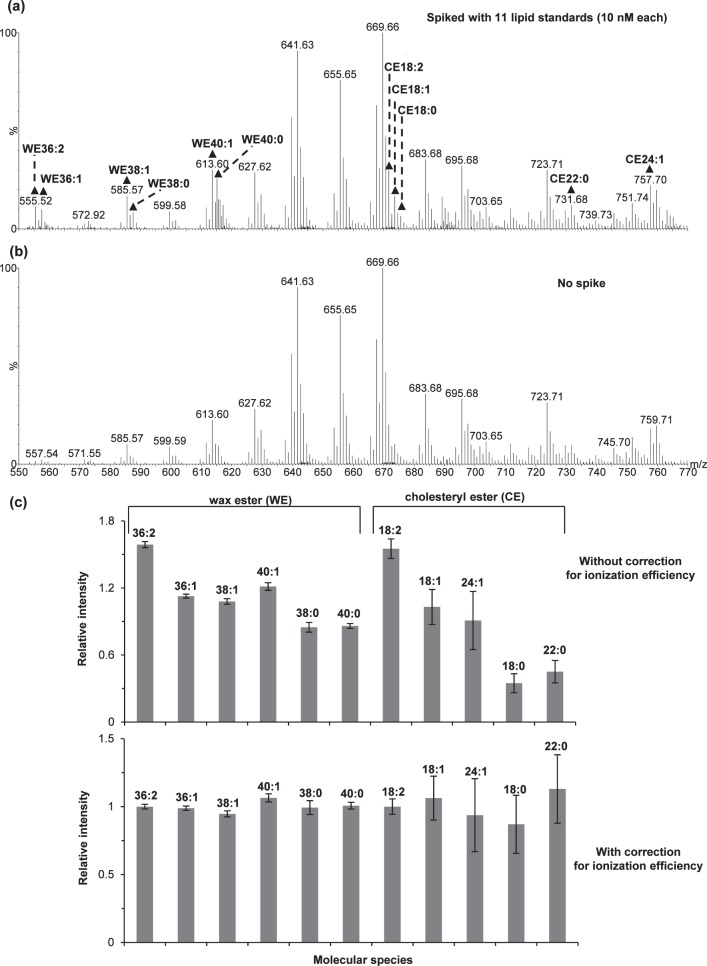
The analysis of the lipid standards spiked in meibum samples (Fig. 7) again supported our previous observation that the ionization efficiency of each species was primarily dependent on the class and saturation degree of the lipids while independent of the carbon chain length. The correction factors were determined as 0.85, 1.14, 1.59, 1.83, and 2.16 for WEs with 0, 1, 2, 3, and 4 double bonds, respectively; and 0.40, 0.97, 1.55, and 2.12 for CEs with 0, 1, 2, and 3 double bonds, respectively. After performing all the corrections including the correction for ionization efficiency, the relative intensity was essentially the same (Fig. 7c). Therefore, the average normalized peak intensity was used for internal calibration.
Figure 7.
Relative responses of a set of internal standards spiked in meibum samples: representative electrospray ionization mass spectra for one meibum sample (∼1.7 μM total lipids) in the (a) presence and (b) absence of spiking, and (c) comparison of the relative peak intensities (versus the average of the 11 lipids) for the lipid standards without and with the correction for ionization efficiency. The intensities in (c) had been corrected for overlapping with isotopic peaks of different species, isotopic distribution, and overlapping with the endogenous meibum lipid peaks. Shown is the average for two meibum samples with the error bars indicating one standard deviation. The experiment was performed at a flow rate of 40 μL/min, a desolvation temperature of 250°C, an additive of 10 μM sodium iodide, and an acquisition time of less than 6 minutes. See text for other experimental details.
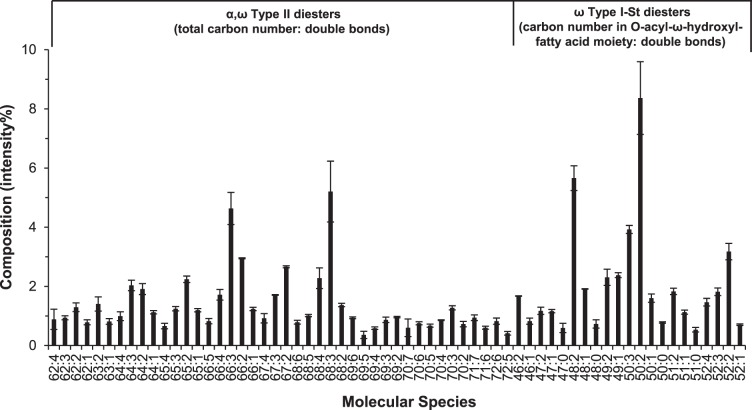
Using these internal standards, quantitative information for the major neutral lipids in meibum was obtained. A total of 51 intact WEs and 31 intact CEs were individually quantified (Tables 2, 3, Fig. 8). The measured concentrations of these WEs and CEs were in the ranges of 1 to 140 nM and 2 to 90 nM, respectively, which were within the linear range shown in Figure 6. The quantitative results were summarized and compared with literature reports2,4,8,32,55,56 in Table 4. The sum of the molarity of WEs and CEs in the meibum working solution was approximately 1.6 μM. Assuming the summed molar percentage for these two types of esters to the total lipids is the same as the summed mass percentage (i.e., 88 ± 4%), then the total lipids in the solution were 1.8 ± 0.1 μM, which agrees with our initial estimate of 1.7 μM for total lipids in the solution. Due to the lack of commercially available DE standards, we did not determine the ionization efficiency of different types of DEs and thus absolute quantitative information for the DEs in meibum was not obtained directly. However, the factors of overlapping and isotopic distribution were corrected (Fig. 9) and these corrected peak intensities provided information on their relative concentration in meibum samples. The correction for ionization efficiency could also be estimated (see details in the Discussion section).
Table 2.
Measured WE Concentrations in Meibum Solutions Containing Approximately 1.3 μg/mL Total Lipids
|
Species |
m/z |
Concentration, nM |
Species |
m/z |
Concentration, nM |
| 32:2 | 499.46 | 3.7 ± 1.6 | 43:3 | 651.62 | 3.8 ± 0.5 |
| 32:3 | 525.47 | 1.3 ± 0.2 | 43:2 | 653.63 | 18 ± 2 |
| 34:2 | 527.49 | 5 ± 2 | 43:1 | 655.65 | 100 ± 11 |
| 36:2 | 555.52 | 1.7 ± 0.1 | 43:0 | 657.66 | 35.8 ± 1.0 |
| 36:1 | 557.54 | 3.4 ± 0.1 | 44:4 | 663.62 | 2.8 ± 0.5 |
| 37:1 | 571.55 | 4.3 ± 0.4 | 44:3 | 665.63 | 11.9 ± 1.9 |
| 37:0 | 573.56 | 4.6 ± 0.4 | 44:2 | 667.65 | 59 ± 9 |
| 38:2 | 583.55 | 3.8 ± 0.5 | 44:1 | 669.66 | 134 ± 3 |
| 38:1 | 585.57 | 13.1 ± 1.3 | 44:0 | 671.64 | 21.2 ± 0.2 |
| 38:0 | 587.58 | 4.7 ± 0.1 | 45:3 | 679.64 | 3.2 ± 0.3 |
| 39:2 | 597.57 | 2.2 ± 0.3 | 45:2 | 681.66 | 10.4 ± 0.4 |
| 39:1 | 599.58 | 12 ± 2 | 45:1 | 683.68 | 50 ± 3 |
| 39:0 | 601.6 | 8.1 ± 0.9 | 46:4 | 691.64 | 2.6 ± 0.7 |
| 40:3 | 609.57 | 2.4 ± 0.3 | 46:3 | 693.66 | 8.0 ± 1.6 |
| 40:2 | 611.59 | 10.6 ± 1.6 | 46:2 | 695.68 | 31 ± 4 |
| 40:1 | 613.6 | 31 ± 2 | 46:1 | 697.69 | 25.7 ± 0.9 |
| 40:0 | 615.61 | 15.1 ± 0.2 | 47:2 | 709.69 | 6.0 ± 0.1 |
| 41:3 | 623.58 | 1.5 ± 0.3 | 47:1 | 711.71 | 15.9 ± 1.2 |
| 41:2 | 625.6 | 6.3 ± 0.9 | 48:3 | 721.7 | 9.3 ± 1.4 |
| 41:1 | 627.62 | 38 ± 5 | 48:2 | 723.71 | 30 ± 4 |
| 41:0 | 629.63 | 29.1 ± 1.0 | 48:1 | 725.72 | 10.7 ± 0.6 |
| 42:4 | 635.58 | 2.0 ± 0.5 | 49:2 | 737.72 | 4.3 ± 0.4 |
| 42:3 | 637.6 | 10 ± 2 | 50:4 | 747.7 | 3.1 ± 0.3 |
| 42:2 | 639.62 | 51 ± 10 | 50:3 | 749.72 | 6.6 ± 1.0 |
| 42:1 | 641.63 | 115 ± 10 | 50:2 | 751.74 | 14 ± 3 |
| 42:0 | 643.64 | 34.3 ± 1.1 | Total | 1030 ± 23 | |
Table 3.
