Abstract
Vγ2Vδ2 T cells in human peripheral blood recognize phosphoantigen and play important roles in host defense and immunoregulation. The TCR is required for Vγ2Vδ2 T cell responses to phosphoantigen, but less is known about soluble or cell-associated costimulatory molecules. In this study, we show that human Vγ2Vδ2 T cell responses to phosphoantigen, including activation, proliferation, cytokine production, and tumor cell cytotoxicity, require TNF-α binding to its receptor, with a preference for TNFR2. Because stimulated Vγ2Vδ2 cells also produce TNF-α, this may be a positive control mechanism to sustain the response. Impaired proliferation in the presence of TNF-α or TNFR blocking agents was partially rescued by a TLR2 agonist, Pam3Cys. Our studies demonstrate that TNF-α plays a critical role in regulating human Vγ2Vδ2 T cell immune responses.
The γδ T cells represent a distinct lymphocyte subset characterized by TCR possessing unique structural and Ag-binding properties (1, 2). In human peripheral blood, γδ T cells are 1–10% of total T cells, with the majority (>80%) expressing the Vγ2Vδ2 (also termed Vγ9Vδ2) TCR (hereafter referred to as Vδ2 T cells) and ~75% of them having the Vγ2-Jγ1.2 rearrangement (3). Vδ2 T cells are expanded in vivo in the context of various infectious diseases, such as tuberculosis (4), legionellosis (5), tularemia (6), brucellosis (7), malaria (8), mononucleosis (9), Listeria (10), or Salmonella (11). The stimulating bacterial Ags are low m.w., nonpeptidic compounds termed phosphoantigens (12, 13) that are related to isoprenoid biosynthesis.
In contrast to αβ T cells, γδ cells in humans often lack CD4 or CD8 expression, recognize phosphoantigens in a MHC-unrestricted manner, and do not require Ag processing by professional APCs (12). Similar to NK cells, Vδ2 T cells express MHC I receptors, including the inhibitory CD94/NKG2 complexes, and killer Ig-like receptors (14, 15) that are involved in tumor recognition and cytolysis. The potently cytotoxic subset in humans is identified by cell surface expression of polysialylated CD56 (16). Vδ2 T cells display a range of innate effector functions, including the rapid secretion of chemokines MIP-α, MIP-β, or RANTES (17, 18) and cytokines, including TNF-α and IFN-γ (19, 20). After phosphoantigen stimulation, Vδ2 cells express markers of cyto-toxicity (21) and contribute to adaptive immunity by providing B cell help and promoting dendritic cell (DC)3 maturation (22, 23). We know that TCR is required for responses to phosphoantigen, but less is known about soluble or cell-associated costimulatory molecules. In this study, we focus on TNF-α and its role in Vδ2 cell activation.
TNF-α was identified originally as a product of lymphocytes or macrophages that caused lysis of tumor cells (24). It is now recognized as a cytokine that is critically required for several biological processes. The pleiotropic actions of TNF-α include stimulating cell growth and differentiation, promoting inflammation, or inducing cell death mechanisms (25, 26). TNF-α exerts its effects by binding to cell surface receptor TNFR1 (also known as p55TNFR, p60, CD120a, or TNFRSF1a) or TNFR2 (also known as p75TNFR, p80, CD120b, or TNFRSF1b) (26, 27). Bound TNFRs mediate the assembly of distinct adaptor protein complexes that regulate signaling processes, including kinase or phosphatase activation, lipase stimulation, and protease induction (26).
TNF-α also has a role in the pathogenesis of inflammatory or immune-mediated diseases, such as rheumatoid arthritis, psoriatic arthritis, and Crohn’s disease (28, 29); therapeutic agents that block TNF-α activity are in clinical use for these and other conditions (30–32). Despite impressive efficacy for treating inflammatory diseases, TNF-α inhibitor therapy carries substantial risk for serious complications, including reactivation of latent tuberculosis (31, 33). Tuberculosis is of particular importance in this study because Vδ2 cells respond in vitro (34) and in vivo (35) to mycobacteria and may be important for protection against disease.
In previous studies, TNF-α or its receptors were reported to affect certain aspects of immunity, including DC maturation/recruitment (36), along with αβ T cell priming (37), proliferation (38), recruitment (39), and function (40). Although the mechanisms remain incompletely defined, TNF-α seems critical for efficient T cell responses (41, 42). In the mouse, the cell response to LPS involves TNF-α that potently stimulates γδ T cells expressing the p75 TNFR (43). In this work, we studied the effect of TNF-α on human cells, to define the role for this costimulatory cytokine in Vγ2Vδ2 cell responses to phosphoantigen.
Materials and Methods
PBMC and tumor cell lines
Whole blood was obtained from healthy human volunteers who provided written informed consent. Total lymphocytes were separated from heparinized peripheral blood by density gradient centrifugation (Ficoll-Paque; Amersham Biosciences). PBMC and TU167 cells were cultured in RPMI 1640 supplemented with 10% FBS (Life Technologies), 2 mMol/L L-glutamine, and penicillin-streptomycin (100 U/ml and 100 mg/ml, respectively); for Daudi B cells (CCL-213; American Type Culture Collection), 4.5 g/L glucose, 1.5 g/L NaHCO3, 10 mMol/L HEPES, and 1 mMol/L sodium pyruvate were added.
In vitro proliferation assays
PBMC (5 × 105 cells/well) were cultured in 12-well plates with complete medium, 15 μM isopentyl pyrophosphate (IPP; Sigma-Aldrich), and 100 U/ml human rIL-2 (Tecin, Biological Resources Branch, National Institutes of Health). In some experiments, anti-TNF-α (clone 28401), anti-TNFR1 (clone 16803), anti-TNFR2 (clone 22210), anti-IFN-γ (clone 25723) blocking Abs (R&D Systems), human rTNF-α (Invitrogen), or the synthetic lipoprotein Pam3Cys-SK4 (Pam3Cys; EMC) were added at varying concentrations. Fresh complete medium and 100 U/ml IL-2 were added every 3 days. The γδ T cell proliferation was measured by staining for CD3 and Vδ2, then defining the percentage of γδ T cells within the total lymphocyte population at days 0, 4, 7, and 10 by flow cytometry. The Vδ2 cell numbers were calculated from the total cell count and the subset frequency in lymphocytes.
