SUMMARY
The effects of dietary modulation of brain DHA content on outcomes after TBI were examined in a juvenile rat model. Long-Evans rats with normal or diet-induced decreases in brain DHA were subjected to a controlled cortical impact or sham surgery on postnatal day 17. Rats with the greatest decreases in brain DHA had the poorest sensorimotor outcomes after TBI. Ccl2, Gfap, and Mmp 9 mRNA levels, and MMP-2 and −9 enzymatic activities were increased after TBI regardless of brain DHA level. Lesion volume was not affected by brain DHA level. In contrast, TBI-induced Timp1 expression was lower in rats on the Deficient diet and correlated with brain DHA level. These data suggest that decreased brain DHA content contributes to poorer sensorimotor outcomes after TBI through a mechanism involving modulation of Timp1 expression.
INTRODUCTION
Traumatic brain injury (TBI) is one of the leading causes of acquired disability and death in children under 5 years of age [1]. Children in this age group tend to have poorer outcomes than adults after sustaining a severe TBI. Adverse outcomes can include sensorimotor deficits, difficulties with long-term memory, attention, language, problem solving, and managing stress and emotions, as well as increased risk for epilepsy and aging-related diseases such as Alzheimer’s and Parkinson’s [2–5].
Worsened outcomes in children are likely due to the unique features of the pediatric population that make the pathophysiology of juvenile TBI different from that of adults; for example, young children have greater brain plasticity, less white matter myelination, higher brain water content, and reduced skull rigidity [6–8]. Accordingly, it is imperative that juvenile TBI be studied independently of adult TBI using an agespecific model [9].
The n-3 polyunsaturated fatty acids (PUFA) are a major component of neuronal membranes and accumulate in the brain through early childhood. PUFAs influence cellular function by forming the micro-environment around membrane-bound proteins, modifying lipid rafts [10, 11] and modulating gene expression through activation of transcription factors (e.g., PPAR and RXR) [12]. Additionally, PUFAs are precursors for lipid-derived signaling molecules such as leukotrienes, prostaglandins, and the more recently discovered anti-inflammatory and inflammation-resolving lipoxins, resolvins, maresins and protectins [13–15].
Docosahexaenoic acid [DHA, 22:6n-3] is derived from the essential fatty acid α-linolenic acid [18:3n-3] and represents approximately 15% of total lipids in the brain [16]. DHA accumulates in the brain during late gestation and early childhood, and is supplied by the mother to the fetus in utero and via breast milk after birth [17, 18]. DHA accumulation in the brain is a function of the quantity of n-3 PUFAs in the diet, which is notably low in the Western diet [19]. Although DHA deficiency does not result in gross developmental pathology [20], adequate DHA accumulation is essential for optimal brain and visual development and function [21, 22].
N-3 PUFAs and their metabolites have anti-inflammatory effects in neural and non-neural tissues [23]. In animal models, administration of DHA or consuming a diet high in n-3 PUFAs, has been beneficial in various types of neuronal injuries including TBI [24–28], although a worsened outcome has also been reported [29]. However, very little is known about what role endogenous brain DHA has in neuroprotection after TBI. Controlled cortical impact (CCI), a model of traumatic brain injury, causes a rapid and sustained increase in free fatty acids in the brain [30]. We can thus hypothesize that populations lacking a full complement of endogenous n-3 PUFAs, such as young children consuming a Western diet, will be likely to have fewer of these fatty acids released after TBI and thus a poorer outcome. Accordingly, we investigated the effects of low dietary n-3 PUFA content, and consequently lower brain DHA levels, on outcomes of TBI in a juvenile rat model. Severity and persistence of sensorimotor outcomes was assessed, as well as expression of representative cellular mediators involved in the various pathophysiological processes initiated by TBI.
Previous studies have shown that maternal diets deficient in n-3 PUFAs decrease the DHA content of the offspring and that this effect increases when animals are maintained on n-3-PUFA-deficient diets for multiple pregnancies or generations [31– 33]. The technique of breeding multiple litters from a single dam on a deficient diet has been used extensively by our lab [31, 32, 34] to produce pups with varying degrees of brain DHA without the confound of using multiple n-3 PUFA deficient diets to produce multiple levels of brain DHA content. This diet and breeding procedure allows us to determine the dose-response effects of brain DHA on content on the outcomes of TBI while avoiding potentially confounding effects of using different diets.
We will show that worsened sensorimotor outcomes following TBI are associated with lower brain DHA level and that these functional deficits correlate with mRNA expression levels of tissue inhibitor of matrix metalloproteinase-1 (Timp1), indicating a potential mechanism for decreased brain DHA-induced sensorimotor deficits after TBI.
MATERIALS AND METHODS
All experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee.
Animals and husbandry
Long-Evans rats were housed in a temperature- and humidity-controlled facility with a 14–10 hour light-dark cycle (on at 06:00 h) with ad libitum access to food and water. Breeding stock (females 75–85 days; male proven breeders; Harlan Laboratories, Inc. Indianapolis, IN) were obtained a minimum of 5 days prior to the beginning of the experiment and were handled regularly. Males and females were maintained on a standard laboratory rodent diet (#8604, Harlan Laboratories, Inc., Indianapolis, IN) until mating. At the time of mating, breeding pairs were placed on one of two purified diets (Control or Deficient). Mated females were singly housed and maintained on their diet through two consecutive litters, allowing one week recovery time after weaning the first litter and mating for the second litter. Litters were culled to eight pups with preference for males on postnatal day (PND) one. Pups randomly selected from each litter cohort received either a controlled cortical impact (CCI) injury or sham surgery on PND 17 and were returned to the dam until weaning. To control for litter effects, each pup within a treatment group was from a different litter. Pups were weaned on PND 20 onto their mothers’ respective diet and housed in groups of two to four, TBI and sham-injured together, for the remainder of the study. To eliminate possible maternal influences or genetic inheritance on outcomes, no treatment group or endpoint contained more than one pup from a single litter [35].
Male rat pups (n = 5–12/group, depending on endpoint, and each from a different litter) were subjected to controlled cortical impact TBI or sham surgery on PND 17. Those used for sensorimotor testing were tested 1, 7, 14, 21, and 28 days after surgery and then euthanized on day 28 by transcardial perfusion under pentobarbital anesthesia followed by the removal of the brain for determination of contusion volume. A second cohort of rats were euthanized on day 1 (28 hrs) or day 7 after surgery by decapitation; these rats were not used for behavioral testing. Brains from these rats were rapidly removed and the dissected on ice. The frontal cortex was snap frozen for later fatty acid analysis. The injured motor cortex was dissected into two halves, with the rostral half being snap frozen for later zymographic analysis and the caudal half preserved in RNAlater (Life Technologies/Ambion, Gaithersburg, MD) for mRNA analysis.
Experimental diets
The Control diet was AIN-93G (Teklad, Indianapolis, IN, which is formulated with unhydrogenated soybean oil (70 g/kg). It meets all current nutrient standards for rat pregnancy and growth [36]. The Deficient diet was a custom prepared pelleted diet (Teklad) that is identical to the Control diet except it was prepared with safflower oil (66.5 g/kg) and soybean oil (3.5 g/kg). Fatty acid composition of the diets is shown in Table 1.
Table 1.
Fatty acid composition of the Control and Deficient diets.
| Fatty acid/Diet | Control | Deficient |
|---|---|---|
| Content in diet (g/kg) | ||
| 14:0 | 0.29 | 1.23 |
| 16:0 | 9.84 | 7.23 |
| 18:0 | 6.42 | 5.84 |
| 20:0 | 0.51 | 0.19 |
| 22:0 | 0.33 | 0.39 |
| 24:0 | ND | ND |
| 16:1 | ND | 0.10 |
| 18:1 | 13.39 | 10.99 |
| 20:1n-9 | 0.06 | 0.16 |
| 18:2n-6 | 28.68 | 37.20 |
| 18:3n-3 | 5.32 | 0.54 |
Diet fatty acid composition was determined by gas chromatography using PUFA 1 and 2 and Supelco 37 standards. ND: Not detected
Controlled cortical impact
Rats were anesthetized with isoflurane (induction, 3.0%; maintenance, 2.0%) and stabilized in a Cunningham stereotaxic frame (Stoelting, Wood Dale, IN). A 4 × 4 mm craniotomy was performed with a burr drill, lateral (right side) to the mid-sagittal suture, with the center at the following coordinates: AP = 0, ML = 2.5 from bregma. The impactor device consisted of a linear motor device (the impactor) with a stainless steel tip, power supply and microprocessor controller (Linmot, Zurich, Switzerland), a Plexiglas table, and a stand for the linear motor device made with an adjustable manipulator (Kopf, Tujunga, CA), which allowed precise positioning of the impactor [37– 39]. . The position of the impactor and tip (3.0 mm-diameter tip with a flat face and slightly rounded edge) was carefully adjusted to be centered within the craniotomy and tangential to the dural surface. The impactor tip was lowered in 0.05 mm increments until the tip just contacted the dura (by visual inspection under surgical microscope at 40× magnification). The cortical impact was initiated through the instrument’s graphical user interface with the following parameters: 3.0 mm depth, 1.5 m/sec strike velocity, 300 msec contact time. The contact area included motor (M1, M2) and sensory (S1FL, S1HL) cortical areas [40, 41]. After the impact, the scalp was closed with 6-0 silk suture, anesthesia was discontinued, and the animal temperature was maintained at 37° C until recovery of locomotion.