Measured CE Concentrations in Meibum Solutions Containing Approximately 1.3 μg/mL Total Lipids
|
Species |
m/z |
Concentration, nM |
Species |
m/z |
Concentration, nM |
| 16:0 | 647.58 | 12 ± 2 | 25:0 | 773.73 | 88 ± 5 |
| 17:0 | 661.59 | 18 ± 5 | 26:2 | 783.72 | 4.6 ± 0.2 |
| 18:1 | 673.61 | 14 ± 4 | 26:1 | 785.73 | 17.9 ± 1.5 |
| 18:0 | 675.61 | 15 ± 2 | 26:0 | 787.74 | 85 ± 9 |
| 19:0 | 689.63 | 19 ± 5 | 27:0 | 801.76 | 53 ± 3 |
| 20:1 | 701.64 | 16 ± 4 | 28:2 | 811.74 | 3.8 ± 0.1 |
| 20:0 | 703.65 | 44 ± 5 | 28:1 | 813.76 | 16.1 ± 1.1 |
| 21:1 | 715.66 | 5 ± 1 | 28:0 | 815.77 | 27 ± 4 |
| 21:0 | 717.66 | 35 ± 7 | 29:0 | 829.78 | 23 ± 3 |
| 22:1 | 729.67 | 17 ± 3 | 30:3 | 837.76 | 2.1 ± 0.3 |
| 22:0 | 731.68 | 41 ± 2 | 30:2 | 839.77 | 3.8 ± 0.1 |
| 23:1 | 743.68 | 4.2 ± 0.8 | 30:1 | 841.79 | 21.3 ± 0.6 |
| 23:0 | 745.70 | 35 ± 5 | 32:3 | 865.78 | 2.8 ± 0.1 |
| 24:1 | 757.70 | 31 ± 3 | 32:2 | 867.80 | 3.8 ± 0.1 |
| 24:0 | 759.71 | 77 ± 2 | 32:1 | 869.82 | 12.4 ± 0.2 |
| 25:1 | 771.71 | 5.5 ± 0.1 | Total | 754 ± 19 | |
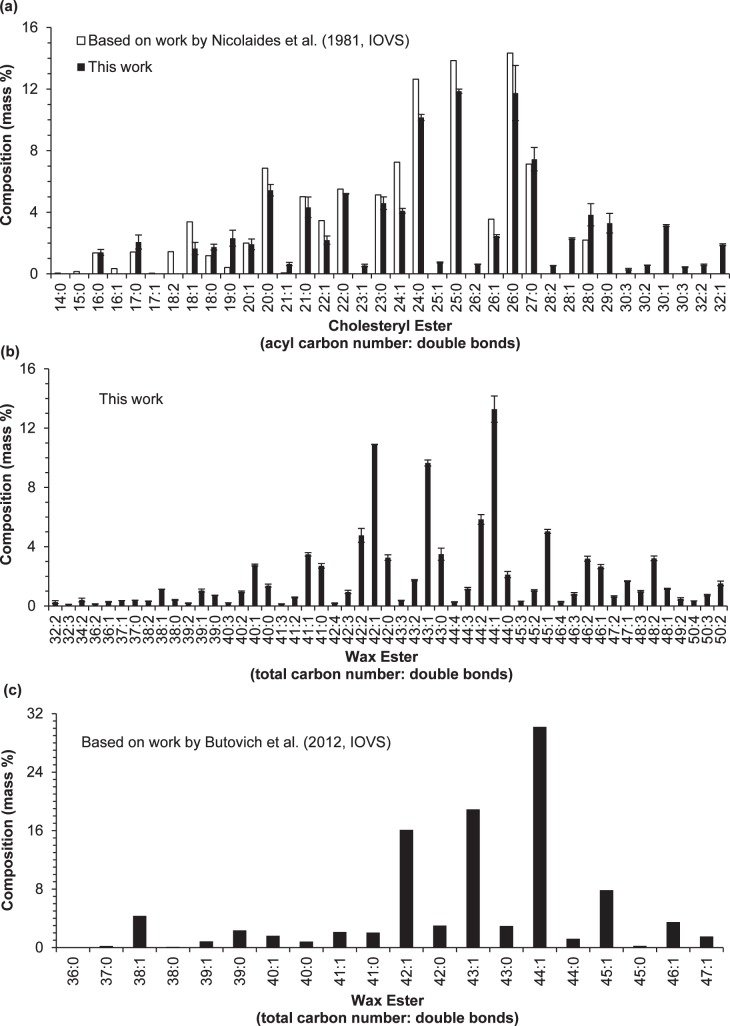
Figure 8.
Comparison of the composition (mass%) of CEs and WEs determined in this work with two previous reports. (a) Side-by-side comparison of the composition of CEs determined in this work with that determined by Nicolaides et al.4; (b) the composition of WEs determined in this work; and (c) the composition of WEs determined by Butovich et al.56 The bar graphs for the two reports4,56 were generated from the data provided in the two references (no standard deviation information was reported). The compositions determined in this work were the average of two samples; the error bars indicate one standard deviation. The experimental conditions used in this experiment were identical to those described in Figure 7.
Table 4.
Summary of the Quantitative Results Determined for WEs, CEs, and DEs in Two Meibum Samples
|
WE |
CE |
DE |
||||
|
This Study |
Literature Reports |
This Study |
Literature Reports |
This Study |
Literature Reports |
|
| Mole | ||||||
| Total moles, μmol/mg meibum | 0.77 ± 0.07 | Not reported | 0.56 ± 0.03 | Not reported | α,ω Type II: 0.034 ± 0.003 | Not reported |
| ω Type I-St: 0.039 ± 0.004 | ||||||
| Saturated, % | 15 ± 1 | Not reported | 76 ± 2 | 4:1 (saturated: unsaturated)55 | α,ω Type II: 0 ω Type I-St: 9 ± 1 | Not reported |
| Unsaturated, % | 85 ± 1 | Not reported | 24 ± 1 | α,ω Type II: 100 | Not reported | |
| ω Type I-St: 91 ± 3 | ||||||
| Mass | ||||||
| Total meibum lipids, % | 48 ± 4 | 34.964 | 40 ± 2 | 29.504 | α,ω Type II: 3.4 ± 0.1 | Not reported |
| 51.1 ± 3.62 | 39.4 ± 3.12 | ω Type I-St: 4.3± 0.1 | ||||
| 41 ± 856 | 3132 | sum: 7.7 ± 0.1 | 2.3 ± 0.82 | |||
| 25.2 ± 2.68 | 66.8 ± 3.48 | 8.374 | ||||
| Saturated, % | 14 ± 1 | 18 ± 256 | 75 ± 2 | 77.224 | α,ω Type II: 0 | Not reported |
| ω Type I-St: 9 ± 1 | ||||||
| Unsaturated, % | 86 ± 1 | 82 ± 256 | 25 ± 1 | 21.52 (1.26 unidentified)4 | α,ω Type II: 100 | Not reported |
| ω Type I-St: 91 ± 3 | ||||||
Figure 9.
Distribution profile of DEs in meibum samples. Error bars indicate one standard deviation. The compositions (intensity%) were calculated after correcting for peak overlapping and isotopic distribution. The experimental conditions used in this experiment were identical to those described in Figure 7.
Comparison of Relative Amount of Molecular Species Within the Same Classes of Clinical Meibum Samples
As mentioned above, the relative ionization efficiency of lipids with the same degree of saturation within the same class of lipids was essentially the same. For some samples, particularly from dry eye subjects, much less sample was available because decreased meibum secretion is common in dry eye. Therefore, there could be more deviations in the length measurements of meibum in collection capillary tubes. However, for these samples, the absolute mass or moles of each species was not required to determine the difference between sample groups. Instead, lipid profiling based on relative ionization efficiency was used for determining the differences. The patterns observed for the compositions of WEs and CEs based on constant relative ionization efficiency were quite similar to those observed in the experiment based on internal standards, which validated the effectiveness of this method.
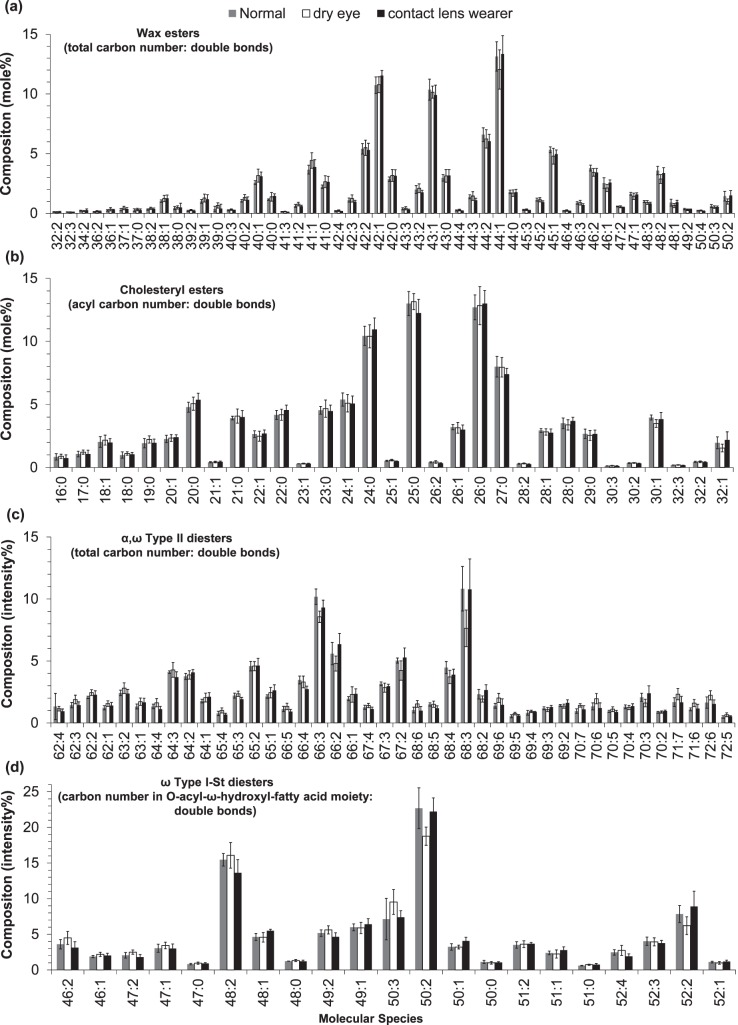
The representative spectra for three groups of samples were compared (Supplementary Fig. S2); the relative intensity distributions of major molecular lipid species within the same lipid classes for three groups of samples are shown (Fig. 10). The lipid profiles include 145 molecular species consisting of 51 WEs, 31 CEs, 42 α, ω Type II DEs, and 21 ω Type I-St DEs. Several highly unsaturated (the number of double bonds ≥ 5) α, ω Type II DEs were assigned based on accurate mass and distribution trend. However, the possibility that some of these α, ω Type II DEs could overlap with ω Type I-St DEs of the same elemental compositions was not excluded. In general, there was no major difference within each class across different groups except two downregulated lipid peaks for dry eye samples: 68:3 α,ω Type II DEs and 52:2 ω Type I-St DEs.