Purifying Vδ2+ cells
The Vδ2+ subsets from fresh PBMC or PBMC expanded with IPP and IL-2 were purified with a MultiSort Kit (Miltenyi Biotec), according to the manufacturer’s instructions. Cells were stained with PE-conjugate Vδ2 Abs for 10 min on ice. The Vδ2-PE-labeled cells were washed and incubated with anti-PE MicroBeads for 15 min on ice, then separated in a magnetic field. We achieved 90–98% purity after magnetic bead separation, as measured by flow cytometry.
Cytotoxicity assay
A nonradioactive, fluorometric cytotoxicity assay with calcein-acetoxym-ethyl (Molecular Probes) was used to measure cytotoxicity against Daudi B cell or TU167 squamous cell tumor lines. Expanded γδ cells (effector cells) were treated with anti-TNFR1 or anti-TNFR2 blocking Abs for 1 h at 37°C and washed to remove the Ab. Daudi B cells or TU167 cells (target cells) were labeled for 15 min with 2 mMol/L calcein-acetoxymethyl at 37°C and then washed once with PBS. Cells were combined at various E:T ratios in 96-well, round-bottom microtiter plates (Corning Glass) and incubated at 37°C in 5% CO2 for 4 h; assays were performed in triplicate. In some cases, neutralizing anti-TNF-α Abs (5 μg/ml) were added. After incubation, supernatants were transferred to a 96-well flat-bottom micro-titer plate, and calcein content was measured using a Wallac Victor2 1420 multichannel counter (l485/535 nm). Percent specific lysis was calculated as follows: (test release − spontaneous release)/(maximum release − spontaneous release) × 100.
Flow cytometry
Unless noted, cells were stained with fluorophore-conjugated mAbs from BD Biosciences. Generally, 3 × 105–5 × 105 cells were washed, resuspended in 50–100 μl of RPMI 1640, and stained with mouse anti-human Vδ2-PE clone B6, mouse anti-human CD3-FITC clone UCHT1, mouse anti-human CD3-allophycocyanin clone UCHT1, mouse anti-human CD69-PE clone FN50, mouse anti-human CD107a-FITC clone H4A3, and isotype controls, including rabbit anti-mouse IgG1-FITC clone X40, IgG1-PE clone X40, and IgG1-allophycocyanin clone X40. For detecting intracellular IFN-γ or TNF-α, cells were stained with Vδ2-PE, then fixed, permeabilized, and incubated for 45 min at 4°C with mouse anti-human IFN-γ-allophycocyanin clone B27 or mouse anti-human TNF-α-allophycocyanin clone MAb11. Intracellular staining solutions were obtained from the Cytofix/Cytoperm Kit (BD Biosciences). Data for at least 1 × 104 lymphocytes (gated on the basis of forward- and side-scatter profiles) were acquired for each sample on a FACSCalibur flow cytometer (BD Biosciences). All samples were analyzed using FlowJo software (Tree Star). For stimulation before staining, PBMC or Vδ2 T cells were stimulated with IPP, anti-γδ-TCR Ab (clone B1.1; eBiosciences), Daudi B cells, or TU167 cells for 2–18 h. For intracellular staining, the protein transport inhibitor brefeldin A (BD Biosciences) was added at the beginning of stimulation or 4 h before staining. In some experiments, Vδ2 T cells were treated with anti-TNFR1 or anti-TNFR2 blocking Abs for 1 h at 37°C before stimulation.
Detecting cytokines by ELISA
Human IFN-γ in culture supernatants was detected with a human IFN-γ ELISA kit (R&D Systems), according to the manufacturer’s directions. Human TNF-α in culture supernatants was detected with a human TNF-α ELISA kit (R&D Systems), according to the manufacturer’s directions.
Statistical analysis
Differences among groups were analyzed by Student’s t test. Value of p < 0.05 was considered to be significant.
Results
Vδ2 T cells, but not CD4, CD8, B cells, monocytes, or NK cells, produce TNF-α in IPP-stimulated PBMC
It was shown before that Vδ2 T cells produce TNF-α after IPP stimulation (21, 44). To show whether this is specific to Vδ2 cells, PBMC were stimulated with IPP (15 μM/ml) in the presence of 10 μg/ml brefeldin A. After 4 h, cell surface markers and intracellular cytokines were stained with anti-Vδ2, anti-CD3, anti-CD4, anti-CD8, anti-CD14, anti-CD20, anti-CD56, anti-TNF-α, and isotype-matched controls. For flow cytometry analysis, the cells were gated on Vδ2+, CD3+CD4+, CD3+CD8+, CD14+ (monocytes), CD20+ (B cells), or CD3−CD56+ (NK cells) cells, and TNF-α expression was analyzed. The results demonstrated that TNF-α production in response to IPP was occurring primarily in Vδ2 T cells and was not substantial among CD3+CD4+, CD3+CD8+, CD14+, CD20+, or CD3−CD56+ cells in these cultures (Fig. 1).
FIGURE 1.
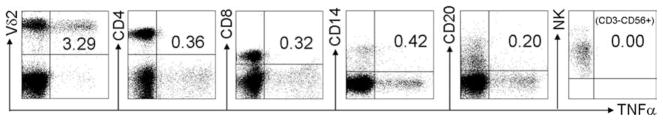
Vδ2 T cells, but not CD4, CD8, B cells, monocytes, or NK cells, produce TNF-α in IPP-stimulated PBMC. PBMC were stimulated with IPP (15 μM/ml) in the presence of 10 μg/ml brefeldin A. After stimulation for 4 h, the cell surface markers and intracellular cytokine were stained with anti-Vδ2, anti-CD3, anti-CD4, anti-CD8, anti-CD14, anti-CD20, anti-CD56, anti-TNF-α, and isotype-matched controls. The expression of TNF-α was analyzed by flow cytometry. Data are representative of three experiments yielding similar results.