Sham procedures involving the use of a trephine or drill to produce craniotomy have been shown to cause brain injury distinct from that caused by impact [42]. While cortical damage induced by TBI significantly outweighs damage caused by the craniotomy [43] to avoid potential experimental confounds, the sham surgery consisted of a scalp incision with no craniotomy or impact from the CCI device.
All rats received 0.05 mg/kg of buprenorphine approximately thirty minutes after surgery and again 24 hours after surgery, after day 1 behavioral testing was completed. The administration of buprenorphine analgesia after CCI surgery was required by the Institutional Animal Care and Use Committee. Buprenorphine is a μ-opioid receptor partial agonist and is thus analgesic, but not anti-inflammatory. Opioid analgesics are clinically contraindicated for use in TBI because they can increase intracranial pressure thus exacerbating the effects of injury; however, craniotomy required for the CCI likely minimizes this effect. Furthermore, the administration of buprenorphine to all treatments groups ensures that any effects it may produce are controlled for.
Sensorimotor testing
All behavioral testing occurred between 09:00 and 12:00 h in a brightly lit room specifically reserved for rodent behavioral testing. Animals were allowed to acclimate to the behavioral testing facility in their home cages for approximately 15 min before beginning testing. Each test was performed by each animal on 1, 7, 14, 21 and 28 days after surgery in a repeated-measures design.
Motor activity and function
Motor activity function was assessed using force-plate actometers as previously described [44]. Rats were placed in the actometer chambers (42 × 28 cm) enclosed in a dark, sound-attenuating cabinet. Behavior was recorded for 20 min. A small wall-mounted fan in each cabinet provided background noise and air circulation. Data were analyzed for total distance traveled, number of low mobility bouts (≥10 sec within a 20-mm radius), and low mobility distance (distance traveled during bouts of low mobility) as previously described by Fowler et al. [45] and validated in our juvenile TBI model [44]. The low mobility parameters assess the number of instances the rats spend time moving while confined within a small area (bouts of low mobility) and the amount of movement within the 20-mm radius (low mobility distance). Low mobility parameters are typically used as an index of stereotyped behavior [45], but can also be used to measure other types of movements such as grooming which are decreased in adult rats after TBI [46].
Spontaneous forelimb elevation (cylinder) test
Rats were placed in a glass cylinder (standard laboratory beaker or cylindrical vase [Living Bright, Inc.]) scaled to the size of the rat as previously described [44]. Rats were observed for spontaneous rearings during a 5-min observation session. One session was performed on the day prior to surgery to control for possible pre-injury limb preference. During each session, the numbers of wall rearings using the left only, right only, or both left and right forelimbs were recorded. The laterality score was calculated using a method described by Schallert et. al [47]. Briefly, the forelimb laterality was calculated as: (number of right only - number of left only) / (number of right only + number of left only + number of both together) such that a higher laterality score indicates a preference for the forelimb ipsilateral to the injured hemisphere.
Quantitative real time PCR
Cortical tissue surrounding the site of injury was dissected on ice at the time of euthanasia and preserved in RNAlater (Life Technologies/Ambion, Gaithersburg, MD) at 4°C until total RNA was to be isolated. Total RNA was isolated from the tissue by homogenizing it in 1 ml of Trizol reagent (Life Technologies/Gibco BRL, Gaithersburg, MD) per 100 mg of tissue weight. The RNA was isolated using a Trizol (Life Technologies/Ambion) phenol-chloroform extraction according to the manufacturer’s protocol and precipitated with 75% isopropyl alcohol overnight at −20°C. mRNA quality was determined using a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE) with good quality being an OD 260/280 greater than 1.8. mRNA quality was further verified using a Agilent Bioanalyzer 2011(Agilent Technologies, Inc., Santa Clara, CA). First strand cDNA was synthesized using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Carlsbad, CA) per the manufacturer’s protocol using a PTC-100 Peltier Thermal Cycler (MJ Research, Waltham, MA). Exon spanning, genespecific primers (Table 2) were prepared using the NCBI’s Primer-BLAST [48] and purchased from Integrated DNA Technologies, Inc. (Coralville, IA). Primer specificity was determined by the presence of a single peak in the melt curve.
Table 2.
Quantitative real-time PCR primers.
| Gene | Accession No. | Forward | Reverse | Slope | Y- Intercept |
R2 | Efficiency |
|---|---|---|---|---|---|---|---|
| B2m | NM_012512.2 | TGCTTGCCATTCAGAAAACTCC | TTGAGGTGGGTGGAACTGAG | −3.1 | 22.41 | 0.99 | 2.23 |
| Cc12 | NM_031530.1 | TGTCTCAGCCAGATGCAGTTAAT | TCCAGCCGACTCATTGGGAT | −3.265 | 27.08 | 0.99 | 2.06 |
| Gfap | NM_017009.2 | GCGTCTGGACCAGCTTACTAC | TTTCATCTTGGAGCTTCTGCCT | −3.6 | 21.53 | 0.99 | 1.78 |
| Mmp2 | NM_031054.2 | GGAGCTCTATGGGCCCTCCCC | GTGGCCACCAGCAAGGGACC | −3.205 | 27.89 | 0.99 | 2.05 |
| Mmp9 | NM_031055.1 | GTGACACCGCTCACCTTCAC | GCGTGTGCCAGTAGACCATC | −3.245 | 29.06 | 0.99 | 2.03 |
| Timp1 | NM_53819.1 | GATATGTCCACAAGTCCCAGAACC | CCACAGCCAGCACTATAGGTCTTT | −3.43 | 25.5 | 0.99 | 1.95 |
The qPCR reactions were prepared using 10 ng of RNA-equivalent cDNA, 125 nM of each forward and reverse primer, and 1× iQ SYBR Green Supermix in 96 well plates and run on a iCycler iQ real-time PCR system (Bio-Rad, Hercules, CA). The thermal cycle conditions were as follows: 30 s at 50°C, 8 min 30 s at 95°C, followed by 95°C for 15 s then 65°C for 30 s for 40 cycles, then 95°C for 1 min, 55°C for 1 min, and a melt curve beginning at 55°C and increasing 0.5°C every 10 s until 100°C.
Relative gene expression was calculated using the 2−ΔΔCt method [49]. Data are expressed as fold change in gene expression compared to the reference gene beta-2-microglobulin, which was experimentally determined to be the most stably expressing gene in our experimental model and brain region of interest [50].
Gelatin zymography
Injured cortical tissue was dissected on ice and homogenized in lysis buffer (50 mM Tris-HCl, 200 mM NaCl, 5 mM CaCl2, 0.02% Brij-35, pH 8) and centrifuged. MMP-2 and MMP-9 were purified from the supernatant using gelatin Sepharose 4B affinity media (GE Healthcare Life Sciences, Pittsburgh, PA) for one hour at 4°C. MMP-2 and MMP-9 were eluted from the Sepharose 4B using the lysis buffer containing 10% DMSO. Samples were loaded with a zymogram sample buffer (Bio-Rad, Hercules, CA) onto a 7.5% polyacrylamide gel containing 1 mg/ml porcine gelatin. Samples were run in a tris-glycine SDS running buffer (25 mM Tris Base, 192 mM Glycine, 0.1% SDS, pH 8.3) at 100V until the dye front reached the bottom of the gel. Gels were rinsed 2X in rinse buffer (50 mM Tris, 5 mM CaCl2, 2.5% Triton X-100, pH 8) for 30 minutes, 1X in incubation buffer (50 mM Tris, 5 mM CaCl2, pH 8) at room temperature and then in fresh incubation buffer overnight at 37°C. Bands were visualized by staining the gels with Coomassie brilliant blue stain (2.5 mg/ml) for several hours. After destaining, gels were digitized and bands analyzed for color density using NIH ImageJ software [51]. Band density is expressed as a percentage of the density of the positive controls, human recombinant MMP-2 and MMP-9 (Anaspec, Freemont, CA).
Brain total phospholipid fatty acid composition
Brain total phospholipid fatty acid composition was analyzed as previously described [31]. Briefly, phospholipids were extracted from frontal cortex and isolated by thin layer chromatography. The phospholipids were then transmethylated with boron trifluoride methanol (Sigma, St. Louis, MO) to produce fatty acid methyl esters. Individual fatty acid methyl esters were analyzed using a Varian 3400 gas chromatograph with an SP-2330 capillary column (30 m, Supelco, Inc., Belfonte, PA), using helium as the carrier gas. Peaks were identified via comparison to authentic standards (PUFA 1 and 2 and Supelco 37Supelco, Inc. and 22:5n-6, Nu-Chek Prep, Elysian, MN) and corrected for response factors. Individual fatty acids were expressed as weight percent of total fatty acids on the basis of peak area.