Figure 10.
Distribution profiles of four lipid classes in three groups of meibum samples: (a) WEs, (b) CEs, (c) α,ω Type II DEs, and (d) ω Type I-St DEs. The three groups were normal (four samples); dry eye (five samples); and contact lens wearers who also displayed dry eye symptoms (five samples). Error bars indicate one standard deviation. The compositions (mole%) for (a, b) were calculated after correcting for peak overlapping, isotopic distribution, and relative ionization efficiency; while the compositions (intensity%) for (c, d) were calculated after correcting for peak overlapping and isotopic distribution. See text for experimental details.
Discussion
The analysis of lipid composition of human medium has a long history.7 Previously, meibum lipids were identified and quantitated by a series procedures including separation of a certain class of lipids from meibum, hydrolysis of the separated class of lipids, derivation of the resulted fatty acids and/or fatty alcohols, followed by detection with gas chromatography (GC, also known as gas-liquid chromatography)–flame ionization detection (FID). A great deal of solid information, particularly fatty acid/fatty alcohol components of WEs and CEs, on meibum lipid composition has been reported as early as 1981.4 However, intact lipid composition of meibum is probably more important and its study (including detection and quantitation of these intact lipids) is made possible only with the recent development of mass spectrometry.7
Unfortunately, it is challenging to quantitate intact meibum lipids and current knowledge of molecular lipid species in meibum is still limited. There are at least two major issues related to current studies on meibum lipids by mass spectrometry: low detection sensitivity and inaccurate quantitation. For the first issue, for instance, as early as 1981 approximately 50 sterol ester species have been quantitated based on the approach including the procedures of hydrolysis and derivation,4 while most recent studies on intact lipids only attempted to quantitate a maximum of 20 of these esters.8,32,55 For another example, as many as 57 fatty acid moieties and 38 fatty alcohol moieties of WEs have been quantified as early as 1981,4 whereas a most recent study only report quantitation of 20 types of WE molecular species (isobaric species were not able to be quantified individually and reported as a group instead) with none of the polyunsaturated WEs included.56 Another major issue relative to the study of human meibum is inaccurate quantitation, for which there are several concerns. First, almost all quantitation has been performed in the absence of internal standards.32,55,56 However, “the effects of the variations of chromatographic separation conditions and/or ESI-MS conditions” can “dramatically alter the detected absolute ion counts of a particular species.”47 This statement applies to APCI-MS57 and probably GC-MS as well. Secondly, curve fitting based on the external responses of a limited number of standards was used to predict the responses of numerous lipid species of interest in meibum, a complex mixute.55,56 The accuracy of this prediction is doubtful considering ionization efficiency affected by the continuous changes of mobile phase composition (HPLC), column temperature (GC), and the number and amount of coeluted species. This prediction is even more problematic58 when 10 parameters were used for the curve fitting.56 Thirdly, the quantitation is often based on fragments of lipids,32,55 lacking the important molecular weight information to confirm their identities.
To address these issues, we have worked out an efficient way for highly sensitive detection and reliable quantitation of intact meibum lipids. By using NaI additive for ESI detection, we increased the detection sensitivity and minimized dissociation of meibum lipids; while by quantifying meibum lipids based on intact lipid peaks and a set of 11 internal standards without the need of curve fitting, we increased the quantitation reliability.
NaI Additive for Highly Sensitive ESI-MS Detection of Intact Meibum Lipids
By using NaI additive for ESI-MS analysis of meibum lipids, we dramatically “cleaned up” the spectra (Fig. 4) and significantly increased detection sensitivity. In this work, the major meibum lipid species detected in positive ion mode were WEs, CEs, and DEs, consistent with our earlier report.6 The most abundant meibum lipid species previously reported, fatty acid amides,59 were not detected in our work and recent studies suggest that abundant fatty acid amides detected in meibum likely result from plastic additive during the experimental process.7 Compared with previous reports,2,4,8 less relative quantity of triacylglycerols was detected. The reason could be that our capillary collection method took up much less lipid unrelated to meibum, such as those originated from skin.
Due to the ubiquitous presence of sodium ions, neutral lipids in meibum samples detected by ESI-MS in the presence of ammonium ions were composed of adducts of ammonium ions, protons, and sodium ions. The coexistence of multiadducts for the lipids in meibum samples resulted in decreased sensitivity and complicated spectra. One way to avoid the multi-adducts issue for the analysis of meibum samples is to intentionally add an appropriate concentration of sodium salt to the meibum sample. Sodium salt additives have been previously reported to be beneficial for the analysis of various types of analytes by ESI-MS.33 These previous studies mainly analyzed relatively polar species such as carbohydrates, where the additives were sodium acetate or chloride and the solvents were mixtures of water and organic solvent or methanol only.60–65 In our study, NaI appeared to be ideal for the detection of these neutral lipids in the mixture of chloroform and methanol. NaI has been used as the additive by two groups for enhanced ESI-MS detection of polar plasmenyl phosphatidylethanolamine66 and phosphatidylethanolamine67 and one group for nonpolar triacylglycerols68; however, no rationale for choosing NaI over other sodium salts as the additive was offered by these groups. We attribute such enhancement primarily to two reasons. First, NaI has higher solubility in the solvent mixture. The solubility of NaI in methanol is 78% to 83% at 25°C,69 which is much higher than that of sodium chloride (1.4% at 25°C)69 and sodium acetate (<2.4% in 98.4% aqueous methanol at 8–19°C).70 Since methanol is more polar than chloroform, the solubility of the additive salts in the chloroform/methanol mixture is most likely lower than in methanol alone. If the solubility is not high enough, the salts may precipitate with the evaporation of the solvent during the ionization process. Additionally, the order of the gas phase affinity for anions to sodium ions is probably in the order of acetate > chloride > iodide,71 and thus iodide will have minimum withdrawal effect on the sodium ions that have attached to an ester group (such as in WEs and CEs); therefore, NaI is a better additive than the other sodium salts for neutral lipids.
The addition of NaI resulted in the formation of only one type of adduct and simplified spectra leading to high signal-to-noise-ratio peaks. In addition, the higher stability of sodiated peaks also made quantitation more reliable. Unlike ammoniated lipids, the highly stable sodiated lipids did not readily dissociate, allowing for analysis at relatively high desolvation temperatures and high cone voltages, which both provide distinct advantages for MS analysis. High desolvation temperatures result in increased ionization efficiency (especially at relatively high flow rates) while high cone voltages increase the relative intensities of species of higher m/z (usually lipid peaks) than those of lower m/z species (usually impurity peaks). High cone voltages and desolvation temperatures both decrease formation of cation-linked dimer peaks and/or other noncovalent complexes. With these optimized conditions, high signal-to-noise ratio detection of more than 145 lipid species was achieved within 1 second for meibum samples containing ∼2.7 μg/mL lipids (Supplementary Fig. S3); the total amount of lipids corresponded to ∼1.8 ng, suggesting that detection required an average of less than 13 pg for one species and demonstrating the extreme sensitivity of this method.
The optimum amount of NaI depended on the lipid concentrations in the meibum sample solutions, the specific instrument parameters, and possibly the type of mass spectrometer that was used. Under the experimental conditions in this study, the optimum concentration of lipids in meibum solutions was ∼1.3 to 13 μg/mL and the molar ratio of NaI to lipids was ∼6:1 to 10:1. The signal-to-noise ratios of the lipid peaks for meibum samples containing ∼1.3 μg/mL lipids appeared to be similar to the samples containing ∼13 μg/mL lipids; however, for meibum samples containing lower concentration of lipids, there was often more interference from higher relative concentrations of impurities. Therefore, extreme care was required to minimize impurities. For samples containing low concentrations of lipids, the amount of chloroform required to dissolve low polarity lipids can be significantly reduced. For instance, as low as 1.3% chloroform in the mixture of chloroform/methanol was adequate for dissolving meibum samples containing ∼2.7 μg/mL lipids. Chloroform is known to easily introduce contaminants and using this minimum amount of chloroform significantly decreased contamination potential. When no chloroform at all (methanol only) was used, WE and CE peaks were consistently demonstrated for meibum samples containing ∼2.7 μg/mL of total lipids. However, the intensity of DE peaks decreased significantly (data not shown), suggesting that the DEs are very hydrophobic and at minimum, a small percentage of low polarity solvent such as chloroform is required to dissolve DEs.
When using chloroform, it is difficult to completely eliminate impurity peaks (Fig. 4a). The relatively high intensity low molecular weight impurity peaks (m/z 393.31, m/z 245.09, m/z 413.28, and m/z 323.16) were likely introduced from glass capillary tubes during the pipetting process as these peaks significantly decreased after repeatedly rinsing with clean solvent(s). With internal calibration using known identity peaks, the accurate m/z values for these unknown peaks were determined to be m/z 393.2959, m/z 245.0770, m/z 413.2658, and m/z 323.1469. Previous publications and the potential element compositions for these m/z values suggest that these peaks probably correspond to sodiated ions of dioctyl adipate72 or bis(2-ethylhexyl) adipate73 (C22H42O4Na, theoretical m/z 393.2981); diethyl phthalate73 (C12H14O4Na, theoretical m/z 245.0790); diisooctyl phthalate72,73 (C24H38O4Na, theoretical m/z 413.2668); and tripropylene glycol diacrylate (C15H24O6Na, theoretical m/z 323.1471), respectively. Interestingly, these species contain two to three esters groups, which may explain their high intensities: sodium ions can easily attach to ester groups as shown for the neutral meibum lipids discussed in this report. The corresponding mass accuracies were −5.6 ppm, −8.1 ppm, −2.4 ppm, and −0.6 ppm.
