Abstract
Background
High tumor dissemination (HTD) is a major risk factor for serious morbidity after primary ovarian cancer (OC) surgery, particularly in medically compromised patients. We performed a pilot study of whether CT findings could predict extent of disease and surgical complexity necessary in advanced OC.
Methods
Preoperative CT images for patients with advanced OC from 1997–2003 were evaluated for rigorously defined disease-related findings and compared to both the findings at exploration and the required surgical procedures. Associations were assessed by the chi-square test.
Results
Forty-six cases met inclusion criteria. Mean age was 66.4 y, and 76% had residual disease (RD) 1cm or less. CT and surgical findings correlated (sensitivity/specificity) as follows: diaphragm disease (48%/100%); surface liver (100%/93%); omental cake (72%/65%); any sigmoid involvement (54%/100%); ascites (44%/100%); extra-pelvic large bowel involvement (29%/91%). When diaphragm disease and omental cake were present, HTD was found in all cases (positive predictive value and specificity=100%, sensitivity 48%). For CT findings of liver, large bowel and spleen involvement there was a strong trend toward resection (p=0.001, p=0.06 and p=0.06, respectively).
Conclusions
The findings of diaphragm disease and omental cake on CT scan are highly predictive for high tumor dissemination (HTD) and thus likelihood of extensive surgery required to achieve low residual disease. In addition, multiple CT findings correlate strongly with the need for higher surgical complexity which should facilitate preoperative planning and/or triage to specialized centers. These preliminary data suggest specific CT findings can be used to optimize treatment planning.
Keywords: Ovarian Neoplasms, Outcome Assessment, Radiologic Imaging, Cytoreduction
INTRODUCTION
Epithelial ovarian carcinoma (EOC) is the fifth leading cause of cancer death in women. More than 70% of patients present with advanced disease (stage III or IV), and long-term survival rates are low (10–30%) [1]. The current standard of care for newly diagnosed ovarian carcinoma is primary surgical cytoreduction followed by platinum-based chemotherapy [2]. Multiple studies have shown that lowest residual disease after cytoreductive surgery improves response to chemotherapy as well as survival [3, 4].
Obviously, there are some patients for whom radical cytoreductive surgery may present an unacceptably high level of perioperative complications, making them poor candidates for this approach [5]. We previously published a multi-institutional study which defined subgroups of patients at highest risk for major morbidity and mortality after primary maximal cytoreductive surgery and adjuvant chemotherapy. Within a consecutive group of 576 cases of advanced stage EOC, a cohort of 38 (6.6% of cohort) were identified who were at very high risk for poor short-term outcomes. Specifically women with i) high initial tumor dissemination (HTD) or stage IV disease, plus ii) poor performance or nutritional status, plus iii) age ≥75 years, had a 63.6% risk of serious morbidity and demonstrated limited survival benefit from maximal cytoreduction. [6]. In addition to our prior work, multiple other studies have confirmed the impact of nutritional status and endogenous patient risk factors on surgical morbidity in general [7–9]. While many factors such as age, performance status, frailty and nutrition can be determined prior to surgery, tumor dissemination (and thus extent of surgery required for complete cytoreduction) is more difficult to assess preoperatively.
Our hypothesis is that preoperative imaging can be used to identify patients with HTD or stage IV disease. When combined with other salient preoperative risk-factors, this information could be used in the initial triage and treatment planning of OC patients. This is in stark contrast to the use of CT scans to predict successful surgical debulking. Several studies have failed to show that CT findings can predict cytoreductive surgery outcomes across centers [10–17]. Subjective interpretation of CT findings and difficulty with standardized definitions have both been cited as minor contributors to this. However, the primary reason predictive models fail to perform well in different clinical centers is the inherent variability in the rates of cytoreduction across institutions. A model developed in one setting with a rate of ‘optimal cytoreduction’ of 45%, has limited relevance when tested in a setting with a rate of 85%. Additionally, most studies have focused on predictors of suboptimal cytoreduction, instead of attempting to predict either extent of disease or type of procedures required to remove disease (surgical complexity)[15]. We reasoned that it is these latter variables that are most directly relevant to preoperative assessment of suitability for surgery or type of surgical expertise best suited to perform such surgery. To address this, we sought to determine whether specific findings available on routine preoperative CT could predict extent of disease dissemination and/or extent of surgery necessary for cytoreduction. Secondarily we compared sensitivity and specificity of CT for identifying disease in specific locations as found at primary exploration.