Lesion volume
Twenty-eight days after surgery, rats were deeply anesthetized with pentobarbital and transcardially perfused with cold 1X PBS followed by 4% phosphate buffered formaldehyde (PBF, pH. 7.4). The brains were removed and post-fixed in 4% PBF for several days then cryoprotected in 30% sucrose in PBS for 3 days and stored at −80°C until sectioning. Frozen sections were cut at 50 µm through the site of impact collecting every tenth section. Sections were mounted on gelatin-subbed slides and dehydrated in graded ethanol and stained with cresyl violet before being coverslipped. The area of intact tissue of the ipsilateral and contralateral hemispheres were measured using ImageJ [52] from macro-level digitized images of each section. The total tissue loss was calculated using the following equations modified from [53]: (contralateral tissue area - ipsilateral intact tissue area) * section thickness * distance between sections = Subvolume. Total lesion volume =Σ Subvolume (Section1 + Section2 + …Sectionn).
Statistical analysis
Normally distributed data were analyzed for effects of injury (TBI or sham-injured) and diet (Control or Deficient) by repeated measures ANOVA with factors of TBI, diet, and day after injury (1–28 days after injury) (SYSTAT, v.12). Time after injury was analyzed as the repeated measure for the sensorimotor function studies. Outliers identified by SYSTAT were discarded from subsequent analyses. Data excluded as outliers were from the qPCR studies and are as follows: one animal in the 1st litter Control TBI group was excluded from all mRNA analyses. In addition, one 1st litter Control Sham excluded from the Gfap analyses; one 1st litter Control Sham, one 1st litter Deficient Sham, and one 1st litter Deficient TBI were excluded from the Ccl2 analyses; one 1st litter, one 1st litter Control Sham, one 1st litter Deficient Sham, one 1st litter Deficient TBI, and one 2nd litter Deficient Sham were excluded from the Mmp9 analyses; and one 1st litter Control Sham, one 1st litter Control TBI, one 1st litter Deficient Sham, one 2nd litter Control TBI, and one 2nd litter Deficient TBI were excluded from the Timp1 analyses.
Post-hoc comparisons were made using 1-way ANOVA and the Fisher’s Least Significant Difference test. In two cases of the real-time PCR analysis (Ccl2, 1st litter; Gfap, 1st litter), data were not normally distributed and instead were analyzed by the Kruskal-Wallis nonparametric ANOVA with post-hoc comparisons made using Dunn’s Multiple Comparisons test. A significant difference was assumed if P<0.05. Because the experimental design required the production pups from 1st and 2nd litters from the same dam, testing of 1st and 2nd litter pups was performed as separate cohorts. Consequently, with the exception of the brain fatty acid data, comparison of the effects in the 1st and 2nd litter is limited to qualitative comparisons.
RESULTS
Effects of diets and breeding procedures on brain phospholipid fatty acid composition
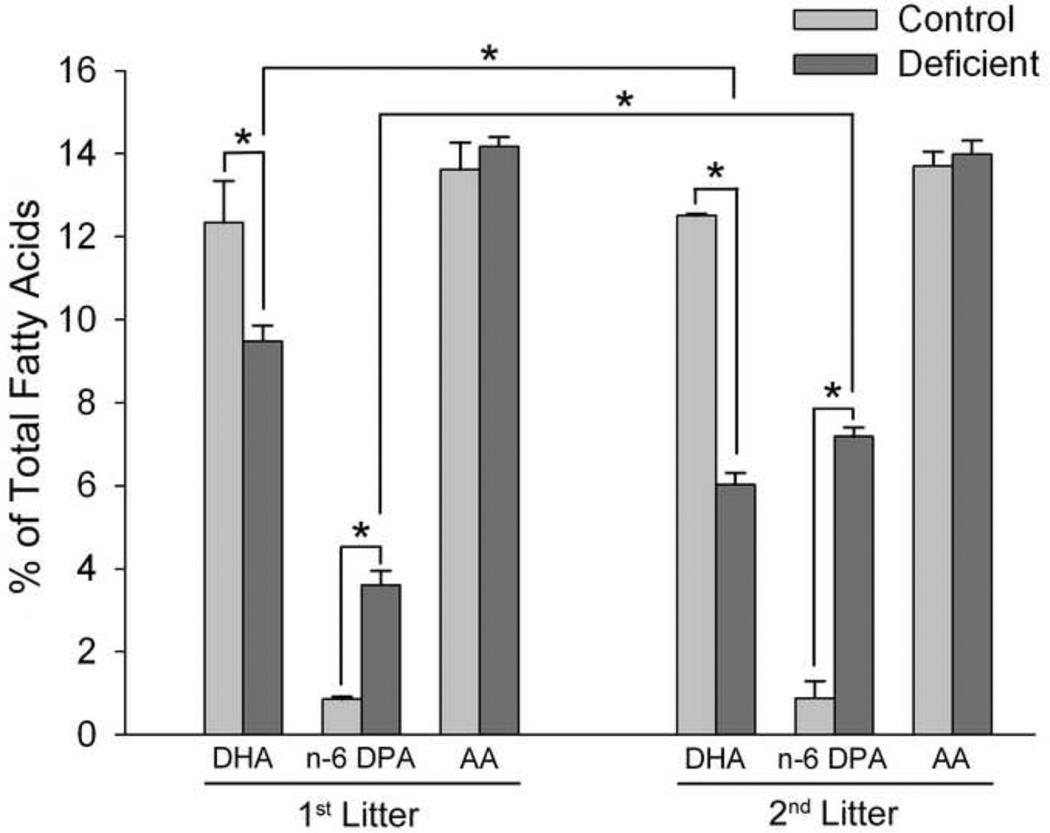
In agreement with previous studies [32], 1st litter rats raised on the Deficient diet had a 25% decrease in brain DHA compared to those raised on the Control diet (P<0.01) (Figure 1). Brain DHA content of 2nd litter of rats raised on the Deficient diet was decreased 54% (P<0.001 v. Control diet, P<0.001 v. 1st litter Deficient diet). This decrease in DHA content was accompanied by compensatory increases in the n-6 fatty acid docosapentaenoic acid [n-6 DPA, 22:5n-6] and no alteration in arachidonic acid [20:4n-6] content, in agreement with previous studies [54].
Figure 1. Effects of diet and breeding protocols on brain total phospholipid fatty acid composition.
Data are presented as the mean ± SEM (1st litter: n = 4–7/group; 2nd litter: n=8–9/group selected at random from the total sample pool with equal numbers of samples from sham and injured groups). *P<0.05 by ANOVA and Fisher’s LSD test.
Effects of diets and breeding procedures on TBI-induced sensorimotor deficits
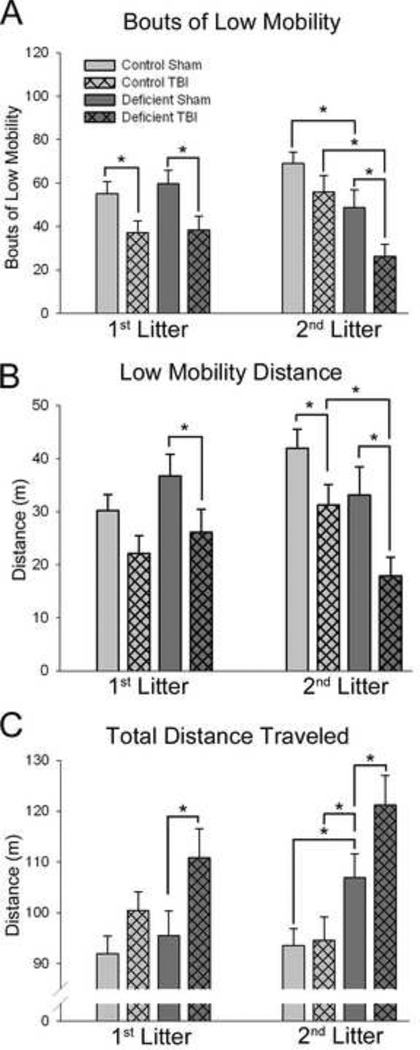
TBI resulted in altered locomotor activity on day 1 after injury as assessed using the force-plate actometer (Figure 2). TBI-induced locomotor deficits returned to nearsham levels after day 1 and therefore, data for subsequent test days are not shown.
Figure 2. Effects of TBI on locomotor function in rats with diet- and breeding-induced decreases in brain DHA content.
Data are presented as the mean ± SEM (n=11–12/group). Bouts of Low Mobility are defined as ≥10 sec spent within a 20-mm radius. Low mobility distance is the distance traveled during bouts of low mobility. All data presented are for the entire 20-minute test session on day 1 after surgery. *P<0.05 by ANOVA and Fisher’s LSD test.