The absence of TNF-α impairs IPP-stimulated Vδ2 T cell proliferation, activation, and IFN-γ production
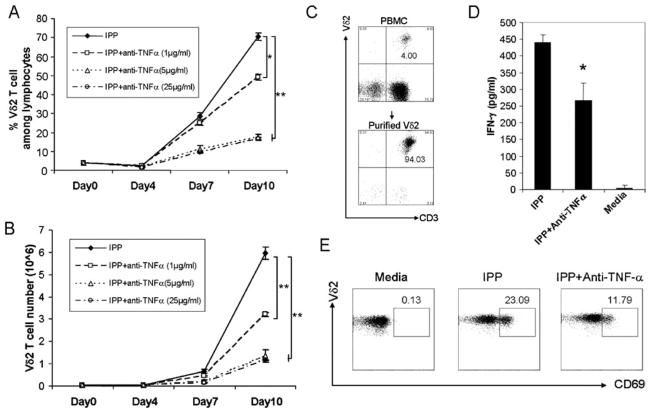
To test whether TNF-α plays an important role in Vδ2 T cell proliferation, TNF-α neutralizing Abs were added to IPP/IL-2-treated cultures, and cells were cultured for 10 days. IPP-driven Vδ2 T cell expansion (both frequency and absolute number) was inhibited significantly by TNF-α neutralizing Abs in a dose-dependent manner (Fig. 2, A and B).
FIGURE 2.
The absence of TNF-α impairs IPP-stimulated Vδ2 T cell proliferation, activation, and IFN-γ production. A and B, Anti-TNF-α blocking Ab at different doses was added to IPP/IL-2-treated cultures, and cells were cultured for 10 days. Both Vδ2 frequency (A) and absolute number (B) were detected every 3 days. The cultures were set up in triplicate. C, Purified Vδ2 T cells by magnetic beads from PBMC are shown. D, Purified Vδ2 T cells stimulated by IPP with or without anti-TNF-α blocking Ab. After stimulation for 18 h, the level of IFN-γ in cell-free supernatant was detected by ELISA. E, Fresh PBMC were stimulated by IPP with or without anti-TNF-α blocking Ab, and the activation marker (CD69) expression on Vδ2 T cells was analyzed by flow cytometry. *, p < 0.01; **, p < 0.001. Data are representative of three experiments yielding similar results.
The impact of TNF-α on cytokine production and activation marker expression was tested next. Vδ2 T cells were purified (Fig. 2C) and stimulated by IPP with or without anti-TNF-α neutralizing Abs. After 18 h, the levels of IFN-γ and TNF-α in cell-free supernatants were detected by ELISA, and expression of the activation marker CD69 on Vδ2 T cells was analyzed by flow cytometry. The ELISA results revealed low levels of cell-free IFN-γ when Vδ2 cells were cultured with medium only; IPP stimulation markedly enhanced IFN-γ production. The absence of TNF-α significantly reduced IFN-γ production ( p < 0.01; Fig. 2D). The flow cytometry results showed that IPP induced CD69 expression on Vδ2 T cells, but activation marker expression was reduced when TNF-α was blocked (Fig. 2E). There was no effect on proliferation of an isotype-matched Ab against IFN-γ (Fig. 3). Exogenous TNF-α added to IPP-stimulated PBMC increased Vδ2 T cell proliferation (Fig. 4) above levels seen normally with IPP stimulation alone.
FIGURE 3.
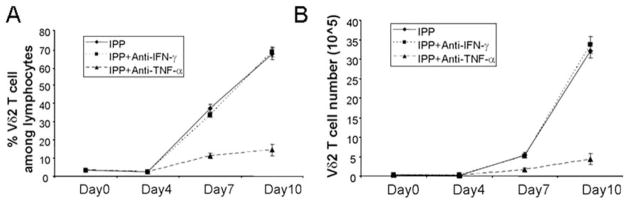
Specific inhibition of Vδ2 T cell proliferation by TNF neutralizing Abs. Anti-TNF-α and anti-IFN-γ Ab (5 μg/ml) were added to IPP/IL-2-treated cultures, and cells were cultured for 10 days. Both Vδ2 frequency (A) and absolute number (B) were measured every 3 days. The cultures were set up in triplicate. Data are representative of three experiments yielding similar results.
FIGURE 4.
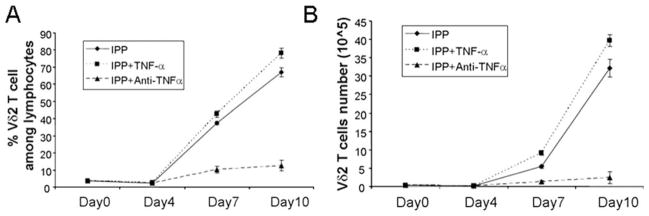
Exogenous TNF-α increased Vδ2 T cell proliferation. TNF-α (50 ng/ml) was added to IPP/IL-2-treated cultures, and cells were cultured for 10 days. Both Vδ2 frequency (A) and absolute number (B) were measured every 3 days. The cultures were set up in triplicate. Data are representative of three experiments yielding similar results.