Contrary to our observation that sodiated lipid peaks are inevitable in ESI analysis of meibum samples, Butovich et al.74 report that exclusively protonated lipid peaks (no sodiated lipid peaks at all) were observed in their ESI analysis of meibum that was dissolved in methanol/chloroform mixture in the absence of any additive. This is surprising because in ESI, ions are generated in the form of adducts of protons, ammonium or sodium ions in solution phase36; in the absence of any intentionally added additives, sodium adducts are expected to be the dominant (if not the only) adduct form due to the ubiquitous presence of sodium ions in glass and/or solvents,75 particularly for neutral lipids such as WEs, due to the high affinity of sodium ions to the less polar ester group within these neutral lipids.15 In contrast, for APCI, ionization occurs in gas phase and neutral molecules are ionized through proton transfer76; as a result, protonation is often the only ionization form.15
Use of 11 Internal Standards for Reliable Quantitation of Meibum Lipids
In our study, absolute and relative quantitation of WEs and CEs in meibum was determined from the ratio of normalized intensities for peaks of interest to the average normalized intensity of a set of internal standards (six WEs and five CEs). The principle of quantitating WEs and CEs via this approach was based on the observation that their ionization efficiency was independent of the acyl or alcohol carbon chain length but predominantly determined by the class (WEs or CEs) of the lipids and the saturation level of the carbon chain.
Our finding that ionization efficiency (of WEs and CEs) was independent of the lipid carbon chain length is consistent with previous reports on ESI analysis of phosphatidylcholines,52 various types of glycerophospholipids,77 galactosylceramides,53 and ceramides.54 The ionization efficiency's independence of the carbon chain length was also apparent in Figure 4 of a reference on CEs, although a discussion of those data was absent in the text of the reference.31 It has been suggested that the polar head group of lipids provides the greatest contribution to ionization efficiency while the stability of the lipid ions is rarely affected by their acyl chain properties.38 In contrast, the dependence of ionization efficiency on carbon chain length has also been reported in two studies, one for phospholipids51 and the other for triacylglycerols.46 For the former study, one possible explanation is that solutions reported in that paper51 might contain a small amount of water introduced via the addition of ammonium hydroxide, resulting in a higher chance of aggregation for lipids with a higher carbon number; while for the latter report on triacylglycerols, the charge adduction to one of the three neighboring ester groups might be influenced by hydrophobic interaction and/or steric hindrance between the closely adjacent three acyl chains.
The dependency of ionization efficiency on the saturation level of the carbon chain has also been reported for other lipids: polyunsaturated species of phospholipids at relatively high concentrations (10 μM) were reported to give 40% higher intensity compared with saturated ones.51 Similar results were obtained in our experiments. We spiked 10 nM of each of the 11 internal standards into ∼1.7 μM meibum lipid solutions and found that the addition of each double bond in WEs and CEs increased the ionization efficiency by approximately 38% and 115%, respectively, compared with their saturated species.
No calibration curve was needed for quantitation in this study and thus the relevant errors were eliminated. Our approach is different from the conventional method based on external calibration curve. Approaches similar to ours, but typically use only one internal standard for each class, have been reported in numerous studies.49,50,52–54 The use of internal standards is beneficial as “the effects of the variations of chromatographic separation conditions and/or ESI-MS conditions much less affect the relative ion counts of the species obtained by normalizing to the ion counts of the internal standard detected under identical conditions.”47 We should point out that people have started to realize the importance of internal standards for quantitation of meibum lipids; however, in that work they used only one internal standard for each class without knowing the effect of saturation level and chain length of the lipids on ionization efficiency.8 In contrast, we used 11 internal standards of different chain length and saturation level for quantitation. As a result, our approach is more reliable in quantitation.
Absolute Quantitative Analysis of WEs and CEs in Meibum
With the increased detection sensitivity for intact lipids, compared with the previous reports on quantitation of a maximum of 20 CEs8,32,55 or 20 types of WEs (isobaric species were not able to be quantified individually and reported as a group) with no polyunsaturated WEs included56, we have quantitated the absolute amount of 51 intact WEs (including 30 polyunsaturated WEs) and 31 intact CEs. For the first time, we reported the total moles of WEs and CEs per mg meibum along with the corresponding mole percentages of saturated and unsaturated species for WEs. The molar ratio of CEs to WEs was determined as 0.73 ± 0.07 in our work, similar to a recently reported value of 0.57 ± 0.09 based on NMR analysis.78 In addition, we have estimated the absolute amount of 42 α,ω Type II DEs and 21 ω Type I-St DEs, which have never been reported previously. Table 4 summarizes our findings.
Mass Percentages of WEs and CEs in Meibum
In our work, the mass percentage of WEs and CEs of total meibum lipids were determined as 48 ± 4% and 40 ± 2%, respectively. These results are compared with literature reports that ranged from 25.2 ± 2.6%8 to 51.1 ± 3.6%2 for WEs and from 29.50%4 to 66.8 ± 3.4%8 for CEs (Table 4).
Our results were in close agreement with the work by Mathers and Lane (51.1 ± 3.6% and 39.4 ± 3.1% for WEs and CEs, respectively),2 which is based on thin layer chromatography and GC-MS; however, the detailed experimental information in that work is not available.2 Nicolaides et al.4 determined the mass percentages as 34.96% and 29.50% for WEs and CEs, respectively. Their quantitation was based on column chromatography and weighing. Interestingly, they reported the percentage of polar lipids as 16.04%, but mentioned, “with extremely soft expression of live human lids, polar lipids decreased.” Apparently different meibum collection methods could be one of the reasons for the different percentages observed. Despite the difference in absolute percentages, the calculated relative percentages (WEs versus CEs) were quite close between our work (1.2 ± 0.1) and theirs (1.19). Quantitative study of meibum lipids by ESI-MS coupled with HPLC has also been reported, where the percentages of WEs and CEs in normal meibum were determined to be 25.2 ± 2.6% and 66.8 ± 3.4%, respectively; however, as Lam et al. point out,8 that study may contain errors in quantitation due to the use of one internal standard for each class and the matrix effect resulting from different retention times.
Unlike the others reporting the mass percentages of WEs56 and CEs32 based on the same experimental setup, Butovich et al. determine the percentages of WEs and CEs in two different setups. In the study on WEs using GC-MS,56 Butovich et al. report the mass percentage of WEs as 41 ± 8% (in the Abstract) and 40 ± 10% (in the Discussion). However, these results are questionable due to the curve fitting with 10 parameters58 and their inability to detect polyunsaturated WEs.56 In addition, it is well known that GC has many limitations, including sample decomposition/isomerization for the analysis of complex lipids (such as WEs).79 While in the other study on CEs using LC-MS, the mass percentage of CEs is determined as 31%.32 However, the number of CEs detected in that work is only 20, possibly resulting in underestimation; the lack of internal standard also decreases the reliability of the quantitation.
Molecular Compositions of CEs and WEs in Meibum
In addition to comparing the total amounts of WE and CE types of lipids in meibum, it is also interesting to compare the molecular compositions within each type determined in our work and literature reports.
Our determination of the composition of molecular CE species demonstrated a strikingly similar pattern to the results Nicolaides et al.4 report using the method of hydrolysis and derivation followed by GC-FID (Fig. 8a). Since it has been reported that the sterol in meibum is predominantly cholesterol (over 97.5%),80 the composition of fatty acyl in the steryl esters that Nicolaides et al.4 report is considered similar in composition to CEs and therefore directly compared with our analysis of intact CEs. The percentages of isobaric CEs that Nicolaides et al.4 report is summed from those containing fatty acid moieties of normal, iso, and anteiso chains as well as with different double bond positions for unsaturated species. Due to the limitation of GC,79 many long chain and/or polyunsaturated species reported by us were not detected by them. This may also explain the slightly diminished relative intensity of the low molecular weight CE species we reported. Importantly, in our work intact lipids instead of fatty acid moieties were quantitated.
The composition of CEs is also reported by Butovich using APCI-LC-MS in negative detection mode55 and positive detection mode.32 In negative mode, only 20 individual intact CEs were quantified. The top four most abundant CEs detected (i.e., C24:0, C25:0, C26:0, and C27:0 CEs55) were the same as our results. However, other species in that report appeared underestimated, likely caused by high background and/or low signal-to-noise ratio; the effect of carbon chain length on ionization efficiency in that study could also make it difficult to perform accurate ionization efficiency correction. When positive detection mode was used, only 8 individual CEs were quantified, along with 6 other mixtures, each containing one saturated and one unsaturated species.32
By using another approach, HPLC coupled with ESI-MS, Lam et al. quantitate 20 CE species.8 In that work, the composition of CEs with mono-unsaturated fatty acyl chain appears to be overestimated in the absence of correction for ionization efficiency of lipid species.
Compared with the quantitation of a maximum of 20 CE molecular species in these previous reports,8,32,55 a total of 31 individual intact CEs were quantified in our work, suggesting that our method resulted in greater sensitivity.
Quantitation of individual intact WEs in meibum has been previously reported by Butovich et al. based on GC-MS.56 However, the number of quantified WEs was only 20; many species, particularly polyunsaturated ones, were missing (Fig. 8c). In contrast, we quantified 51 WEs including eight saturated, 13 monounsaturated, and 30 polyunsaturated WEs (Fig. 8b).
Saturation Degree of CEs and WEs in Meibum
The degrees of saturation of meibum lipids have been reported to be critical factors related to the health of the eye.81,82 Therefore, it is of interest for researchers to know the amount of saturated and unsaturated lipids in meibum samples. Saturation degree of CEs and WEs in human meibum determined in this work was compared with literature reports.