In rats raised on the Control diet, TBI resulted in a decrease in the number of low mobility bouts (P<0.05) in agreement with our previous findings and/or decreased low mobility distance [44] (P<0.05) but no difference in total distance traveled. Both 1st and 2nd litter rats raised on the Deficient diet exhibited decreases in both bouts of low mobility (P<0.05), low mobility distance (P<0.05) and increased total distance traveled (P<0.05) after TBI.
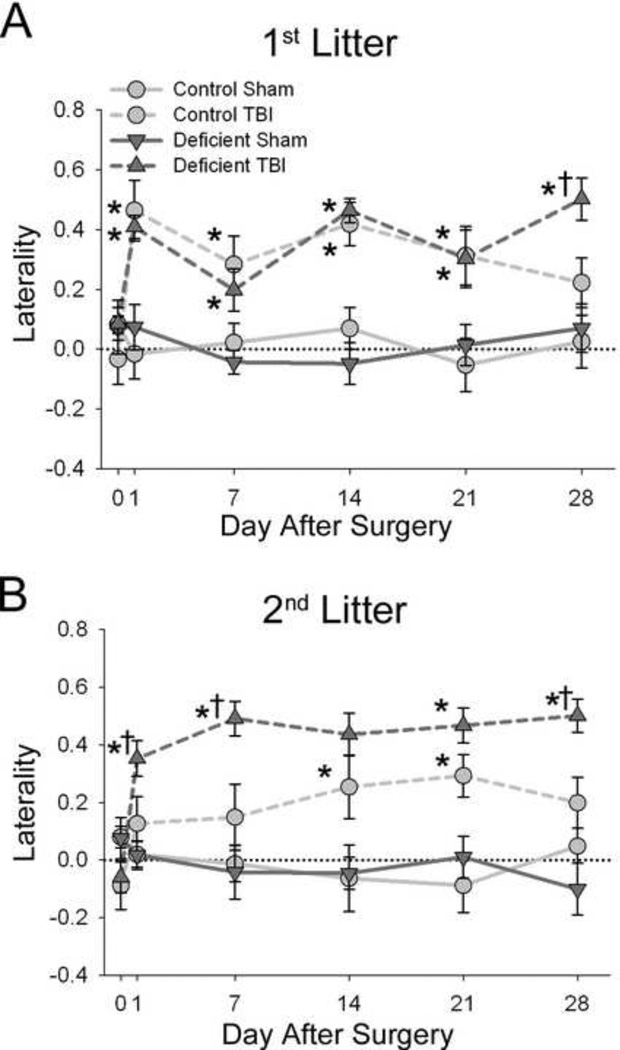
In the spontaneous forelimb elevation (cylinder) test, all injured rats exhibited a preference for using the ipsilateral limb that persisted throughout the 28-day testing period (P<0.01) (Figure 3). The effects of TBI on forelimb preference in 1st litter rats raised on the Deficient diet were not different from those raised in the Control diet.However, in the 2nd litter, injured rats raised on the Deficient diet had greater sustained preference for the forelimb ipsilateral to the injury than injured rats fed the Control diet (P<0.05).
Figure 3. Effects of TBI on forelimb preference in rats with diet- and breeding-induced decreases in brain DHA content.
Laterality was calculated as: (number of right only - number of left only) / (number of right only + number of left only + number of both together) such that a higher laterality score indicates a preference for the forelimb ipsilateral to the injured hemisphere. Data are presented as the mean ± SEM (n=11– 12/group). *P<0.05 v. Sham, †P<0.05 v. Control diet-TBI by ANOVA and Fisher’s LSD test.
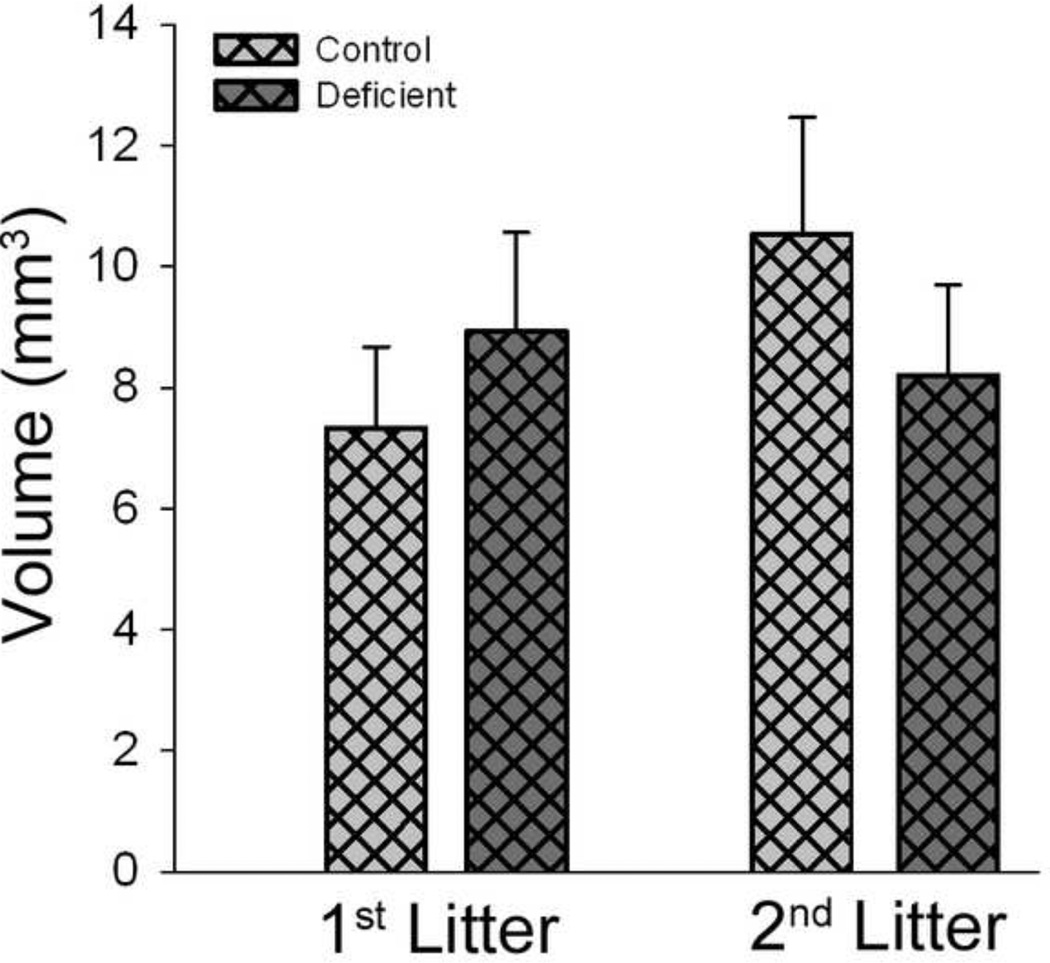
Effects of diets and breeding procedures on lesion volume
TBI caused a significant lesion assessed 28 days after surgery. Lesion volume was not different between rats raised on the Control and Deficient diets for either the 1st or 2nd litters (Figure 4).
Figure 4. Effects of diet- and breeding-induced decreases in brain DHA content on TBI-induced lesion volume 28 days after injury.
Data are presented as the mean ± SEM (n = 5–9/ group). Variation in sample size resulted from technical difficulties that resulted in loss of samples randomly from all groups. No significant differences were indicated by ANOVA.
Effects of diets and breeding procedures on Ccl2, Gfap, Mmp9, Mmp2, and Timp1 mRNA expression
Levels of mRNA were measured on day 1 (28 hrs) and day 7 after TBI. Significant alterations in expression of these mediators was observed primarily on day 1, with mRNA levels returning to, or near, levels observed in sham-injured rats on day 7. Accordingly, data for day 1 only are presented.
Chemokine CC ligand-2 (Ccl2) mRNA was up-regulated approximately 60-fold on day 1 after TBI in all injured rats, regardless of diet or litter (P<0.001) (Figure 5A). In the 1st litter, there was a slight increase in Ccl2 expression in injured rats fed the Deficient diet but this was not statistically significant. In the 2nd litter, there was a significant effect of diet such that injured rats fed the Deficient diet expressed less Ccl2 than did injured rats fed the Control diet (P<0.01).