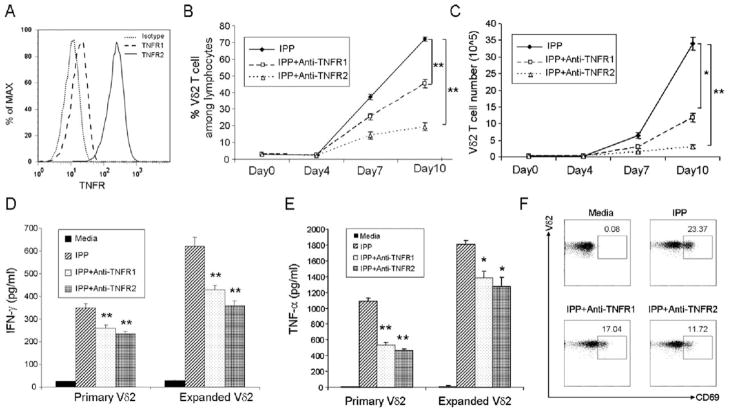
TNFR2 on Vδ2 T cells is mainly responsible for enhancing T cell proliferation, activation, and cytokine production
Two distinct receptors, TNFR1 and TNFR2, mediate the multiple effects of TNF-α. We detected expression of both receptors on Vδ2 T cells, but TNFR2 was expressed at higher levels than TNFR1 (Fig. 5A). To determine whether blocking either TNFR1 or TNFR2 would affect Vδ2 cell functions, the receptors were blocked individually with neutralizing Abs and cells were stimulated with IPP, as described above. Blocking both TNFR1 and TNFR2 significantly reduced proliferation (Fig. 5, B and C), CD69 expression (Fig. 5F), IFN-γ (Fig. 5D), and TNF-α (Fig. 5E) production by Vδ2 cells in response to IPP. When blocked individually, TNFR2 had a greater impact on Vδ2 cell responses. These results indicated that TNFR2 was the preferred costimulatory receptor on Vδ2 T cells similar to what was reported for murine γδ T cells (43).
FIGURE 5.
TNFR2 on Vδ2 T cell is mainly responsible for enhancing T cell proliferation, activation, and cytokine production. A, TNFR1 and TNFR2 expression on Vδ2 T cells was assessed by flow cytometry. B and C, Anti-TNFR1 or anti-TNFR2 blocking Abs were added to IPP/IL-2-treated cultures at an optimized concentration (5 μg/ml), and cells were cultured for 10 days. Both Vδ2 frequency (B) and absolute number (C) were detected every 3 days. The cultures were set up in triplicate. D and E, Purified Vδ2 T cells from primary PBMC or IPP-expanded PBMC were treated with or without anti-TNFR1 or anti-TNFR2 blocking Abs and stimulated by IPP. After stimulation for 18 h, the level of IFN-γ (D) and TNF-α (E) in cell-free supernatant was detected by ELISA. F, Fresh PBMC were treated with or without anti-TNFR1 or anti-TNFR2 blocking Abs and stimulated by IPP. The activation marker (CD69) expression on Vδ2 T cells was analyzed by flow cytometry. *, p < 0.01; **, p < 0.001. Data are representative of three experiments yielding similar results.
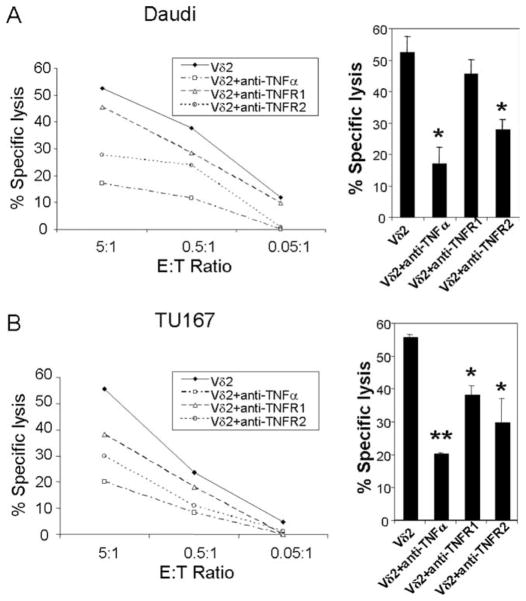
The absence of TNF-α or TNF-α/TNFR2 signal impairs Vδ2 cell cytotoxicity against tumor cells
Using the TNF-α or TNF-α/TNFR blocking conditions, we tested the effect on Vδ2 cell cytotoxicity. Tumor cell lines Daudi and TU167 were used. The results demonstrated that TNF-α is important for Vδ2 T cell cytotoxicity. When TNF-α was blocked during the cytotoxicity assay, specific lysis for both Daudi (Fig. 6A) and TU167 cells (Fig. 6B) was reduced significantly. Vδ2 T cell cytotoxicity was also impaired when TNFR2 was blocked and the Ab was removed by washing before effector cells were added to targets. These data indicated that TNF-α and the signal mediated by its receptors on Vδ2 T cells are important for tumor cytotoxicity.
FIGURE 6.
The absence of TNF-α or TNF-α/TNFR2 signal impairs Vδ2 T cell cytotoxicity against tumor cells. A and B, The cytotoxicity function of Vδ2 T cells against Daudi (A) or TU167 (B) with or without anti-TNF-α, anti-TNFR1, or anti-TNFR2 blocking Abs was evaluated at different E:T ratio in triplicate. The statistical significance of specific lysis compared with Vδ2 T cells at E:T = 5:1 was analyzed. *, p < 0.01; **, p < 0.001. Data are representative of three experiments yielding similar results.
Tumor cells stimulate TNF-α production, but not CD107a expression by Vδ2 T cells
TNF-α, granzyme, and perforin are important factors for Vδ2 cell cytotoxicity. We showed before that Vδ2 T cells are granzyme B and perforin positive (45). In response to TCR ligands (IPP or anti-γδ-TCR Ab), Vδ2 T cells produce TNF-α and increase surface expression of CD107a (45). In this study, we show that Vδ2 cells produce TNF-α after simulation by Daudi B cells or TU167 cells at a ratio of 1:1. We also know that Daudi cells stimulate Vδ2 cell proliferation (21), which is consistent with the higher levels of TNF-α-producing cells compared with TU167, which is a good target, but a poor stimulator. In contrast, tumor cells did not increase the expression of CD107a, irrespective of TNF-α production (Fig. 7). These data suggest that TNF-α or other factors may be more important than granzyme release for Vδ2 cell cytotoxicity.