In our work, the mass percentages (mole percentages) of saturated, monounsaturated, and polyunsaturated species for CEs were determined as 75.4 ± 2.5% (75.9 ± 2.5%); 21.6 ± 0.6% (21.3 ± 0.7%); and 3.0 ± 0.1% (2.8 ± 0.1%), respectively. Note the mole percentages indicated in parentheses are almost identical to the mass percentages. Our results were almost the same as those determined using GC of sterol esters (after hydrolysis and derivation) by Nicolaides et al.,4 which were 77.22%, 20.08%, and 1.44% for saturated, monounsaturated, and diunsaturated species, respectively (1.26% unidentified). Due to the difficulty in detecting polyunsaturated species by GC,56 only one polyunsaturated sterol ester was reported in that work4; in contrast, we quantitated six polyunsaturated species. One additional advantage of our method compared with that work was that the species analyzed in our work were intact lipids instead of fatty acid moieties (after hydrolysis and derivation).
The mass percentages of saturated and unsaturated CEs is not reported in either of the two recent papers by Butovich.32,55 Instead, a molar ratio 4:1 of saturated to unsaturated CEs is reported,55 which is significantly higher than the ratio of 3.15 (± 0.04):1 in our study. The reported higher ratio in that work could be caused by their lacking of internal standards, difficulty in accurate determination of ionization efficiency of 20 species from curve fitting of six species, and much fewer CEs detected in that work.
In addition to CEs, the mass percentages (mole percentages) of saturated, monounsaturated, diunsaturated, and other polyunsaturated species for WEs were also determined in our work, which are 14.5 ± 0.5% (14.9 ± 0.5%); 53.4 ± 0.9% (53.6 ± 0.9%); 25.3 ± 0.7% (24.9 ± 0.7%); and 6.8 ± 0.2% (6.6 ± 0.2%), respectively. Interestingly, much less information was available for WEs.
The mass percentages of saturated and unsaturated intact WEs is not specifically reported by Nicolaides et al. as they investigate hydrolyzed WEs; however, saturated fatty acyl and fatty alcohol in WEs is reported by the authors as 22.49% and 77.02%, respectively.4 Assuming that the mass percentages of the saturated fatty acyl and fatty alcohol distribute randomly across all the WE molecular species, the mass percentage of saturated WEs is estimated as 17.32% by combining these two values, which is a little higher than what we determined.
The mass percentage of saturated and unsaturated WEs that Butovich reports is 18 ± 2% and 82 ± 2%, respectively.56 However, contradictory values appear in that same paper. For instance, from Table 2, “Wax Ester Composition of a Representative Sample of Human Meibum,” the saturated and unsaturated WEs could be easily calculated as 31% and 69%, respectively.56 Their use of 10 parameters for curve fitting and inability to detect polyunsaturated WEs makes the numbers questionable.58
Absolute Quantitative Analysis of Two Types of DEs in Meibum
The amount of DEs were previously reported to account for 2.3 ± 0.8%2 and 8.37%4 of human meibum. It has been reported DEs in human meibum are composed of α,ω Type II DEs and ω Type I-St DEs.6,42 However, quantitation of these individual types of DEs in human meibum has never been reported before. Direct determination of the amount of DEs is not available in this work due to the lack of commercially available DE standards.
However, the amount of individual DE molecular species can be estimated based on an interesting observation in Figures 5 and 7 that the ionization efficiency of the same degree of unsaturated WEs and CEs is quite close. It seems reasonable to assume the ionization efficiency of closely related species such as DEs and WEs is similar. The correction factor for ionization efficiency of the DEs may be considered the same as those of WEs with the same number of double bonds, but with the addition of 0.85 and 0.40 (corresponding to correction factors for saturated WEs and CEs, respectively) to account for the additional ester group in α,ω Type II DEs and ω Type I-St DEs, respectively.
By comparing the normalized peak intensities for each molecular species of these two types DEs and summed normalized peak intensities for WEs in the same run, along with the determined total moles 0.77 ± 0.07 μmol WEs per mg meibum, each of a total of 63 molecular species of DEs including 42 α,ω Type II DEs and 21 ω Type I-St DEs were quantitated. The total moles of α,ω Type II DEs and ω Type I-St DEs in meibum were determined as 0.034 ± 0.003 and 0.039 ± 0.004 μmol per mg meibum, respectively. After taking into consideration of the molecular weights for each species, the corresponding mass percentages α,ω Type II DEs and ω Type I-St DEs in human meibum were determined as 3.4 ± 0.1% and 4.3 ± 0.1%, respectively. Interestingly, the sum of the percentages for the two types of DEs is 7.7 ± 0.1%, which is close to 8.37% DEs in human meibum as Nicolaides et al. reported.4 A similar percentages of α,ω Type II DEs and ω Type I-St DEs in deer meibum were reported as 2.3% and 5%, respectively, in addition to 1.3% α, Type I DEs (which is composed of fatty acid, α-hydroxy fatty acid, and fatty alcohol moieties).42
No study has been reported previously on the percentages of saturated and unsaturated DEs in meibum samples. In our work, α,ω Type II DEs detected were exclusively unsaturated; while for ω Type I-St DEs, the mass percentages of saturated and unsaturated were determined as 9 ± 1% and 91 ± 3%, respectively. The corresponding mole percentages are the same as for mass percentages.
Relative Quantitative Analysis of WE, CEs, and DEs in Clinical Meibum Samples
Under similar experimental conditions, the variation in relative ionization efficiencies within the same lipid class, including WEs and CEs, appeared insignificant. The experiment on clinical samples was performed more than 2 years ago (when quantitation was not our focus) before our attempt on absolute quantitation. However, by assuming the relative ionization efficiency of the lipids species within the same class (WEs or CEs) being the same for the two experiments, the mole percentages of saturated and unsaturated CEs and WEs were quite similar. For instance, the mole percentages of saturated and unsaturated CEs in normal patient meibum were determined to account for 73 ± 1% and 27 ± 1%, respectively; while the mole percentages of saturated and unsaturated WEs were determined to be 13 ± 1% and 87 ± 1%, respectively. These results were consistent with our experiment on absolute quantitation of meibum lipids by comparing the MS spectra in the presence and absence of lipid standards, where the saturated and unsaturated CEs were determined to be 76 ± 2% and 24 ± 1%, respectively, and the saturated and unsaturated WEs were determined to be 14 ± 1% and 86 ± 1%, respectively.
The profiles of four types of lipids (WEs, CEs, α,ω Type II DEs, and ω Type I-St DEs) across three groups of meibum samples were compared by using corrections for peak overlapping and isotopic distribution as well as ionization efficiency. Two abundant DE peaks, 68:3 α,ω Type II DEs and 52:2 ω Type I-St DEs, appeared to be downregulated for dry eye samples. It seems unlikely that these esters directly cause dry eyes as polar lipids are believed to be the main species to maintain meibum stability.3,6,79 However, these species may suggest a deficiency in related polar lipids from which they were synthesized. These two species have been determined to be mainly composed of 18:1 fatty acid/32:1 diol/18:1 fatty acid, and 18:1 fatty acid/32:1 ω hydroxyl fatty acid/cholesterol,6 and thus their deficiency might result from downregulated corresponding polar O-acyl-ω hydroxyl fatty acid (18:1/32:1). This hypothesis remains to be confirmed.
The relative peak intensities of the molecular species determined for meibum lipids may be helpful in providing a clue to the source of these lipids. It is interesting to note that several molecular species in each class exhibit significantly higher peak intensities than others (Fig. 10). These high intensity species include six WE peaks (42:1, 43:1, 44:1, 45:1, 44:2, and 42:2); four CE peaks (24:0, 25:0, 26:0, and 27:0), two α,ω Type II DE peaks (66:3 and 68:3), and two ω Type I-St DE peaks (48:2 and 50:2). A close examination of the abundant species of WEs and CEs suggests that the synthesis of these two types of esters is related. From the MS/MS analysis of these abundant WEs, the fatty acyl moieties were mainly 18:1 in WEs 42:1, 43:1, 44:1, and 45:1, and of approximately equal amount for 18:1 and 18:2 in WEs 42:2 and 44:2.6 Thus, the corresponding fatty alcohol moieties in the six most abundant WE peaks were 24:0, 25:0, 26:0, and 27:0, which were of the same carbon chain lengths as those of fatty acyl moieties in the abundant CEs. The similarity observed suggested that the synthesis of the two classes of lipids was likely derived from the same pool of fatty acids and involved the conversion of fatty acids to fatty alcohols for WEs.83 Likewise, the syntheses of α,ω Type II DEs and ω Type I-St DEs also appeared to be related. The two most abundant species were 66:3 and 68:3 for α,ω Type II DEs, and 48:2 and 50:2 for ω Type I-St DEs. The major fatty acid moiety for these two types of DEs in steer meibum42 was reported as 18:1, while the composition of steer meibum was in general close to that of human meibum.4 Thus, the corresponding α, ω diol moieties in α,ω Type II DEs and ω-hydroxy fatty acid moieties in ω Type I-St DEs were most likely of the same chain length (i.e., 30:1 and 32:1). The similarity suggests that α,ω diol moieties and ω-hydroxy fatty acid moieties may also come from the same pool of derivatized fatty acids, where α,ω diols are likely converted from ω-hydroxy fatty acids via similar enzymatic reactions as suggested for WEs. This hypothesis is consistent with the downregulation of both 68:3 α,ω Type II DEs and the related 52:2 ω Type I-St DEs in dry eye samples.
Advantages of Direct Infusion ESI-MS Using NaI Additive and Internal Standards for Quantitation of Meibum Lipids
Direct infusion ESI-MS analysis of meibum samples was selected for use in this quantitation study and many more molecular lipid species were quantitated compared with literature reports. This approach, including the uses of NaI additive and internal standards, offers several advantages detailed as follows.