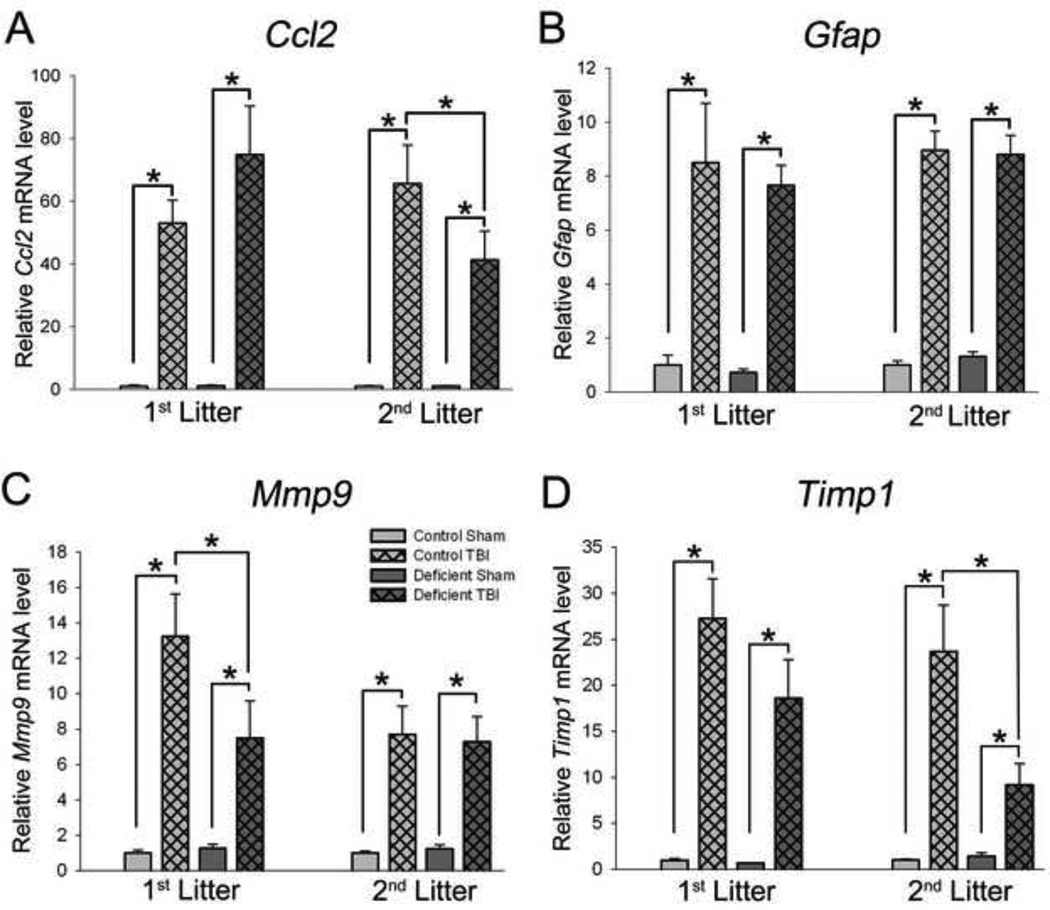
Figure 5. Effects of TBI on relative expression mRNAs encoding Ccl2 (A), Gfap (B), Mmp9 (C), and Timp1 (D) in rats with diet- and breeding-induced decreases in brain DHA content.
Data are presented as the mean ± SEM (1st litter: n=6–13/group, 2nd litter: n=6–9/group). *P<0.05 by ANOVA and Fisher’s LSD test (Ccl2, 2nd litter; Gfap; Mmp9, Timp1) or Kruskal-Wallis nonparametric ANOVA and Dunn’s Multiple Comparisons test (Ccl2, 1st litter; Gfap, 1st litter).
TBI increased glial fibrillary acidic protein (Gfap) mRNA level on day 1 after TBI and average of 8-fold in the 1st litter (P<0.001) and an average of 9-fold in the 2nd litter (P<0.001) (Figure 5B). There was no influence of diet on Gfap expression after TBI.
Matrix metalloproteinase-9 (Mmp9) gene expression was induced approximately 9-fold after TBI in all rats on day 1 after injury (P<0.001). The Deficient diet resulted in less expression of Mmp9 after TBI than those on the Control diet in 1st litter pups (P<0.05), but not in 2nd litter pups (Figure 5C).
Matrix metalloproteinase-2 (Mmp2) mRNA level was not affected by TBI or diet on day 1 after injury in either litter (data not shown).
Tissue inhibitor of matrix metalloproteinases-1 (Timp1) mRNA level was affected by TBI in the 1st litter and by both TBI and diet in the 2nd litter (Figure 5D). In the 1st litter, injured rats fed the Control diet had a 27-fold increase in Timp1 expression (P<0.001) while those fed the Deficient diet had a 19-fold increase in Timp1 mRNA level after TBI (P<0.001 v. sham, P=0.056 v. TBI, Control diet). Second litter pups fed the Control diet exhibited a 24-fold increase of Timp1 expression (P<0.001 vs. sham), similar to that observed in the 1st litter. In 2nd litter rats fed the Deficient diet, TBI resulted in an increase in Timp1 expression of only 9-fold (P<0.05 v. Sham, P<0.001 v. TBI, Control diet).
Effects of diets and breeding procedures on enzymatic activity of MMP-2 and MMP-9
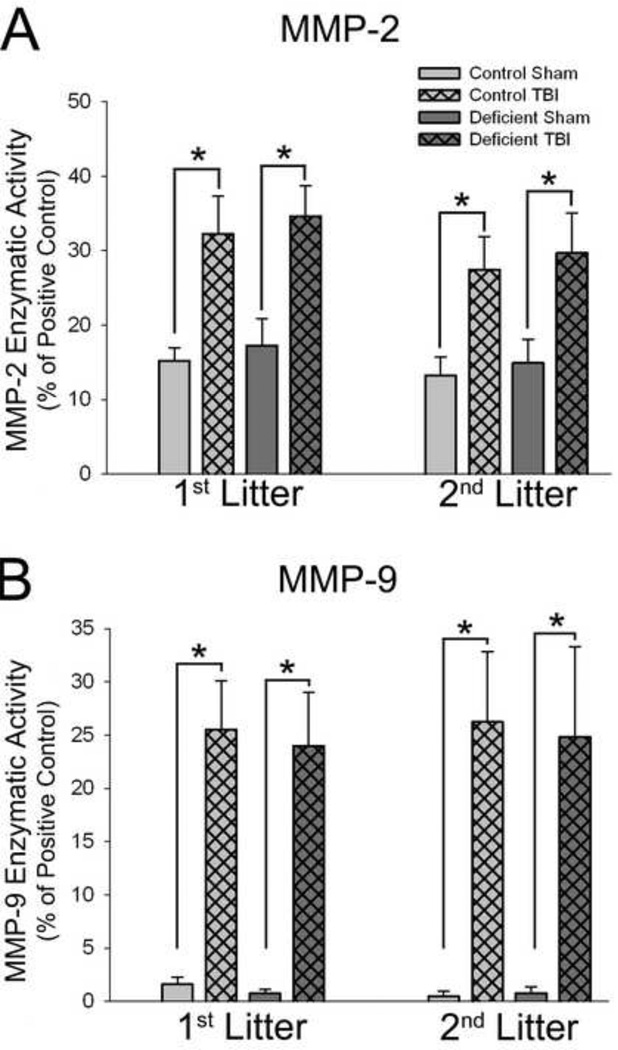
Enzymatic activity of MMP-2 was increased roughly 2-fold in 1st and 2nd litter pups on day 1 after TBI compared to shams (P<0.001), but there was no effect of diet (Figure 6A). TBI-induced increases in MMP-2 activity persisted through day 7 after injury in both litters with no effect of diet (data not shown).
Figure 6. Effects of TBI on MMP-2 and MMP-9 enzymatic activities in rats with diet- and breeding-induced decreases in brain DHA content.
Data are presented as the mean ± SEM (n=6–8/ group). *P<0.05 by ANOVA and Fisher’s LSD test.
MMP-9 activity was increased roughly 25-fold on day 1 after injury in 1st and 2ndlitter injured rats (Figure 6B) regardless of diet treatment (P<0.001, 1st litter; P<0.001,2nd litter). TBI-induced increases in MMP-9 activity remained elevated slightly above sham levels on day 7 after injury (data not shown).
DISCUSSION
This study examined the effects of an n-3 PUFA-deficient diet and the resulting diet-induced decreases in brain DHA content on sensorimotor and biochemical recovery from traumatic brain injury in a juvenile model. The use of diet and breeding protocols enabled the assessment of the dose-response effects of brain DHA content on the effects of TBI independently of the effects of dietary n-3 fatty acid content.
Effects of TBI
In concordance with the site of injury in the motor cortex, sensorimotor function was used as the key assessment of functional outcome. Deficits were observed in all groups using sensorimotor tests previously validated for use with this CCI model of juvenile TBI [44]. These included decreased low mobility movement on day after injury (Figure 2) consistent with reports of decreased grooming after TBI [46] and a persistent preference for the forelimb ipsilateral to the injury (Figure 3). TBI also resulted in a notable lesion at 28 days after injury, indicating significant cell loss (Figure 4).
TBI also increased mRNA expression of several mediators involved in the pathophysiological processes initiated by TBI on day 1 after injury including Ccl2, Gfap, Mmp9, and Timp1 (Figure 5). TBI also increased enzymatic activity of MMP-9 and MMP-2 (Figure 6). These increases are consistent with the known increases in protein levels for these mediators after TBI and are supportive of the roles they play in inflammation, glial cell activation and degradation of the blood brain barrier that occur in this time frame after TBI [55].