MATERIALS AND METHODS
In compliance with the Minnesota Statute for Use of Medical Information in Research, only patients who consented to the use of their medical records were included in the analysis. Institutional Review Board approval was obtained from the Mayo Foundation. Inclusion criteria included cases of EOC (including tubal and primary peritoneal cancers) with advanced stage (IIIC or IV) undergoing primary surgery at Mayo Clinic, Rochester, Minnesota between the years of 1997 and 2003. During this time surgical procedures were performed by one of seven gynecologic oncologists. Cases were required to have preoperative imaging with CT scanning (using both oral and intravenous contrast) to include the lower lungs to below the pelvis, and to have sufficient information in the operative note to allow adequate assignment of tumor dissemination. For this preliminary investigation, we selected 50 patients meeting the above criteria.
Electronic copies of CT scans archived using Digital Imaging and Communications in Medicine (Rosslyn, VA) software were assessed by one radiologist at our institution (BK). The radiologist was blinded as to surgical procedures performed, surgical findings and outcomes. Default slice thickness on the CT scanners at our institution was 5mm during the years of study.
Factors evaluated on all scans and their definitions were: a) diffuse peritoneal thickening (DPT) (≥4mm of disease identified in any of the following areas: lateral colic gutters, lateral conal fascia, anterior abdominal wall, diaphragm, pelvic peritoneal reflections); b) large-volume ascites (present on at least 2/3 of CT scan sections); c) large bowel involvement (sigmoid or non-sigmoid); d) diaphragmatic disease (small volume <1cm or large volume >1cm); e) splenic involvement (surface or hilar); f) hepatic involvement (surface or parenchymal); g) bulky omental disease; h) pleural space/extra-abdominal disease (including pleural effusion).
Demographic information, preoperative CA-125, operative time, findings at the time of surgery, surgical procedures performed, surgical complexity score as previously defined[6], amount of residual disease (RD), volume of ascites and estimated blood loss (EBL) were abstracted from clinical records. RD was classified as: no gross; ≤ 1cm; or > 1cm. For the purposes of this study, high tumor dissemination (HTD) was defined as previously outlined: carcinomatosis including bulky nodules (nodules greater than 4 cm or plaques) on the diaphragm surface and on the mesentery or with liver metastases.
Data was summarized using standard statistical statistics. Associations were evaluated using the chi-square test. Logistic regression was used to identify a combination of CT parameters associated with HTD. All calculated P values were two-sided and P values less than 0.05 were considered statistically significant. Statistical analysis was performed using the SAS version 9.2 software package (SAS Institute, Inc.; Cary, NC).
RESULTS
Among the 50 patients selected, 4 were subsequently excluded because of inadequate CT scan information. The demographics of the 46 cases meeting inclusion criteria are shown in Table 1. Twenty-six patients (57%) presented with stage IIIC disease, and the remainder (43%) with stage IV disease. The majority of patients (89%) had high grade lesions and serous histology (72%). The mean operating time was 228 minutes (range: 40–470 minutes). Among the 15 patients with RD > 1cm the mean (SD) operating room time was 178.6 (35.9) minutes with a range of 120–240 minutes. At the conclusion of surgery RD was as follows: 54% RD=0; 76% RD≤1 (including those with RD=0); 24% RD>1.
Table 1.