FIGURE 7.
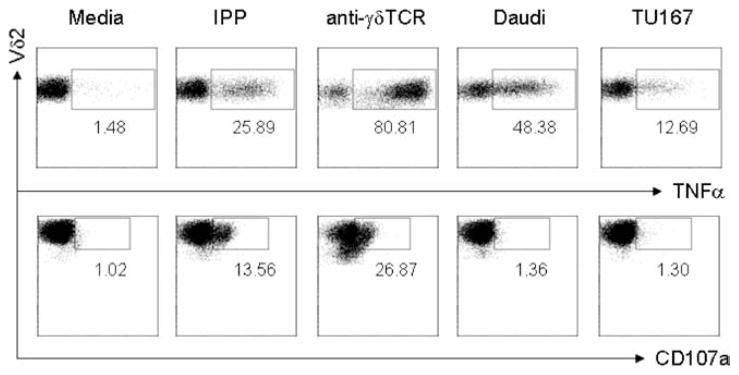
Tumor cells stimulate TNF-α production, but not CD107a expression on Vδ2 T cells. IPP-expanded Vδ2 T cells were stimulated with medium, IPP, anti-γδ-TCR Ab, Daudi, or TU167 cells, respectively. To detect TNF-α, 10 μg/ml brefeldin A was added. Two hours later, cells were collected and stained with Vδ2, CD107a, or TNF-α Abs and analyzed by flow cytometry. Data are representative of three experiments yielding similar results.
Pam3Cys, a TLR2 agonist, can rescue Vδ2 T cell proliferation and IFN-γ production after blocking TNF-α
Our previous work showed that Vδ2 cells respond to the TLR2 agonist (Pam3Cys), but require coincident TCR stimulation (45). In this study, we tested whether Pam3Cys can rescue IPP-stimulated Vδ2 cell proliferation in the absence of TNF-α signaling. The results showed that Pam3Cys partly increased the proliferative response to IPP (Fig. 8, A and B), indicating that TLR triggering could compensate for the costimulatory function of TNF-α. Furthermore, to rule out that Pam3Cys might be acting through monocytic or DC in the PBMC culture, we tested this TLR2 agonist on purified Vδ2 T cell and measured the effect on IFN-γ production. The Pam3Cys rescued IFN-γ production in the presence of blocking Ab against TNF-α (Fig. 8C), showing that signaling through TLR2 partly substituted for TNF-α in Vδ2 T cell activation.
FIGURE 8.
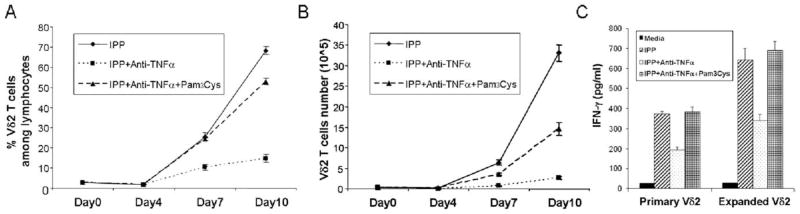
Pam3Cys, a TLR2 agonist, can rescue Vδ2 T cell proliferation and IFN-γ production after blocking TNF-α. A and B, Anti-TNF-α blocking Ab (5 μg/ml) or Pam3Cys (10 μg/ml) was added to IPP/IL-2-treated cultures, and cells were cultured for 10 days. Both Vδ2 frequency (A) and absolute number (B) were detected every 3 days. The cultures were set up in triplicate. C, Purified Vδ2 T cells from primary PBMC or IPP-expanded PBMC were treated with or without anti-TNF-α blocking Abs or Pam3Cys and stimulated by IPP. After stimulation for 18 h, the level of IFN-γ in cell-free supernatant was detected by ELISA. Data are representative of three experiments yielding similar results.
Discussion
Human Vδ2 T cells play important roles in host defense, but have Ag recognition and immunoregulation mechanisms distinct from conventional T cells. In the present study, we focused on the impact of TNF-α on Vδ2 T cell function. The Vδ2 T cell responses to Ag stimulation, including activation, proliferation, cytokine production, and tumor cell cytotoxicity, were impaired significantly when TNF-α or its receptor was blocked. It is well known that Vδ2 T cells produce TNF-α after TCR stimulation; in this study, we showed that Vδ2 T cells are the major source of TNF-α in IPP-stimulated PBMC. Now we show that TNF-α is a positive regulator of human Vδ2 T cells similar to what was reported for murine γδ T cells (43). Another cytokine, IL-2, is also required for IPP-driven Vδ2 T cells expansion in vitro (21, 46) and in vivo (47), but is not produced by Vδ2 cells and must be supplied exogenously. Stimulated Vδ2 cells are the primary source of TNF-α in our PBMC cultures, so an exogenous source is not required. The co-stimulatory signal is important for TCR-dependent activation and cytokine production by Vδ2 T cells.
The TNF-α signal is mediated by binding to TNFRs. TNFRs comprise a superfamily of proteins with at least 41 members that are characterized by sequence homology within the extracellular domains. This group includes TNFRs, Fas, CD40, the low-affinity nerve growth factor receptor, TRAIL receptors, RANK, and other death and decoy receptors (48). TNF ligand achieves all its different cellular and pathological effects by binding to TNFR1 and TNFR2 (26). Our studies demonstrated that both TNFR1 and TNFR2 were expressed on Vδ2 T cells, but TNFR2 was expressed at a higher level. Further studies using neutralizing Abs showed that TNFR2 is mainly responsible for mediating TNF-α effects on Vδ2 T cells. Although this can be explained partly by the relative expression levels, it might be due to the different characteristics and cellular consequences of these two different TNFRs and their signaling pathways (26).