Effects of diet and breeding procedures
Our lab has extensively used the technique of breeding multiple litters from a single dam on a DHA-deficient diet [31, 32, 34] to produce pups with varying degrees of brain DHA. This allows us to determine the dose-response effects of reduced brain DHA using a single diet, and therefore avoids potentially confounding effects of using different diets, which alone can cause changes in basal gene expression and mask potential treatment effects [56]. Furthermore, although maternal parity produces some effects on maternal behavior [57, 58], each litter has its own control group, thus controlling for this potential confound. Likewise, although maternal diet does affect maternal neurochemistry, we have observed no differences in reproductive outcomes [59] nor have we observed any notable differences in maternal behavior (unpublished observation). Critically, for this study, any potential effects of differences in maternal behavior are likely to be minimal compared to the effects of the TBI.
In agreement with previous studies using these diets to manipulate brain DHA [32], 1st litter and 2nd litter of pups raised on the Deficient diet had decreases in brain DHA content of 25% and 54% DHA, respectively, compared to pups raised on the Control diet (Figure 1). This graded effect on offspring brain DHA content occurs because consumption of the Deficient diet by the dam while gestating and nursing the 1st litter results in depletion of maternal stores of n-3 PUFAs, and thus an even greater failure to deliver DHA to the 2nd litter even though dietary n-3 content is the same for both litters [32, 60].
In sham-injured rats, the Deficient diet produced an increase in locomotor activity (Figure 2) in agreement with previous studies indicating an increase in activity in rats with decreased brain DHA [34, 61–63]. However, there were no effects of diet in either 1st or 2nd litter sham rats on any of the other endpoints examined in this study.
Effects of diet and breeding protocols on TBI outcomes
Functional outcomes after TBI were poorer in rats with decreased brain DHA content. Specifically, injured rats with either a 25% or 54% decrease in brain DHA exhibited greater distance traveled in the locomotor test, in addition to the fewer bouts of low mobility and decreased low mobility distance also observed after TBI in rats raised on the Control diet. This indicates that the decreases in low mobility after TBI observed in rats raised on the Deficient diet was of sufficient magnitude that it also resulted in greater total distance traveled compared to those fed the Control diet (Figure 2) and thus indicates a poorer locomotor outcome in both 1st and 2nd litter rats raised on the Deficient diet. Effects of TBI on locomotor activity were primarily observed on day 1 after injury [46], suggesting that this test is likely best interpreted as a measure of acute behavioral effects after TBI, rather than of long-term functional outcome. This effect was similar after TBI in both litters raised on the Deficient diet, suggesting that augmented early functional response to TBI is the result of the low n-3 PUFA content of the Deficient Diet. Alternatively, this effect may result more specifically from a reduction in the percentage of DHA in brain phospholipids, but may be maximal in the rats with a 25% decrease in brain DHA, and thus an additional effect was not observed in rats with a 54% decrease.
In contrast to the effects on locomotor activity, TBI produced persistent deficits in forelimb preference, indicating that this parameter reflects longer-term functional outcome. Rats with a 25% decrease in brain DHA had forelimb deficits similar to those of rats fed the Control diet, whereas rats with a 54% decrease in brain DHA had greater forelimb deficits than their respective controls (Figure 3). Since the Deficient diet was identical for the 1st and 2nd litters, this suggests that the brain DHA levels, rather than simply dietary n-3 fatty acid content, are of primary importance in influencing long-term sensorimotor outcomes after TBI.
To assess the cellular mechanisms by which variation in dietary and/or brain DHA content might influence functional outcome after TBI, the effects of the diet and breeding treatments were assessed on representative measures of the various pathobiological processes induced by TBI. Final lesion size after TBI was not different between groups (Figure 4), indicating that differences in neuronal cell loss do not underlie the differences in functional outcomes. Likewise, induction MMP-2 and −9 activities after TBI was not affected by low dietary and/or brain DHA (Figure 6). Several cellular markers of injury were altered by the diet and breeding protocol, such as mRNA levels of Ccl2 and Mmp9; however, the effects were not consistent with the diet treatment between litters, nor did they correlate with brain DHA levels, suggesting that they do not play a primary role in the poorer functional outcomes in rats with lower brain DHA levels (Figure 5). Thus, these data suggest that changes in these mediators are not primary contributors to the observed differences in outcome.
TBI-induced expression of Timp1, however, was directly related to brain DHA content and correlated with sensorimotor outcomes in the spontaneous forelimb elevation test (Figure 5D). Specifically, rats with a 25% decrease in brain DHA tended toward expressing less Timp1 after TBI than rats on the Control diet. Furthermore, although the experimental design allows only qualitative comparison, rats with a 54% decrease in brain DHA expressed even less Timp1 after TBI than rats with a 25% decrease in brain DHA. This correlation between brain DHA content and Timp1 expression suggests a possible mechanism for brain DHA-mediated improvement in functional outcomes. Another study, however, reported decreased Timp1 mRNA and protein expression in the thalamus 12–72 hrs after weight drop injury to the parietal cortex of PND7 rats [64]. This difference in Timp1 response after injury may be due to differences in age at the time of injury, injury model, and/or tissue examined. The relationship between Timp1 mRNA level and brain DHA content suggests a potential role for Timp1 that must be examined in future experiments.
Matrix metalloproteinases-2 and −9 are proteases that are rapidly induced after TBI and other neuroinflammatory conditions [65, 66]. Unlike Mmp9, Mmp2 is constitutively expressed and is primarily regulated at the level of enzyme-activation [67, 68], in agreement with our measured mRNA levels after TBI. Early after injury, MMPs initiate apoptotic cell death and degrade the extracellular matrix, contributing to opening the blood brain barrier and facilitating vasogenic edema [66]. Consistent with the present findings, peak expression and activity of MMPs occurs approximately 24 hours after juvenile TBI [64].
Tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) is one of a family of four endogenous MMP inhibitors [69]. All four TIMP family members are expressed in the brain [70]. TIMP-1, which has the broadest substrate specificity, inhibits MMPs in a 1:1 ratio by binding to the MMP active site [71]. TIMP-1 also has anti-apoptotic and growth factor properties that are independent of its MMP-inhibiting ability [72–74], and are thought to occur through interactions with its cell surface receptor, CD63 [68]. In this study, the decreased induction of Timp1 expression after TBI in rats with either a 25% or 54% decrease in brain DHA, which would be anticipated to lead to decreased levels of TIMP-1 protein, was not associated with altered MMP-2 or MMP-9 enzymatic activity (Figure 6). Accordingly, it may be hypothesized that the anti-apoptotic and/or growth factor properties of TIMP-1, rather than inhibition of MMP activity, may provide neuroprotection after TBI in rats with sufficient levels of brain DHA, and that the decreased Timp1 expression observed in rats with decreased brain DHA is contributing to worsened functional outcomes.
Conclusions
Diet-induced decreases in brain DHA content resulted in worsened sensorimotor outcomes after TBI compared to rats with adequate levels of brain DHA. The poorest long-term function was observed in rats with the greatest decrease in brain DHA, suggesting that brain DHA level, rather than dietary n-3 PUFA content, is of greatest importance in influencing the ultimate outcomes after TBI. Timp1 mRNA level after TBI correlated with brain DHA content, suggesting that lower Timp1 expression, as a result of decreased brain DHA, contributes to poorer sensorimotor outcomes, most likely through its anti-apoptotic and/or growth factor activities. Thus, the present data indicate a novel mechanism by which PUFAs modulate a response to neural injury, in addition to serving as a precursor for many anti-inflammatory molecules [13]. Furthermore, these data suggest that diet regulates brain development and the ability of the brain to respond to injury. Therefore, young children may be provided with greater protection against the deleterious effects of TBI by ensuring optimal accretion and maintenance of brain DHA levels throu
ACKNOWLEDGMENTS
The authors thank Andrew Ralya, Ph.D. and Patrick Gregg for technical assistance. Supported by NIH R03 MH059939 (BL), P30 HD02528 (BL, NEJB), P20 RR016475 (BL), T32 ES007079 (KLR), T32 HD007523 (KLR), and the University of Kansas Medical Center Biomedical Research Training Program (KLR)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kristin L. Russell, Email: krussell@kumc.edu.
Nancy E. J. Berman, Email: nberman@kumc.edu.
Beth Levant, Email: blevant@kumc.edu.