Summary of Patient Characteristics.
| Characteristic | Total (N=46) |
|---|---|
| Age at surgery (years) | |
| Mean (SD) | 66.4 (11.2) |
| Range | 39–82 |
| Stage, n (%) | |
| III | 26 (57%) |
| IV | 20 (43%) |
| Grade, n (%) | |
| 1 | 1 (2%) |
| 2 | 1 (2%) |
| 3 | 41 (89%) |
| Unknown | 3 (7%) |
| Histology, n (%) | |
| Serous | 33 (72%) |
| Mucinous | 1 (2%) |
| Endometrioid | 4 (9%) |
| Clear cell | 3 (7%) |
| Seroanaplastic | 3 (7%) |
| Brenner | 1 (2%) |
| Unknown | 1 (2%) |
| Operating room time (minutes) * | |
| Mean (SD) | 228.4 (83.2) |
| Range | 40–470 |
| Amount of residual disease (RD), n (%) | |
| No gross | 25 (54%) |
| <=1cm | 35 (76%) |
| >1cm | 11 (24%) |
| High Tumor Dissemination (HTD) | 25 (54%) |
The percentages may exceed 100% due to rounding.
We first evaluated the sensitivity and specificity of preoperative CT findings and location of disease noted at the time of surgery (Table 2). In general, a CT finding of no disease at a specific location was specific for lack of disease at that site by surgical exploration; however the sensitivity at most sites was low. Specifically, the absence of CT findings at the following sites correlated very well with the operative findings: diaphragmatic (specificity 100%) or pleural effusion as predictive of no diaphragm disease (specificity 87%); ascites present on ≥ 2/3 of CT scan sections (specificity 100%); sigmoid colon (specificity 100%); DPT (specificity 88%); extra-pelvic large bowel (specificity 91%); splenic (specificity 96%). However, the corresponding sensitivity at most sites was modest ranging from 29% (extra-pelvic large bowel involvement) to 65% (pleural effusion). Not surprisingly the sensitivity for parenchymal liver disease, surface liver disease and splenic involvement was quite high (sensitivity 100%), as surgery on these organs would typically be planned based upon preoperative diagnosis of disease.
Table 2.
Sensitivity and Specificity for CT Predictors of Surgical Findings.
| CT Finding | Surgical Finding | Sensitivity % (n/N) | Specificity % (n/N) |
|---|---|---|---|
| Diaphragm (any involvement) | Same | 48% (15/31) | 100% (15/15) |
| Pleural effusion | Diaphragm | 65% (20/31) | 87% (13/15) |
| Ascites on 2/3 of images | Ascites | 44% (16/36) | 100% (10/10) |
| Any sigmoid involvement | Same | 54% (21/39) | 100% (7/7) |
| Omental cake | Same | 72% (21/29) | 65% (11/17) |
| Splenic involvement | Same | 100% (1/1) | 96% (43/45) |
| Surface liver | Same | 100% (1/1) | 93% (42/45) |
| DPT | Mesenteric disease | 36% (13/36) | 90% (9/10) |
| Sigmoid encasement | Same | 33% (13/39) | 100% (7/7) |
| Large bowel involvement | Same | 29% (10/35) | 91% (10/11) |
| Any liver (surface or parenchymal) disease | Parenchymal liver | 100% (1/1) | 64% (29/45) |
| DPT | Carcinomatosis | 34% (13/38) | 88% (7/8) |
DPT, Diffuse peritoneal thickening.
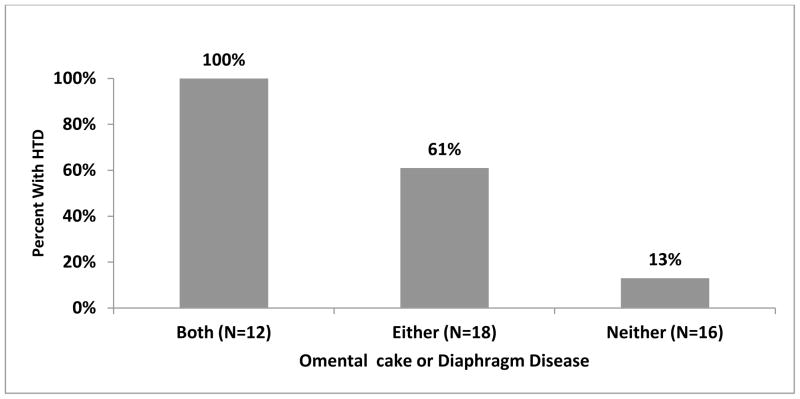
We tested the ability of CT scan to predict high tumor dissemination (HTD) (Table 3). Overall, 25 cases (54%) had HTD. Multiple findings were significantly associated with HTD by univariate analysis. Specifically, omental cake (P<0.001), diaphragmatic disease (P<0.001), liver disease (P=0.004), DPT (P=0.005), sigmoid involvement (P=0.006), ascites on at least 2/3 of images (P=0.008), and pleural effusion (P=0.017) were predictive of HTD. When we allowed all of the CT parameters to compete in a logistic regression model to predict HTD (Figure 1), the two strongest predictors were the presence of bulky omental disease and any diaphragmatic disease. The presence of both findings had a positive predictive value of 100% (12/12), although this only captured 12 out of 25 cases with HTD (sensitivity 48%). Comparing cases with HTD who were identified by CT scan vs. those missed by CT scan, the rate of RD≤1cm was 50% vs. 46%, respectively.