The TNF-α/TNFR signal might be substituted by other costimulatory factors that induce similar signaling pathways. Published studies demonstrated that TNF-α binding to its receptors triggers a series of intracellular events that ultimately result in the activation of two major transcription factors, NF-κB and c-Jun (26), which are important for cell growth and activation. Alternately, TLR signaling involves a family of five adaptor proteins that couple to downstream protein kinases that ultimately lead to activation of NF-κB and members of the IFN-regulatory factor family. We know that Vδ2 T cells respond to TLR2 agonist (Pam3Cys) with coincident TCR stimulation (45), and we showed in this study that Pam3Cys partly rescued Vδ2 T cell proliferation in the absence of TNF-α. This suggests the importance of NF-κB signaling pathway in initiating Vδ2 T cell proliferation.
Blocking TNF-α or its receptors significantly impaired Vδ2 T cell cytotoxicity against tumor cells. This may be due to the combined effects of blocking cell proliferation and reducing production of TNF-α itself. This shows that TNF-α signaling is required for effector functions, including high-level TNF-α release.
Our studies may have clinical significance for anti-TNF-α therapy. Recognition of the central role for TNF-α in the pathophysiology of inflammation led to the development of TNF-α inhibitors that have revolutionized the therapeutic approaches to autoimmune and inflammatory diseases (31). The initial clinical experience with TNF-α inhibitors demonstrated impressive efficacy against rheumatoid arthritis, but carried a risk for tuberculosis (31, 33). Tuberculosis appeared commonly within the first few months of use, frequently was disseminated or atypical in presentation, and resulted in high death rates. These cases were believed due, in most cases, to reactivation of latent mycobacterial infection (32).
Several lines of evidence suggest that Vδ2 T cells are involved in the protective immune response against tuberculosis. The Vδ2 cells expand in response to nonpeptidic Ags from mycobacterium (34, 49), and they accumulate in bacillus Calmette-Guerin-vaccinated and purified protein derivative skin test-positive individuals with latent tuberculosis infection (35, 50). Vδ2 T cell effector functions, such as TNF-α and IFN-γ secretion, cytotoxicity, and a capacity to reduce the viability of extracellular and intracellular mycobacteria, have all been associated with protective immunity (16, 51, 52). We hypothesize that the impairment of Vδ2 T cells might be an important reason for the high risk of tuberculosis in recipients of anti-TNF-α therapy. It may be important to consider supportive therapies, including agonists for TLR2 or similar agents, to overcome the block to TNF-α signaling. These adjunctive agents may restore the protective effects of Vδ2 T cells against tuberculosis and other opportunistic infections that arise when TNF-α signaling is interrupted.
Acknowledgments
We thank Cheryl Armstrong for technical assistance. We are grateful to Cristiana Cairo and Jean-Saville Cummings for critical reading of the manuscript.
Footnotes
This work was supported by Public Health Service Grant AI077059 (to C.D.P.).
Abbreviations used in this paper: DC, dendritic cell; IPP, isopentyl pyrophosphate.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Shin S, El-Diwany R, Schaffert S, Adams EJ, Garcia KC, Pereira P, Chien YH. Antigen recognition determinants of γδ T cell receptors. Science. 2005;308:252–255. doi: 10.1126/science.1106480. [DOI] [PubMed] [Google Scholar]
- 2.Carding SR, Egan PJ. Gammaδ T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 3.Evans PS, Enders PJ, Yin C, Ruckwardt TJ, Malkovsky M, Pauza CD. In vitro stimulation with a non-peptidic alkylphosphate expands cells expressing Vγ2-Jγ1.2/Vδ2 T-cell receptors. Immunology. 2001;104:19–27. doi: 10.1046/j.0019-2805.2001.01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balbi B, Valle MT, Oddera S, Giunti D, Manca F, Rossi GA, Allegra L. T-lymphocytes with γδ+ Vδ2+ antigen receptors are present in increased proportions in a fraction of patients with tuberculosis or with sarcoidosis. Am Rev Respir Dis. 1993;148:1685–1690. doi: 10.1164/ajrccm/148.6_Pt_1.1685. [DOI] [PubMed] [Google Scholar]
- 5.Kroca M, Johansson A, Sjostedt A, Tarnvik A. Vγ9Vδ2 T cells in human legionellosis. Clin Diagn Lab Immunol. 2001;8:949–954. doi: 10.1128/CDLI.8.5.949-954.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poquet Y, Kroca M, Halary F, Stenmark S, Peyrat MA, Bonneville M, Fournie JJ, Sjostedt A. Expansion of Vγ9Vδ2 T cells is triggered by Francisella tularensis-derived phosphoantigens in tularemia but not after tularemia vaccination. Infect Immun. 1998;66:2107–2114. doi: 10.1128/iai.66.5.2107-2114.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertotto A, Gerli R, Spinozzi F, Muscat C, Scalise F, Castellucci G, Sposito M, Candio F, Vaccaro R. Lymphocytes bearing the γδ T cell receptor in acute Brucella melitensis infection. Eur J Immunol. 1993;23:1177–1180. doi: 10.1002/eji.1830230531. [DOI] [PubMed] [Google Scholar]
- 8.Roussilhon C, Agrapart M, Guglielmi P, Bensussan A, Brasseur P, Ballet JJ. Human TCR γδ+ lymphocyte response on primary exposure to Plasmodium falciparum. Clin Exp Immunol. 1994;95:91–97. doi: 10.1111/j.1365-2249.1994.tb06020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassan J, Feighery C, Bresnihan B, Whelan A. Elevated T cell receptor γδ+ T cells in patients with infectious mononucleosis. Br J Haematol. 1991;77:255–256. doi: 10.1111/j.1365-2141.1991.tb07990.x. [DOI] [PubMed] [Google Scholar]