REFERENCES
- 1.Faul M, Xu L, Wald M, Coronado V. Traumatic Brain Injury in the United States:Emergency Department visits, Hospitalizations and Deaths 2002–2006, Atlanta(GA): Centers for Disease Control and Prevention. National Center for Injury Prevention and Control. 2010 [Google Scholar]
- 2.Anderson V, Brown S, Newitt H, Hoile H. Long-term outcome from childhood traumatic brain injury: intellectual ability, personality and quality of life, Neuropsychology. 2011;25:176–184. doi: 10.1037/a0021217. [DOI] [PubMed] [Google Scholar]
- 3.Davis AS, Dean RS. Assessing sensory-motor deficits in pediatric traumatic brain injury. Appl Neuropsychol. 2010;17:104–109. doi: 10.1080/09084281003708951. [DOI] [PubMed] [Google Scholar]
- 4.Ylvisaker M, Todis B, Glang A, Urbanczyk B, Franklin C, DePompei R, Feeney T, Maxwell NM, Pearson S, Tyler JS. Educating students with TBI: themes and recommendations. J Head Trauma Rehabil. 2001;16:76–93. doi: 10.1097/00001199-200102000-00009. [DOI] [PubMed] [Google Scholar]
- 5.National Institute of Neurologic Disorders and Stroke. Traumatic Brain Injury: Hope Through Research, in, National Institutes of Health, Bethesda, MD. 2002 [Google Scholar]
- 6.Pinto PS, Poretti A, Meoded A, Tekes A, Huisman TA. The unique features of traumatic brain injury in childern. Review of the characteristics of the pediatric skull and brain, mechanisms of trauma, patterns of injury, complications and their imaging findings--part 1. J Neuroimaging. 2012;22:e1–e17. doi: 10.1111/j.1552-6569.2011.00688.x. [DOI] [PubMed] [Google Scholar]
- 7.Maxwell WL. Traumatic brain injury in the neonate, child and adolescent human: an overview of pathology. Int J Dev Neurosci. 2012;30:167–183. doi: 10.1016/j.ijdevneu.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Pinto PS, Meoded A, Poretti A, Tekes A, Huisman TA. The unique features of traumatic brain injury in childern. review of the characteristics of the pediatric skull and brain, mechanisms of trauma, patterns of injury, complications and their imaging findings--part 2. J Neuroimaging. 2012;22:e18–e41. doi: 10.1111/j.1552-6569.2011.00690.x. [DOI] [PubMed] [Google Scholar]
- 9.Prins ML, Hovda DA. Developing experimental models to address traumatic brain injury in children. Journal of neurotrauma. 2003;20:123–137. doi: 10.1089/08977150360547053. [DOI] [PubMed] [Google Scholar]
- 10.Salem N, Jr, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system Lipids. 2001;36:945–959. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- 11.Shaikh SR. Biophysical and biochemical mechanisms by which dietary N-3 polyunsaturated fatty acids from fish oil disrupt membrane lipid rafts. The Journal of nutritional biochemistry. 2012;23:101–105. doi: 10.1016/j.jnutbio.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan SA, Vanden Heuvel JP. Role of nuclear receptors in the regulation of gene expression by dietary fatty acids (review) The Journal of nutritional biochemistry. 2003;14:554–567. doi: 10.1016/s0955-2863(03)00098-6. [DOI] [PubMed] [Google Scholar]
- 13.Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol. 2008;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. The Journal of experimental medicine. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual antiinflammatory and pro-resolution lipid mediators, Nature reviews. Immunology. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinclair AJ. Long-chain polyunsaturated fatty acids in the mammalian brain. Proc Nutr Soc. 1975;34:287–291. doi: 10.1079/pns19750051. [DOI] [PubMed] [Google Scholar]
- 17.Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Intrauterine fatty acid accretion rates in human brain: implications for fatty acid requirements. Early Hum Dev. 1980;4:121–129. doi: 10.1016/0378-3782(80)90015-8. [DOI] [PubMed] [Google Scholar]
- 18.Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Extrauterine fatty acid accretion in infant brain: implications for fatty acid requirements. Early Hum Dev. 1980;4:131–138. doi: 10.1016/0378-3782(80)90016-x. [DOI] [PubMed] [Google Scholar]
- 19.Simopoulos AP. Evolutionary aspects of diet: the omega-6/omega-3 ratio and the brain. Mol Neurobiol. 2011;44:203–215. doi: 10.1007/s12035-010-8162-0. [DOI] [PubMed] [Google Scholar]
- 20.Gordon N. Nutrition and cognitive function. Brain & development. 1997;19:165–170. doi: 10.1016/s0387-7604(96)00560-8. [DOI] [PubMed] [Google Scholar]
- 21.McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain development function: potential implications for the pathogenesis and prevention of psychopathology, Prostaglandins, leukotrienes, and essential fatty acids. 2006;75:329–349. doi: 10.1016/j.plefa.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Salem N, Jr, Moriguchi T, Greiner RS, McBride K, Ahmad A, Catalan JN, Slotnick B. Alterations in brain function after loss of docosahexaenoate due to dietary restriction of n-3 fatty acids. J Mol Neurosci. 2001;16:299–307. doi: 10.1385/JMN:16:2-3:299. discussion 317-221. [DOI] [PubMed] [Google Scholar]
- 23.Orr SK, Trepanier MO, Bazinet RP. n-3 Polyunsaturated fatty acids in animal models with neuroinflammation, Prostaglandins, leukotrienes, and essential fatty acids. 2012 doi: 10.1016/j.plefa.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Javierre C, Vidal J, Segura R, Lizarraga MA, Medina J, Ventura JL. The effect of supplementation with n-3 fatty acids on the physical performance in subjects with spinal cord injury. J Physiol Biochem. 2006;62:271–279. doi: 10.1007/BF03165756. [DOI] [PubMed] [Google Scholar]
- 25.Pan HC, Kao TK, Ou YC, Yang DY, Yen YJ, Wang CC, Chuang YH, Liao SL, Raung SL, Wu CW, Chiang AN, Chen CJ. Protective effect of docosahexaenoic acid against brain injury in ischemic rats. The Journal of nutritional biochemistry. 2009;20:715–725. doi: 10.1016/j.jnutbio.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Huang WL, King VR, Curran OE, Dyall SC, Ward RE, Lal N, Priestley JV, Michael-Titus AT. A combination of intravenous and dietary docosahexaenoic acid significantly improves outcome after spinal cord injury. Brain : a journal of neurology. 2007;130:3004–3019. doi: 10.1093/brain/awm223. [DOI] [PubMed] [Google Scholar]
- 27.Bailes JE, Mills JD. Docosahexaenoic acid reduces traumatic axonal injury in a rodent head injury model. Journal of neurotrauma. 2010;27:1617–1624. doi: 10.1089/neu.2009.1239. [DOI] [PubMed] [Google Scholar]
- 28.Wu A, Ying Z, Gomez-Pinilla F. Omega-3 fatty acids supplementation restores mechanisms that maintain brain homeostasis in traumatic brain injury. Journal of neurotrauma. 2007;24:1587–1595. doi: 10.1089/neu.2007.0313. [DOI] [PubMed] [Google Scholar]
- 29.Yang DY, Pan HC, Yen YJ, Wang CC, Chuang YH, Chen SY, Lin SY, Liao SL, Raung SL, Wu CW, Chou MC, Chiang AN, Chen CJ. Detrimental effects of post-treatment with fatty acids on brain injury in ischemic rats. Neurotoxicology. 2007;28:1220–1229. doi: 10.1016/j.neuro.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Homayoun P, Parkins NE, Soblosky J, Carey ME, Rodriguez de, Turco EB, Bazan NG. Cortical impact injury in rats promotes a rapid and sustained increase in polyunsaturated free fatty acids and diacylglycerols. Neurochem Res. 2000;25:269–276. doi: 10.1023/a:1007583806138. [DOI] [PubMed] [Google Scholar]
- 31.Levant B, Ozias MK, Jones KA, Carlson SE. Differential effects of modulation of docosahexaenoic acid content during development in specific regions of rat brain. Lipids. 2006;41:407–414. doi: 10.1007/s11745-006-5114-6. [DOI] [PubMed] [Google Scholar]
- 32.Ozias MK, Carlson SE, Levant B. Maternal parity and diet (n-3) polyunsaturated fatty acid concentration influence accretion of brain phospholipid docosahexaenoic acid in developing rats. The Journal of nutrition. 2007;137:125–129. doi: 10.1093/jn/137.1.125. [DOI] [PubMed] [Google Scholar]
- 33.Favreliere S, Barrier L, Durand G, Chalon S, Tallineau C. Chronic dietary n-3 polyunsaturated fatty acids deficiency affects the fatty acid composition of plasmenylethanolamine and phosphatidylethanolamine differently in rat frontal cortex, striatum, and cerebellum, Lipids. 1998;33:401–407. doi: 10.1007/s11745-998-0221-y. [DOI] [PubMed] [Google Scholar]
- 34.Levant B, Zarcone TJ, Fowler SC. Developmental effects of dietary n-3 fatty acids on activity and response to novelty. Physiology & behavior. 2010;101:176–183. doi: 10.1016/j.physbeh.2010.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wainwright PE. Issues of design and analysis relating to the use of multiparous species in developmental nutritional studies. The Journal of nutrition. 1998;128:661–663. doi: 10.1093/jn/128.3.661. [DOI] [PubMed] [Google Scholar]