First, it is sensitive. A minimal amount of sample is needed for the analysis and the capillary collection method is sufficient. In this study, for absolute quantitation, 55 nL or 47 μg meibum was collected for each sample; while for relative quantitation of the clinical samples, 8 to 23 nL or 7 to 20 μg meibum was collected for each sample. Each of collected meibum samples was typically dissolved in 100 μL chloroform/methanol mixture per 13.4 μg meibum as the stock solution and the working solution was prepared by 50- or 100-fold dilution of this stock solution. Typically 100 μL working solution, which corresponded to a maximum of 0.268 μg, was sufficient for quantitation. In contrast, for the LC-MS and GC-MS methods reported, the typical amount (and much more to make the solution) of meibum injected to the systems was 1 to 10 μL of 0.1 to 1 mg/mL solution, or 0.1 to 2 μg,10,32,55,56 which sounds just a little more than what we need. However, taking into consideration the potential evaporation of solvents, the accuracy of pipetting, and the extra solution in sample vials to avoid syringe needle drawing up air or touching the bottom of the glass, 50 μL (even more for absolute quantitation) is likely the minimum to prepare the solution, which probably requires more than 50 μg. With this minimum amount, the microcapillary collection method used in our work, which is painless and with less likely contamination from surrounding tissues,11 will be impossible for these LC-MS and GC-MS methods.
Second, it is reliable. This is due to the following four factors:
Constant experimental condition. The invariable experimental condition makes it much easier to correct matrix effect in MS analysis. The observation of ionization efficiency independent of carbon chain length further simplifies the quantitation and no curve fitting is needed. In contrast, in LC-MS and GC-MS, the constant changes in mobile phase composition or column temperature with retention time (also run to run) inevitably affects the ionization efficiency of species eluted at different times, which makes it difficult to accurately predict the responses of many more lipid species of interest based on the responses of a limited number of external standards. As many as 10 parameters have been applied for the prediction,56 which cast doubt on its reliability to match the actual responses.58
Minimal ionization suppression. The use of very low concentrations of lipids (∼1.7 μM of total lipids) and additive (e.g., 10 μM) greatly reduces the ion suppression effect to negligible levels. In contrast, typically 5 to 10 mM ammonium formate or NH4Ac is used as the solution additive for MS analysis of lipids15,16,18,19,23,26,84; the high concentration additive alone could cause significant ion suppression,39 regardless if the MS analysis is coupled with front-end chromatography separation or not. In addition, generally a much higher concentration of lipids was applied to HPLC-MS or GC-MS system and the enrichment of the same lipid species by HPLC or GC could increase lipid-lipid interaction within the same lipid.39 For instance, a concentration of 0.1 to 1 mg/mL meibum solution, which corresponded to approximately 125 to 1250 μM, was reported to be applied to HPLC-MS and GC-MS systems.32,55
Direct detection of intact molecular species avoids potential false discoveries. Compared with APCI and EI, ESI is a much softer ionization method13 and in-source dissociation is typically insignificant and even negligible.14 As demonstrated in this work, the use of NaI additive further increases the stability of labile lipids such as CEs. As a result, intact molecules can be easily directly monitored and quantitated. In contrast, quantitation of meibum lipids by APCI and EI is often indirectly determined (from fragments of the lipids),32,55 which adds uncertainty in quantitation: the molecular weight information for confirming the identity of lipids is missing, while the dissociation efficiency of different species may be different, which increases difficulty in curve fitting of the responses for species of interest that are different from the standards.
Internal standards. The use of internal standards makes corrections for the sample matrix effects and instrument variations during the mass spectrometry analysis.
Third, it is rapid. The lipid profile can be generated in less than a minute versus hours as reported in literature (several runs for each type of lipids with 30 to 60 minutes for each run),32,55,56 which is hundreds of times faster.
Lastly, it is comprehensive. This approach allows all of the lipid species to be displayed in one spectrum, which makes it easier to get a complete profile of the lipid composition. In addition, it avoids potential problems associated with solvents (such as chloroform) that are incompatible with HPLC systems but necessary for the detection of certain lipids such as DEs.
It is worth pointing out that MS analysis alone is not sufficient for identifying lipid species. For unknown samples, the lipid species should be first identified using MS/MS prior to quantitative analysis with the method described herein. The identification helps to eliminate false discovery. To that end, we previously identified the lipid species in meibum using MS/MS,6 but those results are not reiterated in this report.
Comments on Quantitation of Isobaric Species of Meibum Lipids
One criticism of the direct infusion method is that it cannot separate isobaric peaks in meibum samples. It is true that there could be multiple isomers of lipids for a peak of the same m/z including normal, iso, anteiso isomers of fatty acids/fatty alcohols, and different position of double bonds. Even though isobaric species of meibum esters cannot be separated on our instrument used in this study, they could be at least partially separated by mass spectrometers including ion mobility feature.85 Alternatively, the isobaric species of interest could be further analyzed by other methods such as LC-MS, when in the future, the separation of these species is possible. Focusing the study on these species of interest will minimize the total analysis time.
Interestingly, we have not seen convincing evidence on separation of isobaric intact meibum lipids, including the well-studied CEs8,32,55 and WEs.8,32,56 Theoretically, methods such as GC or HPLC could separate isobaric lipids of various natures. Indeed, after hydrolysis and conversion of fatty acid moieties of meibum lipids to methyl esters, isobaric species including straight chain, iso, and anteiso methyl esters were able to be separated.4 However, for intact lipids, which are the interest of current studies on meibum composition, it is a different story. It is much more difficult to achieve the separation of intact lipids of various natures (including straight and branched chain) since the difference between their retention times on LC/GC columns is often negligible.
All three papers on quantitation CEs8,32,55 did not mention at all the detection of different isobaric species of the same m/z values. On the contrary, in one study, the method for detection of CEs in positive detection mode even could not separate many CEs with different carbon chain lengths and had to report quantitation of two CEs of different m/z values as a group.32
For the other three papers on WEs, only one mentioned separation of isobaric species.10 They claimed to have separated isomers of the WEs with m/z 643 and m/z 641 by HPLC (Fig. 7 of that reference). However, if read carefully, those peaks are actually the WEs of a different m/z: in Figure 7b, for the three peaks claimed to be the isomers of WE with m/z 643, two of them with the retention times 12.1 and 13.7 minutes actually correspond to WEs with m/z 645 and 647, respectively; similarly, in Figure 7a, for the four peaks claimed to be isobaric species of WE with m/z 641, three of them with the retention time of 10.4, 12.0, and 13.7 minutes were the WEs with m/z of 643, 645, and 647, respectively. These peaks of different m/z can be detected when the peak width for the selected ion is not set narrow enough.
For the report using GC-MS approach,56 no separation of any isomeric peaks were really shown. Figure 10 of that report was misleading. It gave the readers the impression that they were able to separate the m/z 620 WEs into three isomers. However, on careful read of the legend, one would know the three spectra (B, C, D)56 actually correspond to the same chromatographic peak shown in A56 (41.08–41.12 minutes; some variation in retention time is normal). The other peaks in Figure 10A56 are probably species with a chain length longer than the species corresponding to the 41.12-minute peak. Moreover, the important molecular ions (m/z 620) of claimed WE of interest were not shown; instead, the spectra B, C, and D56 suggest the 41.12 chromatographic peak most likely contained a mixture of WEs with three different m/z values (563.5, 577.6, and 591.7), which is consistent with the fact that the WE with m/z 618.7 (Fig. 10E)56 eluted out later (41.71 minute), as generally the longer the chain of WEs, the later the WEs eluted out (please see Fig. 3B of Butovich et al.56). It seems their identification of WEs was based on predicted retention time, which may vary from run to run and thus cannot identify an unknown species correctly. On the other hand, it is questionable how the branched chain (iso and anteiso) WEs could be identified in that work based on the fragmentation patterns of branched chain fatty acid methyl esters. It is known that the fragmentation pattern of WEs is significantly different from methyl esters.86 In addition, in a recent study of a series of straight-chain and branched chain WE standards, the authors stated “The mass spectra of WEs with mono-methyl branching near the end or in the middle of the FA chain closely resembled those of straight-chain esters and showed only slight variations in the peak intensities.”87 Indeed, the claim of detection of anteiso branched compound (Fig. 11 of Butovich et al.56) is unconvincing: the peaks m/z 213.3 and 241.3 with m/z 28 apart were labeled as (M − 58 + H) and (M − 28 + H), respectively, which were actually m/z 30 apart. In fact, their quantitation of WEs was based on isobaric species of same m/z instead of each isomer peak (Table 1 of Butovich et al.56), which again suggests these peaks could not be separated by Butovich et al.56
Conclusions
Highly sensitive quantitative profiling of neutral lipids was rapidly obtained for meibum samples. This was achieved by first optimizing the experimental conditions including using NaI additive and appropriate instrument parameters, followed by appropriate data analysis including corrections for peak overlapping, isotopic distribution, and ionization efficiency. The correction for ionization efficiency was performed for WEs and CEs and estimated for DEs. One interesting finding in our work is that the ionization efficiency of WEs and CEs was independent of their carbon chain lengths under the optimized conditions, which makes it much easier to quantitate many more molecular lipid species with a few internal standards. It is possible that the ionization efficiency's independence of carbon chain length extends to other classes of meibum lipids including DEs; on the other hand, the ionization efficiency may be influenced by the number of double bonds in other types of meibum lipids as well. These hypotheses remain to be confirmed with commercially available standards and more conclusive quantitation of these other types of meibum lipids will be obtained in the future.
Supplementary Material
Acknowledgments
The authors thank the OSU Contact Lens and Tear Film Lab research optometrists for meibum collection and Jeremy Keirsey at the Campus Chemical Instrument Center of The Ohio State University for proofreading and critical comments.
Supported by National Institutes of Health (NIH) Grant NEI R01EY015519.