- 10.Jouen-Beades F, Paris E, Dieulois C, Lemeland JF, Barre-Dezelus V, Marret S, Humbert G, Leroy J, Tron F. In vivo and in vitro activation and expansion of γδ T cells during Listeria monocytogenes infection in humans. Infect Immun. 1997;65:4267–4272. doi: 10.1128/iai.65.10.4267-4272.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hara T, Mizuno Y, Takaki K, Takada H, Akeda H, Aoki T, Nagata M, Ueda K, Matsuzaki G, Yoshikai Y, et al. Predominant activation and expansion of Vγ9-bearing γδ T cells in vivo as well as in vitro in Salmonella infection. J Clin Invest. 1992;90:204–210. doi: 10.1172/JCI115837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Constant P, Davodeau F, Peyrat MA, Poquet Y, Puzo G, Bonneville M, Fournie JJ. Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka Y, Morita CT, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 14.Poccia F, Cipriani B, Vendetti S, Colizzi V, Poquet Y, Battistini L, Lopez-Botet M, Fournie JJ, Gougeon ML. CD94/NKG2 inhibitory receptor complex modulates both anti-viral and anti-tumoral responses of polyclonal phosphoantigen-reactive Vγ 9Vδ2 T lymphocytes. J Immunol. 1997;159:6009–6017. [PubMed] [Google Scholar]
- 15.Battistini L, Borsellino G, Sawicki G, Poccia F, Salvetti M, Ristori G, Brosnan CF. Phenotypic and cytokine analysis of human peripheral blood γδ T cells expressing NK cell receptors. J Immunol. 1997;159:3723–3730. [PubMed] [Google Scholar]
- 16.Alexander AA, Maniar A, Cummings JS, Hebbeler AM, Schulze DH, Gastman BR, Pauza CD, Strome SE, Chapoval AI. Isopentenyl pyrophosphate-activated CD56+ γδ T lymphocytes display potent antitumor activity toward human squamous cell carcinoma. Clin Cancer Res. 2008;14:4232–4240. doi: 10.1158/1078-0432.CCR-07-4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poccia F, Battistini L, Cipriani B, Mancino G, Martini F, Gougeon ML, Colizzi V. Phosphoantigen-reactive Vγ9Vδ2 T lymphocytes suppress in vitro human immunodeficiency virus type 1 replication by cell-released antiviral factors including CC chemokines. J Infect Dis. 1999;180:858–861. doi: 10.1086/314925. [DOI] [PubMed] [Google Scholar]
- 18.Tikhonov I, Deetz CO, Paca R, Berg S, Lukyanenko V, Lim JK, Pauza CD. Human Vγ2Vδ2 T cells contain cytoplasmic RANTES. Int Immunol. 2006;18:1243–1251. doi: 10.1093/intimm/dxl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subauste CS, Chung JY, Do D, Koniaris AH, Hunter CA, Montoya JG, Porcelli S, Remington JS. Preferential activation and expansion of human peripheral blood γδ T cells in response to Toxoplasma gondii in vitro and their cytokine production and cytotoxic activity against T. gondii-infected cells. J Clin Invest. 1995;96:610–619. doi: 10.1172/JCI118076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Das H, Kamath A, Bukowski JF. Human Vγ2Vδ2 T cells produce IFN-γ and TNF-α with an on/off/on cycling pattern in response to live bacterial products. J Immunol. 2001;167:6195–6201. doi: 10.4049/jimmunol.167.11.6195. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Deetz CO, Zapata JC, Cairo C, Hebbeler AM, Propp N, Salvato MS, Shao Y, Pauza CD. Vaccinia virus inhibits T cell receptor-dependent responses by human γδ T cells. J Infect Dis. 2007;195:37–45. doi: 10.1086/509823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brandes M, Willimann K, Lang AB, Nam KH, Jin C, Brenner MB, Morita CT, Moser B. Flexible migration program regulates γδ T-cell involvement in humoral immunity. Blood. 2003;102:3693–3701. doi: 10.1182/blood-2003-04-1016. [DOI] [PubMed] [Google Scholar]
- 23.Leslie DS, Vincent MS, Spada FM, Das H, Sugita M, Morita CT, Brenner MB. CD1-mediated γ/δ T cell maturation of dendritic cells. J Exp Med. 2002;196:1575–1584. doi: 10.1084/jem.20021515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci USA. 1975;72:3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calzascia T, Pellegrini M, Hall H, Sabbagh L, Ono N, Elford AR, Mak TW, Ohashi PS. TNF-α is critical for antitumor but not antiviral T cell immunity in mice. J Clin Invest. 2007;117:3833–3845. doi: 10.1172/JCI32567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacEwan DJ. TNF receptor subtype signalling: differences and cellular consequences. Cell Signal. 2002;14:477–492. doi: 10.1016/s0898-6568(01)00262-5. [DOI] [PubMed] [Google Scholar]
- 27.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 28.McDevitt H, Munson S, Ettinger R, Wu A. Multiple roles for tumor necrosis factor-α and lymphotoxin α/β in immunity and autoimmunity. Arthritis Res. 2002;4(Suppl 3):S141–S152. doi: 10.1186/ar570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kollias G. TNF pathophysiology in murine models of chronic inflammation and autoimmunity. Semin Arthritis Rheum. 2005;34:3–6. doi: 10.1016/j.semarthrit.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Deng GM, Zheng L, Chan FK, Lenardo M. Amelioration of inflammatory arthritis by targeting the pre-ligand assembly domain of tumor necrosis factor receptors. Nat Med. 2005;11:1066–1072. doi: 10.1038/nm1304. [DOI] [PubMed] [Google Scholar]
- 31.Winthrop KL, Yamashita S, Beekmann SE, Polgreen PM. Mycobacterial and other serious infections in patients receiving anti-tumor necrosis factor and other newly approved biologic therapies: case finding through the Emerging Infections Network. Clin Infect Dis. 2008;46:1738–1740. doi: 10.1086/587989. [DOI] [PubMed] [Google Scholar]