Table 3.
Correlation of CT Findings with High Tumor Dissemination (HTD)
| CT Finding |
|
|
|---|---|---|
| HTD n (%) | P-value† | |
| Omental Cake | <0.001 | |
| Yes (N=27) | 21 (78%) | |
| No (N=19) | 4 (21%) | |
| Diaphragmatic disease (any) | <0.001 | |
| Yes (N=15) | 14 (93%) | |
| No (N=31) | 11 (35%) | |
| Liver disease (any) | 0.004 | |
| Yes (N=17) | 14 (82%) | |
| No (N=29) | 11 (38%) | |
| DPT | 0.005 | |
| Yes (N=14) | 12 (86%) | |
| No (N=32) | 13 (41%) | |
| Sigmoid involvement (any) | 0.006 | |
| Yes (N=21) | 16 (76%) | |
| No (N=25) | 9 (36%) | |
| Ascites on 2/3 of images | 0.008 | |
| Yes (N=16) | 13 (81%) | |
| No (N=30) | 12 (40%) | |
| Pleural effusion | 0.017 | |
| Yes (N=22) | 16 (73%) | |
| No (N=24) | 9 (38%) | |
DPT, Diffuse peritoneal thickening.
Two-sided chi-square test.
Figure 1.
Predictive Value of Independent CT Predictors of Tumor Volume at Primary Surgery
When certain CT findings were compared with corresponding surgical procedures (e.g. stripping of pelvic peritoneum, splenectomy, omentectomy, diaphragm resection), the following relationships were observed. Among the 4 patients with liver disease (surface or parenchymal) noted on CT, 1 (25%) had a liver resection, compared to none of the remaining 42 patients without liver disease noted (p=0.001). The presence of extra-pelvic large bowel disease on CT was strongly associated with the need for large bowel resection at the time of surgery (36% (4/11) vs. 11% (4/25), p=0.06), though this was just outside of statistical significance, and likewise those with spleen disease on CT were more likely to have a splenectomy (33% (2/6) vs. 8% (3/40), p=0.06). Of the patients who had both omental cake and diaphragmatic disease (n=12) versus those who had neither omental cake nor diaphragmatic disease (n=16), 50% and 25% respectively required either spleen, liver, diaphragm, or colon surgery of any kind. For patients who received extensive surgery (i.e. spleen, liver, diaphragm, colon) the rate of RD≤1cm was 83%.
DISCUSSION
Determining the extent of disease in patients with advanced ovarian cancer is a challenge faced by every gynecologic oncologist in clinical practice. Accurate assessment is an important prerequisite for selecting the best treatment strategy, ascertaining patient fitness for surgery and triaging to expert centers or involving other surgical specialties when specific procedures are indicated. Thus it is critical to evaluate available preoperative testing. In our previous study we showed that HTD (along with endogenous patient risk factors such as age, performance status, and nutritional status) was a significant predictor of major perioperative complications after primary cytoreductive surgery [6]. Therefore, to make decisions regarding operability, extent of disease along with patient risk factors are both necessary. We sought to determine preoperative CT findings which will define patients who have HTD or will require specific surgical procedures to remove disease. In the present study we observed that the presence of both bulky omental disease and any diaphragm disease on CT scan correlate well with HTD, and thus likely complex surgery. Additionally, disease at specific sites was predictive of a higher likelihood for extended surgical procedures such as large bowel or liver resection or splenectomy.