Disclosure: J. Chen, None; K.B. Green, None; K.K. Nichols, None
References
- 1. Cory CC, Hinks W, Burton JL, Shuster S. Meibomian gland secretion in the red eyes of rosacea. Br J Dermatol. 1973; 89: 25–27 [DOI] [PubMed] [Google Scholar]
- 2. Mathers WD, Lane JA. Meibomian gland lipids, evaporation, and tear film stability. Adv Exp Med Biol. 1998; 438: 349–360 [DOI] [PubMed] [Google Scholar]
- 3. McCulley JP, Shine W. A compositional based model for the tear film lipid layer. Trans Am Ophthalmol Soc. 1997; 95: 79–88; discussion 88–93 [PMC free article] [PubMed] [Google Scholar]
- 4. Nicolaides N, Kaitaranta JK, Rawdah TN, Macy JI, Boswell FM III, Smith RE. Meibomian gland studies: comparison of steer and human lipids. Invest Ophthalmol Vis Sci. 1981; 20: 522–536 [PubMed] [Google Scholar]
- 5. Tiffany JM. Individual variations in human meibomian lipid composition. Exp Eye Res. 1978; 27: 289–300 [DOI] [PubMed] [Google Scholar]
- 6. Chen J, Green-Church KB, Nichols KK. Shotgun lipidomic analysis of human meibomian gland secretions with electrospray ionization tandem mass spectrometry. Invest Ophthalmol Vis Sci. 2010; 51: 6220–6231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Butovich IA. Lipidomics of human meibomian gland secretions: Chemistry, biophysics, and physiological role of meibomian lipids. Prog Lipid Res. 2011; 50: 278–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lam SM, Tong L, Yong SS, et al. Meibum lipid composition in Asians with dry eye disease. PLoS One. 2011; 6: e24339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dougherty JM, McCulley JP. Analysis of the free fatty acid component of meibomian secretions in chronic blepharitis. Invest Ophthalmol Vis Sci. 1986; 27: 52–56 [PubMed] [Google Scholar]
- 10. Butovich IA, Wojtowicz JC, Molai M. Human tear film and meibum. Very long chain wax esters and (O-acyl)-omega-hydroxy fatty acids of meibum. J Lipid Res. 2009; 50: 2471–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Green-Church KB, Butovich I, Willcox M, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on tear film lipids and lipid-protein interactions in health and disease. Invest Ophthalmol Vis Sci. 2011; 52: 1979–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murphy RC, Fiedler J, Hevko J. Analysis of nonvolatile lipids by mass spectrometry. Chem Rev. 2001; 101: 479–526 [DOI] [PubMed] [Google Scholar]
- 13. Zaikin VG, Halket JM. Derivatization in mass spectrometry--8. Soft ionization mass spectrometry of small molecules. Eur J Mass Spectrom (Chichester, Eng). 2006; 12: 79–115 [DOI] [PubMed] [Google Scholar]
- 14. Chen J, Green-Church KB, Nichols KK. Author response: On the presence of (O-acyl)-omega-hydroxy fatty acids and their esters in human meibomian gland secretions. Invest Ophthalmol Vis Sci. 2011; 52: 1894–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cai SS, Syage JA. Comparison of atmospheric pressure photoionization, atmospheric pressure chemical ionization, and electrospray ionization mass spectrometry for analysis of lipids. Anal Chem. 2006; 78: 1191–1199 [DOI] [PubMed] [Google Scholar]
- 16. Camera E, Ludovici M, Galante M, Sinagra JL, Picardo M. Comprehensive analysis of the major lipid classes in sebum by rapid resolution high-performance liquid chromatography and electrospray mass spectrometry. J Lipid Res. 2010; 51: 3377–3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cheng CF, Gross ML. Complete structural elucidation of triacylglycerols by tandem sector mass spectrometry. Anal Chem. 1998; 70: 4417–4426 [DOI] [PubMed] [Google Scholar]
- 18. Duffin K, Obukowicz M, Raz A, Shieh JJ. Electrospray/tandem mass spectrometry for quantitative analysis of lipid remodeling in essential fatty acid deficient mice. Anal Biochem. 2000; 279: 179–188 [DOI] [PubMed] [Google Scholar]
- 19. Duffin KL, Henion JD, Shieh JJ. Electrospray and tandem mass spectrometric characterization of acylglycerol mixtures that are dissolved in nonpolar solvents. Anal Chem. 1991; 63: 1781–1788 [DOI] [PubMed] [Google Scholar]
- 20. Fitzgerald M, Murphy RC. Electrospray mass spectrometry of human hair wax esters. J Lipid Res. 2007; 48: 1231–1246 [DOI] [PubMed] [Google Scholar]
- 21. Hutchins PM, Barkley RM, Murphy RC. Separation of cellular nonpolar neutral lipids by normal-phase chromatography and analysis by electrospray ionization mass spectrometry. J Lipid Res. 2008; 49: 804–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hvattum E. Analysis of triacylglycerols with non-aqueous reversed phase liquid chromatography and positive ion electrospray tandem mass spectrometry. Rapid Commun Mass Spectrom. 2001; 15: 187–190 [DOI] [PubMed] [Google Scholar]
- 23. Kalo P, Kuuranne T. Analysis of free and esterified sterols in fats and oils by flash chromatography, gas chromatography and electrospray tandem mass spectrometry. J Chromatogr A. 2001; 935: 237–248 [DOI] [PubMed] [Google Scholar]
- 24. Liebisch G, Binder M, Schifferer R, Langmann T, Schulz B, Schmitz G. High throughput quantification of cholesterol and cholesteryl ester by electrospray ionization tandem mass spectrometry (ESI-MS/MS). BBA-Mol Cell Biol Lipids. 2006; 1761: 121–128 [DOI] [PubMed] [Google Scholar]
- 25. McAnoy AM, Wu CC, Murphy RC. Direct qualitative analysis of triacylglycerols by electrospray mass spectrometry using a linear ion trap. J Am Soc Mass Spectrom. 2005; 16: 1498–1509 [DOI] [PubMed] [Google Scholar]
- 26. Murphy RC, James PF, McAnoy AM, Krank J, Duchoslav E, Barkley RM. Detection of the abundance of diacylglycerol and triacylglycerol molecular species in cells using neutral loss mass spectrometry. Anal Biochem. 2007; 366: 59–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sommer U, Herscovitz H, Welty FK, Costello CE. LC-MS-based method for the qualitative and quantitative analysis of complex lipid mixtures. J Lipid Res. 2006; 47: 804–814 [DOI] [PubMed] [Google Scholar]
- 28. Hsu FF, Turk J. Electrospray ionization multiple-stage linear ion-trap mass spectrometry for structural elucidation of triacylglycerols: assignment of fatty acyl groups on the glycerol backbone and location of double bonds. J Am Soc Mass Spectrom. 2010; 21: 657–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mirabaud S, Rolando C, Regert M. Molecular criteria for discriminating adipose fat and milk from different species by NanoESl MS and MS/MS of their triacylglycerols: application to archaeological remains. Anal Chem. 2007; 79: 6182–6192 [DOI] [PubMed] [Google Scholar]
- 30. Santos S, Schreiber L, Graca J. Cuticular waxes from ivy leaves (Hedera helix L.): Analysis of high-molecular-weight esters. Phytochem Analysis. 2007; 18: 60–69 [DOI] [PubMed] [Google Scholar]
- 31. Bowden JA, Albert CJ, Barnaby OS, Ford DA. Analysis of cholesteryl esters and diacylglycerols using lithiated adducts and electrospray ionization-tandem mass spectrometry. Anal Biochem. 2011; 417: 202–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Butovich IA. Cholesteryl esters as a depot for very long chain fatty acids in human meibum. J Lipid Res. 2009; 50: 501–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gao SM, Zhang ZP, Karnes HT. Sensitivity enhancement in liquid chromatography/atmospheric pressure ionization mass spectrometry using derivatization and mobile phase additives. J Chromatogr B. 2005; 825: 98–110 [DOI] [PubMed] [Google Scholar]
- 34. Anacleto JF, Pleasance S, Boyd RK. Calibration of ion spray mass-spectra using cluster ions. Org Mass Spectrom. 1992; 27: 660–666 [Google Scholar]
- 35. Wang GD, Cole RB. Effect of solution ionic-strength on analyte charge-state distributions in positive and negative-ion electrospray mass-spectrometry. Anal Chem. 1994; 66: 3702–3708 [Google Scholar]
- 36. Cech NB, Enke CG. Practical implications of some recent studies in electrospray ionization fundamentals. Mass Spectrom Rev. 2001; 20: 362–387 [DOI] [PubMed] [Google Scholar]
- 37. Li M, Zhou Z, Nie H, Bai Y, Liu H. Recent advances of chromatography and mass spectrometry in lipidomics. Anal Bioanal Chem. 2011; 399: 243–249 [DOI] [PubMed] [Google Scholar]
- 38. Han X, Gross RW. Shotgun lipidomics: electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom Rev. 2005; 24: 367–412 [DOI] [PubMed] [Google Scholar]
- 39. Han X, Yang K, Gross RW. Multi-dimensional mass spectrometry-based shotgun lipidomics and novel strategies for lipidomic analyses. Mass Spectrom Rev. 2012; 31: 134–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kinsinger CR, Apffel J, Baker M, et al. Recommendations for mass spectrometry data quality metrics for open access data (corollary to the Amsterdam principles). Mol Cell Proteomics. 2011; 10:O111.015446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Murphy RC, Gaskell SJ. New applications of mass spectrometry in lipid analysis. J Biol Chem. 2011; 286: 25427–25433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nicolaides N, Santos EC. The di- and triesters of the lipids of steer and human meibomian glands. Lipids. 1985; 20: 454–467 [DOI] [PubMed] [Google Scholar]
- 43. Nicolaides N, Santos EC, Papadakis K. Double-bond patterns of fatty acids and alcohols in steer and human meibomian gland lipids. Lipids. 1984; 19: 264–277 [DOI] [PubMed] [Google Scholar]