- 32.Iseman MD, Fischer A. Tumor necrosis factor-α at the intersection of mycobacterial immunity and pathogenesis: an important new address in medicine. Clin Infect Dis. 2008;46:1741–1742. doi: 10.1086/587990. [DOI] [PubMed] [Google Scholar]
- 33.Ellerin T, Rubin RH, Weinblatt ME. Infections and anti-tumor necrosis factor α therapy. Arthritis Rheum. 2003;48:3013–3022. doi: 10.1002/art.11301. [DOI] [PubMed] [Google Scholar]
- 34.Havlir DV, Ellner JJ, Chervenak KA, Boom WH. Selective expansion of human γδ T cells by monocytes infected with live Mycobacterium tuberculosis. J Clin Invest. 1991;87:729–733. doi: 10.1172/JCI115053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li B, Rossman MD, Imir T, Oner-Eyuboglu AF, Lee CW, Biancaniello R, Carding SR. Disease-specific changes in γδ T cell repertoire and function in patients with pulmonary tuberculosis. J Immunol. 1996;157:4222–4229. [PubMed] [Google Scholar]
- 36.Trevejo JM, Marino MW, Philpott N, Josien R, Richards EC, Elkon KB, Falck-Pedersen E. TNF-α-dependent maturation of local dendritic cells is critical for activating the adaptive immune response to virus infection. Proc Natl Acad Sci USA. 2001;98:12162–12167. doi: 10.1073/pnas.211423598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bromberg JS, Chavin KD, Kunkel SL. Anti-tumor necrosis factor antibodies suppress cell-mediated immunity in vivo. J Immunol. 1992;148:3412–3417. [PubMed] [Google Scholar]
- 38.Boyman O, Hefti HP, Conrad C, Nickoloff BJ, Suter M, Nestle FO. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-α. J Exp Med. 2004;199:731–736. doi: 10.1084/jem.20031482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sata M, Walsh K. TNFα regulation of Fas ligand expression on the vascular endothelium modulates leukocyte extravasation. Nat Med. 1998;4:415–420. doi: 10.1038/nm0498-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kafrouni MI, Brown GR, Thiele DL. The role of TNF-TNFR2 interactions in generation of CTL responses and clearance of hepatic adenovirus infection. J Leukocyte Biol. 2003;74:564–571. doi: 10.1189/jlb.0103035. [DOI] [PubMed] [Google Scholar]
- 41.Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumor necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 42.Marino MW, Dunn A, Grail D, Inglese M, Noguchi Y, Richards E, Jungbluth A, Wada H, Moore M, Williamson B, et al. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci USA. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lahn M, Kalataradi H, Mittelstadt P, Pflum E, Vollmer M, Cady C, Mukasa A, Vella AT, Ikle D, Harbeck R, et al. Early preferential stimulation of γδ T cells by TNF-α. J Immunol. 1998;160:5221–5230. [PubMed] [Google Scholar]
- 44.Li H, Peng H, Ma P, Ruan Y, Su B, Ding X, Xu C, Pauza CD, Shao Y. Association between Vγ2Vδ2 T cells and disease progression after infection with closely related strains of HIV in China. Clin Infect Dis. 2008;46:1466–1472. doi: 10.1086/587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deetz CO, Hebbeler AM, Propp NA, Cairo C, Tikhonov I, Pauza CD. γ Interferon secretion by human Vγ2Vδ2 T cells after stimulation with antibody against the T-cell receptor plus the Toll-like receptor 2 agonist Pam3Cys. Infect Immun. 2006;74:4505–4511. doi: 10.1128/IAI.00088-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hebbeler AM, Cairo C, Cummings JS, Pauza CD. Individual Vγ2-Jγ1.2+ T cells respond to both isopentenyl pyrophosphate and Daudi cell stimulation: generating tumor effectors with low molecular weight phosphoantigens. Cancer Immunol Immunother. 2007;56:819–829. doi: 10.1007/s00262-006-0235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sicard H, Ingoure S, Luciani B, Serraz C, Fournie JJ, Bonneville M, Tiollier J, Romagne F. In vivo immunomanipulation of Vγ9Vδ2 T cells with a synthetic phosphoantigen in a preclinical nonhuman primate model. J Immunol. 2005;175:5471–5480. doi: 10.4049/jimmunol.175.8.5471. [DOI] [PubMed] [Google Scholar]
- 48.Inoue J, Ishida T, Tsukamoto N, Kobayashi N, Naito A, Azuma S, Yamamoto T. Tumor necrosis factor receptor-associated factor (TRAF) family: adapter proteins that mediate cytokine signaling. Exp Cell Res. 2000;254:14–24. doi: 10.1006/excr.1999.4733. [DOI] [PubMed] [Google Scholar]
- 49.Kabelitz D, Bender A, Schondelmaier S, Schoel B, Kaufmann SH. A large fraction of human peripheral blood γ/δ+ T cells is activated by Mycobacterium tuberculosis but not by its 65-kD heat shock protein. J Exp Med. 1990;171:667–679. doi: 10.1084/jem.171.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoft DF, Brown RM, Roodman ST. Bacille Calmette-Guerin vaccination enhances human γδ T cell responsiveness to mycobacteria suggestive of a memory-like phenotype. J Immunol. 1998;161:1045–1054. [PubMed] [Google Scholar]
- 51.Thoma-Uszynski S, Stenger S, Modlin RL. CTL-mediated killing of intracellular Mycobacterium tuberculosis is independent of target cell nuclear apoptosis. J Immunol. 2000;165:5773–5779. doi: 10.4049/jimmunol.165.10.5773. [DOI] [PubMed] [Google Scholar]
- 52.Dieli F, Troye-Blomberg M, Ivanyi J, Fournie JJ, Krensky AM, Bonneville M, Peyrat MA, Caccamo N, Sireci G, Salerno A. Granulysin-dependent killing of intracellular and extracellular Mycobacterium tuberculosis by Vγ9/Vδ2 T lymphocytes. J Infect Dis. 2001;184:1082–1085. doi: 10.1086/323600. [DOI] [PubMed] [Google Scholar]