Models using CT to predict successful cytoreductive surgery have been developed, but have not performed well when extrapolated to other centers[15]. For example, Fujwara et al. reported an association of implants in the small or large bowel mesentery, DPT, infrarenal para-aortic or pelvic lymph node involvement, omental caking, ascites fluid, and bowel encasement (≥2cm) with suboptimal cytoreduction in two separate models[18]. Simultaneously, Gerestein et al. examined 115 patients and found DPT and the presence of ascites to be most predictive[19]. Gemer et al. performed validation measurements of several studies[10, 12–14] and found lack of confirmation of their metrics[20]. In addition to utilizing CT scan findings to predict suboptimal cytoreduction, others have attempted to predict ability to achieve an optimal cytoreduction using various preoperative tests, including non-CT imaging and CA-125[12, 13, 15, 21–25]. There are inherent problems extrapolating models to predict the success or non-success of a debulking surgery to other centers. Primary amongst these is lack of uniform rates of cytoreduction across centers, along with lack of patient tolerance of aggressive surgery in some cases [26]. A model using CT scan to predict extent of disease should be absent these issues and could potentially be more generalizable.
We determined that diaphragm involvement, pleural effusion, large-volume ascites, sigmoid involvement, and omental cake were significantly associated with findings at primary surgery. These findings as well as any liver disease or diffuse peritoneal thickening were correlated with HTD, defined in the aforementioned study as carcinomatosis including bulky nodes (nodules greater than 4cm or plaques) on the diaphragm surface and on the mesentery, or with liver metastases. In addition, we report that omental caking and diaphragmatic disease on CT were the strongest predictors of HTD and thus secondarily predictors of extent of surgery required. When combined with a nutritional assessment or performance status and age, these preoperative CT findings of extensive disease can be useful in predicting women at highest risk of morbidity and mortality from primary surgery.
This preliminary study had several strengths. First, all CT scans were performed in a single institution and reviewed by a blinded radiologist with special expertise in this imaging modality. Second, surgeries were performed at one institution by gynecologic oncologists with advanced surgical training and the ability to reliably assess the upper abdomen. After each procedure the supervising surgeon recorded his or her operative findings on a form designed for this purpose, thus eliminating the risk of missed findings or procedures.
One weakness of this study was its retrospective nature. In order to truly determine the ideal treatment path for patients with high-volume disease, a prospective trial may be warranted. In addition, this preliminary study was designed to investigate an important topic in gynecologic oncology. Making firmer recommendations regarding preoperative CT scan and its use in treatment counseling will require examination of a larger cohort, which we plan to undertake. Finally, we did not evaluate every possible CT parameter, and more recent imaging technology including spiral CT with thinner sections and multiplanar image reconstructions may have increased the sensitivity of CT. We encourage other investigators to apply this model and refine it to their own cohorts to help define the best predictors.
Our results contribute to what is perhaps one of the most elusive aspects of gynecologic oncology: how to treat ovarian cancer patients without harming them further or significantly reducing their quality and/or quantity of life. The ability to accurately predict extent of tumor dissemination preoperatively will add needed information to the complicated equation which must weigh patient risk factors, extent of disease and treatment center expertise to individualize for the best treatment plan.
Highlights.
CT findings of diaphragm disease and omental cake are highly predictive of HTD.
Multiple CT findings correlate strongly with the need for higher surgical complexity.
Information gained using preoperative CT can help triage patients to appropriate surgical centers or alternative primary therapy.
Acknowledgments
Supported in part by the Mayo Clinic Ovarian SPORE (CA136393) and the Andersen Foundation (AM) and R01CA148747 (WC).