- 44. Nicolaides N, Santos EC, Papadakis K, Ruth EC, Muller L. The occurrence of long chain alpha, omega-diols in the lipids of steer and human meibomian glands. Lipids. 1984; 19: 990–993 [DOI] [PubMed] [Google Scholar]
- 45. Lewis RW. The densities of three classes of marine lipids in relation to their possible role as hydrostatic agents. Lipids. 1970; 5: 151–153 [DOI] [PubMed] [Google Scholar]
- 46. Han X, Gross RW. Quantitative analysis and molecular species fingerprinting of triacylglyceride molecular species directly from lipid extracts of biological samples by electrospray ionization tandem mass spectrometry. Anal Biochem. 2001; 295: 88–100 [DOI] [PubMed] [Google Scholar]
- 47. Yang K, Han X. Accurate quantification of lipid species by electrospray ionization mass spectrometry—meets a key challenge in lipidomics. Metabolites. 2011; 1: 21–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Swiss-Prot Group, Swiss Institute of Bioinformatics Isotopident. Available at: http://education.expasy.org/student_projects/isotopident/htdocs/. Accessed July 26, 2013 [Google Scholar]
- 49. Cifkova E, Holcapek M, Lisa M, et al. Nontargeted quantitation of lipid classes using hydrophilic interaction liquid chromatography-electrospray ionization mass spectrometry with single internal standard and response factor approach. Anal Chem. 2012; 84: 10064–10070 [DOI] [PubMed] [Google Scholar]
- 50. Han XL, Gross RW. Quantitative analysis and molecular species fingerprinting of triacylglyceride molecular species directly from lipid extracts of biological samples by electrospray ionization tandem mass spectrometry. Anal Biochem. 2001; 295: 88–100 [DOI] [PubMed] [Google Scholar]
- 51. Koivusalo M, Haimi P, Heikinheimo L, Kostiainen R, Somerharju P. Quantitative determination of phospholipid compositions by ESI-MS: effects of acyl chain length, unsaturation, and lipid concentration on instrument response. J Lipid Res. 2001; 42: 663–672 [PubMed] [Google Scholar]
- 52. Deeley JM, Mitchell TW, Wei XJ, et al. Human lens lipids differ markedly from those of commonly used experimental animals. BBA-Mol Cell Biol Lipids. 2008; 1781: 288–298 [DOI] [PubMed] [Google Scholar]
- 53. Han X, Cheng H. Characterization and direct quantitation of cerebroside molecular species from lipid extracts by shotgun lipidomics. J Lipid Res. 2005; 46: 163–175 [DOI] [PubMed] [Google Scholar]
- 54. Han XL. Characterization and direct quantitation of ceramide molecular species from lipid extracts of biological samples by electrospray ionization tandem mass spectrometry. Anal Biochem. 2002; 302: 199–212 [DOI] [PubMed] [Google Scholar]
- 55. Butovich IA. Fatty acid composition of cholesteryl esters of human meibomian gland secretions. Steroids. 2010; 75: 726–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Butovich IA, Arciniega JC, Lu H, Molai M. Evaluation and quantitation of intact wax esters of human meibum by gas-liquid chromatography-ion trap mass spectrometry. Invest Ophthalmol Vis Sci. 2012; 53: 3766–3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jessome LL, Volmer DA. Ion suppression: a major concern in mass spectrometry. LC GC N Am. 2006; 24: 498–510 [Google Scholar]
- 58. Hawkins DM. The problem of overfitting. J Chem Inf Comput Sci. 2004; 44: 1–12 [DOI] [PubMed] [Google Scholar]
- 59. Nichols KK, Ham BM, Nichols JJ, Ziegler C, Green-Church KB. Identification of fatty acids and fatty acid amides in human meibomian gland secretions. Invest Ophthalmol Vis Sci. 2007; 48: 34–39 [DOI] [PubMed] [Google Scholar]
- 60. Kohler M, Leary JA. Lc/Ms/Ms of carbohydrates with postcolumn addition of metal chlorides using a triaxial electrospray probe. Anal Chem. 1995; 67: 3501–3508 [DOI] [PubMed] [Google Scholar]
- 61. Karlsson KE. Cationization in electrospray microcolumn liquid chromatography mass spectrometry. J Chromatogr A. 1998; 794: 359–366 [Google Scholar]
- 62. Schneider RP, Lynch MJ, Ericson JF, Fouda HG. Electrospray ionization mass-spectrometry of semduramicin and other polyether ionophores. Anal Chem. 1991; 63: 1789–1794 [DOI] [PubMed] [Google Scholar]
- 63. Volmer DA, Lock CM. Electrospray ionization and collision-induced dissociation of antibiotic polyether ionophores. Rapid Commun Mass Spectrom. 1998; 12: 157–164 [DOI] [PubMed] [Google Scholar]
- 64. Ma YC, Kim HY. Determination of steroids by liquid chromatography mass spectrometry. J Am Soc Mass Spectrom. 1997; 8: 1010–1020 [Google Scholar]
- 65. Jemal M, Almond RB, Teitz DS. Quantitative bioanalysis utilizing high-performance liquid chromatography electrospray mass spectrometry via selected-ion monitoring of the sodium ion adduct [M+Na](+). Rapid Commun Mass Spectrom. 1997; 11: 1083–1088 [DOI] [PubMed] [Google Scholar]
- 66. Brooks S, Clark GT, Wright SM, et al. Electrospray ionisation mass spectrometric analysis of lipid restructuring in the carp (Cyprinus carpio L.) during cold acclimation. J Exp Biol. 2002; 205: 3989–3997 [DOI] [PubMed] [Google Scholar]
- 67. Hunt AN, Clark GT, Attard GS, Postle AD. Highly saturated endonuclear phosphatidylcholine is synthesized in situ and colocated with CDP-choline pathway enzymes. J Biol Chem. 2001; 276: 8492–8499 [DOI] [PubMed] [Google Scholar]
- 68. Steenhorst-Slikkerveer L, Louter A, Janssen HG, Bauer-Plank C. Analysis of nonvolatile lipid oxidation products in vegetable oils by normal-phase high-performance liquid chromatography with mass spectrometric detection. J Am Oil Chem Soc. 2000; 77: 837–845 [Google Scholar]
- 69. Stenger VA. Solubilities of various alkali metal and alkaline earth metal compounds in methanol. J Chem Eng Data. 1996; 41: 1111–1113 [Google Scholar]
- 70. Schiavon G. On the solubility of sodium acetate in aqueous alcohol. Gazz Chim Ital. 1902; 32: 4 [Google Scholar]
- 71. Pearson RG. Hard and soft acids and bases HSAB, part 1: fundamental principles. J Chem Educ. 1968; 45: 581–587 [Google Scholar]
- 72. Keller BO, Sui J, Young AB, Whittal RM. Interferences and contaminants encountered in modern mass spectrometry. Anal Chim Acta. 2008; 627: 71–81 [DOI] [PubMed] [Google Scholar]
- 73. Ende M, Spiteller G. Contaminants in mass spectrometry. Mass Spectrom Rev. 1982; 1: 29–62 [Google Scholar]
- 74. Butovich IA, Uchiyama E, McCulley JP. Lipids of human meibum: mass-spectrometric analysis and structural elucidation. J Lipid Res. 2007; 48: 2220–2235 [DOI] [PubMed] [Google Scholar]
- 75. Leitner A, Emmert J, Boerner K, Lindner W. Influence of solvent additive composition on chromatographic separation and sodium adduct formation of peptides in HPLC-ESI MS. Chromatographia. 2007; 65: 649–653 [Google Scholar]
- 76. Carroll DI, Dzidic I, Horning EC, Stillwell RN. Atmospheric-pressure ionization mass-spectrometry. Appl Spectrosc Rev. 1981; 17: 337–406 [Google Scholar]
- 77. Han XL, Yang K, Yang JY, Fikes KN, Cheng H, Gross RW. Factors influencing the electrospray intrasource separation and selective ionization of glycerophospholipids. J Am Soc Mass Spectrom. 2006; 17: 264–274 [DOI] [PubMed] [Google Scholar]
- 78. Shrestha RK, Borchman D, Foulks GN, Yappert MC, Milliner SE. Analysis of the composition of lipid in human meibum from normal infants, children, adolescents, adults, and adults with meibomian gland dysfunction using (1)H-NMR spectroscopy. Invest Ophthalmol Vis Sci. 2011; 52: 7350–7358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Butovich IA. The meibomian puzzle: combining pieces together. Prog Retin Eye Res. 2009; 28: 483–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Harvey DJ, Tiffany JM, Duerden JM, Pandher KS, Mengher LS. Identification by combined gas chromatography-mass spectrometry of constituent long-chain fatty acids and alcohols from the meibomian glands of the rat and a comparison with human meibomian lipids. J Chromatogr. 1987; 414: 253–263 [DOI] [PubMed] [Google Scholar]
- 81. Borchman D, Foulks GN, Yappert MC, et al. Human meibum lipid conformation and thermodynamic changes with meibomian-gland dysfunction. Invest Ophthalmol Vis Sci. 2011; 52: 3805–3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Joffre C, Souchier M, Gregoire S, et al. Differences in meibomian fatty acid composition in patients with meibomian gland dysfunction and aqueous-deficient dry eye. Br J Ophthalmol. 2008; 92: 116–119 [DOI] [PubMed] [Google Scholar]
- 83. Cheng JB, Russell DW. Mammalian wax biosynthesis—I. Identification of two fatty acyl-coenzyme A reductases with different substrate specificities and tissue distributions. J Biol Chem. 2004; 279: 37789–37797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Murphy RC, Fitzgerald M, Barkley RM. Neutral lipidomics and mass spectrometry. In: Griffiths WJ. ed Metabolomics, Metabonomics and Metabolite Profiling. Cambridge, UK: The Royal Society of Chemistry; 2008; 161–194 [Google Scholar]
- 85. Kanu AB, Dwivedi P, Tam M, Matz L, Hill HH Jr. Ion mobility-mass spectrometry. J Mass Spectrom. 2008; 43: 1–22 [DOI] [PubMed] [Google Scholar]
- 86. Ryhage R, Stenhagen E. Mass spectrometry in lipid research. J Lipid Res. 1960; 1: 361–390 [PubMed] [Google Scholar]
- 87. Urbanova K, Vrkoslav V, Valterova I, Hakova M, Cvacka J. Structural characterization of wax esters by electron ionization mass spectrometry. J Lipid Res. 2012; 53: 204–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.