- 36.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. The Journal of nutrition. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 37.Onyszchuk G, Al-Hafez B, He YY, Bilgen M, Berman NE, W.M.Brooks A. mouse model of sensorimotor controlled cortical impact: characterization using longitudinal magnetic resonance imaging behavioral assessments and histology. J Neurosci Methods. 2007;160:187–196. doi: 10.1016/j.jneumeth.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bilgen M. A new device for experimental modeling of central nervous system injuries. Neurorehabil Neural Repair. 2005;19:219–226. doi: 10.1177/1545968305278635. [DOI] [PubMed] [Google Scholar]
- 39.Narayana PA, Grill RJ, Chacko T, Vang R. Endogenous recovery of injured spinal cord: longitudinal in vivo magnetic resonance imaging. Journal of neuroscience research. 2004;78:749–759. doi: 10.1002/jnr.20275. [DOI] [PubMed] [Google Scholar]
- 40.Sherwood NM, Timiras PS. A Stereotaxic Atlas of the Developing Rat Brain. Berkeley and Los Angeles: University of California Press, Ltd; 1970. [Google Scholar]
- 41.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press, Inc; 1986. [Google Scholar]
- 42.Cole JT, Yarnell A, Kean WS, Gold E, Lewis B, Ren M, McMullen DC, Jacobowitz DM, Pollard HB, O’Neill JT, Grunberg NE, Dalgard CL, Frank JA. Craniotomy:true sham for traumatic brain injury or a sham of a sham? Journal of neurotrauma. 2011;28:359–369. doi: 10.1089/neu.2010.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu JC, Chen KY, Yo YW, Huang SW, Shih HM, Chiu WT, Chiang YH, Shiau CY. Different sham procedures for rats in traumatic brain injury experiments induce corresponding increases in levels of trauma markers. The Journal of surgical research. 2013;179:138–144. doi: 10.1016/j.jss.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 44.Russell KL, Kutchko KM, Fowler SC, Berman NE, Levant B. Sensorimotor behavioral tests for use in a juvenile rat model of traumatic brain injury: assessment of sex differences. J Neurosci Methods. 2011;199:214–222. doi: 10.1016/j.jneumeth.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fowler SC, Birkestrand BR, Chen R, Moss SJ, Vorontsova E, Wang G, Zarcone TJ. A force-plate actometer for quantitating rodent behaviors: illustrative data on locomotion, rotation, spatial patterning, stereotypies, and tremor. J Neurosci Methods. 2001;107:107–124. doi: 10.1016/s0165-0270(01)00359-4. [DOI] [PubMed] [Google Scholar]
- 46.Grossman KJ, Goss CW, Stein DG. Sickness behaviors following medial frontal cortical contusions in male rats. Behavioural brain research. 2011;217:202–208. doi: 10.1016/j.bbr.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 48.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden T. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13:134. doi: 10.1186/1471-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using realtime quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 50.Harris JL, Reeves TM, Phillips LL. Injury modality, survival interval, and sample region are critical determinants of qRT-PCR reference gene selection during long-term recovery from brain trauma. Journal of neurotrauma. 2009;26:1669–1681. doi: 10.1089/neu.2009.0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abramoff MD, Magalhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- 52.Rasband WS. ImageJ,in, National Institutes of Health, Bethesda, MD, USA. 1997–2012 [Google Scholar]
- 53.Coggeshall RE. A consideration of neural counting methods. Trends Neurosci. 1992;15:9–13. doi: 10.1016/0166-2236(92)90339-a. [DOI] [PubMed] [Google Scholar]
- 54.Galli C, Trzeciak H, Paoletti R. Effects of dietary fatty acids on the fatty acid composition of brain ethanolamine phosphoglyceride: reciprocal replacement of n-6 and n-3 polyunsaturated fatty acids. Biochim Biophys Acta. 1971;248:449–454. [Google Scholar]
- 55.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. British journal of anaesthesia. 2007;99:4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 56.Kozul CD, Nomikos AP, Hampton TH, Warnke LA, Gosse JA, Davey JC, Thorpe JE, Jackson BP, Ihnat MA, Hamilton JW. Laboratory diet profoundly alters gene expression and confounds genomic analysis in mouse liver and lung. Chemico-biological interactions. 2008;173:129–140. doi: 10.1016/j.cbi.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 57.Wright LL, Bell RW, Schreiber HL, Villescas R, Conely L. Interactive effects of parity and pup stress on the maternal behavior of Rattus norvegicus. Developmental psychobiology. 1977;10:331–337. doi: 10.1002/dev.420100407. [DOI] [PubMed] [Google Scholar]
- 58.Whishaw IQ, Kolb B. A Handbook with Tests. New York, NY: Oxford University Press; 2005. The Behavior of the Laboratory Rat. [Google Scholar]
- 59.Levant B, Ozias MK, Davis PF, Winter M, Russell KL, Carlson SE, Reed GA, McCarson KE. Decreased brain docosahexaenoic acid content produces neurobiological effects associated with depression: Interactions with reproductive status in female rats. Psychoneuroendocrinology. 2008;33:1279–1292. doi: 10.1016/j.psyneuen.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Levant B, Ozias MK, Carlson SE. Diet (n-3) polyunsaturated fatty acid content and parity interact to alter maternal rat brain phospholipid fatty acid composition. The Journal of nutrition. 2006;136:2236–2242. doi: 10.1093/jn/136.8.2236. [DOI] [PubMed] [Google Scholar]
- 61.Levant B, Radel JD, Carlson SE. Decreased brain docosahexaenoic acid during development alters dopamine-related behaviors in adult rats that are differentially affected by dietary remediation. Behavioural brain research. 2004;152:49–57. doi: 10.1016/j.bbr.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 62.Levant B, Ozias MK, Carlson SE. Sex-specific effects of brain LC-PUFA composition on locomotor activity in rats. Physiology & behavior. 2006;89:196–204. doi: 10.1016/j.physbeh.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 63.Vancassel S, Blondeau C, Lallemand S, Cador M, Linard A, Lavialle M, Dellu-Hagedorn F. Hyperactivity in the rat is associated with spontaneous low level of n-3 polyunsaturated fatty acids in the frontal cortex. Behavioural brain research. 2007;180:119–126. doi: 10.1016/j.bbr.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 64.Sifringer M, Stefovska V, Zentner I, Hansen B, Stepulak A, Knaute C, Marzahn J, Ikonomidou C. The role of matrix metalloproteinases in infant traumatic brain injury. Neurobiology of disease. 2007;25:526–535. doi: 10.1016/j.nbd.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 65.Jia F, Pan YH, Mao Q, Liang YM, Jiang JY. Matrix metalloproteinase-9 expression and protein levels after fluid percussion injury in rats: the effect of injury severity and brain temperature. Journal of neurotrauma. 2010;27:1059–1068. doi: 10.1089/neu.2009.1067. [DOI] [PubMed] [Google Scholar]
- 66.Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158:983–994. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gottschall PE, Deb S. Regulation of matrix metalloproteinase expressions in astrocytes microglia and neurons. Neuroimmunomodulation. 1996;3:69–75. doi: 10.1159/000097229. [DOI] [PubMed] [Google Scholar]
- 68.Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. The Journal of biological chemistry. 1995;270:5331–5338. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- 69.Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochimica et biophysica acta. 2010;1803:55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pagenstecher A, Stalder AK, Kincaid CL, Shapiro SD, Campbell IL. Differential expression of matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase genes in the mouse central nervous system in normal and inflammatory states. The American journal of pathology. 1998;152:729–741. [PMC free article] [PubMed] [Google Scholar]
- 71.Gomis-Ruth FX, Maskos K, Betz M, Bergner A, Huber R, Suzuki K, Yoshida N, Nagase H, Brew K, Bourenkov GP, Bartunik H, Bode W. Mechanism of inhibition of the human matrix metalloproteinase stromelysin-1 by TIMP-1. Nature. 1997;389:77–81. doi: 10.1038/37995. [DOI] [PubMed] [Google Scholar]
- 72.Gardner J, Ghorpade A. Tissue inhibitor of metalloproteinase (TIMP)-1: the TIMPed balance of matrix metalloproteinases in the central nervous system. Journal of neuroscience research. 2003;74:801–806. doi: 10.1002/jnr.10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hayakawa T, Yamashita K, Tanzawa K, Uchijima E, Iwata K. Growth-promoting activity of tissue inhibitor of metalloproteinases-1 (TIMP-1) for a wide range of cells. A possible new growth factor in serum. FEBS letters. 1992;298:29–32. doi: 10.1016/0014-5793(92)80015-9. [DOI] [PubMed] [Google Scholar]
- 74.Jourquin J, Tremblay E, Bernard A, Charton G, Chaillan FA, Marchetti E, Roman FS, Soloway PD, Dive V, Yiotakis A, Khrestchatisky M, Rivera S. Tissue inhibitor of metalloproteinases-1 (TIMP-1) modulates neuronal death, axonal plasticity, and learning and memory. The European journal of neuroscience. 2005;22:2569–2578. doi: 10.1111/j.1460-9568.2005.04426.x. [DOI] [PubMed] [Google Scholar]