Footnotes
Authors’ Disclosure of Potential Conflicts of Interests: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hennessy BT, Coleman RL, Markman M. Ovarian cancer. Lancet. 2009;374:1371–82. doi: 10.1016/S0140-6736(09)61338-6. [DOI] [PubMed] [Google Scholar]
- 2.Morgan RJ, Jr, Alvarez RD, Armstrong DK, Boston B, Chen LM, Copeland L, Fowler J, Gaffney DK, Gershenson D, Greer BE, Grigsby PW, Havrilesky LJ, Johnston C, Lancaster JM, Lele S, Matulonis U, O’Malley D, Ozols RF, Remmenga SW, Sabbatini P, Schink J, Teng N. Ovarian cancer. Clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network: JNCCN. 2008;6:766–94. doi: 10.6004/jnccn.2008.0058. [DOI] [PubMed] [Google Scholar]
- 3.McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, Clarke-Pearson DL, Davidson M. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. The New England journal of medicine. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 4.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM, Baergen R. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2003;21:3194–200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 5.Aletti GD, Dowdy SC, Podratz KC, Cliby WA. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. American journal of obstetrics and gynecology. 2007;197:676, e1–7. doi: 10.1016/j.ajog.2007.10.495. [DOI] [PubMed] [Google Scholar]
- 6.Aletti GD, Eisenhauer EL, Santillan A, Axtell A, Aletti G, Holschneider C, Chi DS, Bristow RE, Cliby WA. Identification of patient groups at highest risk from traditional approach to ovarian cancer treatment. Gynecologic oncology. 2011;120:23–8. doi: 10.1016/j.ygyno.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Aletti GD, Santillan A, Eisenhauer EL, Hu J, Aletti G, Podratz KC, Bristow RE, Chi DS, Cliby WA. A new frontier for quality of care in gynecologic oncology surgery: multi-institutional assessment of short-term outcomes for ovarian cancer using a risk-adjusted model. Gynecologic oncology. 2007;107:99–106. doi: 10.1016/j.ygyno.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 8.Geisler JP, Linnemeier GC, Thomas AJ, Manahan KJ. Nutritional assessment using prealbumin as an objective criterion to determine whom should not undergo primary radical cytoreductive surgery for ovarian cancer. Gynecologic oncology. 2007;106:128–31. doi: 10.1016/j.ygyno.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Moreno Elola-Olaso A, Davenport DL, Hundley JC, Daily MF, Gedaly R. Predictors of surgical site infection after liver resection: a multicentre analysis using National Surgical Quality Improvement Program data. HPB: the official journal of the International Hepato Pancreato Biliary Association. 2012;14:136–41. doi: 10.1111/j.1477-2574.2011.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson BE, Rosenfield AT, Schwartz PE. Preoperative abdominopelvic computed tomographic prediction of optimal cytoreduction in epithelial ovarian carcinoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1993;11:166–72. doi: 10.1200/JCO.1993.11.1.166. [DOI] [PubMed] [Google Scholar]
- 11.Meyer JI, Kennedy AW, Friedman R, Ayoub A, Zepp RC. Ovarian carcinoma: value of CT in predicting success of debulking surgery. AJR American journal of roentgenology. 1995;165:875–8. doi: 10.2214/ajr.165.4.7676985. [DOI] [PubMed] [Google Scholar]
- 12.Bristow RE, Duska LR, Lambrou NC, Fishman EK, O’Neill MJ, Trimble EL, Montz FJ. A model for predicting surgical outcome in patients with advanced ovarian carcinoma using computed tomography. Cancer. 2000;89:1532–40. doi: 10.1002/1097-0142(20001001)89:7<1532::aid-cncr17>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 13.Dowdy SC, Mullany SA, Brandt KR, Huppert BJ, Cliby WA. The utility of computed tomography scans in predicting suboptimal cytoreductive surgery in women with advanced ovarian carcinoma. Cancer. 2004;101:346–52. doi: 10.1002/cncr.20376. [DOI] [PubMed] [Google Scholar]
- 14.Qayyum A, Coakley FV, Westphalen AC, Hricak H, Okuno WT, Powell B. Role of CT and MR imaging in predicting optimal cytoreduction of newly diagnosed primary epithelial ovarian cancer. Gynecologic oncology. 2005;96:301–6. doi: 10.1016/j.ygyno.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 15.Axtell AE, Lee MH, Bristow RE, Dowdy SC, Cliby WA, Raman S, Weaver JP, Gabbay M, Ngo M, Lentz S, Cass I, Li AJ, Karlan BY, Holschneider CH. Multi-institutional reciprocal validation study of computed tomography predictors of suboptimal primary cytoreduction in patients with advanced ovarian cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25:384–9. doi: 10.1200/JCO.2006.07.7800. [DOI] [PubMed] [Google Scholar]
- 16.Risum S, Hogdall C, Loft A, Berthelsen AK, Hogdall E, Nedergaard L, Lundvall L, Engelholm SA. Prediction of suboptimal primary cytoreduction in primary ovarian cancer with combined positron emission tomography/computed tomography--a prospective study. Gynecologic oncology. 2008;108:265–70. doi: 10.1016/j.ygyno.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Jung DC, Kang S, Kim MJ, Park SY, Kim HB. Multidetector CT predictors of incomplete resection in primary cytoreduction of patients with advanced ovarian cancer. European radiology. 2010;20:100–7. doi: 10.1007/s00330-009-1533-0. [DOI] [PubMed] [Google Scholar]
- 18.Fujwara K, Yoshino K, Enomoto T, Fujita M, Ueda Y, Miyatake T, Kimura T, Muraji M, Fujita H, Hori M. Usefulness of computed tomography in predicting cytoreductive surgical outcomes for ovarian cancer. Archives of gynecology and obstetrics. 2011;284:1501–7. doi: 10.1007/s00404-011-1864-3. [DOI] [PubMed] [Google Scholar]
- 19.Gerestein CG, Eijkemans MJ, Bakker J, Elgersma OE, van der Burg ME, Kooi GS, Burger CW. Nomogram for suboptimal cytoreduction at primary surgery for advanced stage ovarian cancer. Anticancer research. 2011;31:4043–9. [PubMed] [Google Scholar]
- 20.Gemer O, Gdalevich M, Ravid M, Piura B, Rabinovich A, Gasper T, Khashper A, Voldarsky M, Linov L, Ben Shachar I, Anteby EY, Lavie O. A multicenter validation of computerized tomography models as predictors of non- optimal primary cytoreduction of advanced epithelial ovarian cancer. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2009;35:1109–12. doi: 10.1016/j.ejso.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Chi DS, Venkatraman ES, Masson V, Hoskins WJ. The ability of preoperative serum CA-125 to predict optimal primary tumor cytoreduction in stage III epithelial ovarian carcinoma. Gynecologic oncology. 2000;77:227–31. doi: 10.1006/gyno.2000.5749. [DOI] [PubMed] [Google Scholar]
- 22.Memarzadeh S, Lee SB, Berek JS, Farias-Eisner R. CA125 levels are a weak predictor of optimal cytoreductive surgery in patients with advanced epithelial ovarian cancer. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society. 2003;13:120–4. doi: 10.1046/j.1525-1438.2003.13019.x. [DOI] [PubMed] [Google Scholar]
- 23.Berchuck A, Iversen ES, Lancaster JM, Dressman HK, West M, Nevins JR, Marks JR. Prediction of optimal versus suboptimal cytoreduction of advanced-stage serous ovarian cancer with the use of microarrays. American journal of obstetrics and gynecology. 2004;190:910–25. doi: 10.1016/j.ajog.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Ricke J, Sehouli J, Hach C, Hanninen EL, Lichtenegger W, Felix R. Prospective evaluation of contrast-enhanced MRI in the depiction of peritoneal spread in primary or recurrent ovarian cancer. European radiology. 2003;13:943–9. doi: 10.1007/s00330-002-1712-8. [DOI] [PubMed] [Google Scholar]
- 25.Risum S, Loft A, Hogdall C, Berthelsen AK, Hogdall E, Lundvall L, Nedergaard L, Engelholm SA. Standardized FDG uptake as a prognostic variable and as a predictor of incomplete cytoreduction in primary advanced ovarian cancer. Acta oncologica. 2011;50:415–9. doi: 10.3109/0284186X.2010.500296. [DOI] [PubMed] [Google Scholar]
- 26.Elattar A, Bryant A, Winter-Roach BA, Hatem M, Naik R. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane database of systematic reviews. 2011:CD007565. doi: 10.1002/14651858.CD007565.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]